Abstract
The 2005 ISHLT rejection grading system merged grades 1A, 1B and 2 into a single grade (1R), assuming equivalent prognostic significance. We hypothesized that recurrent 1B acute cellular rejection (ACR) is associated with adverse outcomes. Data on all heart transplant recipients at our center from 1990–2007 were reviewed. Patients were excluded if they had more than one grade ≥3A/2R biopsy in the first 6 weeks or any grade ≥3A/2R biopsies during the first year thereafter. Patients with ≥2 grade 1B biopsies from 6 weeks-1 year were classified as “recurrent 1B.” Outcomes were freedom from late (>1 year) ACR (grade ≥3A/2R), coronary artery disease (CAD), re-transplantation/death, and a composite end-point. Sixty-two patients (53 non-recurrent 1B, 9 recurrent 1B) met inclusion criteria. In univariate analyses, recurrent 1B status was associated with decreased freedom from late ACR (p<0.001), CAD (p=0.004), and the composite outcome (p<0.001). There was no difference in freedom from re-transplantation/death (p=0.48). After controlling for demographic differences between the groups, recurrent 1B status was independently associated with late ACR (HR 5.90; p=0.002) and the composite outcome (HR 4.52; p=0.002). These data suggest that further study of the impact of removal of the 1B classification from the ISHLT grading scheme is warranted.
Keywords: acute rejection, cardiac transplantation, pediatrics
INTRODUCTION
In 2005, the ISHLT revised its cardiac allograft rejection grading scheme (1). Biopsies showing mild focal (grade 1A), mild diffuse (1B), and focal moderate (2) acute cellular rejection (ACR) according to the 1990 ISHLT criteria (2) were merged into a single category (grade 1R). This assumes equivalent prognostic significance of these grades, a fact which has not been well demonstrated. Though studies have shown that recurrent moderate or severe rejection (grade ≥3A/2R) predicts worse long term outcomes (3, 4), the effect of recurrent low grade rejection is unclear. Therefore, we sought to investigate the effect of recurrent mild diffuse rejection (grade 1B) on medium and long-term outcomes in a pediatric heart transplant population.
PATIENTS AND METHODS
This project was approved by the Institutional Review Board of the University of Pittsburgh. Data on all cardiac transplant recipients at our institution between January 1990 and December 2007 were reviewed. Patients were included if they had no more than a single grade ≥3A/2R endomyocardial biopsy (EMB) in the first 6 weeks after transplant (early ACR) and at least 4 EMBs between 6 weeks and 1 year after transplant with none graded ≥3A/2R during this time. Subjects were then classified based on the frequency of grade 1B ACR during the 6 week to 1 year post transplant period. Those with two or more episodes of grade 1B ACR that comprised at least 25% of biopsies during this time were classified as “recurrent 1B”. The remainder was classified as “non-recurrent 1B.” All EMB grades were abstracted from the medical record and all patients were clinically well at the time of these biopsies. Those who died or were re-transplanted during the first year after transplantation were excluded from the analysis.
Outcomes assessed were freedom from late (>1 year) ACR (grade ≥3A/2R), coronary artery disease (CAD), re-transplantation/death, and a composite end-point of these outcomes. CAD was routinely assessed by coronary angiography in patients weighing ≥15kg beginning 1 year after transplant and repeated on a biannual basis. CAD diagnosed at autopsy was also included in the analysis.
All patients received tacrolimus based immunosuppression. Prior to our routine use of thymoglobulin induction therapy in 2004, patients received intravenous or oral tacrolimus within 24 hours of transplantation and were maintained on tacrolimus while corticosteroids were tapered over the first 6 months after transplant. Since 2004 we have used thymoglobulin induction with delayed initiation of tacrolimus until 2 – 3 days after transplantation. These patients were quickly tapered off steroids after transplant (5 –7 days) and were maintained on tacrolimus plus adjunctive therapy (either sirolimus or mycophenolate mofetil).
During the study period, surveillance EMBs were usually performed 6–8 times in the first year after transplant (at 7 to 14 days, 1, 2, 4, 6 – 7, 9 – 10, and 12 months). Infants or patients with limited central venous access typically had less frequent surveillance EMBs while those with grade 1B ACR on any biopsy often had follow-up EMB earlier than those with EMB grades 0 or 1A.
Characteristics of the study groups were compared by the Wilcoxon rank-sum test or Fisher’s exact test. Univariate analyses of time-to-event outcomes were assessed by the Kaplan-Meier technique using log-rank tests to determine significance. Multivariable analyses of these outcomes were performed using Cox proportional hazard modeling. Each model included clinically relevant confounders as well as variables that were significant to p≤0.1 in univariate analyses. All tests employed a two-sided alpha of 0.05. Statistical analyses were performed using Stata 10.1 (StataCorp LP, College Station, TX).
RESULTS
Patients
Sixty-two of 159 patients (39%) who underwent orthotopic cardiac transplantation met eligibility criteria. Reasons for exclusion were as follows: grade ≥3A/2R rejection between 6 weeks and 1 year after transplant (n=58), fewer than 4 EMBs between 6 weeks and 1 year (n=18), more than a single ≥3A/2R EMB prior to 6 weeks after transplant (n=10), death prior to 1 year after transplant (n=8), and loss to follow-up prior to 1 year after transplant (n=3). Of the 8 deaths in the first post transplant year, 2 died of complications of PTLD, and 1 each died of progressive pulmonary vein stenosis, heart failure due to accelerated graft vasculopathy, primary graft failure, early post-transplant sepsis, and severe acute rejection due to non-compliance. The cause of death of 1 patient could not be ascertained.
Median age at transplantation was 4.6 years (range 13 days to 20.8 years) and median length of follow-up was 5.1 years (4.2 months to 19.7 years). Seventeen of the study patients (27%) were transplanted between 1990 and 1999 and 31 patients (50%) received thymoglobulin induction. The non-recurrent 1B group consisted of 53 patients (86%) whereas the remaining 9 patients (14%) comprised the recurrent 1B group.
Baseline demographics
Group characteristics are shown in table 1. Median age at transplantation and the proportion of patients with early ACR were greater in the recurrent 1B group (p=0.05 and p=0.01, respectively). Non-white race and male sex were also more common in the recurrent 1B group; however these differences did not reach statistical significance (p=0.1 and p=0.28, respectively). There were no significant differences in duration of follow-up, underlying diagnosis, pre-transplant allosensitization (DTT treated CDC-PRA >10%), proportion with a positive CDC crossmatch, and proportion receiving induction therapy.
Table 1.
Group characteristics
| Non-recurrent 1B group (n=53) |
Recurrent 1B group (n=9) |
p-value | |
|---|---|---|---|
| Age at transplant (years) | 4.04 (0.04 – 19.86) | 10.66 (1.45 – 20.75) | 0.05 |
| Follow-up (years) | 4.73 (0.35 – 19.65) | 5.84 (4.46 – 11.41) | 0.88 |
| Male gender (%) | 31 (59) | 3 (33) | 0.28 |
| Race | |||
| White (%) | 41 (77) | 4 (44) | 0.1 |
| Non-white (%) | 12 (23) | 5 (56) | |
| Underlying diagnosis | |||
| Cardiomyopathy (%) | 33 (62) | 4 (44) | 0.47 |
| Non-Cardiomyopathy (%) | 20 (38) | 5(56) | |
| Pre-allosensitizeda (%) | 15 (28) | 3 (33) | 0.71 |
| Positive CDC crossmatch | 2 (4) | 1 (11) | 0.38 |
| Induction therapy (%) | 27 (51) | 4 (44) | 0.99 |
| Re-transplant recipients | 3 (6) | 2 (22) | 0.15 |
| Era | |||
| 1990 – 1999 (%) | 16 (30) | 1 (15) | 0.42 |
| 2000 – 2006 (%) | 37 (70) | 8 (89) | |
| Early ACRb (%) | 12 (23) | 6 (67) | 0.01 |
Data presented as median (range) or count (percentage)
ACR, acute cellular rejection;
CDC-PRA >10%;
<6 weeks
Outcomes post transplant
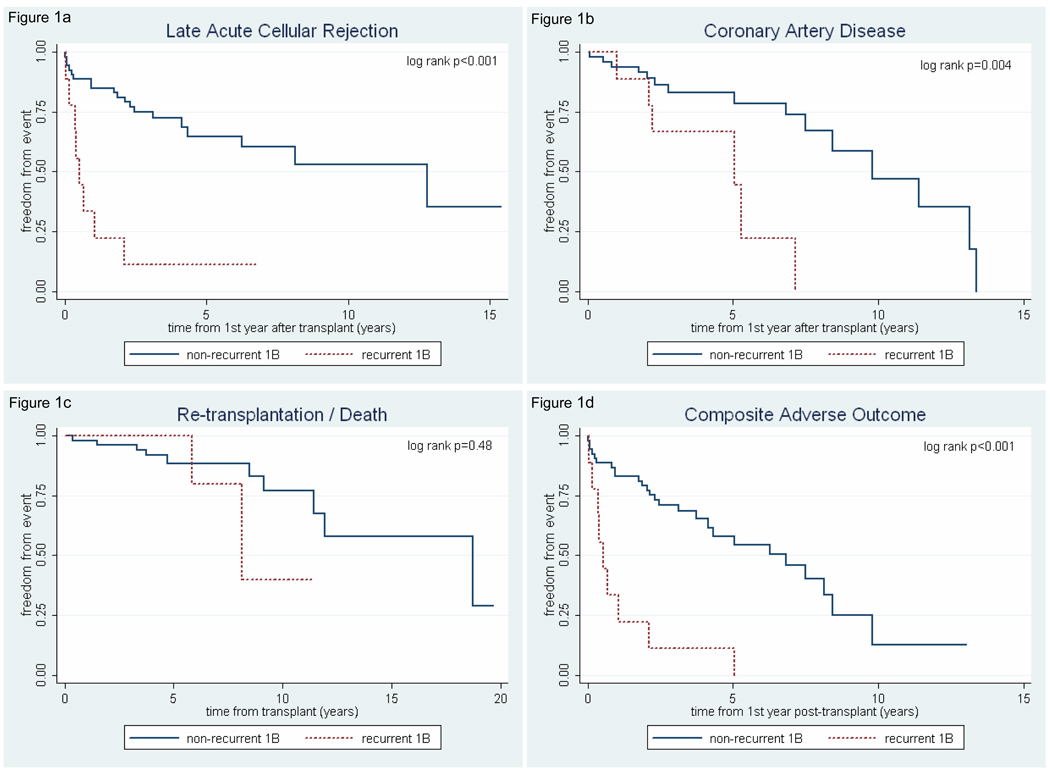
As shown in figure 1, recurrent 1B status was associated with decreased freedom from late acute cellular rejection (p<0.001), CAD (p=0.004), and the composite outcome of late ACR, CAD, or re-transplantation/death (p<0.001). We found no difference in freedom from re-transplantation/death (p=0.48) between the groups (figure 1c).
Figure 1.
Kaplan-Meier curves showing effect of recurrent 1B group status on late post-transplant outcomes. (a) Freedom from late (>1 year) grade ≥3A/2R acute cellular rejection. (b) Freedom from post-transplant coronary artery disease. (c) Freedom from re-transplantation/death. (d) Freedom from composite end-point (late ≥3A/2R ACR, coronary artery disease, or re-transplantation/death).
Using multivariable Cox proportional hazard models, recurrent 1B group status conferred an increased hazard of late ACR (HR 5.90, 95% CI 1.97 – 17.69; p=0.002), after controlling for age at transplant, early ACR, race and sex; and an increased hazard for the composite outcome (HR 4.52, 95% CI 1.65 – 12.37; p=0.003) after controlling for age at transplant, early ACR, race, sex, and primary vs. re-transplant status. There was no independent association of CAD with recurrent 1B status.
DISCUSSION
In our analysis of this retrospective pediatric cohort we have found that recurrent grade 1B ACR between 6 weeks and 1 year after transplantation is independently associated with decreased freedom from late ACR and decreased freedom from the composite endpoint of late ACR, CAD, or re-transplantation/death. We also found a strong association between recurrent 1B status and decreased freedom from CAD by Kaplan-Meier analysis; however this difference was not observed when other risk factors for CAD were considered. These data suggest that the presence of recurrent grade 1B ACR is a clinically important observation that may be associated with poorer late outcomes and questions the wisdom of having removed grade 1B rejection from the revision of the 1990 ISHLT Classification scheme in 2005 (1).
Our results are consistent with data from an adult heart transplant population which found that patients with grades 1B or 2 ACR on more than 20% of their biopsies had worse graft function and lower 3 year survival than patients with at least 80% grade 0 or 1A biopsies or patients with only 1–2 episodes of grade 3A or higher rejection (5). Also, a recent study profiling gene expression in peripheral blood of cardiac allograft recipients showed those with biopsy grade 1B ACR had an elevated AlloMap® score as compared to those with grades 0 and 1A. In fact, the score for grade 1B ACR was comparable to grades ≥3A/2R (6). These data suggest greater immune system activation and more significant sequelae in the setting of grade 1B ACR.
One factor cited for the change in the ISHLT cardiac allograft grading scheme was report of poor inter-observer reproducibility in the classification of biopsies using the 1990 grading scheme (7). However a recent study showed no improvement in inter-observer variability with the current grading scheme (8). Additional factors cited for the change included studies showing that grade 1A, 1B, 2 and some subsets of grade 3 ACR only progress to higher grades on subsequent EMB in 15 to 20% of cases (9–11) and reports demonstrating that lower grades of rejection may resolve without treatment (7, 10–13). However, these reports only assessed the degree of rejection at the next biopsy rather than any long-term clinical outcomes. Therefore, it remains possible that grade 1B ACR, especially if recurrent, has significantly different clinical implications than grade1A during long term follow-up.
In our study, we excluded any patient with more than one EMB ≥3A/2R in the first 6 weeks after transplant as well as those with any acute rejection episode (EMB ≥3A/2R) between 6 weeks and 1 year after transplant since we were interested in the late effects of recurrent first year 1B rejection in the absence of an adverse first year rejection profile. We chose these entry criteria because it has been demonstrated that recurrent early ACR (≥3A/2R), but not a single early ACR episode, is associated with late ACR and coronary artery disease (3, 14). Ideally, we would wish to evaluate a population of patients without any first year acute rejection episodes; however, too few patients would have fulfilled study entry criteria with this strict diagnosis.
Because the timing of transplantation in our study population spanned many years, we felt it was important to assess the groups in terms of era. With both groups having the majority of patients transplanted after 1999 (70% non-recurrent 1B vs. 89% recurrent 1B), it is unlikely that less experienced immunosuppressive management strategies from the early era were a significant contributor to the adverse outcomes we observed in the recurrent grade 1B group. We also considered the use of induction therapy but found no significant difference between the groups.
When interpreting our results, it is necessary to consider a number of significant limitations of our study. Our sample size was small and the data were derived from retrospective chart review. Thus it is possible that there are differences between the groups that were not observed due to the small sample sizes and therefore not accounted for in our analyses. For this reason, we considered race and sex in the multivariable analyses despite the lack of statistical significance between the groups for these characteristics. Also, as a result of our selective inclusion criteria it is possible that our study population may be so restrictive as to limit the generalizability of our findings.
Because nearly all of these biopsies were collected in an era in which it was not routine clinical practice to assess for post-transplant donor-specific antibodies, we unfortunately have no data on the presence of alloantibodies or C4d staining at the time of these biopsies. Clearly, it is possible that patients with recurrent grade 1B EMBs might have increased prevalence of donor specific alloantibodies and we are currently addressing this hypothesis in a prospective fashion. If proven, this would add strength to the argument that recurrent 1B rejection does not have the same clinical implications and long term outcomes as grade 1A rejection and the two should not be merged. It might also support the contention that recurrent 1B rejection should lead to evaluation of the recipient for alloantibodies and evidence of antibody mediated rejection.
Another limitation is that over the 16 year study period, EMBs were reviewed by at least 7 different pediatric pathologists making it difficult to fully control for inter-interpreter grading variability. Nonetheless, all were very experienced in interpretation of pediatric EMB with each reporting at least 100 EMB annually. Furthermore, all EMBs were reviewed in a weekly combined pathology-transplant cardiology conference where consensus was reached on any questionable EMB grades. While this is an important methodological consideration, we believe our findings show that recurrent grade 1B EMBs, as reported by routine clinical evaluation in an experienced pathology department, predicts adverse allograft and patient outcomes.
Finally, while our findings support the contention that recurrent grade 1B ACR does not carry the same prognostic significance as lesser degrees of rejection (1990 ISHLT grades 0 and 1A), we are not simply recommending a return to the 1990 grading scheme as this would not resolve all clinical diagnostic ambiguities that were present within the old grading system (1, 15). For example, while the distinction between grades 1B and 3A was sometimes difficult to make, this problem is not at all solved by the current scheme. Under the 1990 system one could at least delineate grade 1B from grade 1A and thereby indicate a more active (and perhaps more worrisome) biopsy. Under the current ISHLT scheme, one is forced to decide between an even less descriptive grade 1R (encompassing grades 1A, 1B, and 2) or grade 2R. Thus, we suggest that any future grading system maintain a separate category for mild diffuse rejection, as recurrent episodes of this nature appear to portend adverse long-term graft outcomes.
In summary, we have shown in this retrospective pediatric cohort that recurrent grade 1B acute cellular rejection in the first year after cardiac transplantation is independently associated with late acute cellular rejection and the composite end-point of late acute cellular rejection, CAD, and re-transplantation/death. Further study of the impact of removal of the 1B classification from the ISHLT grading scheme seems warranted.
Acknowledgments
Dr. Feingold’s effort on this project was made possible by grant KL2 RR024154 from the National Center for Research Resources (NCRR), a component of the National Institutes of Health (NIH), and NIH Roadmap for Medical Research. Its contents are solely the responsibility of the authors and do not necessarily represent the official view of NCRR or NIH.
Footnotes
DISCLOSURES
No author has a financial interest or other potential conflict of interest related to subject matter or materials mentioned in the manuscript.
AUTHOR CONTRIBUTIONS
Brian Feingold: concept/design, statistics, data analysis/interpretation, drafting article, critical revision of article, approval of article
Claire Irving: data analysis/interpretation, drafting article, critical revision of article, approval of article
Gregory H. Tatum: concept/design, data analysis/interpretation, drafting article, critical revision of article, approval of article
Steven A. Webber: concept/design, data analysis/interpretation, critical revision of article, approval of article
REFERENCES
- 1.Stewart S, Winters GL, Fishbein MC, et al. Revision of the 1990 working formulation for the standardization of nomenclature in the diagnosis of heart rejection. J Heart Lung Transplant. 2005;24:1710–1720. doi: 10.1016/j.healun.2005.03.019. [DOI] [PubMed] [Google Scholar]
- 2.Billingham ME, Cary NR, Hammond ME, et al. A working formulation for the standardization of nomenclature in diagnosis of heart and lung rejection: heart rejection study group. J Heart Lung Transplant. 1990;9:587–593. [PubMed] [Google Scholar]
- 3.Webber SA, Naftel DC, Parker J, et al. Late rejection episodes more than 1 year after pediatric heart transplantation: risk factors and outcomes. J Heart Lung Transplant. 2003;22:869–875. doi: 10.1016/s1053-2498(02)00819-7. [DOI] [PubMed] [Google Scholar]
- 4.Chin C, Naftel DC, Singh TP, et al. Risk factors for recurrent rejection in pediatric heart transplantation: a multicenter experience. J Heart Lung Transplant. 2004;23:178–185. doi: 10.1016/S1053-2498(03)00059-7. [DOI] [PubMed] [Google Scholar]
- 5.Anguita M, Lopez-Rubio F, Arizon JM, et al. Repetitive nontreated episodes of grade 1B or 2 acute rejection impair long-term cardiac graft function. J Heart Lung Transplant. 1995;14:452–460. [PubMed] [Google Scholar]
- 6.Bernstein D, Williams GE, Eisen H, et al. Gene expression profiling distinguishes a molecular signature for grade 1B mild acute cellular rejection in cardiac allograft recipients. J Heart Lung Transplant. 2007;26:1270–1280. doi: 10.1016/j.healun.2007.09.017. [DOI] [PubMed] [Google Scholar]
- 7.Winters GL, McManus BM. Consistencies and controversies in the application of the International Society for Heart and Lung Transplantation working formulation for heart transplant biopsy specimens. Rapamycin cardiac rejection treatment trial pathologists. J Heart Lung Transplant. 1996;15:728–735. [PubMed] [Google Scholar]
- 8.Yang HM, Lai CK, Gjertson DW, et al. Has the 2004 revision of the International Society of Heart and Lung Transplantation grading system improved the reproducibility of the diagnosis and grading of cardiac transplant rejection? Cardiovasc Pathol. 2009;18:198–204. doi: 10.1016/j.carpath.2008.05.003. [DOI] [PubMed] [Google Scholar]
- 9.Winters GL, Loh E, Schoen FJ. Natural history of focal moderate cardiac allograft rejection. Is treatment warranted? Circulation. 1995;91:1975–1980. doi: 10.1161/01.cir.91.7.1975. [DOI] [PubMed] [Google Scholar]
- 10.Lloveras JL, Escourrou G, Delisle MB, et al. Evolution of untreated mild rejection in heart transplant recipients. J Heart Lung Transplant. 1992;11:751–756. [PubMed] [Google Scholar]
- 11.Brunner-LaRocca HP, Sutsch G, Schneider J, Follath F, Kiowski W. Natural course of moderate cardiac allograft rejections (International Society for Heart Transplantation Grade 2) early and late after transplantation. Circulation. 1996;94:1334–1338. doi: 10.1161/01.cir.94.6.1334. [DOI] [PubMed] [Google Scholar]
- 12.Rizeq MN, Masek MA, Billingham ME. Acute rejection: significance of elapsed time after transplantation. J Heart Lung Transplant. 1994;13:862–868. [PubMed] [Google Scholar]
- 13.Milano A, Caforio AL, Livi U, et al. Evolution of focal moderate (International Society for Heart and Lung Transplantation Grade 2) rejection of the cardiac allograft. J Heart Lung Transplant. 1996;15:456–460. [PubMed] [Google Scholar]
- 14.Pahl E, Naftel DC, Kuhn MA, et al. The Impact and Outcome of Transplant Coronary Artery Disease in a Pediatric Population: A 9-year multi-institutional study. J Heart Lung Transplant. 2005;24:645–651. doi: 10.1016/j.healun.2004.03.021. [DOI] [PubMed] [Google Scholar]
- 15.Rodriguez ER. The pathology of heart transplant biopsy specimens: revisiting the 1990 ISHLT working formulation. J Heart Lung Transplant. 2003;22:3–15. doi: 10.1016/s1053-2498(02)00575-2. [DOI] [PubMed] [Google Scholar]