Abstract
Sixteen participants viewed a videotaped tour of 4 houses, highlighting a series of objects and their spatial locations. Participants were tested for memory of object, spatial, and temporal order information while undergoing functional Magnetic Resonance Imaging. Preferential activation was observed in right parahippocampal gyrus during the retrieval of spatial location information. Retrieval of contextual information (spatial location and temporal order) was associated with activation in right dorsolateral prefrontal cortex. In bilateral posterior parietal regions, greater activation was associated with processing of visual scenes, regardless of the memory judgment. These findings support current theories positing roles for frontal and medial temporal regions during episodic retrieval and suggest a specific role for the hippocampal complex in the retrieval of spatial location information
INTRODUCTION
A variety of distinctions have been made to categorize different types of memory. One common distinction is that between episodic and semantic memory (Tulving, 1972; Tulving, 1983; Tulving, 1985). Semantic memory refers to general knowledge about the world – objects, concepts, relations, and facts – when the source or context in which the information was acquired is unknown or irrelevant. Episodic memory, the focus of the present study, represents events such as the first time you drove a car or the day you moved into a new apartment. According to Tulving (1972; 1983; 1985), episodic memory refers to the recollection of an event that is defined by a unique spatial and temporal context. Phenomenologically, retrieval from episodic memory has been described as remembering or re-experiencing a specific event from the past (Tulving & Markowitsch, 1998).
Despite the importance of context for episodic memory, few experimental paradigms simultaneously investigate the three defining components of episodic memory, namely content (objects or items), spatial context, and temporal information. Studies of episodic memory most often focus on the retrieval of items that are learned within a single episode, or some aspect of the source of the information. Source, as the term is typically used in the literature, may refer to multiple types of context, such as spatial, temporal, or physical attributes of the original learning event, depending upon the experimental paradigm. A typical spatial location paradigm entails the presentation of objects in various locations in a grid; participants are tested subsequently for their memory for the objects alone or for the objects and their original locations (Chalfonte, Verfaellie, Johnson, & Reiss, 1996; Kohler, Moscovitch, Winocur, Houle, & McIntosh, 1998; Piggot & Milner, 1993; Smith & Milner, 1981; 1984; 1989). Other typical source paradigms present words, sentences, or other items in different test lists (visual presentation) or different voices (auditory presentation; Glisky, Poster, & Routhieaux, 1995; Glisky, Rubin, & Davidson, 2001; for review, see Johnson, Hashtroudi, Lindsay, 1993). The participant is then tested on his or her memory for the item (a word or sentence) and the source or presentation context (the test list or voice).
Evidence from animal, neuroimaging, and patient studies indicate that medial temporal lobe structures play an important role in encoding and retrieval of episodic memories (for reviews see O’Keefe & Nadel, 1978; Lepage, Habib, & Tulving, 1998). Additionally, there is little question that at least some frontal lobe regions play a critical role in episodic memory retrieval, as discussed in recent reviews of neuroimaging (Fletcher & Henson, 2001), normal aging in older adults (Glisky, 1998), and confabulating patients (Johnson, Hayes, D’Esposito, & Raye, 2000). However, considerable debate remains regarding the specific contributions of medial temporal and frontal subregions to episodic memory. Additionally, current theories of episodic memory either focus on the role of the frontal lobes (Nolde, Johnson, Raye, 1998; Tulving, Kapur, Craik, Moscovitch, & Houle, 1994) or the role of the medial temporal lobes (Lepage et al., 1998; Nadel & Moscovitch, 1997; Squire, 1992), without describing how these regions may interact (but see Glisky, 1998; Moscovitch, 1994).
One step towards an integrated theory of episodic memory retrieval may be to simultaneously assess contributions of frontal and medial temporal regions while isolating retrieval of content from various aspects of context. Several recent neuroimaging studies have investigated different components of episodic memory retrieval, with somewhat inconsistent results. In a positron emission tomography (PET) study, participants studied words that were presented in one of two lists and appeared either on the left or the right side of the screen (Nyberg, McIntosh, Cabeza, Habib, Houle, & Tulving, 1996). Participants were later tested for their memory for the words (items), the study list in which the words appeared (temporal order), and whether the words appeared on the left or right side of the screen (spatial location). The authors reported specific regions of activation that were associated with the retrieval of each episodic component; right middle temporal gyrus and right inferior frontal gyrus for item retrieval, left middle frontal gyrus for location information, and anterior cingulate gyrus for temporal order. Burgess, Maguire, Spiers, and O’Keefe (2001) also investigated this issue using a virtual reality environment. In this functional magnetic resonance imaging (fMRI) study, participants explored a computer generated environment and were presented with objects from one of two characters in either of two spatial locations. During subsequent scanning, participants were then tested for memory for the objects they received, the character who presented the object, and where they received the object. The results indicated activation in bilateral anterior prefrontal cortices during retrieval of both types of context information. Unlike Nyberg et al. (1996), however, they did not find activation in left middle frontal gyrus associated specifically with retrieval of spatial location, but instead reported a network of regions that included right posterior parietal lobe, precuneus, and bilateral parahippocampal gyri. This study did not test for temporal order information.
The goal of the present study was to further address the regional patterns of activation in frontal and medial temporal lobe structures during recollection of specific elements of episodic memory. Like Nyberg et al. (1996), we focused on the defining components of episodic memory, comparing retrieval of item, spatial, and temporal information acquired within a single episode. We propose that while some brain regions will participate similarly in the retrieval of all aspects of episodic memory, retrieval of item, spatial, and temporal information may also rely on more specific regions within the frontal and medial temporal lobes. Like Burgess et al., (2001), we chose to present participants with context-rich materials. Participants in the present study viewed a narrated videotaped tour of four houses in the Tucson area. The tour highlighted a series of objects and their particular locations within a distinctive room in each house. During a subsequent fMRI scan, participants were tested for their memory for the objects, the spatial locations of the objects, and the temporal order in which the objects were presented.
MATERIALS AND METHODS
Participants
Sixteen volunteers with normal vision and hearing who gave informed consent were recruited from the University of Arizona community and screened for contraindications to MRI. Participants were oriented to the fMRI procedure prior to the imaging session and were compensated for their participation. Two subjects were excluded from the neuroimaging analyses based on their behavioral accuracy results, which did not differ significantly from chance. The remaining 14 subjects (9 females and 5 males; mean age, 24 years; range, 20–36 years; mean level of education, 16.2 years) were included in all analyses.
Study materials and procedure
During the study phase, participants were placed supine on the MRI table, fitted with high-resolution goggles and earphones (Resonance Technologies, Inc.), and their heads were stabilized with cushions. They were shown the study material via the goggles while lying outside the scanner. Study material consisted of a videotaped tour of four different houses in the Tucson community, viewed consecutively. Total viewing time was approximately 9 mins. Each tour began with a view of the outside of the house for 5 secs, and then moved inside to a distinctive room, highlighting objects (between 7 and 9 objects per house) and their spatial locations within the room. The tour was narrated to ensure that participants were attending to target objects while never naming them; “Notice what’s on the (coffee table).” After a 2 sec delay (black screen), the tour of the next house began, as described above. In total, participants were presented with 30 target objects. Two study videos were created using the same four house tours and target objects, with the four houses presented in different orders. Presentation of the two study videos was counterbalanced across participants.
Prior to viewing the videotape, participants were told that they would be tested for their memory for the objects, the location of objects, and the order of objects and their locations. It was emphasized that they would not have to remember the order of objects as they were encountered within each house, but that they would be required to remember whether the item was encountered within the first, second, third, or fourth house. They were shown a brief practice videotape and samples of test materials to ensure that they understood the types of information that would later be tested. The entire study videotape was presented to participants twice, because pilot data suggested that recognition rates, particularly for the temporal order condition, were poor after only one presentation of the tour. After viewing the videotape twice, the participants were moved into the bore of the scanner, and sagittal localizer and T1 structural scans were collected (see Imaging Parameters), so that the delay between study and test was approximately 15 mins.
Test materials and procedures
The test phase occurred while participants were undergoing functional scanning. Participants were tested on their memory for the objects, spatial location, and temporal order using a self-paced two-alternative forced choice recognition test. The test list was based on the 30 target objects presented during the videotaped tour, and included 240 trials: 60 object, 60 spatial location, 60 temporal order, and 60 control trials (details of each trial type are described below; see Figure 1 for examples). Trials were presented in quasi-random order with the stipulations that a) an object had to be tested in the object recognition condition before it was tested in either the spatial location or temporal order condition, and b) at least three intervening trials occurred before an object was presented again in any trial type. Halfway through the 120 trial test, participants were given a 90 sec rest break, and then the remaining half of the test trials were presented.
Figure 1.
Examples of test probes. The cue appeared on the screen for 2 seconds, followed by the simultaneous presentation of the two images. The images remained on the screen until the participant made a response.
The general description of a trial was as follows: All trials were preceded by a cue presented for 2 secs that alerted the participant to the particular trial type that would follow. Two still photographs were then presented side-by-side on a white background. It is important to note that all photographs of objects and scenes used to create the test trials were taken from the exact same perspective as they were viewed during the videotaped tour. Participants indicated their choice by pressing one of two response buttons held in their left and right hands, corresponding to the left and right pictures respectively. Correct responses were equally likely to occur on either side of the screen. The test proceeded to the next trial 1.5 secs after the participant made their choice. Thus, a trial lasted 3.5 secs plus the response time of the participant for that particular trial, resulting in jittered total trial times ranging from 4.4 secs to 6.9 secs.
While participants were in the scanner, we presented test stimuli to them via the high-resolution goggles using a PC computer and DMDX (version 2.4.06; Forster & Forster, 2003), a stimulus presentation program which also recorded the timing of the onset of each test stimulus, button press responses, and response times. Onset of scanning was also controlled by DMDX, to allow for exact alignment of recorded stimulus onset times and volume acquisition times for the purpose of event-related analysis.
Memory for object trials consisted of a target object from the videotaped tour presented beside a similar object lure not previously viewed in the videotape. There were 60 object trials that included the 30 target objects, each tested twice, and 30 object lures that were also repeated. However, target and lure pairs were not repeated. Thus, on the first test trial, a target object was presented with a novel object lure. On the second test trial, the target object was presented with a repeated object lure, but the target-lure pair had not previously occurred. Object trials were preceded by the cue “WHICH OBJECT DID YOU SEE?”
Memory for spatial location trials consisted of 30 object-location targets and 60 object-location lures. Lures were created by recombining objects and locations that were viewed during the videotaped tour. As with object trials, object-location targets were tested twice. On one test trial, the target was presented with an object-consistent lure (the same target object was presented but in an incorrect location within the same house). On the other test trial, the target was presented with a landmark-consistent lure (the same location was presented but with an incorrect object from the same house). The participant was required to choose which object-location pair occurred during the tour. Trials were preceded by the cue “WHICH SCENE DID YOU SEE?”
Memory for temporal order trials presented two objects or two scenes that were previously viewed in the videotaped tour, and the participant was required to indicate which object or scene was viewed first. There were 30 object trials and 30 scene trials in the temporal order condition. All trials compared two objects or two scenes that had occurred in different houses. Trials were preceded by the cue “WHICH OBJECT WAS VIEWED FIRST?” or “WHICH SCENE WAS VIEWED FIRST?”
Control Condition stimuli were Fourier transformed images of objects and scenes used in the three memory trial types. No image was identifiable as an object. Two scrambled pictures were presented side-by-side, one overlaid with an ‘X’ and the other overlaid with an ‘O’. Participants were required to determine which picture had an ‘X’ or which picture had an ‘O.’ There were 60 control trials, with 30 trials preceded by the cue “WHICH PICTURE HAS AN ‘X’?” and 30 trials preceded by the cue “WHICH PICTURE HAS AN ‘O’?”
Image acquisition
Images were collected on a 1.5 Tesla whole body scanner (Signa Echo Speed, General Electric, Milwaukee, WI). Total scan time was approximately one hour. A sagittal localizer was collected in order to align a set of T1 weighted set of images (matrix=256×256, TR = 500, TE = min full, FOV = 22, sections=25, 4.5mm, no skip) parallel to the anterior commissure-posterior commissure plane. Following acquisition of the T1 anatomical images, whole brain functional images were acquired in the same anterior-posterior commissure plane using a single-shot spiral sequence (Glover & Lee, 1995; matrix=64, TR=2500, TE=40, sections=25, 4.5mm, no skip). Functional scanning occurred in two runs, and lasted approximately 35 mins. After the completion of the functional scans, a high resolution SPGR series (1.5mm sections covering whole brain, matrix=256×256, flip angle=30, TR=22ms, TE=min full, FOV=25cm) was collected in order to locate anatomical regions of activation and to overlay functional images for co-registration in Talairach space.
Image analysis
Images were corrected for minor head movement using Analysis of Functional NeuroImages (AFNI; Cox, 1996). Data was normalized and smoothed (within-plane, 5 mm) and transformed into standard space (Talairach & Tournoux, 1988). Image data were analyzed using a rapid presentation event-related technique developed and validated by Dale and Buckner (1997; see also Dale, 1999). Only correct responses were included in the event-related analyses. Time windows of a 20 sec post-stimulus onset window and a 5 sec pre-stimulus window were modeled as a linear combination time-invariant hemodynamic response (HDR) with Gaussian noise. HDR estimates for each condition were calculated by simultaneous least-squares fitting across the time windows. The onset time for each test trial stimulus was used as the onset time for the HDR estimation. The estimates of the HDR for the control condition were used as a baseline.
Analyses were conducted as a two-step process, first identifying regions of significant activation, and then extracting and comparing the mean hemodynamic response (HDR) for each experimental condition within each region. In order to identify regions of significant activation, all memory trials (object, spatial, and temporal) were combined and contrasted with the control condition using a group random effects analysis conducted on the co-registered image data sets that included all participants (MGH FS-FAST software; Burock & Dale, 2000). No region was included in further analyses unless it was significant in the group random effects analysis (p<.05). Within regions identified in the group random effects analysis, individual data sets were tested on a voxel by voxel basis using a t-statistic weighted for an ideal HDR (Dale and Buckner, 1997). Voxels that were significant at p<.0001 were included in further analyses for a given individual. Clusters of activation were identified by Talairach coordinates and Brodmann areas were assigned as specified in the Talaraich atlas (Talairach and Tournoux, 1988). While most studies report frontal activations as groups of Brodmann’s areas, regions were separated in this study to determine whether or not a specific frontal region activated differentially depending upon the particular trial type. 1 Medial temporal lobe structures were also carefully separated to investigate differential contributions of hippocampus proper, parahippocampal gyrus, and fusiform gyrus. For instances where the localization of a voxel was equivocal, localization was based on an automated program (Talairach Daemon; Lancaster, Woldorff, Parsons, Liotti, Freitas, Rainey, Kochunov, Nickerson, Mikiten, & Fox, 2000).
Once regions of significant activation were identified, the mean amplitude of HDR was compared across the three memory trial types. For each subject, a mean HDR estimate was created for each memory condition, within each significant region of activation, and output as percent signal change relative to the baseline condition (control task) at 2.5, 5, 7.5, and 10 secs post-stimulus onset. Peak amplitudes for the three memory conditions were analyzed using a repeated measures ANOVA comparing the average of the three peak time points of the HDR: 2.5, 5, and 7.5 secs post-stimulus onset. If a significant main effect of memory condition was observed, follow-up paired-samples t-tests were performed between conditions.
RESULTS
Behavioral Results
Even after two presentations of the video-taped tour, temporal order judgments were more difficult than spatial location judgments, which were in turn more difficult than object recognition (see Table 1). Accuracy and response times were analyzed separately using one-way repeated measures ANOVAs, comparing the three experimental conditions (object, spatial, temporal), and followed up with paired t-tests. Accuracy rates differed significantly, F(2,26)=97.78, p<.001, with higher accuracy for object, followed by spatial location, followed by temporal order (for all pairs, t’s (13) > 3.72, p<.005).
Table 1.
Mean and standard deviations for accuracy scores and response time for object, spatial, and temporal conditions.
| TRIAL TYPE | ACCURACY SCORES (%) | MEAN RESPONSE TIME (ms) |
|---|---|---|
| OBJECT | 93.33 (5.62) | 1447 (285) |
| SPATIAL | 88.45 (5.56) | 1855 (360) |
| TEMPORAL | 72.98 (7.60) | 2045 (609) |
Response times also differed significantly, F(2,26)=23.38, p<.001. Participants responded significantly faster during object trials compared to spatial trials, t(13)=12.7, p<.001 and temporal order trials, t(13)=5.4, p<.001. There was no difference in response time for spatial and temporal trials, t(13)=1.84, ns.
Imaging Results
Results of the random effects analyses indicated significant activation in left and right fusiform gyrus, left and right posterior parietal regions, right hippocampus, and right parahippocampal gyrus. Talairach coordinates for these regions are listed in Figures 2 through 4. Frontal activation included left and right dorsolateral prefrontal cortical regions, and right ventral frontal cortical regions (see Table 2 for details of Brodmann’s areas and Talairach coordinates).
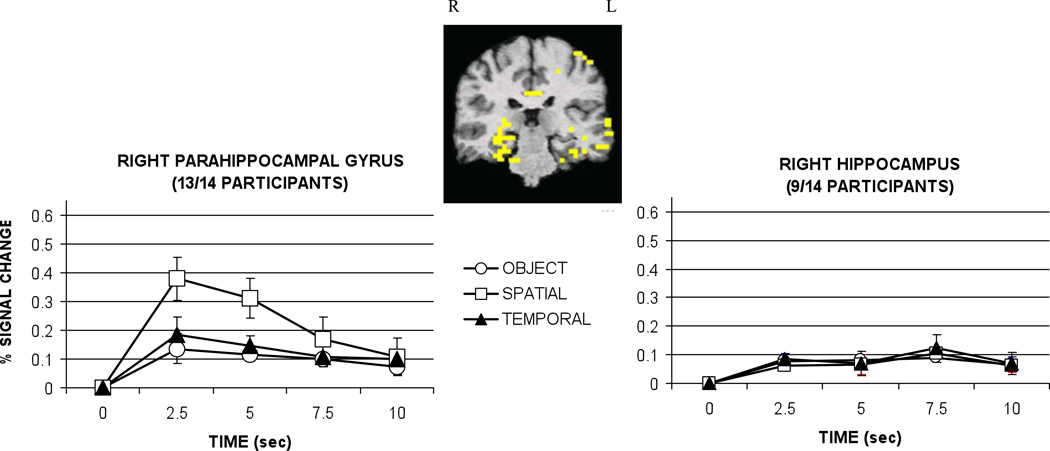
Figure 2.
Hemodynamic responses in right parahippocampal gyrus and right hippocampus graphed as percent signal change relative to baseline (control task) by condition. Talairach coordinates for the center of the activation cluster were located at 24 -35 -9 in the parahippocampal gyrus and 31 -30 -7 in the hippocampus. Error bars represent standard error. Sec = seconds.
Table 2.
Talairach coordinates for regions of frontal lobe activation identified by random effects analysis. Listed are the results of one-way ANOVA and follow-up paired samples t-tests in frontal regions comparing the average peak hemodynamic response between object, spatial, and temporal trials.
| RIGHT FRONTAL LOBE | ||||||
|---|---|---|---|---|---|---|
| Cluster | Talairach | BA | N | MAIN EFFECT | S>O | T>O |
| 1 | 52 30 18 | 46 | 12 | F(2,22)=3.72, p<.05 | t(11)=2.17, p<.05 | t(11)=2.45, p<.05 |
| 45 | 11 | F(2,20)=3.49, p<.05 | t(10)=2.63, p<.05 | t(10)=1.96, p=.08 | ||
| 44 | 8 | F(2,14)=5.52, p<.05 | t(7)=2.64, p<.05 | t(7)=2.63, p<.05 | ||
| 2 | 49 19 34 | 9 | 8 | F(2,14)=3.65, p<.05 | t(7)=2.45, p<.05 | t(7)=2.27, p=.06 |
| 3 | 36 20 45 | 8 | 7 | F(2,12)=4.33, p<.05 | t(6)=2.48, p<.05 | t(6)=2.27, p=.06 |
| 4 | 37 1 54 | 6 | 8 | F(2,14)=7.26, p<.05 | t(7)=2.83, p<.05 | t(7)=3.07, p<.05 |
| 5 | 42 37 −13 | 11 | 7 | F<1,ns | ||
| 47 | 9 | F<1,ns | ||||
| 6 | 37 53 −2 | 10 | 12 | F<1,ns | ||
| LEFT FRONTAL LOBE | ||||||
| Cluster | Talairach | BA | S’s | MAIN EFFECT | S>O | T>O |
| 1 | −43 29 18 | 46 | 11 | F(2,20)=2.79, p=.09 | ||
| 45 | 10 | F(2,18)=2.21, ns | ||||
| 44 | 7 | F(2,12)=1.87, ns | ||||
| 2 | −53 6 37 | 9 | 8 | F(2,14)=2.9, p=.09 | ||
| 3 | −42 1 55 | 6 | 6 | F(2,10)=1.72, ns | ||
| 4 | −34 59 −7 | 10 | 13 | F<1,ns | ||
N indicates the number of participants of 14 included in each subregion analysis. Significant differences were not observed between spatial and temporal conditions in any frontal region. Talairach=Talairach Coordinates of the center of a given cluster, BA=Brodmann’s areas of a given cluster, S=Spatial, T=Temporal, O=Object.
Analyses indicated that some regions were equally involved in the retrieval of each type of information, while others were preferentially involved in the retrieval of contextual (spatial location and temporal order) information, and still others were involved specifically in the retrieval of spatial location. Medial temporal lobe structures will be discussed first, followed by frontal regions. Other areas of activation will be mentioned last.
Medial Temporal Lobe Regions
Retrieval of episodic information was associated with significant activation in two MTL structures, the right parahippocampal gyrus and hippocampus proper, as depicted in Figure 2. The two subregions showed different hemodynamic response patterns for the three trial types.
The most striking finding was the robust activation in right parahippocampal gyrus, observed in 13 of 14 participants. In this region, activation was significantly greater during the retrieval of spatial location information relative to the retrieval of object and temporal information, F(2,24)=5.15, p<.05, all t’s(12)>2.3, p<.05.
Significant activation was also observed in the right hippocampus proper in 9 of 14 participants. The right hippocampus showed equivalent activation during the retrieval of object, spatial locations, and temporal order information, as a main effect for condition F(2,16)<1, ns, was not observed. The difference in pattern of activation between hippocampus and parahippocampal gyrus was confirmed with a repeated-measures ANOVA comparing region (hippocampus, parahippocampal gyrus) and condition (object, spatial, temporal). The analysis yielded a significant interaction, F(2,8)=9.43, p<.01.
Frontal Regions
Table 2 lists all active frontal regions organized by Brodmann’s areas, and follow-up statistical tests comparing activation across memory conditions. Voxels meeting criteria for significance were classified into Brodmann’s areas (BA) using the Talairach atlas (Talairach & Tournoux, 1988). Significant activation was observed bilaterally in anterior and dorsolateral, regions of prefrontal cortex (PFC), and also in right ventral PFC. In the majority of right frontal regions that included dorsolateral PFC and extending into motor regions (BA 44, 45, 46, 6, 8, and 9), greater activation was observed during the retrieval of contextual (spatial and temporal) information than content (object) information. A similar trend was observed across left dorsolateral PFC regions, but differences were not statistically significant. In left and right anterior (BA 10) and right ventral frontal regions (BA 11, 47), no differences in activation were observed between conditions. There were no frontal regions in which we observed significantly greater activation during the retrieval of temporal order information compared to spatial information, or the reverse.
Other regions of activation
Fusiform gyrus
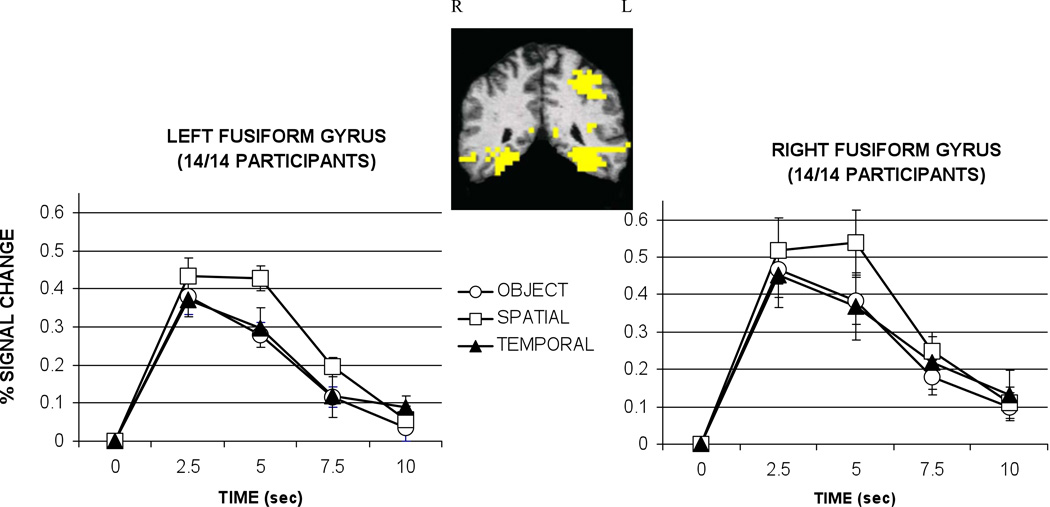
Significant activation was observed in all 14 participants bilaterally in the fusiform gyrus (depicted in Figure 3), and the main effect for condition was significant for the right fusiform, F(2,26)=5.48,p<.01, and the left fusiform, F(2,26)=6.19, p<.01. In both hemispheres significantly greater activation was associated with the retrieval of spatial location compared to object and temporal information, all t’s (13)>2.74, p<.05.
Figure 3.
Hemodynamic responses for left and right fusiform gyrus graphed as percent signal change relative to baseline (control task) by condition. Talairach coordinates for the center of the left and right activation clusters were located at -37 -50 −11 and 32 -50 -13, respectively. Error bars represent standard error. Sec = seconds.
Parietal regions
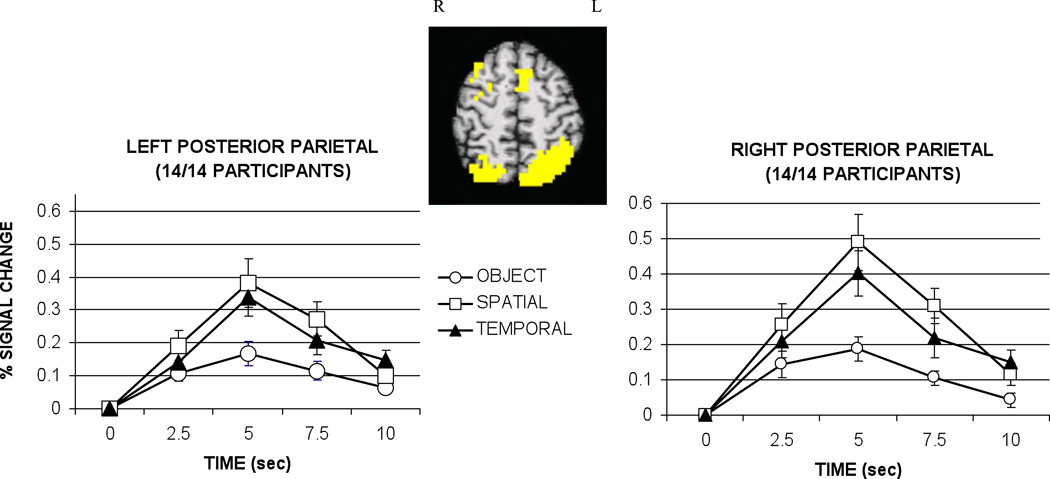
Retrieval of contextual information was associated with significant activation bilaterally in posterior parietal regions in all 14 participants as depicted in Figure 4. The activation in the parietal lobe was extensive, including superior parietal lobule and precuneus as well as portions of the inferior parietal lobule (Brodmann’s Areas 39/40/7). These regions were combined in a single analysis. In the left posterior parietal region, a main effect of condition was observed, F(2,26)=14.07, p<.001. Greater activation in this region was associated with the retrieval of both types of contextual information – spatial location and temporal order – relative to object information, all t’s(13)>3.89, p<.01. No difference in degree of activation was observed during spatial and temporal trials, t(13)=1.70, ns.
Figure 4.
Hemodynamic responses for left and right posterior parietal cortex graphed as percent signal change relative to baseline (control task) by condition. Talairach coordinates for the center of the activation clusters were located at -32 -60 53 on the left and 25 -64 53 on the right. Activation extended through BA 40, 39, and 7 in both the left and right hemispheres. Error bars represent standard error. Sec = seconds.
In the right posterior parietal region, a main effect of condition was also observed, F(2,26)=11.58, p<.001. Greater activation was observed during the spatial and temporal trials compared to object trials. Furthermore, significantly greater activation was associated with the retrieval of spatial locations relative to temporal order information, a pattern not observed in the homologous region on the left, all t’s (13)>2.13, p<.05.
Object recognition repeated trials
One concern in the study design was that target objects and object lures were repeated during the test phase to create 60 test trials. Although the objects and lures were re-paired at second test presentation, it is possible that the second recognition discrimination relied on processes that were more related to source monitoring than to simple object recognition. Since frontal cortical areas in particular have been implicated in source judgments, we compared the peak HDR amplitude for first and second presentation of object recognition in each frontal region. Only one frontal region showed a significant difference, left BA 46, t(9)=3.1, p<.013, where HDR amplitude was greater during the second presentation (mean 0.18% signal change) than first presentation (mean 0.08% signal change). Although this increase was observed in only one frontal region, it raises the possibility that the second presentation of the objects may have relied more heavily on source monitoring processes. However, in all other regions, separating the two object trial types were consistent with results of previous analyses.
Spatial scenes and temporal order scenes
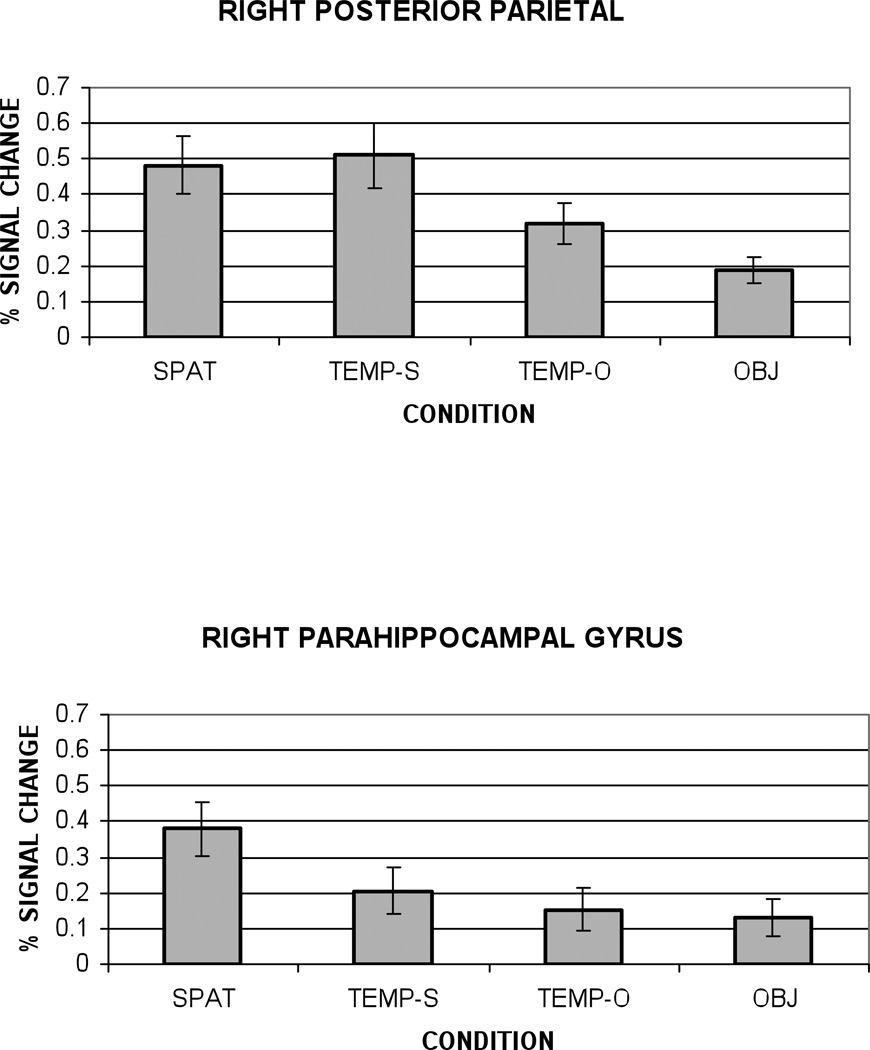
We sought further confirmation that activation in right PHG was specifically associated with retrieval of spatial location information, rather than the visual analysis of complex scenes. Using the temporal order trials, we were able to investigate this issue directly. Recall that there were two subtypes of temporal trials; in half the trials, two objects were presented in a scene, while in the other half of the trials, the two objects were presented alone. If a region responds preferentially to the retrieval of spatial location information, then spatial trials should be signficantly greater than both temporal trial subtypes. If, however, a region is responding preferentially to the visual analysis of a complex scene, then one would predict equivalent activation for all scene trials (spatial and temporal order-scenes), and greater activation for scenes relative to object trials (object and temporal order-objects). These hypotheses were compared in right posterior parietal cortex and right parahippocampal gyrus, as these regions have been implicated in differential processing of scenes and spatial context (Bar & Aminoff, 2003; Burgess et al., 2001).
Figure 5 shows the peak HDR amplitude for each trial subtype in right posterior parietal and right parahippocampal gyrus. Right posterior parietal cortex showed a pattern of activation consistent with the processing of complex visual scenes. Significantly greater peak amplitude activation was observed in conditions that included complex scenes (spatial and temporal order-scenes) relative to trials that presented objects only (objects and temporal order-objects), all t’s (13) > 2.86, p < .01. Importantly, right parahippocampal gyrus responded preferentially to the retrieval of spatial location information. In contrast to parietal cortex, temporal order-scenes, temporal order-objects, and object trials did not differ from one another, all t’s (12) < 1.57, ns. Spatial location trials showed significantly greater activation than both temporal trials and object trials, t’s (12) > 2.36, p < .05.
Figure 5.
Amplitude of the peak hemodynamic response graphed as percent signal change relative to baseline (control task) by condition for right posterior parietal cortex and right parahippocampal gyrus. Error bars represent standard error. SPAT= Spatial condition, TEMP-S=Temporal trials-scenes, TEMP-O=Temporal trials-objects, OBJ=Object trials.
DISCUSSION
To summarize, during episodic retrieval we found regions of activation in medial temporal, frontal, fusiform, and parietal regions consistent with previous neuroimaging studies (Cabeza & Nyberg, 2000; Desgranges, Baron, & Eustache, 1998). Importantly, some regions were equally involved in the retrieval of object, spatial, and temporal information, while others were preferentially involved in the retrieval of both types of contextual information, and still others were involved specifically in the retrieval of spatial location information.
The current results are consistent with a previous fMRI study by Burgess et al. (2001). Both studies tested participants’ memory for objects, spatial locations, and additional non-spatial contextual information – temporal order in the current study and “memory for person” in Burgess et al. (2001). Both studies found preferential activation in right parahippocampal gyrus during retrieval of spatial information relative to object information and non-spatial context. Activation in right and left posterior parietal regions was also greatest during retrieval of spatial context, but unlike right parahippocampal gyrus, this region also responded moderately to non-spatial contextual memories, as greater activation was observed for non-spatial context relative to object information. Finally, both studies found that activation in bilateral dorsolateral prefrontal regions increased during the retrieval of contextual information compared to object retrieval, with no observed differences between spatial and non-spatial context in any frontal region.
Before discussing the results from medial temporal and frontal regions in more detail, it is important to note that the pattern of activation observed did not simply coincide with level of difficulty. Despite behavioral evidence and participant reports that the temporal order condition was the most difficult, no brain region responded preferentially to temporal order information. Activation during temporal order judgments was sometimes equivalent with object retrieval, and sometimes equivalent to spatial location retrieval, depending upon the brain region.
One question that arises from the current study relates to the absence of regions responding preferentially to the retrieval of object or temporal order information. First, although significant activation was observed during object trials relative to the control condition, no region showed activation during object trials that was greater than that observed during temporal and spatial location trials. In the current paradigm, spatial and temporal trials probably also incorporate object recognition as part of the task, making it less likely to observe activation in regions specific to the object-only trials. Second, no region showed a specific response to temporal order trials, with greater activation for temporal trials relative to both object and spatial trials. While frontal regions have been implicated in memory for temporal order (Shimamura, Janowsky, & Squire, 1992), retrieval of temporal order may be confounded with retrieval of spatial information in the current experimental paradigm. To simplify the task, participants may have employed a strategy for temporal trials in which they first thought of the house to which the object belonged, and then the order of the four houses. Using such a strategy, one would not expect to observe obvious differences in the level of activation between spatial and temporal trials. Alternatively, the lack of a frontal region responding to temporal order judgments may also reflect the inadequacy of defining Brodmann areas as identified by atlas-based coordinates. It is possible, given a paradigm that could not rely on a spatially-mediated strategy and better methods for separating functional subfields of frontal lobe clusters, that such temporal order-specific frontal regions would be identified.
Medial temporal lobe regions
Activation was found in both right hippocampus proper and right parahippocampal gyrus (PHG). Hippocampus proper showed a similar pattern of low amplitude activation across object, temporal order, and spatial location conditions. In contrast, activation in adjacent PHG showed a specific response to the spatial location condition. While activation was significant for all three memory conditions, there was a striking increase in amplitude of response during spatial location trials.
Previous imaging studies have implicated PHG in the processing and representation of spatial scenes (Epstein & Kanwisher, 1998; Mellet, Briscogne, Tzourio-Mazoyer, Ghaem, Petit, Zago, Etard, Berthoz, Mazoyer, & Denis, 2000), and for successful encoding of novel information for subsequent memory performance, particularly when encoding includes spatial context (Brewer, Zhao, Desmond, Glover, & Gabrieli, 1998; Davachi, Mitchell, & Wagner, 2003). PHG appears to be particularly important for encoding the location or spatial context of objects in novel large-scale environments. For example, Maguire and colleagues have reported PHG activation during encoding of objects in locations in a virtual environment relative to encoding during exploration of a featureless environment (Maguire, Frackowiak, & Frith, 1996; Maguire, Frith, Burgess, Donnett, & O’Keefe, 1998; see also Aguirre, Detre, Alsop, & D’Espositio,1996). Interestingly, PHG not only mediates the encoding of objects in novel spatial contexts, but it may also play a role in the representation or analysis of long-term spatial contextual associations. A recent fMRI study by Bar and Aminoff (2003) demonstrated that PHG activation was observed when participants viewed objects with differing strengths of contextual associations. Objects that were highly associated with a unique context (e.g., a hardhat) elicited greater activity in PHG than objects without a uniquely associated context (e.g., a book).
The current study suggests that, besides playing a role in the analysis and encoding of spatial context, the PHG also is important for the retrieval of the spatial location of objects. Activation in right PHG was greatest for retrieval of spatial location information – more so than during the retrieval of either object or temporal order information – suggesting that PHG activation was not driven simply by the presentation of complex scenes, but by the specific requirements of the memory task. The secondary analysis separating temporal order trials into scene and object subtypes strengthens this conclusion. The presentation of two complex scenes (as in the temporal order-scene condition) did not, by themselves, increase PHG activation (in contrast to the pattern of activation observed in right posterior parietal cortex). PHG responded preferentially only when subjects were required to remember the combination of scene elements that had been presented at study.
In the present study, the predominant PHG activation was in the right hemisphere, consistent with neuroimaging studies of spatial recognition (Johnsrude, Owen, Crane, Milner, & Evans, 1999) and earlier patient studies that emphasize the importance of right PHG in recognizing object location information. Right temporal lobectomy patients, for example, are impaired in memory for the location of objects but not for the objects themselves (Smith & Milner, 1981; 1984; 1989; Piggot & Milner, 1993). Milner and colleagues attributed this impairment in object location to damage to the hippocampus, although it was noted that damage extended beyond the hippocampus proper to include PHG, and indeed, the severity of object location impairment correlated with the extent of damage. It is possible, therefore, that the critical region underlying the observed deficit was PHG. However, Bohbot, Kalina, Stepankova et al. (1998) tested patients who had undergone thermo-coagulation with a single electrode along the amygdalo-hippocampal axis – a procedure that can potentially lesion single structures of the medial temporal lobe – to alleviate epilepsy. Patients with lesions restricted either to the right hippocampus proper or to the right PHG were equally impaired on an object location memory task. The PHG provides one of the major neocortical inputs to the hippocampus proper; a lesion to the PHG could be considered a functional lesion to the hippocampus.
Alternatively, the PHG and hippocampus proper may play complementary, but different, roles in memory for spatial relations. Burgess, Maguire, & O’Keefe (2002) suggest that PHG is required for the iconic representation of scenes, while the hippocampus is required additionally only when the memory task requires the use of 3D space, such as when an individual is asked to identify a scene from an alternate point of view. The present results are consistent with this hypothesis. Still pictures of objects and scenes presented during the test phase of the study were taken from the same vantage point from which they were originally viewed during the videotaped tour. Subjects therefore were not required to infer information about 3D relations about objects in any of the three test conditions. Although we observed activation in hippocampus proper, the magnitude of that activation was similar across all three test conditions, with no differences between spatial location, temporal order, and object trials.
While there is little doubt that PHG is engaged during the analysis, encoding, and retrieval of spatial location and spatial context, it has been suggested that PHG more generally mediates associative processing of stimuli of any kind, spatial or non-spatial (Eichenbaum, 2001; Bar & Aminoff, 2003). In rat lesion studies, for example, intact PHG is critical for the acquisition of associations between novel odors (Bunsey & Eichenbaum, 1993; Wood, Dudchenko, & Eichenbaum, 1999). In humans, PHG activation has been observed during a task that required subjects to make novel associations between abstract nouns such as calm-illusion (Jackson, Dobbins, & Schacter, 2002). The present study suggests, however, that not all types of non-spatial contextual associations are mediated to the same degree by PHG. Although we observed significant PHG activation during retrieval of temporal order information (which presumably required temporal associations across objects), the magnitude of that activation was no greater than during object retrieval; only the spatial location condition showed preferential activity in PHG. Consistent with the present finding, Burgess et al. (2001) also observed PHG activation associated with retrieval of spatial location, but not with another type of non-spatial source information (which character was holding the object). It remains to be explained why some types of contextual associations engage PHG while others do not, or alternatively, the circumstances under which some non-spatial contexts also engage the PHG.
The finding that the right PHG was most active during retrieval of spatial location has theoretical implications. An ongoing debate exists in the literature on the role of the hippocampal complex during the retrieval of episodic memory (for a review see Nadel, Ryan, Hayes, Gilboa, and Moscovitch, 2003). Multiple Trace Theory (MTT) suggests that the hippocampal complex not only binds disparate pieces of information in the neocortex, but also participates in the storage of contextual information, possibly spatial. An alternative view hypothesizes that the hippocampus merely acts as a pointer to neocortically represented information (Alvarez & Squire, 1994). MTT posits that the hippocampal complex will be preferentially activated during the retrieval of spatial/contextual material (Nadel & Moscovitch, 1997; Nadel, Samsonovitch, Ryan, & Moscovitch, 2000; Nadel et al, 2003). While MTT did not originally specify which structure within the hippocampal complex mediates encoding and retrieval of spatial contextual material, the preponderance of previous literature and the results of the current study suggest that the PHG may serve such a role.
Frontal lobes and their role in context
Researchers agree that the frontal lobes play an integral part during episodic memory retrieval, but there is ongoing debate regarding the laterality of frontal involvement, as well as the specific roles played by various subregions of the frontal cortex. For example, the Hemispheric Encoding/Retrieval Asymmetry model (HERA; Tulving et al., 1994) suggests that retrieval of episodic memories engages right prefrontal regions, while retrieval of semantic memories (evident during encoding of novel material) engages left prefrontal regions. This appears to be true when the episodic task is a simple old/new recognition judgment. In many other studies, frontal activation associated with episodic retrieval is often bilateral, particularly those with more complex or difficult recognition discriminations, including judgments regarding the source of the item being presented (for review, see Nolde et al., 1998).
An alternative model, the Cortical Asymmetry of Reflective Activity (CARA; Nolde et al., 1998), suggests that during memory tasks the right prefrontal cortex is involved with heuristic (automatic) processing of information while the left frontal lobe is recruited for systematic or reflective (deliberate) processing. By this view, simple old/new judgments based on familiarity may be sustained by heuristic processes subserved by right prefrontal cortex. More complex reflective processes necessary for the retrieval of additional information for complex old/new recognition or judgments of source are supported by the additional recruitment of left prefrontal cortical regions.
The results of the current study are generally consistent with the CARA model, since we observed robust activation bilaterally in prefrontal cortex, including BA 10, 46, and 9, regions that have been consistently observed in other complex recognition studies noted in Nolde et al. (1998). Several specific findings, however, warrant discussion. First, the CARA model suggests that simple old/new recognition, such as a forced choice task using one old and one new stimulus, should engage heuristic processes mediated by right prefrontal cortex. In the current study, we used a forced-choice recognition procedure, but our design most likely required greater reflective processing relative to traditional forced-choice tasks. Spatial lures consisted of recombinations of scene components that were all viewed during study, and temporal trials consisted of two scenes, both viewed during study, so these tasks could not simply have involved an assessment of familiarity. Even within the object condition, lures were repeated twice, making reliance on familiarity alone less likely. Performance in each of the three recognition conditions probably involved more complex processing than with a traditional forced choice recognition task, resulting in bilateral activation in PFC.
What is not directly predicted by the CARA model is the finding that right frontal regions, but not left, showed clear differentiations in the degree of activation between context trials (spatial location and temporal order judgments) compared to object-only trials. While we observed a trend for greater activation during context and object-only trials in left prefrontal regions, these differences were not statistically significant. According to the model, the retrieval of contextual information would most likely require additional systematic processing, which should be reflected in left, not right, prefrontal activation. The finding is not necessarily problematic for the CARA model, as it could be argued that spatial and temporal trials also required more heuristic processing than object trials, resulting in greater activation on the right for those conditions. Currently, the CARA model does not specify what particular processes are associated with left and right prefrontal regions, although recent work by Johnson and colleagues has begun to address this issue (Johnson, Mitchell, Raye, & Greene, 2004; Johnson, Raye, Mitchell, Greene, & Anderson, 2003). Based on the design of the current study, it cannot be determined whether or not the increased activation for contextual trials in the right PFC is best explained by increased heuristic processing or increased reflective demand. The present findings are, however, consistent with the general notion that prefrontal regions are important for memory judgments that involve multiple aspects of context and/or source (Fletcher & Henson, 2001; Glisky, 1998). In contrast to PHG, where processing may be more circumscribed, perhaps only related to spatial location context, frontal lobes appear to be involved in multiple aspects of context and/or source.
CONCLUSIONS
In summary, we found that a region of the medial temporal lobe, the right parahippocampal gyrus, was preferentially activated during the retrieval of spatial location information. Retrieval of spatial and temporal contextual information was associated with equivalent activation increases in right prefrontal cortex relative to object recognition. The current study suggests a complex network of brain regions associated with retrieval of episodic memories, and furthermore, that these regions respond differently based on the type of information that is retrieved. The results are consistent with the notion that parahippocampal gyrus participates preferentially in the processing and retrieval of spatial location information, while frontal lobes may mediate retrieval of multiple types of context, including both spatial and temporal information.
ACKNOWLEDGMENTS
This project was completed in partial fulfillment of Scott Hayes’ Master’s degree. This work was supported by the National Institute of Neurological Disorders and Stroke Grant RO1 NS044107-01, the State of Arizona Alzheimer’s Research Center, the McDonnell-Pew Cognitive Neuroscience Program, and the Flinn Foundation Program in Cognitive Science at the University of Arizona. We are grateful to Dr. Elizabeth Glisky for her useful comments and discussion and Dr. Dianne Patterson for technical support. We would like to thank Dr. James T. Enns, Dr. Elizabeth Glisky, Dr. Alfred Kaszniak, Jasmeet Pannu, and Heather Peters for the use of their homes for stimuli collection.
Footnotes
While an atlas-based method for defining Brodmann areas for a given individual is not ideal (see Rajkowski & Goldman-Rakic, 1995), this is an attempt, given the methods currently available, to separate large clusters of frontal activation that may or may not include different functional regions. At best, the method may show that a continguous cluster of active voxels is not homogenous in its response to the different trial types. At least, it will yield a similar pattern of activation for all subregions of a contiguous cluster, as defined by Brodmann area atlas coordinates.
REFERENCES
- Aguirre GK, Detre JA, Alsop DC, D’Esposito M. The parahippocampus subserves topographical learning in man. Cerebal Cortex. 1996;6:823–829. doi: 10.1093/cercor/6.6.823. [DOI] [PubMed] [Google Scholar]
- Alvarez P, Squire LR. Memory consolidation and the medial temporal lobe: A simple network model. Proceedings of the National Academy of Sciences USA. 1994;91:7041–7045. doi: 10.1073/pnas.91.15.7041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bar M, Aminoff E. Cortical analysis of visual context. Neuron. 2003;38:347–358. doi: 10.1016/s0896-6273(03)00167-3. [DOI] [PubMed] [Google Scholar]
- Bohbot VD, Kalina M, Stepankova K, Spackova N, Petrides M, Nadel L. Spatial memory deficits in patients with lesions to the right hippocampus and to the right parahippocampal cortex. Neuropsychologia. 1998;36:1217–1238. doi: 10.1016/s0028-3932(97)00161-9. [DOI] [PubMed] [Google Scholar]
- Brewer JB, Zhao Z, Desmond JE, Glover GH, Gabrieli JD. Making memories: brain activity that predicts how well visual experience will be remembered. Science. 1998;281:1185–1187. doi: 10.1126/science.281.5380.1185. [DOI] [PubMed] [Google Scholar]
- Bunsey M, Eichenbaum H. Critical role of the parahippocampal region for paired-associate learning in rats. Behavioral Neuroscience. 1993;107:740–747. doi: 10.1037//0735-7044.107.5.740. [DOI] [PubMed] [Google Scholar]
- Burock MA, Dale AM. Estimation and detection of event-related fMRI signals with temporally correlated noise: A statistically efficient approach. Human Brain Mapping. 2000;11:249–260. doi: 10.1002/1097-0193(200012)11:4<249::AID-HBM20>3.0.CO;2-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burgess N, Maguire EA, Spiers HJ, O’Keefe J. A temporoparietal and prefrontal network for retrieving the spatial context of lifelike events. NeuroImage. 2001;14:439–453. doi: 10.1006/nimg.2001.0806. [DOI] [PubMed] [Google Scholar]
- Burgess N, Maguire EA, O’Keefe J. The human hippocampus and spatial and episodic memory. Neuron. 2002;35:625–641. doi: 10.1016/s0896-6273(02)00830-9. [DOI] [PubMed] [Google Scholar]
- Cabeza R, Nyberg L. Imaging cognition II: An empirical review of 275 PET and fMRI studies. Journal of Cognitive Neuroscience. 2000;12:1–47. doi: 10.1162/08989290051137585. [DOI] [PubMed] [Google Scholar]
- Chalfonte BL, Verfaellie M, Johnson MK, Reiss L. Spatial location memory in amnesia: Binding item and location information under incidental and intentional encoding conditions. Memory. 1996;4:591–614. doi: 10.1080/741940998. [DOI] [PubMed] [Google Scholar]
- Cox RW. AFNI: software for analysis and visualization of functional magnetic resonance neuroimages. Computers & Biomedical Research. 1996;29:162–173. doi: 10.1006/cbmr.1996.0014. [DOI] [PubMed] [Google Scholar]
- Dale AM. Optimal experimental design for event-related fMRI. Human Brain Mapping. 1999;8:109–114. doi: 10.1002/(SICI)1097-0193(1999)8:2/3<109::AID-HBM7>3.0.CO;2-W. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dale AM, Buckner RL. Selective averaging of rapidly presented individual trials using fMRI. Human Brain Mapping. 1997;5:329–340. doi: 10.1002/(SICI)1097-0193(1997)5:5<329::AID-HBM1>3.0.CO;2-5. [DOI] [PubMed] [Google Scholar]
- Davachi L, Mitchell J, Wagner A. Multiple routes to memory: distinct medial temporal lobe processes build item and source memories. Proceedings of the National Academy of Sciences of the United States of America. 2003;100:2157–2162. doi: 10.1073/pnas.0337195100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Desgranges B, Baron J, Eustache F. The functional neuroanatomy of episodic memory: The role of the frontal lobes, the hippocampal formation, and other areas. Neuroimage. 1998;8:198–213. doi: 10.1006/nimg.1998.0359. [DOI] [PubMed] [Google Scholar]
- Eichenbaum H. The hippocampus and declarative memory: cognitive mechanisms and neural codes. Behavioural Brain Research. 2001;127:199–207. doi: 10.1016/s0166-4328(01)00365-5. [DOI] [PubMed] [Google Scholar]
- Epstein R, Kanwisher N. A cortical representation of the local visual environment. Nature. 1998;392:598–601. doi: 10.1038/33402. [DOI] [PubMed] [Google Scholar]
- Fletcher PC, Henson RNA. Frontal lobes and human memory: Insights from functional neuroimaging. Brain. 2001;124:849–881. doi: 10.1093/brain/124.5.849. [DOI] [PubMed] [Google Scholar]
- Forster KI, Forster JC. DMDX: A windows display program with millisecond accuracy. Behavior Research Methods, Instruments, & Computers. 2003;35:116–124. doi: 10.3758/bf03195503. [DOI] [PubMed] [Google Scholar]
- Glisky EL, Polster MR, Routhieaux BC. Double dissociation between item and source memory. Neuropsychology. 1995;9:229–235. [Google Scholar]
- Glisky EL. Differential contributions of frontal and medial temporal lobes to memory: Evidence from focal lesion and normal aging. In: Raz N, editor. The Other Side of the Error Term. Elsevier Science; 1998. pp. 261–317. [Google Scholar]
- Glisky EL, Rubin SR, Davidson PSR. Source memory in older adults: An encoding or retrieval problem? Journal of Experimental Psychology: Learning, Memory, and Cognition. 2001;27:1131–1146. doi: 10.1037//0278-7393.27.5.1131. [DOI] [PubMed] [Google Scholar]
- Glover GH, Lee AT. Motion artifacts in fMRI: comparison of 2DFT with PR and spiral scan methods. Magnetic Resonance in Medicine. 1995;33:624–635. doi: 10.1002/mrm.1910330507. [DOI] [PubMed] [Google Scholar]
- Jackson O, Dobbins IG, Schacter DL. The ties that bind: an event-related fMRI study of associative encoding. Poster presented at the annual meeting of the Cognitive Neuroscience Society; Poster presented at the annual meeting of the Cognitive Neuroscience Society; San Francisco. 2002. [Google Scholar]
- Johnson MK, Hashtroudi S, Lindsay DS. Source monitoring. Psychological Bulletin. 1993;114:3–28. doi: 10.1037/0033-2909.114.1.3. [DOI] [PubMed] [Google Scholar]
- Johnson MK, Hayes SM, D’Esposito M, Raye CL. Confabulation. In: Grafman J, Boller F, editors. Handbook of Neuropsychology. 2nd ed. Amsterdam, Netherlands: Elsevier Science; 2000. pp. 359–383. [Google Scholar]
- Johnson MK, Mitchell KJ, Raye CL, Greene EJ. An age-related deficit in prefrontal cortical function associated with refreshing information. Psychological Science. 2004;15:127–132. doi: 10.1111/j.0963-7214.2004.01502009.x. [DOI] [PubMed] [Google Scholar]
- Johnson MK, Raye CL, Mitchell KM, Greene EJ, Anderson AW. fMRI evidence for an organization of prefrontal cortex by both type of process and type of information. Cerebral Cortex. 2003;13:265–273. doi: 10.1093/cercor/13.3.265. [DOI] [PubMed] [Google Scholar]
- Johnsrude IS, Owen AM, Crane J, Milner B, Evans AC. A cognitive activation study of memory for spatial relationships. Neuropsychologia. 1999;37:829–841. doi: 10.1016/s0028-3932(98)00136-5. [DOI] [PubMed] [Google Scholar]
- Kohler S, Moscovitch M, Winocur G, Houle S, McIntosh AR. Networks of domain-specific and general regions involved in episodic memory for spatial location and object identity. Neuropsychologia. 1998;36:129–142. doi: 10.1016/s0028-3932(97)00098-5. [DOI] [PubMed] [Google Scholar]
- Lancaster JL, Woldorff MG, Parsons LM, Liotti M, Freitas CS, Rainey L, et al. Automated Talairach atlas labels for functional brain mapping. Human Brain Mapping. 2000;10:120–131. doi: 10.1002/1097-0193(200007)10:3<120::AID-HBM30>3.0.CO;2-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lepage M, Habib R, Tulving E. Hippocampal PET activations of memory encoding and retrieval: The HIPER model. Hippocampus. 1998;8:313–322. doi: 10.1002/(SICI)1098-1063(1998)8:4<313::AID-HIPO1>3.0.CO;2-I. [DOI] [PubMed] [Google Scholar]
- Maguire EA, Burke T, Phillips J, Staunton H. Topographical disorientation following unilateral temporal lobe lesions in humans. Neuropsychologia. 1996;34:993–1001. doi: 10.1016/0028-3932(96)00022-x. [DOI] [PubMed] [Google Scholar]
- Maguire EA, Frackowiak RS, Frith CD. Learning to find your way: a role for the human hippocampal formation. Proceedings of the Royal Society of London. Series B, Biological Sciences. 1996;263:1745–1750. doi: 10.1098/rspb.1996.0255. [DOI] [PubMed] [Google Scholar]
- Maguire EA, Frith CD, Burgess N, Donnett JG, O’Keefe J. Knowing where things are: Parahippocampal involvement in encoding object locations in virtual large-scale space. Journal of Cognitive Neuroscience. 1998;10:61–76. doi: 10.1162/089892998563789. [DOI] [PubMed] [Google Scholar]
- Maguire EA, Henson RNA, Mummery CJ, Frith CD. Activity in prefrontal cortex, not hippocampus, varies parametrically with the increasing remoteness of memories. Cognitive Neuroscience and Neuropsychology. 2001;12:441–444. doi: 10.1097/00001756-200103050-00004. [DOI] [PubMed] [Google Scholar]
- Mellet E, Briscogne S, Tzourio-Mazoyer N, Ghaem O, Petit L, Zago L, Etard O, Berthoz A, Mazoyer B, Denis M. Neural correlates of topographic mental exploration: the impact of route versus survey perspective learning. Neuroimage. 2000;12:588–600. doi: 10.1006/nimg.2000.0648. [DOI] [PubMed] [Google Scholar]
- Moscovitch M. Memory and working with memory: Evaluation of a component process model and comparison with other models. In: Schacter DL, Tulving E, editors. Memory Systems. Cambridge: MIT Press; 1994. pp. 269–310. [Google Scholar]
- Nadel L, Moscovitch M. Memory consolidation, retrograde amnesia, and the hippocampal complex. Current Opinions in Neurobiology. 1997;7:217–227. doi: 10.1016/s0959-4388(97)80010-4. [DOI] [PubMed] [Google Scholar]
- Nadel L, Samsonovich A, Ryan L, Moscovitch M. Multiple trace theory of human memory: Computational, neuroimaging, and neuropsychological results. Hippocampus. 2000;10:352–368. doi: 10.1002/1098-1063(2000)10:4<352::AID-HIPO2>3.0.CO;2-D. [DOI] [PubMed] [Google Scholar]
- Nadel L, Ryan L, Hayes SM, Gilboa A, Moscovitch M. The role of the hippocampal complex in long-term episodic memory. In: Ono T, Matsumoto G, Llinas RR, Berthoz A, Norgren R, Nishijo H, Tamura R, editors. Cognition and Emotion in the Brain: Selected Topics of the International Symposium on Limbic and Association Cortical Systems; 7–12 October 2002; Toyama, Japan. Amsterdam: Elsevier Science; 2003. Excerpta Medica International Congress Series, 1250, pp. 215–235. [Google Scholar]
- Nolde SF, Johnson MK, Raye CL. The role of prefrontal cortex during tests of episodic memory. Trends in Cognitive Science. 1998;2:399–406. doi: 10.1016/s1364-6613(98)01233-9. [DOI] [PubMed] [Google Scholar]
- Nyberg L, McIntosh AR, Cabeza R, Habib R, Houle S, Tulving E. General and specific brain regions involved in encoding and retrieval of events: What, where, and when. Proceedings of the National Academy of Sciences of the United States of America. 1996;93:11280–11285. doi: 10.1073/pnas.93.20.11280. [DOI] [PMC free article] [PubMed] [Google Scholar]
- O’Keefe J, Nadel L. The Hippocampus as a Cognitive Map. Oxford: Oxford University Press; 1978. [Google Scholar]
- Pigott S, Milner M. Memory for different aspects of complex visual scenes after unilateral temporal- or frontal-lobe resection. Neuropsychologia. 1993;31:1–15. doi: 10.1016/0028-3932(93)90076-c. [DOI] [PubMed] [Google Scholar]
- Rajkowski G, Goldman-Rakic PS. Cytoarchitectonic definition of prefrontal areas in the normal human cortex II: Variability in locations of areas 9 and 46 and relation to the Talairach Coordinate system. Cerebral Cortex. 1995;5:323–337. doi: 10.1093/cercor/5.4.323. [DOI] [PubMed] [Google Scholar]
- Schacter DL, Wagner AD. Medial temporal lobe activations in fMRI and PET studies of episodic encoding and retrieval. Hippocampus. 1999;9:7–24. doi: 10.1002/(SICI)1098-1063(1999)9:1<7::AID-HIPO2>3.0.CO;2-K. [DOI] [PubMed] [Google Scholar]
- Shimamura AP, Janowsky JS, Squire LR. Memory for temporal order events in patients with frontal lobe lesions and amnesic patients. Neuropsychologia. 1990;28:803–813. doi: 10.1016/0028-3932(90)90004-8. [DOI] [PubMed] [Google Scholar]
- Smith ML, Milner B. The role of the right hippocampus in the recall of spatial location. Neuropsychologia. 1981;19:781–793. doi: 10.1016/0028-3932(81)90090-7. [DOI] [PubMed] [Google Scholar]
- Smith ML, Milner B. Differential effects of frontal lobe lesions on cognitive estimation and spatial memory. Neuropsychologia. 1984;22:697–705. doi: 10.1016/0028-3932(84)90096-4. [DOI] [PubMed] [Google Scholar]
- Smith ML, Milner B. Right hippocampal impairment in the recall of spatial location: Encoding deficit or rapid forgetting? Neuropsychologia. 1989;27:71–81. doi: 10.1016/0028-3932(89)90091-2. [DOI] [PubMed] [Google Scholar]
- Squire LR. Memory and the hippocampus: a synthesis from findings with rats, monkeys, and humans. Psychological Review. 1992;99:195–231. doi: 10.1037/0033-295x.99.2.195. [DOI] [PubMed] [Google Scholar]
- Talairach J, Tournoux P. Co-planar stereotaxic atlas of the human brain. New York: Times Medical; 1988. [Google Scholar]
- Tulving E. Episodic and semantic memory. In: Tulving E, Donaldson W, editors. Organization of Memory. New York, NY: Academic Press; 1972. pp. 382–403. [Google Scholar]
- Tulving E. Elements of Episodic Memory. New York: Oxford University Press; 1983. [Google Scholar]
- Tulving E. Memory and consciousness. Canadian Psychologist. 1985;26:1–12. [Google Scholar]
- Tulving E, Kapur S, Craik FIM, Moscovitch M, Houle S. Hemispheric encoding/retrieval asymmetry in episodic memory: Positron emission tomography findings. Proceedings of the National Academy of Science. 1994;91:2016–2020. doi: 10.1073/pnas.91.6.2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tulving E, Markowitsch HJ. Episodic and declarative memory: role of the hippocampus. Hippocampus. 1998;8:198–204. doi: 10.1002/(SICI)1098-1063(1998)8:3<198::AID-HIPO2>3.0.CO;2-G. [DOI] [PubMed] [Google Scholar]
- Wood ER, Dudchenko PA, Eichenbaum H. The global record of memory in hippocampal neuronal activity. Nature. 1999;397:613–616. doi: 10.1038/17605. [DOI] [PubMed] [Google Scholar]