Abstract
Excitatory amino acid transporter 2 (EAAT2) is the major glutamate transporter and functions to remove glutamate from synapses. A thiopyridazine derivative has been found to increase EAAT2 protein levels in astrocytes. A structure-activity relationship study revealed that several components of the molecule were required for activity, such as the thioether and pyridazine. Modification of the benzylthioether resulted in several derivatives (7–13, 7–15 and 7–17) that enhanced EAAT2 levels by > 6 fold at concentrations < 5 μM after 24 h. In addition, one of the derivatives (7–22) enhanced EAAT2 levels 3.5 – 3.9 fold after 24 h with an EC50 of 0.5 μM.
Glutamate is a major neurotransmitter in the mammalian central nervous system (CNS) and essential for normal brain function including cognition, memory, and learning. However, the extracellular concentration of glutamate must remain below excitotoxic levels (~ 1 μM) to avoid overstimulation of glutamate receptors, leading to neuronal damage or death.1 Excitotoxicity has been associated with multiple acute neurological conditions such as ischemic stroke, epilepsy, and trauma, as well as chronic adult-onset neurodegenerative disorders such as Alzheimer’s disease and amyotrophic lateral sclerosis (ALS).2–6 One potential approach to preventing excitotoxicity is to enhance glutamate reuptake. Excitatory amino acid transporter 2 (EAAT2) is the major glutamate transporter and functions to remove glutamate from synapses.7 An increase in EAAT2 protein expression and function may provide a means to prevent insufficient glutamate reuptake and consequently reduce neuronal damage.
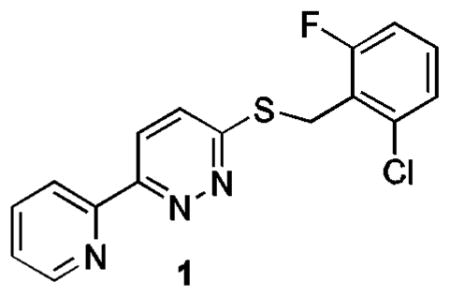
In an effort to identify small molecules that can increase EAAT2 protein expression, a high-throughput screen of approximately 140,000 compounds was previously conducted in our laboratories using a cell-based enzyme-linked immunosorbent assay.8 The hits identified from this screening provide starting points for further optimization in order to arrive at pharmacologically useful molecules for studying the role of EAAT2 in excitotoxicity induced neuronal injury and potentially as therapeutic agents.
The thiopyridazine 1 was confirmed to show a dose-dependent increase in EAAT2 protein levels after 24 h exposure. Herein, we report the structure-activity relationship (SAR) study of 1 for elevating EAAT2 protein levels.
Many of the pyridazine analogues utilized in the SAR study were prepared using the method depicted in Scheme 1. Treatment of ketone 2 with glyoxylic acid (3) and K2CO3 gave 4, which was used directly without purification. It was allowed to react with hydrazine in acetic acid at 100 °C to yield the desired pyridazinone 5 as an off-white solid after recrystallization from ethyl acetate.9 Direct alkylation of 5 gave 8 in good yields. Intermediate 5 was also converted into pyridazinethione 6 in the presence of P2S5 in pyridine at 120 °C.10 Alkylation of 6 provided 7, which could be subsequently oxidized to sulfone 9 with 3-chloroperoxybenzoic acid (m-CPBA) in CH2Cl2.11
Scheme 1.

Reagents and conditions: (a) K2CO3, H2O; (b) NH2NH2, AcOH, 100 °C (20%); (c) P2S5, pyridine, 120 °C (80%); (d) R2Br, K2CO3, DMF (90%); (e) MCPBA, CH2Cl2 (80%).
A series of additional analogues 14–17 was prepared using the methodology outlined in Scheme 2. Recently, 2-pyridyl N-methyliminodiacetic acid (MIDA) boronate (10) has been reported as an air-stable slow-release reagent with high cross-coupling efficiency even with heteroaryl chlorides.12 Therefore, this material was used in palladium-mediated cross-couple reactions with heteroaryl chlorides 11 – 13 to obtain 14 – 16, respectively. In the case of 13 transesterification occurred during the cross-coupling reaction to give the isopropyl ester 16, which was then allowed to react with alkylamines in ethanol at 85 °C to generate amides 17.
Scheme 2.

Reagents and conditions: (a) Pd2(dba)3, XPhos, Cu(OAc)2, K2CO3, DMF/i-PrOH (4/1), 100 °C; (b) H2NR, ethanol, 85 °C (90%).
Finally, several additional analogues designed to evaluate replacement of the pyridazine in 1 with phenyl and pyridyl moieties were prepared using the methodology outlined in Scheme 3. Suzuki coupling of 2-bromopyridine (18) with various boronic acids afforded 19 and 20.13 Treatment of 20 with P2S5 in pyridine at 120 °C provided 21 in 71% yield. Alkylation of 21 with 2-methylbenzyl bromide in the presence of K2CO3 in DMF generated 22.
Scheme 3.
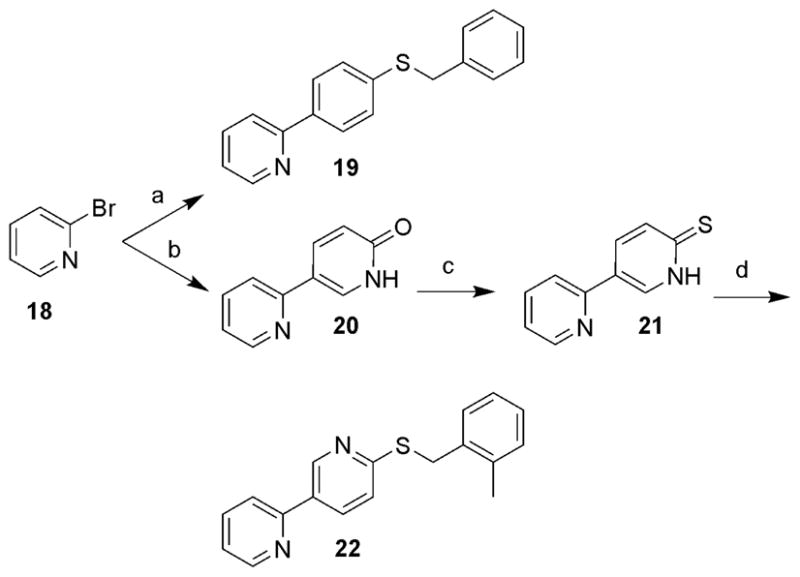
Reagents and conditions: (a) Pd(PPh3)4, Na2CO3, CH3CN/H2O (1/1), 75 °C, 4-benzylthiophenylboronic acid (54%); (b) Pd(PPh3)4, Na2CO3, CH3CN/H2O (1/1), 75 °C, 6-hydroxypyridine-3-boronic acid pinacol ester (70%); (c) P2S5, pyridine, 120 °C (71%); (d) 2-methylbenzyl bromide, K2CO3, DMF (76%).
All of the derivatives of 1 were initially evaluated in PA-EAAT2 cells3 (a primary astrocyte line stably expressing EAAT2 mRNAs) following compound (10 μM) incubation for 4 and 24 h before harvesting and measuring EAAT2 levels by Western blot analysis. The fold increases in EAAT2 protein levels relative to DMSO controls are reported (Tables 1 – 4).
Table 1.
Effects of the modified pyridyl and benzyl substituent on EAAT2 protein levels
 | ||||
|---|---|---|---|---|
| Compound | R1 | R2 | Fold increase for EAAT2a |
|
| 4 h | 24 h | |||
| 1 | 2-Py | 2-Cl-6-F-Bn | nab | 2.0 ± 0.8 |
| 7–1 | 3-Py | 2-Cl-6-F-Bn | na | 2.3 ± 0.8 |
| 7–2 | 4-Py | 2-Cl-6-F-Bn | na | 1.9 ± 0.9 |
| 7–3 | 4-Me-Ph | 2-Cl-6-F-Bn | 1.2 ± 0.2 | 2.3 ± 0.7 |
| 7–4 | 2-Py | 2-Me-Bn | 1.7 ± 0.1 | 3.5 ± 0.3 |
| 7–5 | 3-Py | 2-Me-Bn | 1.5 ± 0.4 | na |
| 7–6 | Ph | 2-Me-Bn | 1.1 ± 0.1 | 2.0 ± 0.4 |
| 7–7 | 2-Py | 2-Cl-Bn | 2.1 ± 1.4 | 4.0 ± 0.3 |
| 7–8 | 3-Py | 2-Cl-Bn | 1.2 ± 0.4 | 1.2 ± 0.3 |
| 7–9 | 4-Py | 2-Cl-Bn | 1.5 ± 0.2 | 1.7 ± 0.3 |
| 7–10 | 2-Py | 4-Me-Bn | 2.1 ± 1.1 | 2.6 ± 0.4 |
| 7–11 | 4-Py | 4-Me-Bn | 1.3 ± 0.1 | 2.4 ± 0.6 |
Compound concentration of 10 μM;
na = not active
Table 4.
Effects of the benzyl group on EAAT2 protein levels
 | |||
|---|---|---|---|
| Compound | R | Fold increase for EAAT2a |
|
| 4 h | 24 h | ||
| 1 | 2-Cl-6-F-Bn | nab | 2.0 ± 0.8 |
| 7–7 | 2-Cl-Bn | 2.1 ± 1.4 | 4.0 ± 0.3 |
| 7–18 | 3-Cl-Bn | 2.1 ± 0.3 | 4.1 ± 0.5 |
| 7–19 | 2,3-di-Cl-Bn | na | 2.4 ± 0.4 |
| 7–20 | 2,5-di-Cl-Bn | 1.4 ± 0.1 | 2.5 ± 0.4 |
| 7–21 | 2,4-di-Cl-Bn | 1.8 ± 0.5 | na |
| 7–22 | 2,6-di-Cl-Bn | 1.5 ± 0.8 | 3.9 ± 0.4 |
| 7–23 | 2-F-Bn | 2.2 ± 0.1 | 3.1 ± 0.2 |
| 7–24 | 4-F-Bn | 2.5 ± 0.6 | 2.6 ± 0.3 |
| 7–16 | 2,4-di-F-Bn | 2.4 ± 1.5 | 3.8 ± 0.8 |
| 7–25 | 2,6-di-F-Bn | 2.1 ± 1.4 | 2.2 ± 0.7 |
| 7–26 | 2,4,6-tri-F-Bn | 1.6 ± 1.0 | 2.4 ± 0.6 |
| 7–4 | 2-Me-Bn | 1.7 ± 0.1 | 3.5 ± 0.3 |
| 7–27 | 3-Me-Bn | 2.0 ± 0.2 | 2.8 ± 0.5 |
| 7–28 | 4-Me-Bn | 2.1 ± 1.1 | 2.6 ± 0.4 |
| 7–29 | 2,4-di-Me-Ph | 1.2 ± 0.3 | 2.2 ± 0.3 |
| 7–17 | 2,4-di-Me-Bn | 1.9 ± 0.8 | 6.4 ± 1.0 |
| 7–13 | 2,6-di-Me-Bn | na | 6.5 ± 1.0 |
| 7–30 | 2,4,6-tri-Me-Bn | 1.7 ± 0.3 | 4.7 ± 0.5 |
| 7–15 | 2-(2-Cl-6-F-phenylethyl) | 1.9 ± 0.7 | 6.7 ± 1.5 |
| 7–31 | 2-(2-Cl-phenylethyl) | 1.7 ± 0.2 | 4.8 ± 0.6 |
| 7–32 | 2-(2-Me-phenylethyl) | 1.2 ± 0.3 | 2.8 ± 0.4 |
| 7–33 | 1-(1,2,3,4-tetrahydronaphthalenyl) | na | 5.3 ± 1.1 |
| 7–14 | 1-(2-Cl-6-F-phenyl)ethyl | 1.7 ± 0.9 | 5.5 ± 1.0 |
| 7–34 | 2-Cl-4-F-Ph | 1.3 ± 0.1 | 2.5 ± 0.7 |
| 7–35 | 2-Cl-4-F-Bn | 1.6 ± 0.6 | 1.7 ± 0.4 |
| 7–12 | Bn | 1.3 ± 0.2 | 3.0 ± 0.5 |
| 7–36 | Et | 1.6 ± 0.7 | 1.9 ± 0.9 |
| 6–1 | H | na | 2.0 ± 0.8 |
Compound concentration of 10 μM;
na = not active
Replacement of the 2-pyridyl with 3-pyridyl (7–1, 7–5 and 7–8), 4-pyridyl (7–2, 7–9 and 7–11) or phenyl (7–6) resulted in equal or reduced activity (Table 1). Removing either one or both of the nitrogen atoms in the pyridazine resulted in loss of activity (Table 2). Collectively, these results suggested that both the 2-pyridyl and the pyridazine were required for enhancing EAAT2 protein expression.
Table 2.
Effects of modifications of the pyridazine on EAAT2 protein levels
 | ||||||
|---|---|---|---|---|---|---|
| Compound | X | Y | Z | R | Fold for increase EAAT2a |
|
| 4 h | 24 h | |||||
| 7–4 | S | N | N | 2-Me-Bn | 1.7 ± 0.1 | 3.5 ± 0.3 |
| 22 | S | C | N | 2-Me-Bn | 1.3 ± 0.6 | nab |
| 8–1 | O | N | N | 2-Me-Bn | 2.0 ± 0.9 | 2.5 ± 0.5 |
| 15 | O | N | C | 2-Me-Bn | 1.5 ± 0.3 | na |
| 7–12 | S | N | N | Bn | 1.3 ± 0.2 | 3.0 ± 0.4 |
| 19 | S | C | C | Bn | 1.2 ± 0.5 | 1.1 ± 0.4 |
Compound concentration of 10 μM;
na = not activ
Next, the sulfur linker was examined (Table 3). Replacing the sulfur with oxygen generally yielded less active derivatives. An analogue containing a NH linker (14) showed weaker activity. Likewise, oxidation of the sulfur to a sulfone (9–1, 9–2, and 9–3) was also detrimental. Finally, replacing the sulfur with an amide moiety (17–1 and 17–2) was not tolerated. Collectively, these results indicated that the sulfur linker was optimal.
Table 3.
Effects of modifications of the sulfur linker on EAAT2 protein levels
 | ||||
|---|---|---|---|---|
| Compound | X | R | Fold increase for EAAT2a |
|
| 4 h | 24 h | |||
| 1 | S | 2-Cl-6-F-Bn | nab | 2.0 ± 0.8 |
| 8–2 | O | 2-Cl-6-F-Bn | 1.1 ± 0.3 | 2.9 ± 0.4 |
| 7–4 | S | 2-Me-Bn | 1.7 ± 0.1 | 3.5 ± 0.3 |
| 8–1 | O | 2-Me-Bn | 2.0 ± 0.9 | 2.5 ± 0.5 |
| 7–13 | S | 2,6-di-Me-Bn | na | 6.5 ± 1.0 |
| 8–3 | O | 2,6-di-Me-Bn | 2.1 ± 1.2 | 3.2 ± 1.4 |
| 7–14 | S | 1-(2-Cl-6-F-phenyl)ethyl | 1.7 ± 0.9 | 5.5 ± 1.0 |
| 8–4 | O | 1-(2-Cl-6-F-phenyl)ethyl | 1.6 ± 0.2 | 3.0 ± 0.7 |
| 7–15 | S | 2-(2-Cl-6-F-phenylethyl) | 1.9 ± 0.7 | 6.7 ± 1.5 |
| 8–5 | O | 2-(2-Cl-6-F-phenylethyl) | 2.2 ± 0.4 | 3.1 ± 1.2 |
| 7–16 | S | 2,4-di-F-Bn | 2.4 ± 1.5 | 3.8 ± 0.8 |
| 8–6 | O | 2,4-di-F-Bn | na | 2.3 ± 0.6 |
| 7–17 | S | 2,4-di-Me-Bn | 1.9 ± 0.8 | 6.4 ± 1.0 |
| 8–7 | O | 2,4-di-Me-Bn | na | 2.0 ± 0.9 |
| 6–1 | S | H | na | 2.0 ± 0.8 |
| 5–1 | O | H | 1.5 ± 0.5 | 1.6 ± 1.0 |
| 14 | NH | 2-Me-Bn | 1.7 ± 1.1 | 2.6 ± 0.5 |
| 9–1 | SO2 | 2-Cl-6-F-Bn | 1.4 ± 0.3 | 2.8 ± 0.4 |
| 9–2 | SO2 | 2-Me-Bn | 2.3 ± 0.4 | 1.1 ± 0.2 |
| 9–3 | SO2 | 2-Cl-Bn | 1.6 ± 0.3 | na |
| 17–1 | CONH | n-Pr | na | 1.2 ± 0.2 |
| 17–2 | CONH | 2-Me-Bn | na | 1.4 ± 0.2 |
Compound concentration of 10 μM;
na = not active
Finally, the benzyl group was examined (Table 4). Compared to 1, 2-chloro, 3-chloro and 2, 6-dichloro substitutions improved the potency by two-fold (7–7, 7–18 and 7–22), but 2, 3-dichloro, 2,4-dichloro and 2, 5-dichloro analogues (7–19, 7–21 and 7–20) were equivalent to 1. In the case of fluorine substitutes, only the 2-fluoro and 2, 4-difluoro derivatives (7–23 and 7–16) demonstrated improved activity. 4-Fluoro, 2, 6-difluoro, and 2, 4, 6-trifluoro analogues (7–24, 7–25, and 2–26) did not result in significant improvement. Replacement of the halogens with methyls (7–4, 7–13, 7–17, and 7–30) gave increased potency. Compounds containing 2-methyl substituted benzyl groups significantly increased EAAT2 protein level, where as the 3- or 4-methylbenzyl derivatives (7–27 and 7–28) were less potent. Increasing the tether length between the phenyl and the sulfur to an ethylene generally yielded more potent analogues (7–15 and 7–31 verses 1 and 7–7, respectively) with one noted exception (compare 7–32 to 7–4). However, truncating the linker resulting in a diarylthioether decreased potency (7–29 verses 7–17). Interestingly, adding substitutes on the carbon linker (7–14 and 7–33) increased activity about 3-fold. Finally, other changes gave compounds with essentially the same potency as 1 (7–12, 7–36, and 6–1).
In order to further characterize twelve of the most active analogues from the initial assessment, they were evaluated in a 6-point dose-response assay (0.1, 0.3, 1.0, 5.0, 10.0, and 30.0 μM). The fold increases at 1 and 10 μM, as well as the EC50 values are summarized in Table 5. In addition, these values were determined relative to two unrelated proteins (e.g. β-actin and GAPDH). Several compounds (7–13, 7–17, 7–31 and 7–18) were able to increase EAAT2 levels > 6-fold at 10 μM compared to β-actin or GAPDH, but with EC50 values in the 2.6 – 3.3 μM range. Analog 7–22 demonstrated the lowest EC50 of 0.5 μM with a maximum increase of EAAT2 levels of 3- to 4-fold.
Table 5.
Fold increase and EC50 values of EAAT2 protein levels of selected analoguesa
 | |||||||
|---|---|---|---|---|---|---|---|
| Compound | R | Fold increase for EAAT2
|
EC50 (μM) | ||||
| 1 μM, 24 h | 10 μM, 24 h | ||||||
| β-actinb | GAPDHc | β-actinb | GAPDHc | β-actinb | GAPDHc | ||
| 7–4 | 2-Me-Bn | 1.0 ± 0.8 | 1.0 ± 0.3 | 3.5 ± 0.3 | 3.1 ± 0.2 | 2.6 ± 0.8 | 1.8 ± 0.3 |
| 7–7 | 2-Cl-Bn | 1.1 ± 0.5 | 1.6 ± 0.4 | 4.0 ± 0.3 | 4.5 ± 0.6 | 3.3 ± 0.5 | 3.2 ± 0.5 |
| 7–13 | 2,6-di-Me-Bn | 2.0 ± 0.2 | 2.0 ± 0.4 | 6.5 ± 1.0 | 6.4 ± 0.9 | 2.6 ± 0.2 | 2.4 ± 0.4 |
| 7–14 | 1-(2-Cl-6-F-phenyl)ethyl | 1.5 ± 0.5 | 1.8 ± 0.4 | 5.5 ± 1.0 | 5.2 ± 1.2 | 3.9 ± 0.5 | 2.4 ± 0.4 |
| 7–17 | 2,4-di-Me-Bn | 2.0 ± 0.3 | 2.2 ± 0.6 | 6.4 ± 1.0 | 6.0 ± 0.9 | 2.7 ± 0.3 | 1.8 ± 0.6 |
| 7–22 | 2,6-di-Cl-Bn | 3.0 ± 0.1 | 3.5 ± 0.0 | 3.9 ± 0.4 | 3.5 ± 0.5 | 0.5 ± 0.1 | 0.5 ± 0.0 |
| 7–16 | 2,4-di-F-Bn | 1.0 ± 0.2 | 1.0 ± 0.5 | 3.8 ± 0.8 | 3.7 ± 0.6 | 2.6 ± 0.2 | 2.6 ± 0.5 |
| 7–30 | 2,4,6-tri-Me-Bn | 1.5 ± 0.4 | 1.6 ± 0.4 | 4.7 ± 0.5 | 4.8 ± 0.6 | 2.4 ± 0.4 | 2.1 ± 0.4 |
| 7–15 | 2-(2-Cl-6-F-phenylethyl) | 1.2 ± 0.3 | 1.2 ± 0.2 | 6.7 ± 1.5 | 5.3 ± 1.2 | 3.3 ± 0.3 | 3.3 ± 0.2 |
| 7–31 | 2-(2-Cl-phenylethyl) | 1.0 ± 0.7 | 1.0 ± 0.1 | 4.8 ± 0.6 | 4.5 ± 0.6 | 4.7 ± 0.8 | 5.1 ± 0.1 |
| 7–33 | 1-(1,2,3,4-tetrahydronaphthalenyl) | 2.4 ± 0.3 | 2.8 ± 0.4 | 5.3 ± 1.1 | 4.5 ± 0.6 | 1.9 ± 0.3 | 1.7 ± 0.4 |
| 7–18 | 3-Cl-Bn | 4.0 ± 0.4 | 4.2 ± 0.1 | 4.1 ± 0.5 | 3.8 ± 0.5 | 1.7 ± 0.4 | 0.5 ± 0.1 |
Data represent the mean values of four separate experiments;
Data standardized by -actin;
Data standardized by GAPDH
In summary, a series of pyridazine derivatives were synthesized and evaluated for increasing EAAT2 protein levels. Several compounds were found to significantly increase EAAT2 protein levels (> 6 fold), such as 7–13, 7–15 and 7–17. Derivative 7–22 increased EAAT2 protein levels to a lower extent (3.5 – 3.9 fold), but with a lower EC50 value (0.5 μM). These compounds will provide useful tools for further assessing the role of glutamate excitotoxicity in cellular systems and potentially in animal models of acute and chronic neurodegeneration. In addition, these probe molecules may also be beneficial in determining the biological mechanisms for regulating EAAT2 levels.
Supplementary Material
Acknowledgments
We are grateful to the National Institutes of Health (U24 NS04933901 and R01 NS064275), the Harvard NeuroDiscovery Center, ALS Therapy Alliance, Inc and Project ALS for financial support.
Footnotes
Supplementary data associated with this article can be found, in the online version, at ().
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References and notes
- 1.Sheldon AL, Robinson MB. Neurochem Int. 2007;51:333. doi: 10.1016/j.neuint.2007.03.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Guo H, Lai L, Butchbath MER, Stockinger MP, Shan X, Bishop GA, Lin CLG. Hum Mol Genet. 2003;12:2519. doi: 10.1093/hmg/ddg267. [DOI] [PubMed] [Google Scholar]
- 3.Tian G, Lai L, Guo H, Lin Y, Butchbach M, Chang Y, Lin CL. J Biol Chem. 2007;282:1727. doi: 10.1074/jbc.M609822200. [DOI] [PubMed] [Google Scholar]
- 4.Hazell A. Neurochem Int. 2007;50:941. doi: 10.1016/j.neuint.2007.04.026. [DOI] [PubMed] [Google Scholar]
- 5.Seifert G, Carmignoto G, Steinhäuser C. Brain Res Rev. 2010;63:212. doi: 10.1016/j.brainresrev.2009.10.004. [DOI] [PubMed] [Google Scholar]
- 6.Tian G, Kong Q, Lai L, Ray-Chaudhury A, Lin CL. J Neurochem. 2010;113:978. doi: 10.1111/j.1471-4159.2010.06661.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Lin Y, Tian G, Roman K, Handy C, Travers JB, Lin CLG, Stephens RL., Jr Am J Physiol Gastrointest Liver Physiol. 2009;296:129. doi: 10.1152/ajpgi.90556.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Colton CK, Kong Q, Lai L, Zhu MX, Seyb KI, Cuny GD, Xian J, Glicksman MA, Lin CLG. J Biomol Screen. 2010;15:653. doi: 10.1177/1087057110370998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Coates WJ, McKillop A. Synthesis. 1993:334–342. [Google Scholar]
- 10.Arakawa K, Miyasaka T, Satoh K. Chem Pharm Bull. 1977;25:299. [Google Scholar]
- 11.Mylari BL, Armento SJ, Beebe DA, Conn EL, Coutcher JB, Dina MS, O’Gorman MT, Linhares MC, Martin WH, Oates PJ, Tess DA, Withbroe GJ, Zembrowsky WJ. J Med Chem. 2005;48:6326. doi: 10.1021/jm050462t. [DOI] [PubMed] [Google Scholar]
- 12.Knapp DM, Gillis EP, Burke MD. J Am Chem Soc. 2009;131:6961. doi: 10.1021/ja901416p. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Ghiron C, Haydar SN, Aschmies S, Bothmann H, Castaldo C, Cocconcelli G, Comery TA, Di L, Dunlop J, Lock T, Kramer A, Kowal D, Jow F, Grauer S, Harrison B, Rosa S, Maccari L, Marquis K, Micco I, Nencini A, Quinn J, Robichaud A, Roncarati R, Scali C, terstappen G, Turlizzi E, Valacchi M, Varrone M, Zalaletti R, Zanelli U. J Med Chem. 2010;53:4379. doi: 10.1021/jm901692q. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


