Abstract
Hormone replacement therapy and selective estrogen receptor modulators have been controversial treatment options for postmenopausal women because of their potential health benefits and/or risks. In this study, we determine the effects of the hormonally active compounds, conjugated equine estrogens (CEE), medroxyprogesterone acetate (MPA), CEE + MPA, and tamoxifen (TAM) on the myometrium of ovariectomized macaques. Immunoexpression of estrogen receptor-α (ERα), progesterone receptor (PR), and Ki-67 in the myometrium is assessed. We found no significant difference in ERα myometrial expression in the CEE, MPA, and CEE + MPA treatment groups, but there was a significant decrease in expression in animals administered TAM versus controls. Conjugated equine estrogen−, TAM−, and CEE + MPA-treated animals had significantly increased expression of PR in myometrial cells and there was no difference in PR expression in cells from MPA-treated animals versus control animals. Myometrial cell proliferation did not significantly differ between the controls and any of the treatment groups, although normalized Ki-67 values were somewhat higher in the CEE and TAM groups. These data suggest that ERα and PR expression in the myometrium is influenced by treatment with hormonally active agents.
Keywords: myometrium, macaques, conjugated equine estrogens (CEE), medroxyprogesterone acetate (MPA), tamoxifen (TAM), steroid receptors, Ki-67
Introduction
Many women rely on hormone replacement therapy (HRT) to alleviate symptoms associated with menopause. There is controversy as to whether estrogenic compounds alone or in conjunction with progestins in HRT contribute to cancer of the endometrium (Lacey et al. 2005) or breast (U.S. Preventive Services Task Force 2005). Also of interest is the effect of tamoxifen (TAM), a synthetic antiestrogenic drug used to treat breast cancer, on the endometrium and myometrium. It has been documented that TAM acts as a partial estrogen agonist on the endometrium and its use may increase the proliferation of endometrial glands, often resulting in endometrial cancer (Bergman et al. 2000; Gielen et al. 2005; Ismail 1994; Kennedy et al. 1999).
The myometrium is the uterine muscular layer and the site of origin for fibroids (uterine leiomyomas), the most common gynecological tumor of women in the United States (Flake et al. 2003). It is generally speculated that ovarian hormones play a role in fibroid development, as this benign neoplasm reportedly occurs after menarche (Marshall et al. 1998), may enlarge during pregnancy (Buttram and Reiter 1981), and often regresses following menopause in women who do not take HRT (Flake et al. 2003; Polatti et al. 2000). There is some debate as to whether treatment with HRT (Polatti et al. 2000; Ylostalo et al. 1996) or TAM (Ugwumadu and Harding 1994) affects the growth of existing fibroids in postmenopausal women or if they may, in fact, be responsible for their hormone-dependent development.
The structure and function of the uterus are highly influenced by the ovarian hormones estrogen and progesterone, which mediate much of their actions directly via specific estrogen receptors, ERα and β, (Kuiper et al. 1997) and progesterone receptors (PR) A and B, (Graham and Clarke 1997), respectively. Estrogen receptors and PR mainly control myometrial function by regulating estrogen- and progesterone-responsive genes, respectively, that are responsible for uterine smooth muscle cell growth, maintenance of gestation, and preparation of the uterus for labor and birth (Hertelendy and Zakar 2004). The significance of exogenous hormones on these specific receptors in the myometrium of postmenopausal women who take long-term HRT or TAM is poorly defined. However, there have been reports on the effects of hormonally active substances on ER and/or PR expression in the breast (Cline et al. 1998; Isaksson et al. 2003; Wood et al. 2004) and endometrium of ovariectomized macaques (Cline et al. 2001; Wang et al. 2002; Wang et al. 2003; Wood et al. 2004), and more recently in the uterus and vagina of postmenopausal women treated for twenty-one days with various hormone therapies (Hanifi-Moghaddam et al. 2008).
The ovariectomized macaque is an excellent animal model for studying reproductive health concerns in postmenopausal women, as the macaque is very similar to humans in regard to female reproductive biology. Endometrial hyperplasia/polyps (Baskin et al. 2002) and breast cancer (Uno 1997) have been identified in these animals. The focus of this work was to assess ERα and PR expression and myometrial cell proliferation in the ovariectomized macaque myometrium following long-term administration of conjugated equine estrogens (CEE), medroxyprogesterone acetate (MPA), CEE + MPA, and TAM.
Materials and Methods
Animals and Treatments
In a previously described study (Cline et al. 1998), 115 adult female cynomolgus macaques (Macaca fascicularis) were imported from Indonesia to the United States. Bilateral ovariectomies were performed on all animals three months prior to treatment with hormonally active agents. The numbers of animals per treatment group were: CEE (n = 25), MPA (n = 19), CEE + MPA (n = 26), and TAM (n = 25). The control ovariectomized (OVX) group consisted of untreated animals (n = 20). The test compounds were administered daily for thirty-six months in the diet at doses equivalent on a caloric basis to 0.625 mg/woman for CEE, 2.5 mg/woman for MPA, and 20 mg/woman for TAM. Animals were housed in social groups of four to six macaques in a facility accredited by the Association for Assessment and Accreditation of Laboratory Animal Care, and the experimental protocols were approved by the Wake Forest University School of Medicine Animal Care and Use Committee. Animals in this study were part of a randomized long-term trial of hormonal therapies in aging females, including assessments of multiple organ systems; the main outcome was cardiovascular disease (Adams et al. 1997; Williams et al. 1997).
Tissue Collection and Processing
At the end of treatment, animals were euthanized by intravenous pentobarbital injection. Uteri were removed, sectioned, immersion-fixed in 4% paraformaldehyde, transferred after twenty-four hours to 70% ethanol, and processed in paraffin for histology. Five-micron–thick sections were cut, and the tissues were either stained with hematoxylin and eosin (H&E) for routine histopathology or used for immunohistochemistry.
Immunohistochemistry
Uterine sections were stained using a biotin–streptavidin staining method for the nuclear antigens, ERα, PR, and Ki-67. The primary mouse monoclonal antibodies NCL-ER-LH2 (Novacastra, Newcastle-upon-Tyne, UK) and NCL-PGR (Novacastra) were used for detection of ERα and PR (A and B proteins), respectively, and NCL-Ki-67 MM1 (Novacastra) was used to detect proliferating myometrial cells. The staining procedure used a streptavidin–biotin–alkaline phosphatase method modified for antigen retrieval from paraffin-embedded tissue by the method described by Cattoretti et al. (1992). The chromogen used was VectorRed (Vector Laboratories, Burlingame, CA), and the immunostained sections were counterstained with hematoxylin. Staining was done in batches of twenty sections. Animals from different treatment groups were randomly placed within each staining batch. Each batch included known positive and negative controls, which were used to accept or reject the batch. All immunostaining was performed using strict standard operating procedures for all procedural steps including antigen retrieval, antibody dilution, incubation time, and chromogen development to minimize uncontrolled variation.
Steroid Hormone Receptor Quickscores
Immunohistochemical evaluation of ERα- and PR-labeled myometrial cells were determined using light microscopy (20× objective) in a blinded manner. All myometrial cells per uterine section were evaluated. A semiquantitative assessment of the immunolabeling was done using a modified quickscore grading method (Detre et al. 1995) as previously described in the myometrium (Dixon et al. 2000). Briefly, the quickscore was determined based on the intensity of the nuclear antibody label (0 = no staining; 1 = weak staining; 2 = intermediate staining; and 3 = strong staining) multiplied by the percentage of myometrial cell nuclei labeling positive for the particular hormone receptor (1 = 0–4%; 2 = 5–19%; 3 = 20–39%; 4 = 40–59%; 5 = 60–79%; and 6 = 80–100%). Estrogen receptor-α and PR quickscores were determined for each section of myometrium available per animal. For those animals in which there were two uterine sections, a mean quickscore was obtained. Nonspecific staining for ERα and PR antibody was occasionally present in the cytoplasm of some myometrial cells from animals in the control and treatment groups. This background staining was not included in the quickscore assessment. Following the evaluation, the quickscores were arranged per treatment group for statistical analysis.
Ki-67
Owing to variations in myometria size per animal, a measurement of the myometrium was obtained using ImageJ (ImageJ v. 1.33u) public domain software. Ki-67-labeled slides were cleaned with an isopropanol solution and then scanned using the Aperio Scanscope T2 Scanner (Aperio Technologies, Inc., Vista, CA), which uses line scanning technology to capture high resolution, seamless digital images of glass slides. After scanning, the slides were viewed using Aperio Imagescope v. 6.25.0.1117 (Aperio Technologies), a digital slide-viewing program. Aperio Imagescope captured images of each uterine section. In a few instances, images were not available for scanning, as the section of uterus was too thick or there was interference by the coverslip. The numbers of animals per group for the Ki-67 evaluation were: CEE, n = 22; MPA, n = 17; CEE + MPA, n = 24; TAM, n = 23; and OVX (n = 20).
Area measurements (mm2) of the myometria were taken using the freehand selection tool to trace the myometrium from the adjacent endometrium. Measurements were calibrated by drawing a straight line across a region of the uterus that corresponded to a known distance using a scale bar.
The entire section of myometrium for each animal was evaluated histologically to determine whether myometrial cell proliferation was present in any treatment group. Ki-67–positive myometrial cell nuclei were assessed using light microscopy (40× objective) in a blinded manner. The number of Ki-67–positive myometrial cell nuclei per animal was recorded. For animals in which there were two uterine sections, an average of the number of Ki-67–positive myometrial cell nuclei and an average of the size of the myometrium was used. Following the evaluation, the uterine measurements and number of Ki-67–positive nuclei were arranged per treatment group for statistical analysis.
Statistical Analysis
For ERα and PR quickscore comparisons of myometrial cell expression, the Mann-Whitney U test with a two-sided p-value was used to compare the mean quickscore for the treatment group versus control group for each hormone receptor.
For evaluating cell proliferation in the myometrium, the number of Ki-67–positive myometrial cell nuclei was divided by the measurement of the myometrium to get a normalized value. The Mann-Whitney U test with a two-sided p-value was used to compare the normalized value means for each treatment group to the control group.
Results
Histology
All H&E–stained myometria were within normal limits for all groups and characterized by smooth muscle layers composed of interlacing bundles of slender to plump myometrial cells with elongated nuclei surrounding prominent vasculature and supported by dense connective tissue. There was no evidence of hyperplasia or neoplasia in any of the myometrial sections examined.
Steroid Hormone Receptor Immunohistochemistry
ERα
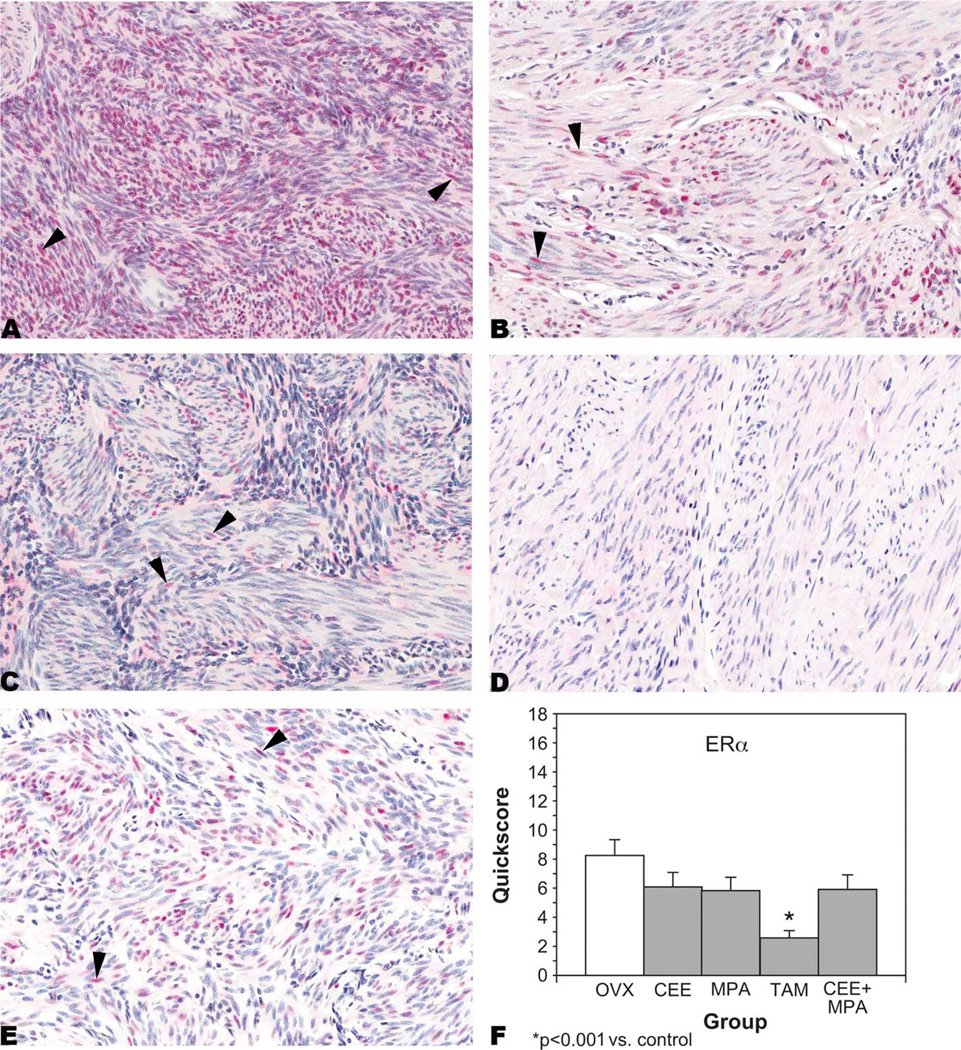
ERα was expressed in the nuclei of myometrial cells from OVX-control (Figure 1A); CEE-treated (Figure 1B); MPA-treated (Figure 1C); TAM-treated (Figure 1D); and CEE + MPA-treated (Figure 1E) animals. Myometrial ERα expression was significantly (p < .001) decreased in the TAM-treated group compared to the control group based on the mean quickscores (Figure 1F). Myometrial ERα expression was also lower in the other treatment groups compared to the control group, but these differences were not statistically significant.
Figure 1.
Estrogen receptor-α (ER-α). Immunohistochemistry. Nuclear staining (arrowheads) in myometrial cells from (A) an ovariectomized (OVX)-control animal, quickscore = 15; (B) a conjugated equine estrogens (CEE)-treated animal, quickscore = 6; (C) a medroxyprogesterone acetate–treated animal, quickscore = 4; (D) a tamoxifen (TAM)-treated animal, quickscore = 0; and (E) a CEE + MPA-treated animal, quickscore = 6. Quickscore graph (F). Note the high nuclear expression of ER-α in OVX-control animals when compared to all treatment groups and the significantly decreased expression of ER-α in myometrial cells from TAM-treated animals.
PR
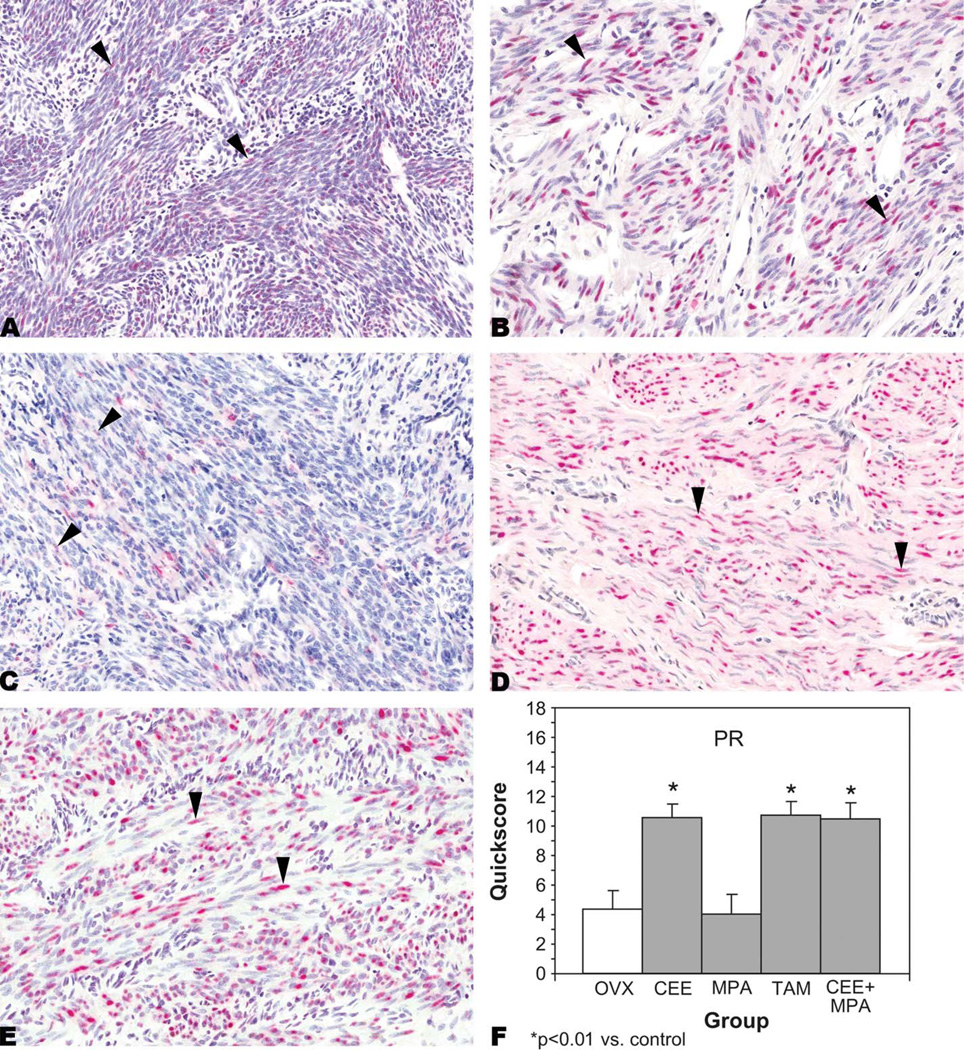
PR was expressed in the nuclei of myometrial cells from OVX-control (Figure 2A); CEE-treated (Figure 2B); MPA-treated (Figure 2C); TAM-treated (Figure 2D); and CEE + MPA-treated (Figure 2E) animals. There were statistically significant (p < .01) increases in expression of PR in myometrial cells from CEE−, TAM−, and CEE + MPA-treated animals when compared to controls (Figure 2F). However, this increase was not observed in MPA-treated animals (Figure 2F).
Figure 2.
Progesterone receptor (PR). Immunohistochemistry. Nuclear staining (arrowheads) in myometrial cells from (A) an ovariectomized (OVX)-control animal, quickscore = 6; (B) a conjugated equine estrogens (CEE)–treated animal, quickscore = 15; (C) a medroxyprogesterone acetate (MPA)–treated animal, quickscore = 4; (D) a tamoxifen (TAM)-treated animal, quickscore = 15; and (E) a CEE + MPA–treated animal, quickscore = 15. Quickscore graph (F). Note significantly increased expression of PR in myometrial cells from CEE−, TAM, and CEE + MPA–treated animals when compared to OVX-controls.
Cell Proliferation Marker Immunohistochemistry
Ki-67
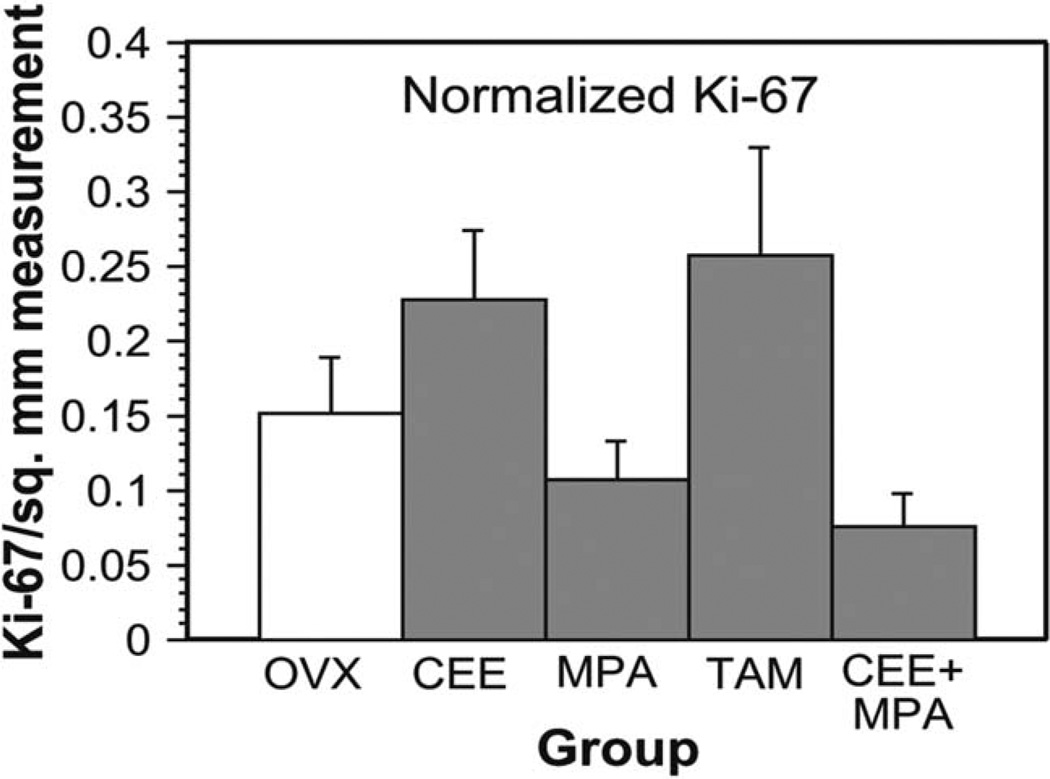
There were few Ki-67–positive myometrial cell nuclei in uterine sections from control (OVX) and treated (CEE, MPA, TAM, and CEE + MPA) animals. Ki-67–positive cells were randomly scattered throughout the myometrium and not localized to any particular region. Though not statistically significant at p < .05, normalized Ki-67 values in CEE-treated and TAM-treated animals were higher than in controls and normalized Ki-67 values in MPA–treated and CEE + MPA-treated animals were lower than in controls (Figure 3).
Figure 3.
Ki-67. Myometrial cell proliferation as represented by normalized Ki-67 values in ovariectomized (OVX)-control animals and animals administered conjugated equine estrogens (CEE), medroxyprogesterone acetate (MPA), tamoxifen (TAM), CEE + MPA.
Discussion
In this study, the expression of the steroid hormone receptors, ERα and PR, and of a marker of proliferation Ki-67 was assessed in the myometrium of ovariectomized macaques following administration of hormonally active compounds similar to those used in HRT (CEE, MPA, or CEE + MPA) or for treatment of breast cancer (TAM) in women. ERβ was not evaluated owing to its reported low expression levels in the nonpregnant myometrium compared to ERα (Andersen 2000). Our results demonstrated that TAM significantly decreased ERα expression in the myometrium when compared to controls, although lower immunoexpression patterns of ERα were observed in the myometrium of animals in the other treatment groups. These data also indicate that ERα is constitutively expressed in the absence of ovarian hormonal stimulation. This result correlates to previous findings in which ERα was immunolocalized in myometrial cell nuclei of control immature OVX Sprague-Dawley rats (Nephew et al. 2000) and in sexually mature control OVX inbred Wistar-derived rats (Varayoud et al. 2005). In addition, constitutive ERα expression was observed in the myometrium of postmenopausal women receiving no hormonal treatment (Hanifi-Moghaddam et al. 2008).
Estrogen receptor-α quickscore means were decreased in animals treated with CEE (an estrogen receptor agonist), MPA (a PR agonist), and CEE + MPA, although not significantly from controls in our study. These results differ from those of postmenopausal women in which increased expression of myometrial ERα was observed following one-dose administration of estradiol dipropionate (Sakaguchi et al. 2003). We hypothesize that this difference may be associated with lower endogenous estrogen concentrations in OVX macaques compared to varying levels of estrogen in postmenopausal women; however, it may also be related to the single dose of estradiol dipropionate given to the postmenopausal women as opposed to daily hormone administration in the macaques for three years.
Progesterone derivatives such as levonorgestrel and MPA have been found to downregulate ERα in the endometrial glands and stroma of women with endometrial hyperplasia (Vereide et al. 2006). Similarly, OVX rats administered progesterone alone demonstrated downregulation of ERα in the luminal epithelium of the endometrium and in the myometrium (Varayoud et al. 2005). The combination of 17-β estradiol (E2) and progesterone in these OVX rats slightly downregulated myometrial ERα protein expression. We report a similar finding, as there was slight downregulation of ERα in the myometrium of macaques that received CEE + MPA. This result supports the theory that ERα expression is cell-type specific (Flouriot et al. 1998) and most likely species specific (Curtis et al. 1997).
In this study, TAM significantly decreased the expression of ERα in the macaque myometrium. TAM is known to function as a partial estrogen agonist in human endometrium (Daniel et al. 1996) and as an estrogen antagonist in the breast (Deroo and Korach 2006). Our findings show that TAM can bind to ERα and downregulate it, suggesting that TAM may be a potential estrogen agonist in the myometrium of OVX macaques. These findings may help explain the reported cases of uterine leiomyosarcoma development in women treated for breast cancer with TAM (Botsis et al. 2006; Deligdisch 2000; McCluggage et al. 1996), as endometrial malignancies in this population of women have been well documented (Cohen et al. 1999; Swerdlow and Jones 2005).
Progesterone receptor expression in the myometrium of animals treated with CEE, TAM, and CEE + MPA was significantly increased when compared to control animals in this study. This finding indicates that PR expression is most likely estrogen induced in animals treated with these compounds. It is well known that E2 can upregulate PR in human breast cancer cells (Eckert and Katzenellenbogen 1982) and that CEE administration increases PR expression in the mammary gland of ovariectomized macaques (Cline et al. 1998). Also, E2 has also been shown to increase progestin binding in the myometrium of immature rats (Ennis and Stumpf 1988). Treatment with E2 increased PR expression in rat myometrial cells (Sahlin et al. 2006). In our study, MPA administration did not alter PR expression in the myometrium when compared to PR expression in OVX controls.
Results from our study show that myometrial cell proliferation was not significantly enhanced by treatment with hormonally active agents. There were higher normalized Ki-67 values in the myometria of CEE-treated and TAM-treated animals when compared to controls. Conversely, there were decreased normalized Ki-67 values from MPA and CEE + MPA-treated animals when compared to controls. Myometrial cell proliferation has been reported to occur in postmenopausal women receiving E2, and E2 + MPA treatments for twenty-one days (Hanifi-Moghaddam et al. 2008). Similarly, in our study, normalized Ki-67 values were increased in macaques receiving CEE. However, these values were decreased in animals receiving both estrogen and progesterone-like compounds (CEE + MPA), indicating a potential difference in macaque and human myometrial cell proliferation sensitivity to various hormones. Alternatively, decreased proliferative activity observed in the myometrium following treatment with MPA alone or in combination with CEE may provide insight into a mechanism for decreased endometrial cancer observed in women on combined oral contraceptives (Kiley and Hammond 2007).
Our findings suggest that ovarian hormone receptors in the myometrium are independently influenced by hormonally active compounds used in HRT or to treat breast cancer, as evidenced by the varying expressions of ERα and PR in myometrial cells compared to reported responses in the endometrial compartment (Cline et al. 2001; Habiba et al. 2000; Mourits et al. 2002). The responses of the uterus to HRT and TAM appear to be cell type specific. The effects of these agents on the macaque myometrium may provide useful information concerning potential myometrial pathology in postmenopausal women who consume these compounds.
Acknowledgments
The authors would like to thank Drs. Mark Hoenerhoff and Arun Pandiri for their critical review of this manuscript, and Ms. Beth Mahler for her expert assistance with imaging. This study was supported in part by the Intramural Research Program of the NIEHS, and Dr. Hill’s work was supported by NIEHS contract #N01-ES-55548. In addition, this study was supported in part by National Heart Lung and Blood Institute grants, HL490852 and HL45666.
Abbreviations
- CEE
conjugated equine estrogens
- E2
17-β estradiol
- ER
estrogen receptor
- ERα
estrogen receptor-α
- ERβ
estrogen receptor-β
- H&E
hematoxylin and eosin
- HRT
hormone replacement therapy
- MPA
medroxyprogesterone acetate
- OVX
ovariectomized
- PR
progesterone receptor
- TAM
tamoxifen
References
- Adams MR, Register TC, Golden DL, Wagner JD, Williams JK. Medroxyprogesterone acetate antagonizes inhibitory effects of conjugated equine estrogens on coronary artery atherosclerosis. Arterioscler Thromb Vasc Biol. 1997;17:217–221. doi: 10.1161/01.atv.17.1.217. [DOI] [PubMed] [Google Scholar]
- Andersen J. Comparing regulation of the connexin43 gene by estrogen in uterine leiomyoma and pregnancy myometrium. Environ Health Perspect. 2000;108:811–815. [PubMed] [Google Scholar]
- Baskin GB, Smith SM, Marx PA. Endometrial hyperplasia, polyps, and adenomyosis associated with unopposed estrogen in rhesus monkeys (Macaca mulatta) Vet Pathol. 2002;39:572–575. doi: 10.1354/vp.39-5-572. [DOI] [PubMed] [Google Scholar]
- Bergman L, Beelen ML, Gallee MP, Hollema H, Benraadt J, van Leeuwen FE. Risk and prognosis of endometrial cancer after tamoxifen for breast cancer. Comprehensive Cancer Centres’ ALERT Group. Assessment of liver and endometrial cancer risk following tamoxifen. Lancet. 2000;356:881–887. doi: 10.1016/s0140-6736(00)02677-5. [DOI] [PubMed] [Google Scholar]
- Botsis D, Koliopoulos C, Kondi-Pafitis A, Creatsas G. Myxoid leiomyosarcoma of the uterus in a patient receiving tamoxifen therapy: A case report. Int J Gynecol Pathol. 2006;25:173–175. doi: 10.1097/01.pgp.0000185407.93308.ce. [DOI] [PubMed] [Google Scholar]
- Buttram VC, Reiter RC. Uterine leiomyomata: Etiology, symptomatology, and management. Fertil Steril. 1981;36:443–445. doi: 10.1016/s0015-0282(16)45789-4. [DOI] [PubMed] [Google Scholar]
- Cattoretti G, Becker MH, Key G, Duchrow M, Schluter C, Galle J, Gerdes J. Monoclonal antibodies against recombinant parts of the Ki-67 antigen (MIB 1 and MIB 3) detect proliferating cells in microwave-processed formalin-fixed paraffin sections. J Pathol. 1992;168:357–363. doi: 10.1002/path.1711680404. [DOI] [PubMed] [Google Scholar]
- Cline JM, Soderqvist G, Register TC, Williams JK, Adams MR, Von Schoultz B. Assessment of hormonally active agents in the reproductive tract of female nonhuman primates. Toxicol Pathol. 2001;29:84–90. doi: 10.1080/019262301301418883. [DOI] [PubMed] [Google Scholar]
- Cline JM, Soderqvist G, von Schoultz E, Skoog L, von Schoultz B. Effects of conjugated estrogens, medroxyprogesterone acetate, and tamoxifen on the mammary glands of macaques. Breast Cancer Res Treat. 1998;48:221–229. doi: 10.1023/a:1005984932268. [DOI] [PubMed] [Google Scholar]
- Cohen I, Azaria R, Fishman A, Tepper R, Shapira J, Beyth Y. Endometrial cancers in postmenopausal breast cancer patients with tamoxifen treatment. Int J Gynecol Pathol. 1999;18:304–309. doi: 10.1097/00004347-199910000-00003. [DOI] [PubMed] [Google Scholar]
- Curtis SW, Shi H, Teng C, Korach KS. Promoter and species specific differential estrogen-mediated gene transcription in the uterus and cultured cells using structurally altered agonists. J Mol Endocrinol. 1997;18:203–211. doi: 10.1677/jme.0.0180203. [DOI] [PubMed] [Google Scholar]
- Daniel Y, Inbar M, Bar-Am A, Peyser MR, Lessing JB. The effects of tamoxifen treatment on the endometrium. Fertil Steril. 1996;65:1083–1089. doi: 10.1016/s0015-0282(16)58318-6. [DOI] [PubMed] [Google Scholar]
- Deligdisch L. Hormonal pathology of the endometrium. Mod Pathol. 2000;13:285–294. doi: 10.1038/modpathol.3880050. [DOI] [PubMed] [Google Scholar]
- Deroo BJ, Korach KS. Estrogen receptors and human disease. J Clin Invest. 2006;116:561–570. doi: 10.1172/JCI27987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Detre S, Saclani Jotti G, Dowsett M. A “quickscore” method for immunohistochemical semiquantitation: Validation for oestrogen receptor in breast carcinomas. J Clin Pathol. 1995;48:876–878. doi: 10.1136/jcp.48.9.876. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dixon D, He H, Haseman JK. Immunohistochemical localization of growth factors and their receptors in uterine leiomyomas and matched myometrium. Environ Health Perspect. 2000;5:795–802. doi: 10.1289/ehp.00108s5795. [DOI] [PubMed] [Google Scholar]
- Eckert RL, Katzenellenbogen BS. Effects of estrogens and antiestrogens on estrogen receptor dynamics and the induction of progesterone receptor in MCF-7 human breast cancer cells. Cancer Res. 1982;42:139–144. [PubMed] [Google Scholar]
- Ennis BW, Stumpf WE. Differential induction of progestin-binding sites in uterine cell types by estrogen and antiestrogen. Endocrinology. 1988;123:1747–1753. doi: 10.1210/endo-123-4-1747. [DOI] [PubMed] [Google Scholar]
- Flake GP, Andersen J, Dixon D. Etiology and pathogenesis of uterine leiomyomas: A review. Environ Health Perspect. 2003;111:1037–1054. doi: 10.1289/ehp.5787. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Flouriot G, Griffin C, Kenealy M, Sonntag-Buck V, Gannon F. Differentially expressed messenger RNA isoforms of the human estrogen receptor-alpha gene are generated by alternative splicing and promoter usage. Mol Endocrinol. 1998;12:1939–1954. doi: 10.1210/mend.12.12.0209. [DOI] [PubMed] [Google Scholar]
- Gielen SC, Kuhne LC, Ewing PC, Blok LJ, Burger CW. Tamoxifen treatment for breast cancer enforces a distinct gene-expression profile on the human endometrium: An exploratory study. Endocr Relat Cancer. 2005;12:1037–1049. doi: 10.1677/erc.1.01046. [DOI] [PubMed] [Google Scholar]
- Graham JD, Clarke CL. Physiological action of progesterone in target tissues. Endocr Rev. 1997;18:502–519. doi: 10.1210/edrv.18.4.0308. [DOI] [PubMed] [Google Scholar]
- Habiba MA, Bell SC, Al-Azzawi F. The effect of hormone replacement therapy on the immunoreactive concentrations in the endometrium of oestrogen and progesterone receptor, heat shock protein 27, and human β-lactoglobulin. Hum Reprod. 2000;15:36–42. doi: 10.1093/humrep/15.1.36. [DOI] [PubMed] [Google Scholar]
- Hanifi-Moghaddam P, Boers-Sijmons B, Klaassens AH, van Wijk FH, Van Ijcken WF, Van der Spek P, Verheul HA, Kloosterboer HJ, Burger CW, Blok LJ. Difference in signalling between various hormone therapies in endometrium, myometrium and upper part of the vagina. Hum Reprod. 2008;23:298–305. doi: 10.1093/humrep/dem366. [DOI] [PubMed] [Google Scholar]
- Hertelendy F, Zakar T. Regulation of myometrial smooth muscle functions. Curr Pharm Des. 2004;10:2499–2517. doi: 10.2174/1381612043383926. [DOI] [PubMed] [Google Scholar]
- Isaksson E, Wang H, Sahlin L, von Schoultz B, Cline JM, von Schoultz E. Effects of long-term HRT and tamoxifen on the expression of progesterone receptors A and B in breast tissue from surgically postmenopausal cynomolgus macaques. Breast Cancer Res Treat. 2003;79:233–239. doi: 10.1023/a:1023925906199. [DOI] [PubMed] [Google Scholar]
- Ismail MT. A prospective study of a monophasic oral contraceptive containing 30 mcg ethinyl oestradiol and 150 mcg desogestrel (Marvelon) Malays J Reprod Health. 1994;12:43–48. [PubMed] [Google Scholar]
- Kennedy MM, Baigrie CF, Manek S. Tamoxifen and the endometrium: Review of 102 cases and comparison with HRT-related and non-HRT-related endometrial pathology. Int J Gynecol Pathol. 1999;18:130–137. [PubMed] [Google Scholar]
- Kiley J, Hammond C. Combined oral contraceptives: A comprehensive review. Clin Obstet Gynecol. 2007;50:868–877. doi: 10.1097/GRF.0b013e318159c06a. [DOI] [PubMed] [Google Scholar]
- Kuiper GG, Carlsson B, Grandien K, Enmark E, Haggblad J, Nilsson S, Gustafsson JA. Comparison of the ligand binding specificity and transcript tissue distribution of estrogen receptors alpha and beta. Endocrinology. 1997;138:863–870. doi: 10.1210/endo.138.3.4979. [DOI] [PubMed] [Google Scholar]
- Lacey JV, Jr, Brinton LA, Lubin JH, Sherman ME, Schatzkin A, Schairer C. Endometrial carcinoma risks among menopausal estrogen plus progestin and unopposed estrogen users in a cohort of postmenopausal women. Cancer Epidemiol Biomarkers Prev. 2005;14:1724–1731. doi: 10.1158/1055-9965.EPI-05-0111. [DOI] [PubMed] [Google Scholar]
- Marshall LM, Spiegelman D, Goldman MB, Manson JE, Colditz GA, Barbieri RL, Stampfer MJ, Hunter DJ. A prospective study of reproductive factors and oral contraceptive use in relation to the risk of uterine leiomyomata. Fertil Steril. 1998;70:432–439. doi: 10.1016/s0015-0282(98)00208-8. [DOI] [PubMed] [Google Scholar]
- McCluggage WG, Varma M, Weir P, Bharucha H. Uterine leiomyosarcoma in patient receiving tamoxifen therapy. Acta Obstet Gynecol Scand. 1996;75:593–595. doi: 10.3109/00016349609054678. [DOI] [PubMed] [Google Scholar]
- Mourits MJE, Ten Hoor KA, van der Zee AGJ, Willemse PHB, de Vries EGE, Hollema H. The effects of tamoxifen on proliferation and steroid receptor expression in postmenopausal endometrium. J Clin Pathol. 2002;55:514–519. doi: 10.1136/jcp.55.7.514. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nephew KP, Long X, Osborne E, Burke KA, Ahluwalia A, Bigsby RM. Effect of estradiol on estrogen receptor expression in rat uterine cell types. Biol Reprod. 2000;62:168–177. doi: 10.1095/biolreprod62.1.168. [DOI] [PubMed] [Google Scholar]
- Polatti F, Viazzo F, Colleoni R, Nappi RE. Uterine myoma in postmenopause: A comparison between two therapeutic schedules of HRT. Maturitas. 2000;37:27–32. doi: 10.1016/s0378-5122(00)00159-6. [DOI] [PubMed] [Google Scholar]
- Sahlin L, Masironi B, Akerberg S, Eriksson H. Tissue- and hormone-dependent progesterone receptor distribution in the rat uterus. Reprod Biol Endocrinol. 2006;4:47. doi: 10.1186/1477-7827-4-47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sakaguchi H, Fujimoto J, Aoki I, Tamaya T. Expression of estrogen receptor alpha and beta in myometrium of premenopausal and postmenopausal women. Steroids. 2003;68:11–19. doi: 10.1016/s0039-128x(02)00111-3. [DOI] [PubMed] [Google Scholar]
- Swerdlow AJ, Jones ME. Tamoxifen treatment for breast cancer and risk of endometrial cancer: A case-control study. J Natl Cancer Inst. 2005;97:375–384. doi: 10.1093/jnci/dji057. [DOI] [PubMed] [Google Scholar]
- Ugwumadu AH, Harding K. Uterine leiomyomata and endometrial proliferation in postmenopausal women treated with the anti-oestrogen tamoxifen. Eur J Obstet Gynecol Reprod Biol. 1994;54:153–156. doi: 10.1016/0028-2243(94)90258-5. [DOI] [PubMed] [Google Scholar]
- Uno H. Age-related pathology and biosenescent markers in captive rhesus macaques. Age. 1997;20:1–13. doi: 10.1007/s11357-997-0001-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- U.S. Preventive Services Task Force. Hormone therapy for the prevention of chronic conditions in postmenopausal women: Recommendations from the U. S. Preventive Services Task Force. Ann Intern Med. 2005;142:855–860. [PubMed] [Google Scholar]
- Varayoud J, Ramos JG, Monje L, Bosquiazzo V, Munoz-de-Toro M, Luque EH. The estrogen receptor alpha sigma3 mRNA splicing variant is differentially regulated by estrogen and progesterone in the rat uterus. J Endocrinol. 2005;186:51–60. doi: 10.1677/joe.1.06099. [DOI] [PubMed] [Google Scholar]
- Vereide AB, Kaino T, Sager G, Arnes M, Orbo A. Effect of levonorgestrel IUD and oral medroxyprogesterone acetate on glandular and stromal progesterone receptors (PRA and PRB), and estrogen receptors (ER-alpha and ER-beta) in human endometrial hyperplasia. Gynecol Oncol. 2006;101:214–223. doi: 10.1016/j.ygyno.2005.10.030. [DOI] [PubMed] [Google Scholar]
- Wang H, Isaksson E, Von Schoultz B, Cline JM, Sahlin L. The effect of long-term treatment with steroid hormones or tamoxifen on oestrogen receptors (alpha and beta) in the endometrium of ovariectomized cynomolgus macaques. J Endocrinol. 2002;175:673–681. doi: 10.1677/joe.0.1750673. [DOI] [PubMed] [Google Scholar]
- Wang H, Isaksson E, Von Schoultz B, Cline JM, Sahlin L. Effect of long-term treatment with steroid hormones or tamoxifen on the progesterone receptor and androgen receptor in the endometrium of ovariectomized cynomolgus macaques. Reprod Biol Endocrinol. 2003;1:7. doi: 10.1186/1477-7827-1-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Williams JK, Wagner JD, Li Z, Golden DL, Adams MR. Tamoxifen inhibits arterial accumulation of LDL degradation products and progression of coronary artery atherosclerosis in monkeys. Arterioscler Thromb Vasc Biol. 1997;17:403–408. doi: 10.1161/01.atv.17.2.403. [DOI] [PubMed] [Google Scholar]
- Wood CE, Register TC, Anthony MS, Kock ND, Cline JM. Breast and uterine effects of soy isoflavones and conjugated equine estrogens in postmenopausal female monkeys. J Clin Endocrinol Metab. 2004;89:3462–3468. doi: 10.1210/jc.2003-032067. [DOI] [PubMed] [Google Scholar]
- Ylostalo P, Granberg S, Backstrom AC, Hirsjarvi-Lahti T. Uterine findings by transvaginal sonography during percutaneous estrogen treatment in postmenopausal women. Maturitas. 1996;23:313–317. doi: 10.1016/0378-5122(96)00993-0. [DOI] [PubMed] [Google Scholar]