Abstract
We identified factors associated with elevated parental perceptions of child vulnerability (PPCV) 12 months after newborn screening (NBS) of 136 children: healthy, normal results (H, n=37), cystic fibrosis carriers (CF-C, n=40), congenital hypothyroidism (CH, n=36), and cystic fibrosis (CF, n=23). Controlling for infant and parent characteristics, mixed logit structural equation modeling showed direct paths to PPCV included parent female sex, CF diagnosis, and high documented illness frequency. PPCV was positively associated with maternal parenting stress. Infants with CF and CF carriers had significantly more documented illness frequency than H group infants. The CH group did not differ significantly from the H group and had no paths to PPCV. Unexpectedly high documented illnesses frequency among infants who are CF carriers warrants further investigation.
Keywords: congenital hypothyroidism, cystic fibrosis, genetic carriers, newborn screening, vulnerable child
Advances in molecular biology have led to rapid expansion of newborn screening (NBS) programs designed to identify pre-symptomatic infants with serious genetic or metabolic disorders (American Academy of Pediatrics Newborn Screening Task Force, 2000). Cystic fibrosis (CF) screening represents one of the most recent additions to NBS panels implemented nationally (National Newborn Screening and Genetics Resource Center, 2009). Although NBS offers significant health benefits for affected infants, it also carries psychosocial risk. While awaiting additional diagnostic testing following positive CF NBS results, parents report symptoms of anxiety and depression (Lewis, Curnow, Ross, & Massie, 2006; Moran, Quirk, Duff, & Brownlee, 2007; Tluczek, Koscik, Farrell, & Rock, 2005). When NBS includes DNA analysis for CF, the procedure also identifies heterozygous carriers who are not expected to have any adverse health effects. Such false-positive CF NBS results, meaning the screening test was positive and the diagnostic test was negative, have been associated with confusion about the meaning of test results (Ciske et al., 2001) and lingering concerns about test accuracy (Tluczek, Orland, & Cavanagh, 2011). Some parents report worrying about their children’s health for as long as 6 years after NBS (Lewis et al., 2006). These findings raised questions about whether the identification of infants as CF carriers contributes to parental stress and perceptions of child vulnerability similar to that described in parents of infants diagnosed with medical conditions requiring treatment. We compared parental perceptions of child vulnerability as a function of the severity of NBS and follow-up diagnostic results including CF diagnosis, congenital hypothyroidism (CH), healthy CF carriers with false-positive CF NBS results, and healthy infants with normal NBS results (H).
Theoretical Basis for Study: Parental Perception of Child Vulnerability
Parental perception of child vulnerability (PPCV) is characterized as parents’ unfounded beliefs that their children are more susceptible to illness or injury than most children (Green & Solnit, 1964; Thomasgard & Metz, 1997). PPCV is postulated to result from a parent’s unresolved loss or grief precipitated by the child having a relatively mild medical diagnosis, previous life-threatening event, or parent’s personal history of loss. Approximately 3–10% of parents in community samples have been classified as having significantly elevated PPCV (Dogan, Ertem, Karaaslan, & Forsyth, 2009; Forsyth, Horwitz, Leventhal, Burger & Leaf, 1996; Gleason & Evans, 2004). These parents tend to have difficulty setting limits, and their children can develop behavior problems or self-perceptions of vulnerability (Gleason & Evans, 2004; Perrin, West, & Culley, 1989; Thomasgard & Metz, 1996). About 20–30% of parents who perceive their children as highly vulnerable also engage in over-protective parenting behaviors that thwart children’s strivings towards separation and individuation (Thomasgard, 1998; Thomasgard & Metz, 1997; Thomasgard, Shonkoff, Metz, & Edelbrock, 1995). Early studies showed PPCV is associated with parental characteristic of anxiety, low income, or low education; child correlates consisted of having a medical condition, previous life-threatening event, premature birth, or being first born (Thomasgard, 1998; Thomasgard & Metz, 1997; Thomasgard et al., 1995). However, more recently researchers found no relationship between PPCV and parent or child demographics (Dogan et al., 2009).
Evidence suggests that abnormal results from neonatal or early childhood screening programs can precipitate parental worry, stress, or hyper-vigilance consistent with elevated PPCV. Examples include parents of infants found to have benign heart murmurs (Bergman & Stamm, 1967), neonatal jaundice (Kemper, Forsyth, & McCarthy, 1989), false-positive newborn hearing screens (Poulakis, Barker, & Wake, 2003), neonatal complications requiring intensive care (De Ocampo, Macias, Saylor, & Katikaneni, 2003), false-positive NBS for metabolic disorders (Gurian, Kinnamon, Henry, & Waisbren, 2006; Waisbren et al., 2003), genetic risk for type 1 diabetes (Kerruish et al., 2007), and school age children with asthma (Spurrier et al., 2000). However, our study is the first to explicitly measure PPCV related to NBS across a range of diagnostic outcomes 1 year following the testing and to characterize direct paths to PPCV.
Methods
This descriptive, cross sectional study was part of a mixed-method longitudinal project conducted between 2002 and 2009 to investigate the psychosocial consequences of abnormal NBS results and their associations with parent-child relationships (Tluczek, Clark, Koscik, & Farrell, 2005). Data in this report were collected during in-home assessments when infants were 12 months old and through a review of medical records. Four groups represented a range in severity of NBS and subsequent diagnostic results: healthy (H), CF carrier (CF-C), congenital hypothyroidism (CH), and cystic fibrosis (CF).
We included CF in this study because it represents the prototype for the use of DNA analysis in state-mandated NBS. The incidental identification of healthy infants who are heterozygote CF carriers, considered false-positive NBS results, represents a psychosocial risk that has been a point of controversy(Ross & Clayton, 2009). We chose CH because NBS for CH is recognized as an exemplary screening program with more than four decades of success in detecting affected neonates and offering early treatment that significantly improves the neurodevelopment of affected infants. CF differs from CH in two important ways. Even with treatment, CF is a life-shortening, progressive illness that usually requires multiple daily medications, specialized treatments, and frequent evaluations by specialists. In contrast, CH has a favorable prognosis and requires a single daily oral medication. Serious complications of CF, such as pulmonary exacerbations, can require hospitalization, whereas thyroid hormone levels in children with CH are monitored on an outpatient basis and are often managed by primary care providers. Healthy infants with normal NBS results were enrolled as controls for the other three groups. Statewide NBS procedures relevant to this study remained constant during the data collection period.
Experts within the CF medical community agree that the presence of only one defective CF gene should not produce physiological changes that cause CF-related symptoms (CF Foundation Website, 2010. http://www.cff.org/AboutCF/). Therefore, if parents of infants in the CF-C group share this physiologically-based perspective, their perceptions of their children’s vulnerability should more closely resemble that of the H group, than the CF group. We hypothesized that CF group parents would be most likely to view their infants as vulnerable to illness, experience high parenting stress, and have infants with higher documented illness frequency. Parent responses in the CH group should fall between the H group and the CF group. However, the psychologically-driven PPCV framework suggests that parents in the CF-C and CF groups could have similar results on measures of PPCV and parenting stress. Using the H group as a reference, we investigated whether abnormal NBS results that were subsequently found to be false-positive results for CF would produce similar PPCV and parenting stress as results that corresponded to a confirmed diagnosis. We also questioned whether the severity of the diagnosis (CF versus CH) was associated with PPCV and parenting stress. We included illness frequency as a potential variable contributing to PPCV, expecting only children with CF to have significantly more documented illnesses than H group infants. Given the mixed results in the literature regarding associations between parent or child characteristics and elevated PPCV (Dogan et al., 2009; Thomasgard, 1998; Thomasgard & Metz, 1997; Thomasgard et al., 1995), we also examined child birth order and sex, as well as parent demographics of sex, age, education, and marital status as potential factors associated with elevated PPCV.
Recruitment
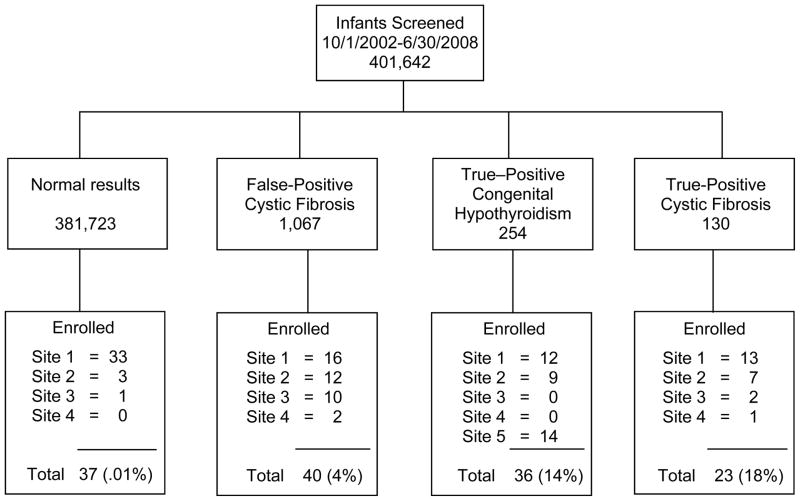
Figure 1 illustrates the recruitment data showing the contributions from each site relative to each study group. Health professionals from each site recruited families with infants less than 6 months old during regularly scheduled appointments in primary and specialty clinics. We enlisted assistance from the Wisconsin State Laboratory of Hygiene (represented as site 5 in Figure 1) to recruit parents of infants with CH because we had documentation that only about half of those infants in our state received care from a pediatric endocrinologist, although almost all infants with CF receive specialty care through CF centers or affiliates. Staff from the State Laboratory sent recruitment materials to primary care providers who distributed them to families with infants diagnosed with CH. All participants provided written informed consent to enroll in this study, approved by the Institutional Review Boards of all four participating medical centers.
FIGURE 1.
Recruitment Algorithm by Site and Study Group
Site 1: American Family Children’s Hospital, Madison, WI; Site 2: Children’s Hospital of Wisconsin, Milwaukee, WI; Site 3: Marshfield Clinic, Marshfield, WI; Site 4: Gundersen Lutheran, La Crosse, WI; Site 5: Wisconsin State Laboratory of Hygiene, Madison, WI.
Patient Sample
A total of 136 mothers and 121 fathers of 136 infants participated in this study: H (n=37), CF-C (n=40), CH (n=36), and CF (n=23). The CF group included five infants with intermediate or normal diagnostic results and/or the presence of two CF mutations suggesting a CF diagnosis. Intermediate diagnostic sweat test results are relatively new phenomena occurring more frequently as a consequence of CF NBS. The long-term prognosis for children with intermediate results remains unclear. We also included one infant with CF identified through prenatal testing plus abnormal NBS results. We included one infant with borderline thyroid levels and one infant with CH who had a false-negative NBS and was subsequently diagnosed at 6 weeks in response to a review of family history. Infants who had serious co-morbid diagnoses, significant perinatal complications, APGAR scores less than 4, or were less than 32 weeks gestation were excluded. To determine sample size, we used a range of subject-to-parameter ratios between 5:1 suggested by Tabachnick and Fidell (1989) and 10:1 recommended by Soeken (2010). Our subject to parameter ratio was approximately 8:1 for the mothers’ and fathers’ data which falls within this generally accepted range and should provide sufficient stability in the estimates.
Measures
Demographic information
Each participating parent completed a self-report form indicating his or her sex, age, ethnicity, educational level, marital status, income, and total number of children. Infant birth order was deduced from the latter information.
Parental perception of child vulnerability
The Child Vulnerability Scale(CVS; Forsyth et al., 1996) is an 8-item self-report that uses a 4-point Likert-type scale (ranging from 0=strongly disagree to 3=strongly agree) to capture parental perception of child vulnerability to illness. Total scores range from 0–24. Scores ≥ 10 suggest elevated PPCV. Although the instrument was standardized with a sample of mothers of children ages 4 to 8 years, it has been used with parents of children as young as 1 month (Dogan et al., 2009). Forsyth et al. (1996) reported total scale and internal consistency of item-total as having reliability (Cronbach alpha=.74; Pearson correlation coefficients=.51 to .68). The Cronbach alpha for the CVS derived from our study sample was .82 suggesting good internal consistency. Significant correlations between the scores on the CVS and the Achenbach Child Behavior Check List (p<.001) and the number of acute care clinic visits (p<.001) support the validity of the CVS (Forsyth et al.). A recent confirmatory factor analysis was conducted on CVS data obtained from 226 parents including mothers, fathers, and custodial grandmothers of children age 8 months to 18 years. Results found a good fit for a single factor of vulnerability (Fedele, Grant, Wolfe-Christensen, Mullins & Ryan, 2010).
Parenting stress
The Parenting Stress Index(PSI; Abidin, 1995) contains 120 self-report items that measure the number and severity of stressors related to parenting children aged 1 month to 12 years. Items are scored on a 5-point Likert-type scale (ranging from 1=strongly disagree to 5=strongly agree). Scores ≥ 85th percentile are within the clinical range. This analysis included only data from the Total Score for the Child Domain because we were interested in child characteristics, rather than other environmental stresses that contributed to parental perception of child vulnerability. We also wanted to limit the number of parameters relative to the sample size. The 50-item Child Domain consists of six subscales: Distractability/Hyperactivity, Adaptability, Reinforces Parent, Demandingness, Mood, and Acceptability. The Total Score for the Child Domain is the sum of all six subscale scores. Alpha coefficients of internal consistency reliability reported in the literature for the Child Domain Total and subscale scores ranged from .59 to .91 (Abidin, 1995). Correlation coefficients for test-retest reliability ranged from .55 to .82 (p<.01). The Cronbach alpha for Child Domain of the current sample was .90 suggesting good internal consistency. Validity of the measure has been estimated in over 200 studies that document significant associations between high parental stress and parent-child relationship disturbances, parenting children with special needs, and other environmental stressors (Abidin, 1995).
Documented illness frequency
Infant illness frequency during the first year of life was measured by compiling data abstracted from infants’ medical records at 12 months. Illness was defined as documented signs or symptoms of alterations in physical health (e.g., fever, cough, diarrhea, rashes) or a medical diagnosis (e.g., otitis media). Health care contacts for primary care, laboratory tests, and re-evaluation of previously documented illnesses were not coded as illnesses. An illness associated with multiple medical contacts was counted as one illness. Pediatric nurses were trained in coding data by conducting practice ratings and comparing their ratings against standardized ratings established a priori until 90% reliability was reached. The primary investigator (PI) performed periodic random reliability checks. To assure accuracy, all medical records were independently reviewed and coded by two researchers. Inconsistencies were resolved through consultation with the PI and consensus ratings (Polit & Beck, 2008).
Analytic Strategy
Sample demographics and major study variables
We assessed maternal and paternal data separately for group differences in age, ethnicity, educational level, marital status, income, and infant sex using both parametric and non-parametric tests. Some of the categorical conditions were small. Therefore, the exact Type I error was obtained using StatXact 8 (Cytel Software Corporation, 2009). Due to the large number of group contrasts in demographic and major study variables, we adjusted the Type 1 error rate using Sidak’s family-wise error (Sidak, 1967).
Structural Equation Model
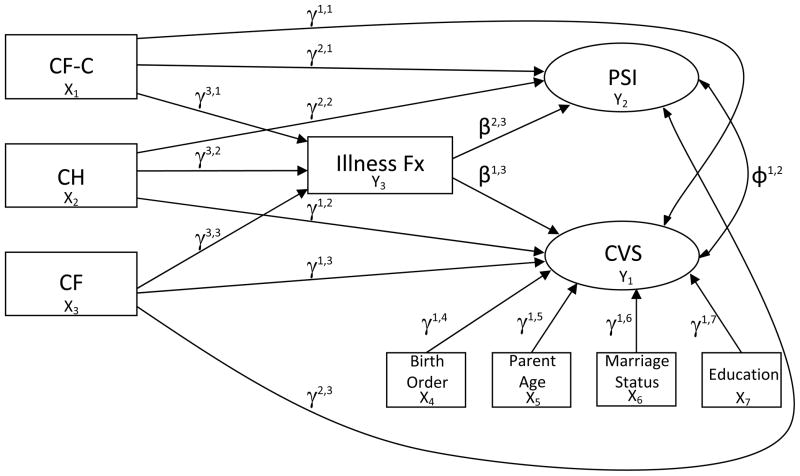
We used a mixed logit and Poisson structural equation strategy to model data from mothers and fathers illustrated in Figure 2. Procedures incorporated continuous data from the PSI, dichotomous cut point for CVS, and k-1 dummy variables of infant group (using the H groups as the reference), along with the count measure of infant illness frequency. The binary data for CVS and count date for illness frequency necessitated this mixed logit and Poisson estimation procedure in the model for the analysis of direct effects. To assess indirect effects on two different estimation procedures, the model was recast into a probit model (MacKinnon, 2008).
Figure 2.
Paths Examined in Mothers’ and Fathers’ Models
CH, congenital hypothyroidism; CF-C, cystic fibrosis carrier; CF, cystic fibrosis; CVS, Child Vulnerability Scale; PSI, Parenting Stress Index; Fx, Frequency.
Due to conflicting previous results found in the literature concerning associations between parent or child characteristics associated with elevated levels of PPCV (Dogan et al., 2009; Thomasgard, 1998; Thomasgard & Metz, 1997; Thomasgard et al., 1995), we added child birth order, parent age, education, and marital status as adjusting covariates in each model. The models for mothers and fathers were constructed separately using Mplus Version 6.1 (Muthén & Muthén, 1998–2010).
RESULTS
Missing Data
An initial appraisal of missing data per subscale for the PSI indicated < 5% total items missing per scale. All missingness met the conditions of missing completely at random (MCAR) based on Little’s test (Little, 1988). The Expectancy Maximum (EM) algorithm with the NORM software was used to impute missing items from the PSI (Schafer, 1999).
Demographics and Major Study Variables
Using Sidak’s family-wise error rate (Sidak, 1967), there were no significant group differences for any parent demographic variables or infant sex. Mean parent age was 30.9 (SD=5.6), most were married (88.4%), European American (95.3%), college educated (56.8%), and had an annual family income ≥ $41,000 (73%). Children of single parents resided with their mothers. Other racial/ethnic backgrounds of participants included African American, Hispanic American, Asian American, and American Indian. Infant sex was fairly evenly divided for the total sample (55% female) and within each group. Although we could not collect demographic data from all families with infants screened in our state during our recruitment period, we could compare our sample to publicly available data for Wisconsin from the United States Census (2010). The racial/ethnic distribution of our sample was consistent with the 2009 data showing 89.4% residents to be non-Hispanic white. Our sample was more highly educated (22.4% of Wisconsin residents have college degrees), but the income of our sample was comparable (median statewide household income in 2008=$52,103).
As detailed in Table 1, significant group differences were found in mothers’ CVS scores. Mothers in the CF group were more likely to have scores above the cut point suggesting elevated PPCV than mothers in the H-group or CH group. Fathers in the CF-C group had significantly lower PSI scores than fathers in H-group. Children with CF had the most documented illnesses followed by the CF-C group, CH group and H-group. However, these differences were not statistically significant in this analysis.
Table 1.
Mother and Father Variables by Study Group
| Variable | Study Groups
|
p-valueb |
|||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| H | CF-C | CH | CF | H vs. CF-C | H vs. CH | H vs. CF | CF-C vs. CF | CH vs. CF-C | CH vs. CF | ||
| Mothers % above cut point | CVSa | 2.70% (1/36) | 15.00% (6/40) | 8.57% (3/35) | 43.48% (10/23) | .39 | .91 | .006* | .101 | .99 | .014* |
| M (SD) | Illness Fx | 4.52 (3.79) | 6.10 (5.02) | 4.67 (3.82) | 6.73 (4.53) | .86 | .99 | .26 | .96 | .91 | .43 |
| M (SD) | PSI | 88.54 (11.15) | 86.21 (17.29) | 91.87 (14.79) | 96.42 (20.61) | .98 | .88 | .35 | .21 | .59 | .91 |
| Fathers % above cut point | CVSa | 8.57% (3/35) | 5.26% (2/38) | 3.13% (1/32) | 21.05% (4/19) | .99 | .99 | .90 | .55 | .99 | .27 |
| M (SD) | Illness Fx | 4.61 (3.88) | 5.83 (4.80) | 4.78 (3.72) | 6.25 (3.66) | .95 | .99 | .43 | .95 | .99 | .59 |
| M (SD) | PSI | 98.70 (12.96) | 87.60 (14.70) | 92.96 (13.96) | 93.87 (15.34) | .006* | .46 | .83 | .64 | .56 | .99 |
Note. H = healthy; CF-C = cystic fibrosis carrier; CH = congenital hypothyroidism; CF = cystic fibrosis; CVS = Child Vulnerability Scale; Fx = frequency; PSI = Parenting Stress Index.
p < .05
Exact probability tests: Test of non-inferiority differences of proportions (Chan, 1998).
Each series of contrasts adjusted using Sidak adjustment.
Structural Equation Model
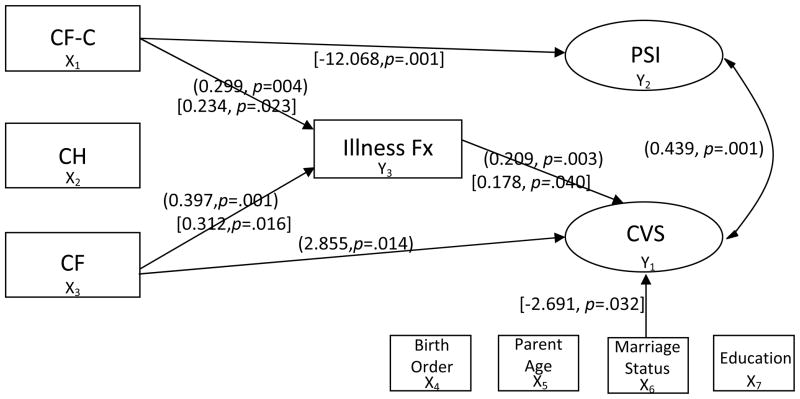
After adjusting for child birth order and maternal demographics, the mixed logit structural equation results for the mothers’ model are illustrated in Figure 3. As indicated by the odds ratio in Table 2, mothers of children with a CF diagnosis were 17 times more likely to have CVS scores above the cut point suggesting elevated PPCV than mothers of healthy children (p=.014). For each one point increase in child illness, mothers were 1.2 times more likely to have CVS scores above the cut than mothers of children with fewer illnesses (p=.003). High maternal CVS scores were associated with high parenting stress (p=.001). Significant findings were in the hypothesized directions. Mothers of healthy infants and infants with CH showed no significant paths to CVS scores suggesting no elevated PPCV. The probit model showed no significant indirect paths to elevated PPCV for the CF group or the CF-C group.
Figure 3.
Significant Paths Using Non-Standardized Parameters in (Mothers’) and [Fathers’] Models
CH, congenital hypothyroidism; CF-C, cystic fibrosis carrier; CF, cystic fibrosis; CVS, Child Vulnerability Scale; PSI, Parenting Stress Index; Fx, Frequency.
Table 2.
Effect Paths For Child Domain of Parenting Stress Index (PSI), Illness Frequency, and Child Vulnerability Scale (CVS) by Parent
| Direct Effect Paths | Model Parameter | Mother
|
Father
|
||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Non-Standardized Parameter | SE | p | 95% CI | OR | Non-Standardized Parameter | SE | p | 95% CI | OR | ||
| Illness Fx → CVS | β1,3 | 0.209* | 0.070 | .003* | [0.072, 0.347] | 1.233 | 0.178* | 0.087 | .040* | [0.008, 0.349] | 1.195 |
| CF–C Group → CVS | γ1,1 | 0.959 | 1.213 | .429 | [−1.419, 3.336] | 2.608 | −1.644 | 1.187 | .166 | [−3.971, 0.683] | 0.193 |
| CH Group → CVS | γ1,2 | 0.766 | 1.255 | .542 | [−1.694, 3.226] | 2.151 | −1.447 | 1.298 | .265 | [−3.991, 1.098] | 0.235 |
| CF Group → CVS | γ1,3 | 2.855* | 1.162 | .014* | [0.578, 5.131] | 17.367 | 1.150 | 0.917 | .210 | [−0.647, 2.946] | 3.157 |
| Birth Order → CVS | γ1,4 | −0.185 | 0.347 | .594 | [−0.865, 0.495] | 0.831 | −0.267 | 0.493 | .588 | [−1.233, 0.699] | 0.766 |
| Parent Age → CVS | γ1,5 | 0.073 | 0.059 | .217 | [−0.043, 0.188] | 1.075 | −0.006 | 0.070 | .934 | [−0.144, 0.132] | 0.994 |
| Marital Status → CVS | γ1,6 | −1.296 | 0.756 | .087 | [−2.778, 0.186] | 0.274 | −2.691* | 1.252 | .032* | [−5.145, −0.236] | 0.068 |
| Education → CVS | γ1,7 | −0.564 | 0.380 | .137 | [−1.309, 0.180] | 1.062 | 0.222 | 0.439 | .621 | [−0.638, 1.083] | 1.249 |
| Illness Fx → PSI | β2,3 | 0.446 | 0.358 | .213 | [−0.256, 1.148] | --- a | 0.225 | 0.374 | .546 | [−0.507, 0.958] | --- a |
| CF–C Group → PSI | γ2,1 | −3.039 | 3.740 | .417 | [−10.370, 4.293] | --- a | −12.068* | 3.492 | .001* | [−18.912,−5.225] | --- a |
| CH Group → PSI | γ2,2 | 3.041 | 3.833 | .428 | [−4.471, 10.553] | --- a | −7.048 | 3.667 | .055 | [−14.236, 0.140] | --- a |
| CF Group → PSI | γ2,3 | 6.711 | 4.441 | .131 | [−1.994, 15.415] | --- a | −6.033 | 4.393 | .170 | [−14.644, 2.579] | --- a |
| CF–C Group → Illness Fx | γ3,1 | 0.299* | 0.103 | .004* | [0.098, 0.501] | --- a | 0.234* | 0.107 | .023* | [0.034, 0.452] | --- a |
| CH Group → Illness Fx | γ3,2 | 0.033 | 0.114 | .776 | [−0.191, 0.256] | --- a | 0.064 | 0.122 | .597 | [−0.174, 0.303] | --- a |
| CF Group → Illness Fx | γ3,3 | 0.397* | 0.118 | .001* | [0.166, 0.629] | --- a | 0.312* | 0.129 | .016* | [0.059, 0.565] | --- a |
|
| |||||||||||
| Correlation | |||||||||||
| PSI ↔ CVS | ϕ1,2 | 0.439* | 0.128 | .001* | [0.183, 0.686] | --- a | 0.144 | 0.178 | .418 | [−0.207, 0.492] | --- a |
|
| |||||||||||
| Indirect Effect Paths | |||||||||||
| CF Group → Illness → CVSb | 0.166 | 0.129 | .198 | [−0.086, 0.419] | --- a | 0.129 | 0.116 | .266 | [−0.099, 0.357] | --- a | |
| CF-C Group → Illness → CVSb | 0.247 | 0.159 | .120 | [−0.065, 0.560] | --- a | 0.146 | 0.159 | .358 | [−0.166, 0.457] | --- a | |
Note. PSI = Parenting Stress Index; CVS = Child Vulnerability Scale; Fx = frequency; CF-C = cystic fibrosis carrier; CH = congenital hypothyroidism; CF = cystic fibrosis; CI = confidence interval; OR = odds ratio.
p < .05
No odds ratios reported for these continuous measures.
Indirect effects for these mixed paths were assessed using a probit model (MacKinnon, 2008).
Child birth order and paternal demographics were adjusted in the results of the fathers’ model, illustrated in Figure 3. As indicated by the odds ratio on Table 2, for each one point increase in child illness, fathers were 1.2 times more likely to report CVS scores above the cut than fathers of children with fewer illnesses (p=.04). Fathers of infants identified as CF carriers reported significantly lower levels of parenting stress than those in the healthy comparison group (p=.001). Married fathers were more likely than single fathers to perceive their children as vulnerable (p=.032). Similar to the mothers’ model, fathers of healthy infants and infants with CH had no significant path findings.
Both models used the same illness data that showed infants with CF (mothers’ model p=.001, fathers’ model p=.016) and those identified as CF carriers (mothers’ model p=.004, fathers’ model p=.023) had significantly more documented illnesses (M=6.74, SD=4.53 and M=6.11, SD=5.03 respectively) than healthy infants with normal NBS results (M=4.53, SD=3.79). There was no significant difference between the frequency of illness in infants with CH (M=4.68, SD=3.82) and the healthy comparison group. Given the unexpectedly high illness frequency in the CF carrier infants, we examined the data for outliers but found none.
Discussion
We acknowledge several limitations in this study. The use of convenience sampling could have produced selection bias. We used the CVS and the recommended cut point of scores above 10 to indicate elevated PPCV. Although researchers recently found the CVS appropriate for use with parents of children the same age as our sample (Fedele et al., 2010), because the instrument was standardized with parents of older children, we must exercise caution in the interpretation of these results. Our sample was demographically homogenous relative to racial/ethnic background, limiting the generalizability of the results. However, our sample was similar to the racial background and socioeconomic characteristics of the sample used to standardize the CVS. Although our overall sample size was adequate, some subgroups were small. Despite significant direct effects between the CF group and CF-C groups on illness frequency and between illness frequencies on CVS scores in both models, no significant indirect effects were found for either study group in either model. This lack of statistical significance can be accounted for by the different procedures used to examine direct versus indirect (mediating) effects. The probit estimate required to examine the indirect effects for mixed distributions was not as sensitive as the procedures use for the direct effects. Given the limitations of this procedure in dealing with different distribution types, illness frequency did not explain elevated PPCV in the CF and CF-C groups. However, a larger sample might have produced significant results that better explain the role of illness frequency relative to elevated PPCV. The small percentage of fathers’ CVS scores above the cut point could have contributed to the limited modeling of father risk. Despite these limitations, the findings add to the evidence-based knowledge of psychosocial outcomes related to NBS and factors contributing to elevated PPCV.
Findings in Table 2 showed that mothers and fathers of infants who had higher documented illness frequencies and mothers of infants with CF were most likely to report perceptions of their children as vulnerable. Overall, mothers were more likely than fathers to experience significant parenting stress associated with elevated PPCV. CF-C group mothers and fathers more closely resembled the CF group regarding their children’s documented illness frequencies than the healthy comparison group. CH group mothers and fathers more closely resembled the healthy comparison group on variables of documented child illness frequencies and PPCV. Furthermore, a CH diagnosis did not lead to elevated PPCV in either model. The percent of CVS scores above the cut point listed in Table 1 indicate that H group parents, CH group parents, and CF-C fathers fell within the 3–10% range found in community samples. CF group parents and CF-C group mothers had higher rates suggesting elevated PPCV.
Given the potentially serious consequences of repeated respiratory infections in children with CF (Accurso, 2008), it is reasonable that parents of infants with CF considered their children more vulnerable and thus their infants had more documented illnesses than healthy infants. However, the higher documented illness frequency in infants identified as CF carriers was unexpected. This finding was statistically significant in the models (Table 2, Figure 3), but not in the group comparisons (Table 1). However, the model offers a more comprehensive representation of the findings than simple contrasts because modeling adjusts for all parameter estimates.
It is not clear whether the presence of one CF mutation poses an actual risk for illness or if parents perceived their children to be vulnerable to illness and sought medical attention more often than parents of infants with normal NBS results. The latter explanation is consistent with our qualitative interviews (Tluczek et al., 2011) in which some parents of infants with false-positive CF NBS results (CF-C group) reported that when their infants acquired respiratory illnesses during the first year of life, they wondered whether the sweat test results had been accurate and their children might actually have CF. Thus, the higher frequency of documented health contacts could represent mildly elevated PPCV. These findings are similar to those of Waisbren et al. (2003) who found that children with false-positive NBS for metabolic disorders were more likely to be hospitalized during the first year than those children with normal NBS results. However, a more recent report from the same research group showed that parents of infants with false-positive NBS results for metabolic disorders utilized health care services no more often than parents of infants with normal NBS results (Lipstein, Perrin, Waisbren, & Prosser, 2009). One potential explanation for this discrepancy might be that false-positive NBS results identifying a defective gene are more likely to engender parental concerns than results derived from other methods.
Another explanation is that the higher illness frequency is real, not an artifact of parents’ perceptions. Support for a biological premise comes from studies that show individuals with one cystic fibrosis transmembrane conductance regulator (CFTR) mutation have higher neonatal immunoreactive trypsinogen levels (Castellani et al., 2005; Gregg et al., 1993; Scotet et al., 2001), higher sweat chloride values (Farrell & Koscik,1996; Parad et al., 2005), and a higher prevalence of sinusitis in adulthood (Wang, Kim, McWilliams, & Cutting, 2005) than non-carriers. There could be physiological implications for CF carriers that are not yet fully understood.
Mothers were more likely than fathers to report stress in parenting children they perceived as vulnerable. Although contemporary fathers actively participate in parenting, mothers still tend to be the primary caregivers of infants (Nyström & Öhrling, 2004). Perhaps the higher frequency and duration of mother-infant interactions provide mothers more opportunities to detect subtle infant cues that could be signs of illness and that, from the mother’s perspective, generates stress.
Sex differences could explain the relationships between fathers’ marital status and report of PPCV as well as reports of low parenting stress in the CF-C group relative to the comparison group of fathers. Given that all infants of the single parents in our study resided with their mothers, these fathers probably had less contact with their children, and therefore less parenting stress, than the married fathers. Following the initial scare of abnormal NBS results, the fathers in the CF-C group might have experienced a deep sense of relief that helped them reframe normative parenting challenges as less taxing than the fathers in the healthy comparison group. This supposition is supported by our earlier report in which parents of infants with false-positive CF NBS results explained how the experience gave them a new perspective about life and an appreciation for their children’s good health (Tluczek et al., 2011).
The lack of paths among the variables for the mothers and fathers of infants with CH suggests that psychosocial sequelae for NBS are a function of the perceived seriousness of the screened condition. These findings are timely, given the recent expansion of NBS programs to include CF (National Newborn Screening and Genetics Resource Center, 2009), and suggest that nurses and other health care providers should remain alert for signs of parental perception to child vulnerability following false-positive NBS results for CF. Additional research is needed to determine whether such on-going concerns are grounded in physiologic changes produced by a single CFTR mutation or a function of parental anxiety. In light of ever increasing application of genetic technologies to screening practices, it is critical that we understand the psychological impacts of such testing and receipt of test results, particularly when screening is mandated by law, and take appropriate measures to ameliorate such iatrogenic effects.
Conclusion
The serendipitous finding of high documented illness frequency among infants who are CF carriers warrants further investigation. This finding could represent an over-utilization of medical services and thus a proxy for mildly elevated levels of PPCV, or as of yet undocumented associated health concerns for CF-C infants. The path to PPCV in the CF group, absence in the CH group, and high documented illness frequency in the CF-C group as well as the CF group suggest that perceived seriousness of a condition identified through NBS is a critical factor in whether parents develop PPCV and associated parenting stress.
Acknowledgments
This project was supported by the National Institute of Child Health and Human Development (K23HD42098), the University of Wisconsin-Madison School of Nursing Research Committee, and the University of Wisconsin-Madison Graduate School, with additional editorial assistance from the Clinical and Translational Science Award (CTSA) program of the National Center for Research Resources, National Institutes of Health (1UL1RR025011).
We wish to thank the participants in this study and the research teams from the University of Wisconsin-Madison, the American Family Children’s Hospital, the Children’s Hospital of Wisconsin, the Marshfield Clinic, the Gunderson-Lutheran Medical Center, and the Wisconsin State Laboratory of Hygiene. Special thanks to Philip M. Farrell, MD, PhD, Karen Pridham, PhD, RN, FAAN, Patricia Becker, PhD, RN, FAAN, and Laura Hogan, PhD for their wisdom in the preparation of this manuscript.
References
- Abidin RR. Parenting stress index: Professional manual. 3. Lutz, FL: Psychological Assessment Resources; 1995. [Google Scholar]
- Accurso FJ. Update in cystic fibrosis 2007. American Journal of Respiratory and Critical Care Medicine. 2008;177:1058–1061. doi: 10.1164/rccm.200801-069UP. [DOI] [PMC free article] [PubMed] [Google Scholar]
- American Academy of Pediatrics Newborn Screening Task Force. Serving the family from birth to the medical home. Newborn screening: A blueprint for the future- A call for a national agenda on state newborn screening programs. Pediatrics. 2000;106:389–422. Retrieved from http://pediatrics.aappublications.org/cgi/content/full/106/2/S1/389. [PubMed]
- Bergman AB, Stamm SJ. The morbidity of cardiac nondisease in schoolchildren. New England Journal of Medicine. 1967;276:1008–1013. doi: 10.1056/NEJM196705042761804. Retrieved from http://www.nejm.org. [DOI] [PubMed]
- Castellani C, Picci L, Scarpa M, Dechecchi MC, Zanolla L, Assael BM, Zacchello F. Cystic fibrosis carriers have higher neonatal immunoreactive trypsinogin values than non-carriers. American Journal of Medical Genetics Part A. 2005;135:142–144. doi: 10.1002/ajmg.a.30470. [DOI] [PubMed] [Google Scholar]
- Chan ISF. Exact tests of equivalence and efficacy with a non-zero lower bound for comparative studies. Statistics in Medicine. 1998;17:1403–1413. doi: 10.1002/(SICI)1097-0258(19980630)17:12<1403::AID-SIM834>3.0.CO;2-Y. [DOI] [PubMed] [Google Scholar]
- Ciske DJ, Haavisto A, Laxova A, Zeng L, Rock M, Farrell PM. Genetic counseling and neonatal screening for cystic fibrosis: An assessment of the communication process. Pediatrics. 2001;107:699–705. doi: 10.1542/peds.107.4.699. [DOI] [PubMed] [Google Scholar]
- Cytel Software Corporation. StatXact8. Cambridge, Mass: Author; 2009. [Google Scholar]
- De Ocampo AC, Macias MM, Saylor CF, Katikaneni LD. Caretaker perception of child vulnerability predicts behavior problems in NICU graduates. Child Psychiatry and Human Development. 2003;34:83–96. doi: 10.1023/A:1027384306827. [DOI] [PubMed] [Google Scholar]
- Dogan DG, Ertem IO, Karaaslan T, Forsyth BW. Perception of vulnerability among mothers of healthy infants in a middle-income country. Child: Care, Health and Development. 2009;35:868–872. doi: 10.1111/j.1365-2214.2009.01015.x. [DOI] [PubMed] [Google Scholar]
- Farrell PM, Koscik RE. Sweat chloride concentrations in infants homozygous or heterozygous for F508 cystic fibrosis. Pediatrics. 1996;97:524–528. Retrieved from http://pediatrics.aappublications.org/ [PubMed]
- Fedele DA, Grant DM, Wolfe-Christensen C, Mullins LL, Ryan JL. An examination of the factor structure of parenting capacity measures in chronic illness populations. Journal of Pediatric Psychology. 2010;35:1083–1092. doi: 10.1093/jpepsy/jsq045. [DOI] [PubMed] [Google Scholar]
- Forsyth BW, Horwitz SM, Leventhal JM, Burger J, Leaf PJ. The child vulnerability scale: An instrument to measure parental perceptions of child vulnerability. Journal of Pediatric Psychology. 1996;21:89–101. doi: 10.1093/jpepsy/21.1.89. Retrieved from http://jpepsy.oxfordjournals.org/ [DOI] [PubMed]
- Gleason TR, Evans ME. Perceived vulnerability: A comparison of parents and children. Journal of Child Health Care. 2004;8:279–287. doi: 10.1177/1367493504047318. [DOI] [PubMed] [Google Scholar]
- Green M, Solnit AJ. Reactions to the threatened loss of a child: A vulnerable child syndrome. Pediatric management of the dying child, Part III. Pediatrics. 1964;34:58–66. Retrieved from http://pediatrics.aappublications.org/ [PubMed]
- Gregg RG, Wilfond BS, Farrell PM, Laxova A, Hassemer D, Mischler EH. Application of DNA analysis in a population-screening program for neonatal diagnosis of cystic fibrosis (CF): Comparison of screening protocols. American Journal of Human Genetics. 1993;52:616–626. Retrieved from http://www.cell.com/AJHG/ [PMC free article] [PubMed]
- Gurian EA, Kinnamon DD, Henry JJ, Waisbren SE. Expanded newborn screening for biochemical disorders: The effect of a false-positive result. Pediatrics. 2006;117:1915–1921. doi: 10.1542/peds.2005-2294. [DOI] [PubMed] [Google Scholar]
- Kemper K, Forsyth B, McCarthy P. Jaundice, terminating breast-feeding, and the vulnerable child. Pediatrics. 1989;84:773–778. Retrieved from http://pediatrics.aappublications.org/ [PubMed]
- Kerruish NJ, Campbell-Stokes PL, Gray A, Merriman TR, Robertson SP, Taylor BJ. Maternal psychological reaction to newborn genetic screening for type 1 diabetes. Pediatrics. 2007;120:e324–e335. doi: 10.1542/peds.2006-1381. [DOI] [PubMed] [Google Scholar]
- Lewis S, Curnow L, Ross M, Massie J. Parental attitudes to the identification of their infants as carriers of cystic fibrosis by newborn screening. Journal of Paediatrics and Child Health. 2006;42:533–537. doi: 10.1111/j.1440-1754.2006.00917.x. [DOI] [PubMed] [Google Scholar]
- Lipstein EA, Perrin JM, Waisbren SE, Prosser LA. Impact of false-positive newborn metabolic screening results on early health care utilization. Genetics in Medicine. 2009;11:716–721. doi: 10.1097/GIM.0b013e3181b3a61e. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Little RJA. A test of missing completely at random for multivariate data with missing values. Journal of the American Statistical Association. 1988;83:1198–1202. Retrieved from http://www.amstat.org/publications/jasa.cfm.
- MacKinnon DP. Introduction to statistical mediation analysis. New York, NY: Erlbaum; 2008. [Google Scholar]
- Moran J, Quirk K, Duff AJA, Brownlee KG. Newborn screening for CF in a regional paediatric centre: The psychosocial effects of false-positive IRT results on parents. Journal of Cystic Fibrosis. 2007;6:250–254. doi: 10.1016/j.jcf.2006.09.001. [DOI] [PubMed] [Google Scholar]
- Muthén LK, Muthén BO. Mplus user’s guide. Los Angeles, CA: Author; 1998–2010. [Google Scholar]
- National Newborn Screening and Genetics Resource Center. National newborn screening status report. 2009 Retrieved from http://genes-r-us.uthscsa.edu/nbsdisorders.pdf.
- Nyström K, Öhrling K. Parenthood experiences during the child’s first year: Literature review. Journal of Advanced Nursing. 2004;46:319–330. doi: 10.1111/j.1365-2648.2004.02991.x. [DOI] [PubMed] [Google Scholar]
- Parad RB, Comeau AM, Dorkin HL, Dovey M, Gerstle R, Martin T, O’Sullivan BP. Sweat testing infants detected by cystic fibrosis newborn screening. Journal of Pediatrics. 2005;147(3 Suppl):S69–S72. doi: 10.1016/j.jpeds.2005.08.015. [DOI] [PubMed] [Google Scholar]
- Perrin EC, West PD, Culley BS. Is my child normal yet? Correlates of vulnerability. Pediatrics. 1989;83:355–363. Retrieved from http://pediatrics.aappublications.org/ [PubMed]
- Polit DF, Beck CT. Nursing research: Generating and assessing evidence for nursing practice. 8. Philadelphia, PA: Lippincott Williams & Wilkins; 2008. [Google Scholar]
- Poulakis Z, Barker M, Wake M. Six month impact of false positives in an Australian infant hearing screening programme. Archives of Disease in Childhood. 2003;88:20–24. doi: 10.1136/adc.88.1.20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ross LF, Clayton EW. Clinical and ethical considerations in managing carrier detection. American Journal of Public Health. 2009;99:1348–1349. doi: 10.2105/AJPH.2009.161554. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schafer JL. NORM: multiple imputation of incomplete multivariate data under a normal model, version 2.03. 1999 software for Windows 95/98/NT, available from www.stat.psu.edu/~jls/misoftwa.html.
- Scotet V, De Braekeleer M, Audrézet MP, Lodé L, Verlingue C, Quéré I, Férec C. Prevalence of CFTR mutations in hypertrypsinaemia detected through neonatal screening for cystic fibrosis. Clinical Genetics. 2001;59:42–47. doi: 10.1034/j.1399-0004.2001.590107.x. [DOI] [PubMed] [Google Scholar]
- Sidak Z. Rectangular confidence regions for the means of multivariate normal distributions. Journal of the American Statistical Association. 1967;62:626–633. Retrieved from http://www.amstat.org/publications/jasa.cfm.
- Soeken KL. Validity of measures. In: Waltz CF, Strickland OL, Lenz ER, editors. Measurement in nursing and health research. 4. New York, NY: Springer; 2010. pp. 163–202. [Google Scholar]
- Spurrier NJ, Sawyer MG, Staugas R, Martin AJ, Kennedy D, Streiner DL. Association between parental perception of children’s vulnerability to illness and management of children’s asthma. Pediatric Pulmonology. 2000;29:88–93. doi: 10.1002/(SICI) 1099-0496(200002)29:2<88::AID-PPUL2>3.0.CO;2-D. [DOI] [PubMed] [Google Scholar]
- Tabachnick BG, Fidell LS. Using multivariate statistics. 2. Cambridge, MA: Harper & Row; 1989. [Google Scholar]
- Thomasgard M. Parental perceptions of child vulnerability, overprotection, and parental psychological characteristics. Child Psychiatry and Human Development. 1998;28:223–240. doi: 10.1023/A:1022631914576. [DOI] [PubMed] [Google Scholar]
- Thomasgard M, Metz WP. The two-year stability of parental perceptions of child vulnerability and parental protectiveness. Journal of Developmental and Behavioral Pediatrics. 1996;17:222–228. [PubMed] [Google Scholar]
- Thomasgard M, Metz WP. Parental overprotection and its relation to perceived child vulnerability. American Journal of Orthopsychiatry. 1997;67:330–335. doi: 10.1037/h0080237. [DOI] [PubMed] [Google Scholar]
- Thomasgard M, Shonkoff JP, Metz WP, Edelbrock C. Parent-child relationship disorders. Part II. The vulnerable child syndrome and its relation to parental overprotection. Developmental and Behavioral Pediatrics. 1995;16:251–256. Retrieved from http://journals.lww.com/jrnldbp/pages/default.aspx. [PubMed]
- Tluczek A, Clark R, Koscik RL, Farrell PM. Mother-infant relationships in the context of neonatal CF diagnosis: Preliminary findings. Pediatric Pulmonology. 2005;28:179–180. Retrieved from http://www.wiley.com/WileyCDA/WileyTitle/productCd-PPUL.html.
- Tluczek A, Koscik RL, Farrell PM, Rock MJ. Psychosocial risk associated with newborn screening for cystic fibrosis: Parents’ experience while awaiting the sweat-test appointment. Pediatrics. 2005;115:1692–1703. doi: 10.1542/peds.2004-0275. [DOI] [PubMed] [Google Scholar]
- Tluczek A, Orland KM, Cavanagh L. Psychosocial consequences of false-positive newborn screen for cystic fibrosis: a relational family system perspective. Qualitative Health Research. 2011;21:174–86. doi: 10.1177/1049732310382919. [Epub 2010 Sep 17] [DOI] [PMC free article] [PubMed] [Google Scholar]
- United States Census Bureau. 2010 Aug 16; Retrieved from http://quickfacts.census.gov/qfd/states/55000.html.
- Waisbren SE, Albers S, Amato S, Ampola M, Brewster TG, Demmer L, … Levy HL. Effect of expanded newborn screening for biochemical genetic disorders on child outcomes and parental stress. JAMA. 2003;290:2564–2572. doi: 10.101/jama.290.19.2564. [DOI] [PubMed] [Google Scholar]
- Wang X, Kim J, McWilliams R, Cutting GR. Increased prevalence of chronic rhinosinusitis in carriers of a cystic fibrosis mutation. Archives of Otolaryngology--Head & Neck Surgery. 2005;131:237–240. doi: 10.1001/archotol.131.3.237. Retrieved from http://archotol.ama-assn.org/ [DOI] [PubMed]