Abstract
Marburg and Ebola viruses cause a severe hemorrhagic disease in humans with high fatality rates. Early target cells of filoviruses are monocytes, macrophages, and dendritic cells. The infection spreads to the liver, spleen and later other organs by blood and lymph flow. A hallmark of filovirus infection is the depletion of non-infected lymphocytes; however, the molecular mechanisms leading to the observed bystander lymphocyte apoptosis are poorly understood. Also, there is limited knowledge about the fate of infected cells in filovirus disease. In this review we will explore what is known about the intracellular events leading to virus amplification and cell damage in filovirus infection. Furthermore, we will discuss how cellular dysfunction and cell death may correlate with disease pathogenesis.
Keywords: Ebola Virus, Marburg Virus, filoviruses, viral replication cycle, target cells, animal models, ultrastructural analysis, virus-cell interaction, bystander apoptosis, cell death
1. Introduction
The members of the filovirus family, Ebola virus (EBOV) and Marburg virus (MARV), cause a severe hemorrhagic fever in infected humans with high fatality rates [1]. Infected individuals who go on to succumb to filovirus infection exhibit dysregulated immune responses (reviewed in two other articles in this issue). This appears to result from several factors, including viral mediated impairment of early innate immune responses and consequent dysregulation of innate immunity [2]. Some reports have suggested that adaptive immune responses may occur [3–6], but it is evident that these fail to clear the disease. Lymphopenia resulting from apoptosis as the infection progresses has also been suggested to contribute to the failure to clear the infection [7]. Studies of both human survivors and murine model systems suggest that a well-regulated cytokine response early in the course of the infection may be critical to the outcome of the disease [8,9].
Ebolaviruses are currently subdivided into four distinct species, Zaire ebolavirus (ZEBOV), Sudan ebolavirus, Tai Forest ebolavirus, and Reston ebolavirus (REBOV), while there is only a single MARV species (Lake Victoria marburgvirus) (ICTV virus taxonomy 2009). Since the Bundibugyo isolate is genetically distinct from the known Ebola viruses, a suggestion has been made to classify it as a new EBOV species, Bundibugyo ebolavirus [10]. The different EBOV species not only show significant molecular differences, they also vary in terms of virulence and pathogenicity. The most pathogenic species in humans is ZEBOV with a case fatality rate of about 80%, followed by Sudan with a case fatality rate of about 50% [11], and Bundibugyo with a fatality rate of about 30% [12]. To date, there are two reported non-fatal human cases of Tai Forest ebolavirus [13,14] and several asymptomatic human cases of REBOV infection [15–17].
The first reported MARV outbreak occurred in Germany and Yugoslavia in 1967 and was caused by infected African green monkeys imported from Uganda [18,19]. Since this outbreak was associated with a case fatality rate of 22%, it was believed for a long time that MARV was less pathogenic than EBOV. However, recent outbreaks of MARV in the Democratic Republic of the Congo in 1998–2000 and in Angola in 2004 were associated with fatality rates up to 90%, indicating that MARV can be as virulent as EBOV [20–22].
Despite the severity of the disease, filoviruses have been regarded as exotic pathogens with fatal outbreaks restricted to Central Africa, and with no major health threat outside of the endemic areas. Knowledge on their biology and pathogenicity consequently remained limited. However, there has been renewed interest given the potential for using filoviruses in bioterrorism attacks and the possibility for infected, asymptomatic persons for bringing the disease to other countries. Indeed, two cases of MARV have been reported in the Netherlands and in the United States, both tourists returning from trips to Uganda [23,24]. Together, the potential for spread outside central Africa has reignited research endeavors to elucidate the biology of the filoviruses and to develop effective therapeutic strategies.
In this review we will describe how filoviruses enter their target cells, replicate their genomes and assemble progeny viruses by exploiting cellular machineries. We will also briefly touch upon the interaction of filoviruses with cellular signaling pathways. Finally, we will discuss the current understanding of the fate of infected and non-infected cells in filovirus infection. In addition, we will present ultrastructural data of infected and non-infected cells, demonstrating the morphological changes in filovirus infection.
2. The Central Players: Virus and Target Cells
2.1. Filovirus Structure
The structure of filovirus particles has been described in detail in [1] and multiple review articles. Briefly, the single stranded non-segmented RNA genome of filoviruses is of negative polarity and contains seven monocistronic genes. It is associated with four viral proteins, the nucleoprotein NP which enwraps the viral RNA, the RNA-dependent RNA polymerase L, the polymerase cofactor VP35, and the transcription factor VP30. The four nucleocapsid proteins are required for replication and transcription of the viral genome (reviewed in [25]). Filovirus genomes encode two matrix proteins, VP40, the functional equivalent of the matrix proteins M of other non-segmented negative-stand RNA viruses, and the minor matrix protein, VP24, which is unique to filoviruses. As a peripheral membrane protein, VP40 is located at the inner side of the virion’s membrane. It mediates budding and viral particle release [26]. The minor matrix protein VP24 is involved in nucleocapsid formation and assembly [27–29] and contributes to the regulation of viral transcription/replication [30,31]. EBOV VP24 and MARV VP40 are considered important virulence factors and play a crucial role in host adaptation. Both proteins block IFN signaling, however, they target different cellular proteins and use different mechanisms to antagonize the IFN response [32–35]. The role of EBOV VP24 and MARV VP40 in the innate immune response to filovirus infection will be discussed in more detail in another article in this issue. Filoviruses possess a single surface protein, the type I transmembrane glycoprotein GP that mediates attachment to target cells, entry, and fusion [36]. The precursor preGP is cleaved in the trans Golgi network by furin or a furin-like protease resulting in two disulfide-linked subunits, GP1and GP2 [37]. Notably, EBOV GP has been implicated in cell damage, which will be discussed in more detail in Section 4.2.2.
EBOV genomes encode an additional protein, the nonstructural soluble form of the glycoprotein, sGP. As GP, sGP is encoded by the fourth gene, but is translated from non-edited mRNA species, while the membrane-bound GP is the result of mRNA editing during transcription [38,39]. sGP is not incorporated into viral particles, but is secreted from infected cells. Although the function of the protein is not fully understood, there is evidence that it acts as an anti-inflammatory factor by protecting the endothelial cell barrier function during infection [40]. Besides sGP, a second soluble GP variant generated by mRNA editing, the small soluble protein (ssGP) has been identified [41,42]. A nonstructural MARV protein comparable to EBOV sGP is not expressed.
2.2. Target Cells in Filovirus Infection
Filoviruses have a broad cell tropism in susceptible host species. Among the target cells supporting viral replication are monocytes, macrophages, dendritic cells (DCs), hepatocytes, adrenal cortical cells, fibroblasts and endothelial cells [43–57].
The earliest events during infection are likely to center around cells of the mononuclear phagocyte system, including monocytes, macrophages, and DCs. These cells not only orchestrate innate and adaptive immune responses [58,59], but also serve as early targets of viral infection [47–49,55,56, 60–66]. It is thought that the early infection of these cells is responsible for the rapid and widespread dissemination of the virus throughout an infected host [67].
Replication of MARV in peritoneal macrophages of guinea pigs can be detected within 24 h after intraperitoneal infection [50]. In African green monkeys, infected Kupffer cells were identified in the liver early in infection, on days 2 or 3 following infection with ZEBOV or MARV. The liver and adrenals are target organs of these viruses, whose parenchyma cells support filovirus replication. Infected hepatocytes and adrenal cortical cells can be detected a day or two after finding infected macrophages. Secondary target cells also include fibroblasts and endothelial cells, which were observed 5 to 7 days after filovirus infection of non-human primates [47,49,50]. Filovirus-infected macrophages, fibroblasts, and endothelial cells were found in all organs examined in these animal models [46–52,55]. The presence of these cell types in virtually all organs may account for the observed filovirus pantropism. Indeed, MARV or ZEBOV can be isolated from any organ or tissue. Besides the typical target cells for ZEBOV and MARV infection in non-human primates, additional target cells were occasionally found in individual animals. These cells included alveolar epithelial cells, bronchial epithelial cells and the cells of endocardial layer [50]. In cynomolgus macaques, REBOV was shown to have similar target tropism, and also to rarely infect alveolar and kidney epithelium as well as adrenal medulla cells [64].
Filoviruses are present in the blood of infected animals and are therefore potentially spread to all parts of the body by blood flow both as free virions as well as within infected monocytes. It is likely that the lymphatics also contribute to the rapid spread of the virus as free viruses and virus-infected DCs. The structure of sinusoids and sinuses in the liver and spleen allows for the direct migration of filoviruses from the blood stream, facilitating the infection of hepatocytes and splenic macrophages. Migration of infected monocytes from blood vessels may deliver the virus into connective tissue where it infects fibroblasts, which then spread progeny viruses by their protrusions to sites far from the main body of the infected cell [46,49,50,68]. It is therefore likely that filoviruses are disseminated in the infected host by multiple different mechanisms including transport of free virus particles by blood and lymphatic fluids, migration of infected monocytes, macrophages and DCs into various tissues, and viral cell-to-cell spread via cell protrusions.
One of the most intriguing features of fatal filovirus infection in animals and humans is that little or no inflammatory cellular response occurs at the sites of viral replication. Accumulation of neutrophils, monocytes, and lymphocytes around infected cells has been rarely observed in infected tissues. The minimal inflammatory cellular response is considered to be a distinctive feature of filovirus infection [45,49,55,60,63,69–71] and may represent a part of the dysregulated immune response observed in fatal cases of EBOV and MARV infection. In non-fatal EBOV infections of guinea pigs, in contrast, a prominent inflammatory response is observed, and infected cells are tightly surrounded by leukocytes forming a substantial barrier which could impair viral dissemination [48,62].
3. What Happens in the Infected Cell?
3.1. Entry: Speak Friend and Enter
The first step that determines if a cell will be a target for infection is the ability of the virus to attach. Filoviruses are able to infect multiple cell types in vivo and in cell culture by exploiting cellular entry machineries. However, some cells are more susceptible to infection than others, and some are not permissive to infection.
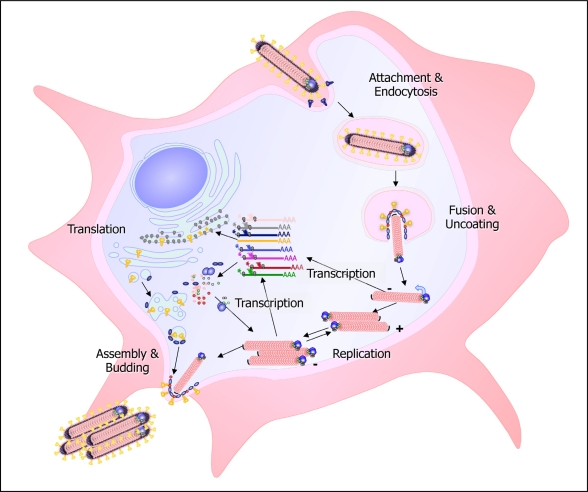
The filovirus replication cycle is depicted in Figure 1. Filoviruses enter the target cells by different uptake mechanisms including lipid raft-dependent and receptor-mediated endocytosis [72–75] and macropinocytosis [76,77]. Receptor binding and attachment to the target cells is mediated by the glycoprotein subunit GP1. A number of cellular proteins have been implicated in filovirus entry, and it is not clear whether there is a “primary” receptor for these viruses. The different co-receptors likely provide access for the virus into different target cells. The folate receptor has been suggested to be a significant filovirus receptor [78]. However, cells lacking folate receptor-α are still permissive for infection [79,80]. An interesting group of glycan binding proteins that enhance filovirus uptake in a cell-specific manner belong to the C-type lectin family. The highly glycosylated GP of both MARV and EBOV is decorated with a set of N- and O-linked glycans [81–83] which, depending on their specific structures, can be recognized by different C-type lectins. This includes asialoglycoprotein receptor on hepatocytes, DC-SIGN and hMGL on macrophages and immature DCs, and L-SIGN and LSECtin on endothelial cells in liver and lymph nodes [84–91]. Another group of proteins involved in filovirus entry are the β1-integrins [92,93]. Integrins are expressed on a wide range of cell types and are involved in the uptake of a variety of different viruses. Interestingly, detailed study of one of these integrins, the α5β1-integrin, has demonstrated that it is not involved in EBOV internalization, but rather in the regulation of endosomal cathepsin required for EBOV fusion [93]. More recently T-cell immunoglobulin and mucin domain 1 (TIM-1) has been suggested as a receptor for EBOV and MARV GP [94]. TIM-1 is not expressed by the primary targets of filoviruses, macrophages and DCs, but is expressed on mucosal epithelial cells, whose role in infection is not clear yet. Another interesting group of proteins involved in EBOV uptake are the members of the Tyro3/Axl/Mer (TAM) receptor family. Ligand-activated TAM receptors are negative regulators of inflammation in macrophages and DCs by upregulating the expression of SOCS1 and SOCS3 proteins in a phospho-STAT1-dependent manner [95]. The TAM receptor Axl serves as a co-receptor for EBOV entry by binding the viral surface protein GP [96]. To bind GP, the TAM receptor ligand binding domain has to be intact [97]. However, it is not known if GP binding leads to the activation of Axl. Recently, Axl has been shown to be involved in receptor-independent uptake of EBOV by macropinocytosis [75].
Figure 1.
Scheme of the filovirus infection cycle. Filoviruses enter the cell by receptor-mediated endocytosis or macropinocytosis. After fusion of the viral and cellular membrane, the nucleocapsid is released into the cytoplasm and serves as a template for transcription and replication. The replicated RNA is encapsidated by the nucleocapsid proteins. The newly synthesized nucleocapsids are transported to the sites of viral release, where budding takes place.
After uptake, the virus particles are internalized into the endosomes, where fusion takes place. Fusion of the viral and cellular membrane is mediated by the fusogenic cleavage product GP2 [98]. To initiate fusion, the proteolytic cleavage of GP1 by the endosomal proteases cathepsin B and cathepsin L is mandatory [99–101]. Interestingly, the cathepsin dependence of virus entry seems to be cell-type specific. While virus entry into Vero cells is dependent on the activity of both cathepsin B and cathepsin L, infection of human DCs by EBOV does not require active cathepsin L [102].
3.2. Replication: The Intruders Take Over
Fusion of the viral and cellular membrane leads to the release of the viral nucleocapsids into the cytoplasm of the infected cell where transcription and replication of the viral genome take place (Figure 1). The nucleocapsid, rather than the naked RNA, serves as the template for both transcription and replication. During transcription, the seven viral genes are sequentially transcribed into monocistronic mRNAs which are capped and polyadenylated and are used for the production of viral proteins. During replication, the encapsidated RNA is copied into full-length positive-sense replicative intermediates, the RNA antigenomes, which are enwrapped by the nucleocapsid proteins. In turn, the antigenomes are used as templates for the synthesis of progeny genomes. The nucleocapsid proteins do not only encapsidate the RNA genomes, they are also essential for replication and transcription. The viral polymerase, consisting of L and VP35, catalyzes replication as well as transcription, including polyadenylation and capping (reviewed in [25]).
The driving force for nucleocapsid formation is NP [103,104]. It has been proposed for EBOV that NP forms helical structures which interact with VP35 and VP24, resulting in the formation of nucleocapsid-like structures [105,106].
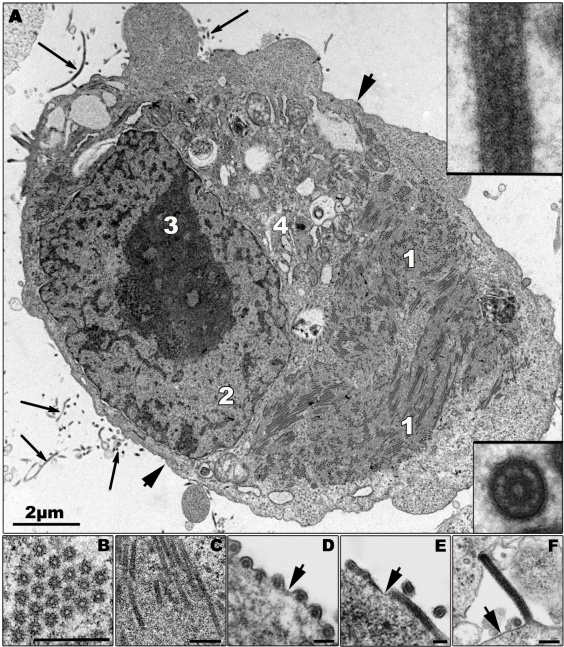
It is believed that the amplification of the viral genome and the assembly of newly synthesized nucleocapsids occur in highly organized regions in the cytoplasm, the viral inclusions [103,106,107] (Figure 2A). The appearance of granular material of average electron density in the cytoplasm of the infected cells at 12 h (MARV) and 9 h (ZEBOV) post infection (p.i.), respectively, is the first morphological sign of viral replication, as revealed by electron microscopic studies following the course of MARV and ZEBOV infection in Vero and MDCK cells [50]. The granular material is closely associated with the surface of the ER and contains viral proteins and RNA (Figure 2C). Tubular structures with an average diameter of 50 nm then appear in the granular material representing the newly synthesized viral nucleocapsids [50,105] (Figures 2B,C). The viral inclusions can be easily detected as large irregularly formed cytoplasmic aggregates by immunofluorescence microscopy [108,109]. To date, it is not known if cellular components are required for inclusion formation.
Figure 2.
Morphological characteristics of Zaire ebolavirus (ZEBOV) replication. (A) Ultrathin section of a ZEBOV-infected Vero cell containing large viral inclusions. The inclusions are composed of granular material (1; also shown in (C)) and rod-like nucleocapsids. Released virions are indicated by long arrows and are shown in the inserts. (B) Cross section of viral inclusion containing nucleocapsids. (C) Longitudinal section of viral inclusion containing nucleocapsids. (D–F) Budding of viral particles. In the initial step of budding a particle can be positioned parallel (D and E, cross and longitudinal sections), or perpendicular (F) to the membrane and then subsequently is enveloped. Short arrows indicate the cellular plasma membrane. 2—nucleus, 3—nucleolus, 4—Golgi zone. Bars in Figures 2B–F correspond to 250 nm. Thick part of frame around cross-sectioned virion corresponds to 120 nm, and thick part of frame around longitudinal section corresponds to 160 nm. Transmission electron microscopy. Cells were fixed at 16 h p.i.
The morphological characteristics of filovirus replication in animal cells in vivo are identical to those observed in cell culture. We analyzed the morphology of inclusion bodies in EBOV and MARV-infected cells of various organs of non-human primates, guinea pigs, mice, and chick embryos, as well as Vero, BHK-13 and MDCK cells and observed that filovirus inclusions, though morphologically heterogeneous, are always composed of granular material and varying numbers of nucleocapsids [50]. A few examples of filovirus infected cells in animal tissues are presented in Figure 3.
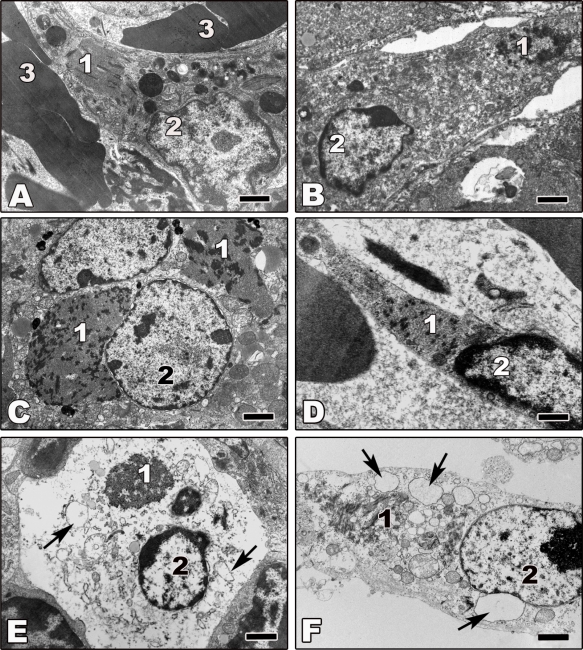
Figure 3.
Transmission electron microscopy of ultrathin sections of tissues from animals experimentally infected with filoviruses. Tissues were fixed at day 5 or 6 p.i. (A) Ultrathin section of liver tissue from ZEBOV-infected rhesus monkey showing an infected macrophage. (B,C) Ultrathin sections of liver tissue from Marburg virus (MARV)-infected guinea pig. Shown are an infected macrophage (B) and an infected hepatocyte (C). (D) Ultrathin section of spleen tissue from ZEBOV-infected African green monkey showing an infected endothelial cell. (E) Ultrathin section of lymphatic node tissue from ZEBOV-infected African green monkey showing a necrotic infected macrophage. (F) Ultrathin section of a necrotic ZEBOV-infected Vero cell showing an infected macrophage. Arrows show vacuolization of endoplasmic reticulum cisterns in cells undergoing non-apoptotic cell death. 1—filovirus inclusions; 2—nucleus; 3—erythrocyte. Bars correspond to 2 μm.
3.3. Exit: Rats Abandon a Sinking Ship
Following assembly, newly synthesized nucleocapsids are transported to the sites of virus budding. Immunoelectron microscopy of EBOV-infected cells revealed that NP and the matrix protein VP40 accumulate in the viral inclusions and are closely associated during viral morphogenesis [110]. Meanwhile, it has been confirmed by multiple studies that the filoviral VP40 protein is the major player in viral budding, though various viral proteins significantly enhance the release of viral particles (reviewed in [111–113]). Importantly, filoviruses exploit the vesicular transport machineries of the infected cell for viral egress, and viral proteins of both MARV and EBOV have been reported to interact with various protein components of the COPII vesicular transport system and the ESCRT machinery (reviewed in [111,114,115]). Viral budding occurs either at intracellular membranes, the multivesicular bodies (MVB) [116], or at the plasma membrane [50,105,117]. In cell culture, MARV particles are preferentially released at filopodia, which may facilitate the infection of neighboring cells [117].
Electron microscopic studies of filovirus-infected cells suggested two models for the release of the long, filamentous viral particles: horizontal budding after lateral association of the nucleocapsids with the plasma membrane or vertical budding [50,105,117] (Figures 2D–F). A recent electron tomography study of MARV-infected cells convincingly reconciled the results into a single model. Welsch and colleagues [118] showed that the budding process is initiated by the lateral association of the intracellular nucleocapsids with the plasma membrane. Starting from one end, the nucleocapsids are then subsequently wrapped by the plasma membrane until the viral particles protrude vertically from the cell surface. The formation of such long filamentous particles (700–900 nm or more) is certainly challenging for the infected cells and may lead to membrane perturbation in the cells and in the released viruses. Notably, the release of infectious filamentous MARV from cultured cells has been reported to peak at early time points post infection (1–2 days p.i.), when the cells were still intact. At late time points (4 d p.i.), most of the infected cells were vesiculated, the released virions were morphologically different, being round or bent, and coincidentally infectivity was decreased [118].
4. To Live or Die: The Fate of Infected and Non-Infected Cells in Filovirus Infection
4.1. Mechanisms of Cell Death
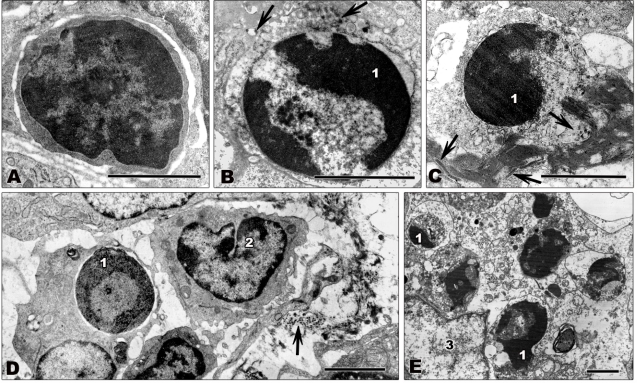
Viruses exploit the host cell for successful replication, potentially leading to death of the infected cell. Since the production of progeny viruses is hampered when the infected cells are destroyed early in infection, many viruses have evolved mechanisms to avoid host cell death, as reflected by a complex interaction between viruses and cell death signaling pathways (reviewed in [119,120]). Different types of cell death, including apoptosis, necrosis, and autophagy, can be described by different morphological and biochemical characteristics (reviewed in [121,122]). Apoptosis or programmed cell death is characterized by shrinking of the dying cell, plasma membrane blebbing, nuclear condensation, and final fragmentation of the cell in apoptotic bodies [123,124]. Tissue lymphocytes from filovirus-infected non-human primates showing typical signs of apoptosis, such as condensation and marginal localization of chromatin, are shown in Figures 4B–E. Biochemically, apoptosis is characterized by activation of caspases. Caspase cleavage can be activated by extrinsic pathways via death receptor signaling induced by TRAIL, Fas/CD95 (Fas), or TNFα or by intrinsic pathways via regulation of cytochrome C efflux from the mitochondria (reviewed in [125–127]). Lymphocyte apoptosis plays an important role in T cell development and control of T cell tolerance [128] and might also play a crucial role in the pathogenesis of filovirus infection as discussed in Section 4.3. In contrast to apoptosis, necrosis is generally described as uncontrolled cell death, but recent findings suggest that it also might be regulated by conserved biochemical mechanisms (reviewed in [129,130]). Necrosis is characterized by swelling of the cell and cellular organelles, membrane blebbing, vacuolization and results in rupture of the plasma membrane [121,124]. Autophagy is a conserved pathway of eukaryotic cells for recycling cellular components. However, extensive cellular stress can lead to autophagic or type II cell death. Autophagic cells feature vacuolization and formation of double membraned vesicles, the autophagosomes, for degradation of cellular content [131,132]. Characteristic features of non-apoptotic cell death, such as vacuolization, swelling, and the lack of chromatin condensation can be seen in Figures 3E,F.
Figure 4.
Transmission electron microscopy of ultrathin sections of lymph node tissue from filovirus-infected African green monkeys infected with ZEBOV (A,C–E) or MARV (B). Tissues were fixed at 4 days p.i. (A) Small lymphocyte showing normal nuclear morphology with large areas of heterochromatin. (B,C) Apoptotic lymphocytes. Typical signs of apoptosis such as chromatin condensation and marginal location of chromatin are visible. (D,E) Apoptotic lymphocytes being engulfed by macrophages. (D) shows initial stages of phagocytosis. The lymphocyte is engulfed by a macrophage. The macrophage shown in (E) contains several destroyed apoptotic lymphocytes. Arrows show filoviral particles. 1—highly condensed heterochromatin; 2—monocyte showing normal nuclear morphology; 3—nucleus of macrophage. Bars correspond to 2 μm.
4.2. Infected Cells
In cell culture, filovirus infection leads to a clear cytopathic effect (CPE) including cell blebbing, cell rounding, vacuolization, and detachment [109,118,133,134]. However, the severity of the observed CPE is dependent on the virus species and the cell line used [54,135,136]. The mechanisms leading to cell death in filovirus infection are far from being understood and the published data are to some extent contradictory. While some studies describe apoptotic cell death for isolated primary human macrophages infected with ZEBOV or Bundibugyo [136,137], others did not observe any signs of apoptosis in filovirus-infected primary human cells including monocytes/macrophages from peripheral blood mononuclear cell cultures, macrovascular endothelial cells, or microvascular endothelial cells [54,138].
Electron microscopic studies and biochemical analyses of tissues from infected animals indicate that infected cells, including macrophages, DCs, hepatocytes, and endothelial cells, do not undergo apoptosis [47–50,55,60,62,63,69,138]. Morphologically, the cells appear normal (Figure 3A–D) or they show signs of necrosis (Figures 3E and F). A few examples of filovirus-infected cells from tissues from infected animals are shown in Figure 3. Although the shown tissue macrophages (Figures 3A,B,E,F), hepatocytes (Figure 3C), and endothelial cells (Figure 3D) are infected, as indicated by the presence of viral inclusions in the cytoplasm of the infected cells, they do not show any signs of apoptosis. Vacuolization of the infected cells, however, indicates that they finally undergo non-apoptotic cell death (Figures 3E,F). Notably, the number of swollen and necrotic cells increases during infection [49,55,62,63,69,138].
Intriguingly, the number of DCs was shown to dramatically decrease early in ZEBOV infection in baboons, African green monkeys, and rhesus macaques, and they were totally absent at 6–7 days p.i. [50]. In contrast, DCs were still present in ZEBOV-infected cynomolgus monkeys on day 6 [55]. These differences might be attributed to species-specific variability in the various non-human primate models for filovirus infection [139].
In non-human primates and guinea pigs, infected hepatocytes develop necrosis not related to the formation of inflammatory foci. The liver is a major target organ for filovirus infection and necrotic hepatocytes appear 3–4 days p.i. in numbers that depend on the infectious dose [44,47,50,60,69]. Notably, significant apoptosis of hepatocytes has been observed in a lethal mouse model for ZEBOV and seems to play an important role in pathogenesis; however, it is not clear if the apoptotic hepatocytes were infected [3].
Extensive filovirus infection may lead to the depletion of cellular stocks and disruption of cellular homeostasis. Morphologically, such depleted cells first show signs of swelling and vacuolization of cellular organelles including the ER, mitochondria and Golgi, suggesting that the cells are unable to maintain a normal water-ion balance. This is followed by cellular swelling, vacuolization and cell rounding, which leads to the formation of vacuolar structures consistent with necrosis in the cytoplasm of infected cells (Figures 3E,F), reflecting edematous conditions within the cells [140]. Whether this is due to damage to ion pumps in the plasma membrane or vacuolization of the membrane resulting in cell permeability as a result of viral budding is unclear. As noted earlier, however, the changes in the host cells are also coincident with changes in the progeny virus morphology and infectivity.
4.2.1. Interaction of Filoviruses with Cellular Pathways
The striking lack of infected apoptotic cells reported in most studies raises the question if filoviruses manipulate signaling pathways involved in apoptosis or cell survival. A first hint pointing in this direction has been given by a recent study showing that EBOV entry leads to the activation of the PI3K/Akt signaling pathway very early in infection, resulting in the activation of Rac1, a regulator of endocytosis and vesicular trafficking [141]. PI3K is embedded in a complex network of signaling cascades regulating cell metabolism, proliferation and survival, and many viruses modulate the PI3K/Akt pro-survival pathway to prevent premature apoptosis of infected cells [142]. The temporal modulation of PI3K activation during viral infection is critical, and different arms of the PI3K signaling network might be activated by distinct viral triggers during the replication cycle. Thus, it has been shown for influenza virus that transient PI3K activation during entry leads to the activation of Rac1, while late in infection, PI3K activation prevents the induction of premature apoptosis [143]. It is currently not known if PI3K/Akt pro-survival signaling cascades are activated at late stages in filovirus infection, but this would be an attractive model to at least partially explain why apoptosis does not seem to be the preferred mechanism of cell death in filovirus infection.
Filoviruses are known to interfere with antiviral signaling pathways. Since the innate immune response to filovirus infection and the corresponding viral countermeasures are the main subject of another review article in this issue, we will focus on the mechanisms involved in the regulation of apoptosis. To combat viral invasion, cells have evolved multiple antiviral defense mechanisms that are activated upon infection and ideally, will lead to the elimination of the viral intruders. The antiviral response is initiated by a cellular detection process mediated by various cellular pattern recognition receptors (PPR) that specifically recognize pathogen-associated molecular patterns (PAMPs). Typical PAMPs of negative-sense RNA viruses are surface proteins and RNA [144,145]. A prominent filovirus PAMP are the 5′triphosphate ends of the genomic RNA which can be sensed by RIG-I, leading to the induction of the type I interferon (IFN) response [146]. However, in cells infected with EBOV, the induction of the type I IFN response via RIG-I activation is blocked by the polymerase cofactor VP35 (reviewed in [147,148]). Recent findings show that RIG-I activation may lead to the induction of mitochondrial-mediated apoptosis, which is triggered by binding of the IFN regulatory factor 3 (IRF-3) to the pro-apoptotic Bax protein. The IRF-3-mediated induction of apoptosis has been considered an important antiviral defense mechanism against RNA viruses [149–151]. In addition, it has been reported that the mitochondrial antiviral signaling protein (MAVS; also known as IPS-1, VISA, or Cardif), an adaptor protein in the RIG-I signaling pathway, is able to activate apoptosis signaling, and this activation can be inhibited by viruses to prevent the induction of apoptosis [152,153]. Although one can speculate that the inhibition of RIG-I signaling by VP35 may prevent the induction of apoptosis in infected cells, no data are available to support this hypothesis. Noteworthy, the activation of PPRs by viral infection or double-stranded (ds) RNA may likewise induce anti-apoptotic signaling through NFκB, resulting in increased host cell survival [154–156] (reviewed in [157]).
The antiviral protein dsRNA-dependent protein kinase R (PKR) senses dsRNA and is a main regulator of IFN signaling and apoptosis. Since dsRNA is formed during replication and transcription of multiple RNA and DNA viruses [158], it is considered to be a major PAMP in viral infection. Upon activation by dsRNA PKR phosphorylates the translation initiation factor eIF2α, leading to translational arrest and apoptosis [159]. Intriguingly, PKR is not activated in ZEBOV-infected cells, and VP35 has been shown to actively block PKR activation [160,161]. It has been reported for various viruses that they inhibit PKR-mediated eIF2α phosphorylation to prevent host cell apoptosis [162–164]. However, it is not known if the inhibition of PKR in EBOV-infected cells blocks the induction of apoptosis.
Toll-like receptors (TLRs) are another important group of PRRs recognizing viral PAMPs [145]. TRL signaling was found to play pro- and anti-apoptotic roles in different cell types highlighting its role in regulation of host cell death [165,166]. The EBOV surface protein GP is recognized by TLR4 and activates TLR4 signaling, including activation of NFκB [167]. It remains unclear, however, if TLR4 signaling is triggered in the context of EBOV infection, since in a recent study, EBOV VP35 was found to interfere with the maturation of DCs induced by lipopolysaccharide, an agonist of TLR4 [168]. VP35 was also shown to inhibit the induction of IFN in murine DCs after treatment with CpG DNA, an agonist for TLR9 [169]. However, a recent study by Leung et al. [170] suggests that cells utilizing TLR-mediated antiviral pathways such as plasmacytoid dendritic cells are less prone to the inhibitory effects of VP35 EBOV than cells relying on RIG-like signaling pathways. In conclusion, the role of TLR signaling in filovirus infection is not well understood and additional studies using filovirus infection models are needed.
4.2.2. The Cytopathic Factor GP
Multiple expression studies have implicated the EBOV surface protein GP in cytotoxicity and cell damage, inducing cell rounding, detachment, and membrane permeabilization [92,171–177], while other EBOV proteins did not induce cell detachment [171]. Expression of GP also leads to a general downregulation of cell surface proteins including adhesion molecules, MHC class I proteins, and EGF receptor [92,174,176,178]. Moreover, GP expression in explanted blood vessels resulted in endothelial cell loss and increased vascular permeability [171].
As mentioned above, GP is proteolytically cleaved in two subunits, GP1 and GP2. Intriguingly, both GP subunits contribute to the observed cytopathogenicity. The cytopathic domain within GP1 has been mapped to the so-called mucin-like domain, a highly O- and N-glycosylated serine-threonine-rich region of about 150 amino acids in length, which is sufficient to cause detachment and downregulation of cellular surface proteins [171,178,179]. Interestingly, the mucin-like domain does not only induce cytopathic effects in cells expressing EBOV GP, but was also shown to induce activation of NFκB and ERK signaling pathways in cells treated with EBOV-like particles [180].
It has been suggested that the GP-induced cytotoxic effects are caused by the interaction of GP with the GTPase dynamin, leading to interference with the intracellular trafficking of cell surface proteins [176]. However, a recent study has questioned the involvement of dynamin in GP-induced CPE [178]. GP has also been shown to modulate the ERK/MAPK signaling cascade by reducing ERK2 activation. This effect is dependent on the mucin-like domain. Since downregulation of active ERK2 leads to a decreased alphaV-integrin expression, which is associated with cell rounding and detachment, it has been suggested that ERK2 signaling cascades are involved in the induction of GP-mediated cytopathic effects [179]. Another possible mechanism of GP-induced cytotoxicity might be the induction of ER stress associated with the unfolded protein response. A recent study reports that ectopically expressed GP containing the mucin-like domain accumulates in the ER, whereas GP lacking the mucin-like domain is distributed throughout the cell and does not localize in the ER. When the membrane-anchored GP2 subunit is expressed in cells in the absence of GP1, it also induces cytopathic effects by enhancing the permeability of the plasma membrane. This effect is mediated by the transmembrane domain [177,181]. If and how the different proposed mechanisms to explain GP-mediated cytopathogenicity are connected, is currently not known.
There is some debate over the ability of GP to induce cell death. Several groups reported that expression of GP, though inducing detachment of the cells, does not lead to cell disruption [172,174], while others observed cell death [171,175,179]. It is possible that the observed differences are due to experimental differences, including GP expression rates, the expression system used, long-term versus short-term expression, and the cell type. Intriguingly, GP has been shown to induce a specific form of apoptosis, anoikis, in primary human cardiac microvascular endothelial cells, while THP-1 cells, a human monocyte–macrophage-derived cell line, did not undergo cell death upon GP expression [175]. Another group has shown that GP-induced cell death is non-apoptotic [179].
Since the described data are based on ectopic expression of GP in the absence of other viral proteins, the question arises: What happens in the infected cells? Volchkov et al. [173] addressed this question by using a recombinant EBOV system. The membrane-anchored full-length GP is synthesized from an edited version of the GP mRNA, which accounts for about 20% of the total GP mRNA. When the editing site was mutated in the viral genome, all GP mRNA molecules were translated into the full-length version of GP, leading to the production of enhanced levels of membrane-anchored GP. The mutant was significantly more cytopathogenic than wild-type virus, indicating that the expression rate of GP in infected cells determines its cytotoxic properties. These results were confirmed by more recent studies showing that low expression rates of GP helped to avoid cytotoxic effects [133]. In addition, there might be other regulatory mechanisms in infected cells to mitigate GP-induced cytotoxicity. As mentioned above, VP35 is able to block various signaling pathways involved in the antiviral response to viral infection and it is conceivable that VP35 also interferes with cellular signaling pathways involved in GP-induced cellular stress.
It remains puzzling that MARV GP does not seem to be cytotoxic when expressed in the absence of other viral proteins, although it is as highly glycosylated as EBOV GP [172]. Also, infection studies in non-human primates revealed that the endothelium remains relatively intact even late in infection, suggesting that the hemorrhagic symptoms in EBOV infection are not caused by EBOV-induced cytolysis of infected endothelial cells, but are rather the result of dysregulated immune mechanisms targeting the vascular system [54,182,183]. Endothelial cells are secondary target cells in filovirus infection and the morphological characteristics of filovirus replication in these cells do not differ from those in other tissues or in cell culture [47,50,51,54,110].
4.3. Non-Infected Cells
Lymphocytes do not support filoviral replication, perhaps because they lack receptors for these viruses [184,185]. Nevertheless, lymphocyte apoptosis is a characteristic feature of filovirus infections and can be observed in both blood and tissues of infected patients and animals [7,55,138,186–188]. Figure 4 shows apoptotic lymphocytes in lymph node tissue from filovirus-infected animals. The apoptotic lymphocytes are not infected and are engulfed by surrounding macrophages. Filovirus-induced lymphocyte apoptosis is also observed in EBOV-infected human PBMCs [137]. The apoptosis of non-infected lymphocytes is not unique to filoviruses. It has been reported in a number of viral infections, including lymphocyte choriomeningitis virus [189], human immunodeficiency virus [190], human herpesvirus 6 [191] and Vaccinia virus infections [192]. Although protection from apoptosis of infected cells is a strategy for survival for a number of viruses [193], induced periods of transient or chronic lymphocytopenia brought about by bystander apoptosis is thought to contribute to the generalized immunosuppression that accompanies some viral infections [192,194].
As mentioned above, apoptosis can be initiated by both extrinsic factors and intrinsic mechanisms, both of which activate caspases and lead to DNA fragmentation and cell death (reviewed in [195]). The best known example of bystander lymphocyte apoptosis in viral infections is in CD4 T cells during HIV infection. Nevertheless, despite years of study, the mechanisms of depletion of these cells remain controversial. However, extrinsic and intrinsic pathways appear to be involved. Although CD4 T cells are a target of HIV, the majority of lymphocytes that are depleted are uninfected, and the depletion of uninfected cells accelerates with disease progression [190,196]. Both viral proteins released from infected cells [197–199] and host-derived proteins [200,201] have been suggested to contribute to CD4 T cell death. More recently, an accumulation of incomplete viral transcripts during abortive infection of resting CD4 T cells has been demonstrated to activate intrinsic pathways that lead to apoptosis during HIV infection [202]. It is unclear which of these mechanisms account for the majority of T cell apoptosis during HIV infection, or if multiple mechanisms are important in HIV pathogenesis. Given that many gaps remain in our understanding of bystander T cell apoptosis in HIV infection, despite years of study, it is not surprising that we would have even less of a universal view of how filoviruses cause bystander cell loss.
In the case of filovirus infection, not only are CD4 T cells depleted, but CD8 T cells and NK cells as well [7,55,186], suggesting that some generalized mechanisms may contribute to lymphocyte apoptosis in filovirus disease. Using a mouse model of EBOV infection, Bradfute et al. [3] suggested that both intrinsic and extrinsic apoptotic pathways may contribute to lymphocyte depletion. If this is also the case for the disease in humans and non-human primates, then it is likely that multiple mechanisms contribute to the generalized lymphocyte depletion. The loss of lymphocytes has been postulated to contribute to the failure to generate fully protective adaptive immune responses in these species [7,203–205]. In fact, extensive lymphocyte apoptosis has been shown to proceed the generation of adaptive responses in humans with fatal disease [7], making this hypothesis all the more plausible.
EBOV-infected cells may secrete TRAIL and increased levels of soluble Fas have been detected in the sera of some EBOV-infected non-human primates [55,206]. Moreover, increased expression levels of TRAIL and Fas mRNA have been observed in peripheral blood mononuclear cells of infected non-human primates [55]. These could trigger conventional extrinsic pathways of apoptosis in susceptible cells, including T cells. This view is supported by an analysis of Fas expression on T cells from patients who survived or succumbed to EBOV infection [188]. In patients who died, a very high proportion of the few remaining CD4 and CD8 T cells were Fas positive in contrast to T cells from survivors. Although Fas can be induced as a result of specific T cell activation [207], it is unlikely that the residual cells in these patients were only antigen-specific T cells.
It is also possible that dysregulated DCs and macrophages could contribute in other ways to lymphocyte apoptosis. Infected DCs and macrophages fail to produce regulated cytokine responses, and they are also impaired in their ability to upregulate costimulatory molecules, such as CD40 and the B7 family member CD86, consistent with their inability to efficiently prime T cells [208,209]. Interestingly, however, other members of the B7 family, including co-inhibitory molecules such as programmed death ligand 1 (PD-L1) are upregulated on infected DCs, and its receptor, programmed death 1 (PD-1) may be upregulated on CD8 T cells in EBOV infections [210]. The interaction of these molecules could lead to apoptosis. The PD-1/PD-L1 pathway is important for controlling T cell tolerance and has been shown to be used by pathogens to down-regulate T cell responses [128,211]. PD-1 signaling results in decreased T cell proliferation and recent findings suggest this might be due to induction of apoptosis via PD-L1 binding [212,213]. While this might explain the depletion of antigen-specific T cell populations, this mechanism is unlikely to explain the generalized lymphopenia observed during filovirus infection, since the infected DCs would not be expected to engage T cells that were not specific for filovirus epitopes sufficiently to trigger PD-1 signaling.
Alternatively, T cell apoptosis may result in part from the dysregulated cytokine responses during filovirus infections. IFNγ helps productively modulate CD8 T cell responses if present at or near the time of T cell activation. However, if not, IFNγ can have pro-apoptotic consequences on T cells [214]. Interestingly, IFNγ is present at high levels in the serum late in fatal filovirus infection [2,162], and these high levels could result in the activation of T cell and/or NK cell apoptotic pathways. Finally, a 17-mer in filovirus GPs, which resembles an immunosuppressive motive found in retroviral envelope proteins, has been reported to induce lymphocyte death and suppression of cytokine responses [215–217]. This is puzzling, because lymphocytes do not bind to the viral GP [184,185]. So the mechanism by which the 17-mer mobilizes lymphocyte death remains unclear.
5. Conclusions
We have learned a lot about the interaction of filoviruses with infected (and non-infected) cells, including identification of target cells, elucidating stages of the replication cycle, cytopathic effects induced by the viruses, and host restriction factors. Nevertheless, there is still a long way to go towards understanding both the cellular and organismal events that lead to the outcome of this devastating disease. There remain major gaps in our understanding of the alterations in signaling pathways in infected cells, the cellular components that contribute to filovirus replication, and the mechanisms that control cell fate in infected cells. Moreover, we remain largely ignorant in our understanding of the mechanisms that impact the innate and adaptive immune system and modulate inflammatory responses during infection. These avenues of inquiry will be crucial for us to have a more complete understanding of the pathogenesis of filoviral disease.
Acknowledgments
The authors thank Kristina Brauburger for the graphic work in Figure 1, Adam Hume for critically reading the manuscript, and Julia Spitsyna for processing of electron micrographs. This work was supported by National Institutes of Health (NIH) grants U01-AI082954 and AI057159 (New England Regional Center of Excellence-Kasper, subaward 149047-0743), and by start-up funds from Boston University.
Footnotes
Conflict of Interest
The authors declare no conflict of interest.
References and Notes
- 1.Kuhn JH. Filoviruses. A compendium of 40 years of epidemiological, clinical, and laboratory studies. Arch Virol Suppl. 2008;20:13–360. [PubMed] [Google Scholar]
- 2.Mohamadzadeh M, Chen L, Schmaljohn AL. How Ebola and Marburg viruses battle the immune system. Nat Rev Immunol. 2007;7:556–567. doi: 10.1038/nri2098. [DOI] [PubMed] [Google Scholar]
- 3.Bradfute SB, Swanson PE, Smith MA, Watanabe E, McDunn JE, Hotchkiss RS, Bavari S. Mechanisms and consequences of ebolavirus-induced lymphocyte apoptosis. J Immunol. 2010;184:327–335. doi: 10.4049/jimmunol.0901231. [DOI] [PubMed] [Google Scholar]
- 4.Ksiazek TG, West CP, Rollin PE, Jahrling PB, Peters CJ. ELISA for the detection of antibodies to Ebola viruses. J Infect Dis. 1999;179:S192–S198. doi: 10.1086/514313. [DOI] [PubMed] [Google Scholar]
- 5.Onyango CO, Opoka ML, Ksiazek TG, Formenty P, Ahmed A, Tukei PM, Sang RC, Ofula VO, Konongoi SL, Coldren RL, et al. Laboratory diagnosis of Ebola hemorrhagic fever during an outbreak in Yambio, Sudan, 2004. J Infect Dis. 2007;196:S193–S198. doi: 10.1086/520609. [DOI] [PubMed] [Google Scholar]
- 6.Towner JS, Rollin PE, Bausch DG, Sanchez A, Crary SM, Vincent M, Lee WF, Spiropoulou CF, Ksiazek TG, Lukwiya M, et al. Rapid diagnosis of Ebola hemorrhagic fever by reverse transcription-PCR in an outbreak setting and assessment of patient viral load as a predictor of outcome. J Virol. 2004;78:4330–4341. doi: 10.1128/JVI.78.8.4330-4341.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Baize S, Leroy EM, Georges-Courbot MC, Capron M, Lansoud-Soukate J, Debre P, Fisher-Hoch SP, McCormick JB, Georges AJ. Defective humoral responses and extensive intravascular apoptosis are associated with fatal outcome in Ebola virus-infected patients. Nat Med. 1999;5:423–426. doi: 10.1038/7422. [DOI] [PubMed] [Google Scholar]
- 8.Baize S, Leroy EM, Georges AJ, Georges-Courbot MC, Capron M, Bedjabaga I, Lansoud-Soukate J, Mavoungou E. Inflammatory responses in Ebola virus-infected patients. Clin Exp Immunol. 2002;128:163–168. doi: 10.1046/j.1365-2249.2002.01800.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Mahanty S, Gupta M, Paragas J, Bray M, Ahmed R, Rollin PE. Protection from lethal infection is determined by innate immune responses in a mouse model of Ebola virus infection. Virology. 2003;312:415–424. doi: 10.1016/s0042-6822(03)00233-2. [DOI] [PubMed] [Google Scholar]
- 10.Kuhn JH, Becker S, Ebihara H, Geisbert TW, Johnson KM, Kawaoka Y, Lipkin WI, Negredo AI, Netesov SV, Nichol ST, et al. Proposal for a revised taxonomy of the family Filoviridae: classification, names of taxa and viruses, and virus abbreviations. Arch Virol. 2010;155:2083–2103. doi: 10.1007/s00705-010-0814-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Hutchinson KL, Rollin PE. Cytokine and chemokine expression in humans infected with Sudan Ebola virus. J Infect Dis. 2007;196:S357–S363. doi: 10.1086/520611. [DOI] [PubMed] [Google Scholar]
- 12.Towner JS, Sealy TK, Khristova ML, Albarino CG, Conlan S, Reeder SA, Quan PL, Lipkin WI, Downing R, Tappero JW, et al. Newly discovered ebola virus associated with hemorrhagic fever outbreak in Uganda. PLoS Pathog. 2008;4:e1000212. doi: 10.1371/journal.ppat.1000212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Formenty P, Hatz C, Le Guenno B, Stoll A, Rogenmoser P, Widmer A. Human infection due to Ebola virus, subtype Cote d'Ivoire: Clinical and biologic presentation. J Infect Dis. 1999;179:S48–S53. doi: 10.1086/514285. [DOI] [PubMed] [Google Scholar]
- 14.Le Guenno B, Formenty P, Boesch C. Ebola virus outbreaks in the Ivory Coast and Liberia, 1994–1995. Curr Top Microbiol Immunol. 1999;235:77–84. doi: 10.1007/978-3-642-59949-1_5. [DOI] [PubMed] [Google Scholar]
- 15.Barrette RW, Metwally SA, Rowland JM, Xu L, Zaki SR, Nichol ST, Rollin PE, Towner JS, Shieh WJ, Batten B, et al. Discovery of swine as a host for the Reston ebolavirus. Science. 2009;325:204–206. doi: 10.1126/science.1172705. [DOI] [PubMed] [Google Scholar]
- 16.Miranda ME, Ksiazek TG, Retuya TJ, Khan AS, Sanchez A, Fulhorst CF, Rollin PE, Calaor AB, Manalo DL, Roces MC, et al. Epidemiology of Ebola (subtype Reston) virus in the Philippines, 1996. J Infect Dis. 1999;179:S115–S119. doi: 10.1086/514314. [DOI] [PubMed] [Google Scholar]
- 17.Rollin PE, Williams RJ, Bressler DS, Pearson S, Cottingham M, Pucak G, Sanchez A, Trappier SG, Peters RL, Greer PW, et al. Ebola (subtype Reston) virus among quarantined nonhuman primates recently imported from the Philippines to the United States. J Infect Dis. 1999;179:S108–S114. doi: 10.1086/514303. [DOI] [PubMed] [Google Scholar]
- 18.Slenczka W, Klenk HD. Forty years of marburg virus. J Infect Dis. 2007;196:S131–S135. doi: 10.1086/520551. [DOI] [PubMed] [Google Scholar]
- 19.Slenczka WG. The Marburg virus outbreak of 1967 and subsequent episodes. Curr Top Microbiol Immunol. 1999;235:49–75. doi: 10.1007/978-3-642-59949-1_4. [DOI] [PubMed] [Google Scholar]
- 20.Feldmann H. Marburg hemorrhagic fever—The forgotten cousin strikes. N Engl J Med. 2006;355:866–869. doi: 10.1056/NEJMp068160. [DOI] [PubMed] [Google Scholar]
- 21.Towner JS, Khristova ML, Sealy TK, Vincent MJ, Erickson BR, Bawiec DA, Hartman AL, Comer JA, Zaki SR, Ströher U, et al. Marburgvirus genomics and association with a large hemorrhagic fever outbreak in Angola. J Virol. 2006;80:6497–6516. doi: 10.1128/JVI.00069-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Bausch DG, Nichol ST, Muyembe-Tamfum JJ, Borchert M, Rollin PE, Sleurs H, Campbell P, Tshioko FK, Roth C, Colebunders R, et al. Marburg hemorrhagic fever associated with multiple genetic lineages of virus. N Engl J Med. 2006;355:909–919. doi: 10.1056/NEJMoa051465. [DOI] [PubMed] [Google Scholar]
- 23.Imported case of Marburg hemorrhagic fever—Colorado, 2008. MMWR Morb Mortal Wkly Rep. 2009;58:1377–1381. [PubMed] [Google Scholar]
- 24.Timen A, Koopmans MP, Vossen AC, van Doornum GJ, Gunther S, van den Berkmortel F, Verduin KM, Dittrich S, Emmerich P, Osterhaus AD, et al. Response to imported case of Marburg hemorrhagic fever, the Netherland. Emerg Infect Dis. 2009;15:1171–1175. doi: 10.3201/eid1508.090051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Mühlberger E. Filovirus replication and transcription. Future Virology. 2007;2:205–215. doi: 10.2217/17460794.2.2.205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Hartlieb B, Weissenhorn W. Filovirus assembly and budding. Virology. 2006;344:64–70. doi: 10.1016/j.virol.2005.09.018. [DOI] [PubMed] [Google Scholar]
- 27.Bamberg S, Kolesnikova L, Möller P, Klenk HD, Becker S. VP24 of Marburg virus influences formation of infectious particles. J Virol. 2005;79:13421–13433. doi: 10.1128/JVI.79.21.13421-13433.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Han Z, Boshra H, Sunyer JO, Zwiers SH, Paragas J, Harty RN. Biochemical and functional characterization of the Ebola virus VP24 protein: Implications for a role in virus assembly and budding. J Virol. 2003;77:1793–1800. doi: 10.1128/JVI.77.3.1793-1800.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Noda T, Halfmann P, Sagara H, Kawaoka Y. Regions in Ebola virus VP24 that are important for nucleocapsid formation. J Infect Dis. 2007;196:S247–S250. doi: 10.1086/520596. [DOI] [PubMed] [Google Scholar]
- 30.Watanabe S, Noda T, Halfmann P, Jasenosky L, Kawaoka Y. Ebola virus (EBOV) VP24 inhibits transcription and replication of the EBOV genome. J Infect Dis. 2007;196:S284–S290. doi: 10.1086/520582. [DOI] [PubMed] [Google Scholar]
- 31.Hoenen T, Jung S, Herwig A, Groseth A, Becker S. Both matrix proteins of Ebola virus contribute to the regulation of viral genome replication and transcription. Virology. 2010;403:56–66. doi: 10.1016/j.virol.2010.04.002. [DOI] [PubMed] [Google Scholar]
- 32.Ebihara H, Takada A, Kobasa D, Jones S, Neumann G, Theriault S, Bray M, Feldmann H, Kawaoka Y. Molecular determinants of Ebola virus virulence in mice. PLoS Pathog. 2006;2:e73. doi: 10.1371/journal.ppat.0020073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Reid SP, Leung LW, Hartman AL, Martinez O, Shaw ML, Carbonnelle C, Volchkov VE, Nichol ST, Basler CF. Ebola virus VP24 binds karyopherin alpha1 and blocks STAT1 nuclear accumulation. J Virol. 2006;80:5156–5167. doi: 10.1128/JVI.02349-05. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Valmas C, Grosch MN, Schümann M, Olejnik J, Martinez O, Best SM, Krähling V, Basler CF, Mühlberger E. Marburg virus evades interferon responses by a mechanism distinct from ebola virus. PLoS Pathog. 2010;6:e1000721. doi: 10.1371/journal.ppat.1000721. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Valmas C, Basler CF. Marburg virus VP40 antagonizes interferon signaling in a species-specific manner. J Virol. 2011;85:4309–4317. doi: 10.1128/JVI.02575-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Feldmann H, Volchkov VE, Volchkova VA, Klenk HD. The glycoproteins of Marburg and Ebola virus and their potential roles in pathogenesis. Arch Virol Suppl. 1999;15:159–169. doi: 10.1007/978-3-7091-6425-9_11. [DOI] [PubMed] [Google Scholar]
- 37.Volchkov VE, Feldmann H, Volchkova VA, Klenk HD. Processing of the Ebola virus glycoprotein by the proprotein convertase furin. Proc Natl Acad Sci U S A. 1998;95:5762–5767. doi: 10.1073/pnas.95.10.5762. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Volchkov VE, Becker S, Volchkova VA, Ternovoj VA, Kotov AN, Netesov SV, Klenk HD. GP mRNA of Ebola virus is edited by the Ebola virus polymerase and by T7 and vaccinia virus polymerases. Virology. 1995;214:421–430. doi: 10.1006/viro.1995.0052. [DOI] [PubMed] [Google Scholar]
- 39.Sanchez A, Trappier SG, Mahy BW, Peters CJ, Nichol ST. The virion glycoproteins of Ebola viruses are encoded in two reading frames and are expressed through transcriptional editing. Proc Natl Acad Sci U S A. 1996;93:3602–3607. doi: 10.1073/pnas.93.8.3602. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Wahl-Jensen V, Kurz SK, Hazelton PR, Schnittler HJ, Stroher U, Burton DR, Feldmann H. Role of Ebola virus secreted glycoproteins and virus-like particles in activation of human macrophages. J Virol. 2005;79:2413–2419. doi: 10.1128/JVI.79.4.2413-2419.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Volchkova VA, Feldmann H, Klenk HD, Volchkov VE. The nonstructural small glycoprotein sGP of Ebola virus is secreted as an antiparallel-orientated homodimer. Virology. 1998;250:408–414. doi: 10.1006/viro.1998.9389. [DOI] [PubMed] [Google Scholar]
- 42.Mehedi M, Falzarano D, Seebach J, Hu X, Carpenter MS, Schnittler HJ, Feldmann H. A new Ebola virus nonstructural glycoprotein expressed through RNA editing. J Virol. 2011;85:5406–5414. doi: 10.1128/JVI.02190-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Bowen ET, Platt GS, Simpson DI, McArdell LB, Raymond RT. Ebola haemorrhagic fever: Experimental infection of monkeys. Trans R Soc Trop Med Hyg. 1978;72:188–191. doi: 10.1016/0035-9203(78)90058-5. [DOI] [PubMed] [Google Scholar]
- 44.Ellis DS, Simpson IH, Francis DP, Knobloch J, Bowen ET, Lolik P, Deng IM. Ultrastructure of Ebola virus particles in human liver. J Clin Pathol. 1978;31:201–208. doi: 10.1136/jcp.31.3.201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Baskerville A, Fisher-Hoch SP, Neild GH, Dowsett AB. Ultrastructural pathology of experimental Ebola haemorrhagic fever virus infection. J Pathol. 1985;147:199–209. doi: 10.1002/path.1711470308. [DOI] [PubMed] [Google Scholar]
- 46.Johnson E, Jaax N, White J, Jahrling P. Lethal experimental infections of rhesus monkeys by aerosolized Ebola virus. Int J Exp Pathol. 1995;76:227–236. [PMC free article] [PubMed] [Google Scholar]
- 47.Ryabchikova E, Strelets L, Kolesnikova L, Pyankov O, Sergeev A. Respiratory Marburg virus infection in guinea pigs. Arch Virol. 1996;141:2177–2190. doi: 10.1007/BF01718224. [DOI] [PubMed] [Google Scholar]
- 48.Ryabchikova E, Kolesnikova L, Smolina M, Tkachev V, Pereboeva L, Baranova S, Grazhdantseva A, Rassadkin Y. Ebola virus infection in guinea pigs: Presumable role of granulomatous inflammation in pathogenesis. Arch Virol. 1996;141:909–921. doi: 10.1007/BF01718165. [DOI] [PubMed] [Google Scholar]
- 49.Ryabchikova EI, Kolesnikova LV, Luchko SV. An analysis of features of pathogenesis in two animal models of Ebola virus infection. J Infect Dis. 1999;179:S199–S202. doi: 10.1086/514293. [DOI] [PubMed] [Google Scholar]
- 50.Ryabchikova E, Price BBS. Ebola and Marburg Viruses: A View of Infection Using Electron Microscopy. Battelle Press; Columbus, Ohio, USA: 2004. [Google Scholar]
- 51.Jaax NK, Davis KJ, Geisbert TJ, Vogel P, Jaax GP, Topper M, Jahrling PB. Lethal experimental infection of rhesus monkeys with Ebola-Zaire (Mayinga) virus by the oral and conjunctival route of exposure. Arch Pathol Lab Med. 1996;120:140–155. [PubMed] [Google Scholar]
- 52.Davis KJ, Anderson AO, Geisbert TW, Steele KE, Geisbert JB, Vogel P, Connolly BM, Huggins JW, Jahrling PB, Jaax NK. Pathology of experimental Ebola virus infection in African green monkeys. Involvement of fibroblastic reticular cells. Arch Pathol Lab Med. 1997;121:805–819. [PubMed] [Google Scholar]
- 53.Wyers M, Formenty P, Cherel Y, Guigand L, Fernandez B, Boesch C, Le Guenno B. Histopathological and immunohistochemical studies of lesions associated with Ebola virus in a naturally infected chimpanzee. J Infect Dis. 1999;179:S54–S59. doi: 10.1086/514300. [DOI] [PubMed] [Google Scholar]
- 54.Geisbert TW, Young HA, Jahrling PB, Davis KJ, Larsen T, Kagan E, Hensley LE. Pathogenesis of Ebola hemorrhagic fever in primate models: Evidence that hemorrhage is not a direct effect of virus-induced cytolysis of endothelial cells. Am J Pathol. 2003;163:2371–2382. doi: 10.1016/S0002-9440(10)63592-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Geisbert TW, Hensley LE, Larsen T, Young HA, Reed DS, Geisbert JB, Scott DP, Kagan E, Jahrling PB, Davis KJ. Pathogenesis of Ebola hemorrhagic fever in cynomolgus macaques: evidence that dendritic cells are early and sustained targets of infection. Am J Pathol. 2003;163:2347–2370. doi: 10.1016/S0002-9440(10)63591-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Zaki SR, Goldsmith CS. Pathologic features of filovirus infections in humans. Curr Top Microbiol Immunol. 1999;235:97–116. doi: 10.1007/978-3-642-59949-1_7. [DOI] [PubMed] [Google Scholar]
- 57.Steele KE, Anderson AO, Mohamadzadeh M. Fibroblastic reticular cell infection by hemorrhagic fever viruses. Immunotherapy. 2009;1:187–197. doi: 10.2217/1750743X.1.2.187. [DOI] [PubMed] [Google Scholar]
- 58.Reis e Sousa C. Toll-like receptors and dendritic cells: for whom the bug tolls. Semin Immunol. 2004;16:27–34. doi: 10.1016/j.smim.2003.10.004. [DOI] [PubMed] [Google Scholar]
- 59.Reis e Sousa C. Activation of dendritic cells: translating innate into adaptive immunity. Curr Opin Immunol. 2004;16:21–25. doi: 10.1016/j.coi.2003.11.007. [DOI] [PubMed] [Google Scholar]
- 60.Murphy FA, Simpson DI, Whitfield SG, Zlotnik I, Carter GB. Marburg virus infection in monkeys. Ultrastructural studies. Lab Invest. 1971;24:279–291. [PubMed] [Google Scholar]
- 61.Bray M, Davis K, Geisbert T, Schmaljohn C, Huggins J. A mouse model for evaluation of prophylaxis and therapy of Ebola hemorrhagic fever. J Infect Dis. 1999;179:S248–S258. doi: 10.1086/514292. [DOI] [PubMed] [Google Scholar]
- 62.Connolly BM, Steele KE, Davis KJ, Geisbert TW, Kell WM, Jaax NK, Jahrling PB. Pathogenesis of experimental Ebola virus infection in guinea pigs. J Infect Dis. 1999;179:S203–S217. doi: 10.1086/514305. [DOI] [PubMed] [Google Scholar]
- 63.Gibb TR, Bray M, Geisbert TW, Steele KE, Kell WM, Davis KJ, Jaax NK. Pathogenesis of experimental Ebola Zaire virus infection in BALB/c mice. J Comp Pathol. 2001;125:233–242. doi: 10.1053/jcpa.2001.0502. [DOI] [PubMed] [Google Scholar]
- 64.Geisbert TW, Jahrling PB, Hanes MA, Zack PM. Association of Ebola-related Reston virus particles and antigen with tissue lesions of monkeys imported to the United States. J Comp Pathol. 1992;106:137–152. doi: 10.1016/0021-9975(92)90043-t. [DOI] [PubMed] [Google Scholar]
- 65.Riabchikova EI, Baranova SG, Tkachev VK, Grazhdantseva AA. The morphological changes in Ebola infection in guinea pigs. Vopr Virusol. 1993;38:176–179. [PubMed] [Google Scholar]
- 66.Geisbert TW, Jaax NK. Marburg hemorrhagic fever: Report of a case studied by immunohistochemistry and electron microscopy. Ultrastruct Pathol. 1998;22:3–17. doi: 10.3109/01913129809032253. [DOI] [PubMed] [Google Scholar]
- 67.Bray M, Geisbert TW. Ebola virus: The role of macrophages and dendritic cells in the pathogenesis of Ebola hemorrhagic fever. Int J Biochem Cell Biol. 2005;37:1560–1566. doi: 10.1016/j.biocel.2005.02.018. [DOI] [PubMed] [Google Scholar]
- 68.Schnittler HJ, Feldmann H. Marburg and Ebola hemorrhagic fevers: Does the primary course of infection depend on the accessibility of organ-specific macrophages? Clin Infect Dis. 1998;27:404–406. doi: 10.1086/517704. [DOI] [PubMed] [Google Scholar]
- 69.Baskerville A, Bowen ET, Platt GS, McArdell LB, Simpson DI. The pathology of experimental Ebola virus infection in monkeys. J Pathol. 1978;125:131–138. doi: 10.1002/path.1711250303. [DOI] [PubMed] [Google Scholar]
- 70.Martini GA, Siegert R. Marburg Virus Disease. Springer; New York, NY, USA: 1971. [Google Scholar]
- 71.Zaki SR, Kilmarx PH. Ebola virus hemorrhagic fever. In: Horsburgh CR, Nelson AM, editors. Pathology of Emerging Infections. ASM (USA); Washington, DC, USA: 1998. [Google Scholar]
- 72.Bavari S, Bosio CM, Wiegand E, Ruthel G, Will AB, Geisbert TW, Hevey M, Schmaljohn C, Schmaljohn A, Aman MJ. Lipid raft microdomains: A gateway for compartmentalized trafficking of Ebola and Marburg viruses. J Exp Med. 2002;195:593–602. doi: 10.1084/jem.20011500. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Empig CJ, Goldsmith MA. Association of the caveola vesicular system with cellular entry by filoviruses. J Virol. 2002;76:5266–5270. doi: 10.1128/JVI.76.10.5266-5270.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Sanchez A. Analysis of filovirus entry into vero e6 cells, using inhibitors of endocytosis, endosomal acidification, structural integrity, and cathepsin (B and L) activity. J Infect Dis. 2007;196:S251–S258. doi: 10.1086/520597. [DOI] [PubMed] [Google Scholar]
- 75.Hunt CL, Kolokoltsov AA, Davey RA, Maury W. The Tyro3 receptor kinase Axl enhances macropinocytosis of Zaire ebolavirus. J Virol. 2011;85:334–347. doi: 10.1128/JVI.01278-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Nanbo A, Imai M, Watanabe S, Noda T, Takahashi K, Neumann G, Halfmann P, Kawaoka Y. Ebolavirus is internalized into host cells via macropinocytosis in a viral glycoprotein-dependent manner. PLoS Pathog. 2010;6 doi: 10.1371/journal.ppat.1001121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Saeed MF, Kolokoltsov AA, Albrecht T, Davey RA. Cellular entry of ebola virus involves uptake by a macropinocytosis-like mechanism and subsequent trafficking through early and late endosomes. PLoS Pathog. 2010;6:pii: e1001110. doi: 10.1371/journal.ppat.1001110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Chan SY, Empig CJ, Welte FJ, Speck RF, Schmaljohn A, Kreisberg JF, Goldsmith MA. Folate receptor-alpha is a cofactor for cellular entry by Marburg and Ebola viruses. Cell. 2001;106:117–126. doi: 10.1016/s0092-8674(01)00418-4. [DOI] [PubMed] [Google Scholar]
- 79.Simmons G, Rennekamp AJ, Chai N, Vandenberghe LH, Riley JL, Bates P. Folate receptor alpha and caveolae are not required for Ebola virus glycoprotein-mediated viral infection. J Virol. 2003;77:13433–13438. doi: 10.1128/JVI.77.24.13433-13438.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Sinn PL, Hickey MA, Staber PD, Dylla DE, Jeffers SA, Davidson BL, Sanders DA, McCray PB., Jr Lentivirus vectors pseudotyped with filoviral envelope glycoproteins transduce airway epithelia from the apical surface independently of folate receptor alpha. J Virol. 2003;77:5902–5910. doi: 10.1128/JVI.77.10.5902-5910.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Sanchez A, Yang ZY, Xu L, Nabel GJ, Crews T, Peters CJ. Biochemical analysis of the secreted and virion glycoproteins of Ebola virus. J Virol. 1998;72:6442–6447. doi: 10.1128/jvi.72.8.6442-6447.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Feldmann H, Will C, Schikore M, Slenczka W, Klenk HD. Glycosylation and oligomerization of the spike protein of Marburg virus. Virology. 1991;182:353–356. doi: 10.1016/0042-6822(91)90680-A. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Feldmann H, Nichol ST, Klenk HD, Peters CJ, Sanchez A. Characterization of filoviruses based on differences in structure and antigenicity of the virion glycoprotein. Virology. 1994;199:469–473. doi: 10.1006/viro.1994.1147. [DOI] [PubMed] [Google Scholar]
- 84.Becker S, Spiess M, Klenk HD. The asialoglycoprotein receptor is a potential liver-specific receptor for Marburg virus. J Gen Virol. 1995;76:393–399. doi: 10.1099/0022-1317-76-2-393. [DOI] [PubMed] [Google Scholar]
- 85.Alvarez CP, Lasala F, Carrillo J, Muniz O, Corbi AL, Delgado R. C-type lectins DC-SIGN and L-SIGN mediate cellular entry by Ebola virus in cis and in trans. J Virol. 2002;76:6841–6844. doi: 10.1128/JVI.76.13.6841-6844.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Simmons G, Reeves JD, Grogan CC, Vandenberghe LH, Baribaud F, Whitbeck JC, Burke E, Buchmeier MJ, Soilleux EJ, Riley JL, et al. DC-SIGN and DC-SIGNR bind ebola glycoproteins and enhance infection of macrophages and endothelial cells. Virology. 2003;305:115–123. doi: 10.1006/viro.2002.1730. [DOI] [PubMed] [Google Scholar]
- 87.Baribaud F, Doms RW, Pohlmann S. The role of DC-SIGN and DC-SIGNR in HIV and Ebola virus infection: can potential therapeutics block virus transmission and dissemination? Expert Opin Ther Targets. 2002;6:423–431. doi: 10.1517/14728222.6.4.423. [DOI] [PubMed] [Google Scholar]
- 88.Marzi A, Gramberg T, Simmons G, Moller P, Rennekamp AJ, Krumbiegel M, Geier M, Eisemann J, Turza N, Saunier B, et al. DC-SIGN and DC-SIGNR interact with the glycoprotein of Marburg virus and the S protein of severe acute respiratory syndrome coronavirus. J Virol. 2004;78:12090–12095. doi: 10.1128/JVI.78.21.12090-12095.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Takada A, Fujioka K, Tsuiji M, Morikawa A, Higashi N, Ebihara H, Kobasa D, Feldmann H, Irimura T, Kawaoka Y. Human macrophage C-type lectin specific for galactose and N-acetylgalactosamine promotes filovirus entry. J Virol. 2004;78:2943–2947. doi: 10.1128/JVI.78.6.2943-2947.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Gramberg T, Hofmann H, Moller P, Lalor PF, Marzi A, Geier M, Krumbiegel M, Winkler T, Kirchhoff F, Adams DH, et al. LSECtin interacts with filovirus glycoproteins and the spike protein of SARS coronavirus. Virology. 2005;340:224–236. doi: 10.1016/j.virol.2005.06.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Matsuno K, Kishida N, Usami K, Igarashi M, Yoshida R, Nakayama E, Shimojima M, Feldmann H, Irimura T, Kawaoka Y, et al. Different potential of C-type lectin-mediated entry between Marburg virus strains. J Virol. 2010;84:5140–5147. doi: 10.1128/JVI.02021-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Takada A, Watanabe S, Ito H, Okazaki K, Kida H, Kawaoka Y. Downregulation of beta1 integrins by Ebola virus glycoprotein: Implication for virus entry. Virology. 2000;278:20–26. doi: 10.1006/viro.2000.0601. [DOI] [PubMed] [Google Scholar]
- 93.Schornberg KL, Shoemaker CJ, Dube D, Abshire MY, Delos SE, Bouton AH, White JM. Alpha5beta1-integrin controls ebolavirus entry by regulating endosomal cathepsins. Proc Natl Acad Sci U S A. 2009;106:8003–8008. doi: 10.1073/pnas.0807578106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Kondratowicz AS, Lennemann NJ, Sinn PL, Davey RA, Hunt CL, Moller-Tank S, Meyerholz DK, Rennert P, Mullins RF, Brindley M, et al. T-cell immunoglobulin and mucin domain 1 (TIM-1) is a receptor for Zaire Ebolavirus and Lake Victoria Marburgvirus. Proc Natl Acad Sci U S A. 2011 doi: 10.1073/pnas.1019030108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Rothlin CV, Ghosh S, Zuniga EI, Oldstone MB, Lemke G. TAM receptors are pleiotropic inhibitors of the innate immune response. Cell. 2007;131:1124–1136. doi: 10.1016/j.cell.2007.10.034. [DOI] [PubMed] [Google Scholar]
- 96.Shimojima M, Takada A, Ebihara H, Neumann G, Fujioka K, Irimura T, Jones S, Feldmann H, Kawaoka Y. Tyro3 family-mediated cell entry of Ebola and Marburg viruses. J Virol. 2006;80:10109–10116. doi: 10.1128/JVI.01157-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Shimojima M, Ikeda Y, Kawaoka Y. The mechanism of Axl-mediated Ebola virus infection. J Infect Dis. 2007;196:S259–S263. doi: 10.1086/520594. [DOI] [PubMed] [Google Scholar]
- 98.Weissenhorn W, Calder LJ, Wharton SA, Skehel JJ, Wiley DC. The central structural feature of the membrane fusion protein subunit from the Ebola virus glycoprotein is a long triple-stranded coiled coil. Proc Natl Acad Sci U S A. 1998;95:6032–6036. doi: 10.1073/pnas.95.11.6032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Chandran K, Sullivan NJ, Felbor U, Whelan SP, Cunningham JM. Endosomal proteolysis of the ebola virus glycoprotein is necessary for infection. Science. 2005;308:1643–1645. doi: 10.1126/science.1110656. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Schornberg K, Matsuyama S, Kabsch K, Delos S, Bouton A, White J. Role of endosomal cathepsins in entry mediated by the Ebola virus glycoprotein. J Virol. 2006;80:4174–4178. doi: 10.1128/JVI.80.8.4174-4178.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Hood CL, Abraham J, Boyington JC, Leung K, Kwong PD, Nabel GJ. Biochemical and structural characterization of cathepsin L-processed Ebola virus glycoprotein: Implications for viral entry and immunogenicity. J Virol. 2010;84:2972–2982. doi: 10.1128/JVI.02151-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Martinez O, Johnson J, Manicassamy B, Rong L, Olinger GG, Hensley LE, Basler CF. Zaire Ebola virus entry into human dendritic cells is insensitive to cathepsin L inhibition. Cell Microbiol. 2010;12:148–157. doi: 10.1111/j.1462-5822.2009.01385.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Kolesnikova L, Mühlberger E, Ryabchikova E, Becker S. Ultrastructural organization of recombinant Marburg virus nucleoprotein: Comparison with Marburg virus inclusions. J Virol. 2000;74:3899–3904. doi: 10.1128/jvi.74.8.3899-3904.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Mavrakis M, Kolesnikova L, Schoehn G, Becker S, Ruigrok RW. Morphology of Marburg virus NP-RNA. Virology. 2002;296:300–307. doi: 10.1006/viro.2002.1433. [DOI] [PubMed] [Google Scholar]
- 105.Noda T, Ebihara H, Muramoto Y, Fujii K, Takada A, Sagara H, Kim JH, Kida H, Feldmann H, Kawaoka Y. Assembly and budding of Ebolavirus. PLoS Pathog. 2006;2:e99. doi: 10.1371/journal.ppat.0020099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Huang Y, Xu L, Sun Y, Nabel GJ. The assembly of Ebola virus nucleocapsid requires virion-associated proteins 35 and 24 and posttranslational modification of nucleoprotein. Mol Cell. 2002;10:307–316. doi: 10.1016/s1097-2765(02)00588-9. [DOI] [PubMed] [Google Scholar]
- 107.Noda T, Aoyama K, Sagara H, Kida H, Kawaoka Y. Nucleocapsid-like structures of Ebola virus reconstructed using electron tomography. J Vet Med Sci. 2005;67:325–328. doi: 10.1292/jvms.67.325. [DOI] [PubMed] [Google Scholar]
- 108.Becker S, Rinne C, Hofsäss U, Klenk H-D, Mühlberger E. Interactions of Marburg virus nucleocapsid proteins. Virology. 1998;249:406–417. doi: 10.1006/viro.1998.9328. [DOI] [PubMed] [Google Scholar]
- 109.Schmidt KM, Schümann M, Olejnik J, Krähling V, Mühlberger E. Recombinant Marburg virus expressing EGFP allows rapid screening of virus growth and real time visualization of virus spread. J Infect Dis. 2011 doi: 10.1093/infdis/jir308. in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Geisbert TW, Jahrling PB. Differentiation of filoviruses by electron microscopy. Virus Res. 1995;39:129–150. doi: 10.1016/0168-1702(95)00080-1. [DOI] [PubMed] [Google Scholar]
- 111.Liu Y, Harty RN. Viral and host proteins that modulate filovirus budding. Future Virol. 2010;5:481–491. doi: 10.2217/FVL.10.33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Dolnik O, Kolesnikova L, Becker S. Filoviruses: Interactions with the host cell. Cell Mol Life Sci. 2008;65:756–776. doi: 10.1007/s00018-007-7406-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Dolnik O, Kolesnikova L, Stevermann L, Becker S. Tsg101 is recruited by a late domain of the nucleocapsid protein to support budding of Marburg virus-like particles. J Virol. 2010;84:7847–7856. doi: 10.1128/JVI.00476-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Roxrud I, Stenmark H, Malerod L. ESCRT & Co. Biol Cell. 2010;102:293–318. doi: 10.1042/BC20090161. [DOI] [PubMed] [Google Scholar]
- 115.Hurley JH, Boura E, Carlson LA, Rozycki B. Membrane budding. Cell. 2010;143:875–887. doi: 10.1016/j.cell.2010.11.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Kolesnikova L, Berghofer B, Bamberg S, Becker S. Multivesicular bodies as a platform for formation of the Marburg virus envelope. J Virol. 2004;78:12277–12287. doi: 10.1128/JVI.78.22.12277-12287.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Kolesnikova L, Bohil AB, Cheney RE, Becker S. Budding of Marburgvirus is associated with filopodia. Cell Microbiol. 2007;9:939–951. doi: 10.1111/j.1462-5822.2006.00842.x. [DOI] [PubMed] [Google Scholar]
- 118.Welsch S, Kolesnikova L, Krahling V, Riches JD, Becker S, Briggs JA. Electron tomography reveals the steps in filovirus budding. PLoS Pathog. 2010;6:e1000875. doi: 10.1371/journal.ppat.1000875. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Lamkanfi M, Dixit VM. Manipulation of host cell death pathways during microbial infections. Cell Host Microbe. 2010;8:44–54. doi: 10.1016/j.chom.2010.06.007. [DOI] [PubMed] [Google Scholar]
- 120.Kaminskyy V, Zhivotovsky B. To kill or be killed: How viruses interact with the cell death machinery. J Intern Med. 2010;267:473–482. doi: 10.1111/j.1365-2796.2010.02222.x. [DOI] [PubMed] [Google Scholar]
- 121.Hotchkiss RS, Strasser A, McDunn JE, Swanson PE. Cell death. N Engl J Med. 2009;361:1570–1583. doi: 10.1056/NEJMra0901217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Fink SL, Cookson BT. Apoptosis, pyroptosis, and necrosis: Mechanistic description of dead and dying eukaryotic cells. Infect Immun. 2005;73:1907–1916. doi: 10.1128/IAI.73.4.1907-1916.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Kerr JF, Wyllie AH, Currie AR. Apoptosis: A basic biological phenomenon with wide-ranging implications in tissue kinetics. Br J Cancer. 1972;26:239–257. doi: 10.1038/bjc.1972.33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Walker NI, Harmon BV, Gobe GC, Kerr JF. Patterns of cell death. Methods Achiev Exp Pathol. 1988;13:18–54. [PubMed] [Google Scholar]
- 125.Gonzalvez F, Ashkenazi A. New insights into apoptosis signaling by Apo2L/TRAIL. Oncogene. 2010;29:4752–4765. doi: 10.1038/onc.2010.221. [DOI] [PubMed] [Google Scholar]
- 126.Degterev A, Boyce M, Yuan J. A decade of caspases. Oncogene. 2003;22:8543–8567. doi: 10.1038/sj.onc.1207107. [DOI] [PubMed] [Google Scholar]
- 127.Burlacu A. Regulation of apoptosis by Bcl-2 family proteins. J Cell Mol Med. 2003;7:249–257. doi: 10.1111/j.1582-4934.2003.tb00225.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Sharpe AH, Wherry EJ, Ahmed R, Freeman GJ. The function of programmed cell death 1 and its ligands in regulating autoimmunity and infection. Nat Immunol. 2007;8:239–245. doi: 10.1038/ni1443. [DOI] [PubMed] [Google Scholar]
- 129.Moquin D, Chan FK. The molecular regulation of programmed necrotic cell injury. Trends Biochem Sci. 2010;35:434–441. doi: 10.1016/j.tibs.2010.03.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Golstein P, Kroemer G. Cell death by necrosis: Towards a molecular definition. Trends Biochem Sci. 2007;32:37–43. doi: 10.1016/j.tibs.2006.11.001. [DOI] [PubMed] [Google Scholar]
- 131.De Duve C, Wattiaux R. Functions of lysosomes. Annu Rev Physiol. 1966;28:435–492. doi: 10.1146/annurev.ph.28.030166.002251. [DOI] [PubMed] [Google Scholar]
- 132.Klionsky DJ. Autophagy: from phenomenology to molecular understanding in less than a decade. Nat Rev Mol Cell Biol. 2007;8:931–937. doi: 10.1038/nrm2245. [DOI] [PubMed] [Google Scholar]
- 133.Alazard-Dany N, Volchkova V, Reynard O, Carbonnelle C, Dolnik O, Ottmann M, Khromykh A, Volchkov VE. Ebola virus glycoprotein GP is not cytotoxic when expressed constitutively at a moderate level. J Gen Virol. 2006;87:1247–1257. doi: 10.1099/vir.0.81361-0. [DOI] [PubMed] [Google Scholar]
- 134.Barrientos LG, Rollin PE. Release of cellular proteases into the acidic extracellular milieu exacerbates Ebola virus-induced cell damage. Virology. 2007;358:1–9. doi: 10.1016/j.virol.2006.08.018. [DOI] [PubMed] [Google Scholar]
- 135.Boehmann Y, Enterlein S, Randolf A, Mühlberger E. A reconstituted replication and transcription system for Ebola virus Reston and comparison with Ebola virus Zaire. Virology. 2005;332:406–417. doi: 10.1016/j.virol.2004.11.018. [DOI] [PubMed] [Google Scholar]
- 136.Gupta M, Goldsmith CS, Metcalfe MG, Spiropoulou CF, Rollin PE. Reduced virus replication, proinflammatory cytokine production, and delayed macrophage cell death in human PBMCs infected with the newly discovered Bundibugyo ebolavirus relative to Zaire ebolavirus. Virology. 2010;402:203–208. doi: 10.1016/j.virol.2010.03.024. [DOI] [PubMed] [Google Scholar]
- 137.Gupta M, Spiropoulou C, Rollin PE. Ebola virus infection of human PBMCs causes massive death of macrophages, CD4 and CD8 T cell sub-populations in vitro. Virology. 2007;364:45–54. doi: 10.1016/j.virol.2007.02.017. [DOI] [PubMed] [Google Scholar]
- 138.Geisbert TW, Hensley LE, Gibb TR, Steele KE, Jaax NK, Jahrling PB. Apoptosis induced in vitro and in vivo during infection by Ebola and Marburg viruses. Lab Invest. 2000;80:171–186. doi: 10.1038/labinvest.3780021. [DOI] [PubMed] [Google Scholar]
- 139.Bente D, Gren J, Strong JE, Feldmann H. Disease modeling for Ebola and Marburg viruses. Dis Model Mech. 2009;2:12–17. doi: 10.1242/dmm.000471. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140.Rosenblum WI. Cytotoxic edema: Monitoring its magnitude and contribution to brain swelling. J Neuropathol Exp Neurol. 2007;66:771–778. doi: 10.1097/nen.0b013e3181461965. [DOI] [PubMed] [Google Scholar]
- 141.Saeed MF, Kolokoltsov AA, Freiberg AN, Holbrook MR, Davey RA. Phosphoinositide-3 kinase-Akt pathway controls cellular entry of Ebola virus. PLoS Pathog. 2008;4:e1000141. doi: 10.1371/journal.ppat.1000141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142.Cooray S. The pivotal role of phosphatidylinositol 3-kinase-Akt signal transduction in virus survival. J Gen Virol. 2004;85:1065–1076. doi: 10.1099/vir.0.19771-0. [DOI] [PubMed] [Google Scholar]
- 143.Ehrhardt C, Ludwig S. A new player in a deadly game: Influenza viruses and the PI3K/Akt signalling pathway. Cell Microbiol. 2009;11:863–871. doi: 10.1111/j.1462-5822.2009.01309.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144.Brennan K, Bowie AG. Activation of host pattern recognition receptors by viruses. Curr Opin Microbiol. 2010;13:503–507. doi: 10.1016/j.mib.2010.05.007. [DOI] [PubMed] [Google Scholar]
- 145.Baum A, Garcia-Sastre A. Induction of type I interferon by RNA viruses: Cellular receptors and their substrates. Amino Acids. 2010;38:1283–1299. doi: 10.1007/s00726-009-0374-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146.Habjan M, Andersson I, Klingstrom J, Schümann M, Martin A, Zimmermann P, Wagner V, Pichlmair A, Schneider U, Mühlberger E, et al. Processing of genome 5′ termini as a strategy of negative-strand RNA viruses to avoid RIG-I-dependent interferon induction. PLoS ONE. 2008;3:e2032. doi: 10.1371/journal.pone.0002032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147.Basler CF, Amarasinghe GK. Evasion of interferon responses by Ebola and Marburg viruses. J Interferon Cytokine Res. 2009;29:511–520. doi: 10.1089/jir.2009.0076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148.Leung DW, Prins KC, Basler CF, Amarasinghe GK. Ebolavirus VP35 is a multifunctional virulence factor. Virulence. 2010;1:526–531. doi: 10.4161/viru.1.6.12984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 149.Chattopadhyay S, Yamashita M, Zhang Y, Sen GC. The IRF-3/Bax-mediated apoptotic pathway, activated by viral cytoplasmic RNA and DNA, inhibits virus replication. J Virol. 2011;85:3708–3716. doi: 10.1128/JVI.02133-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 150.Sharif-Askari E, Nakhaei P, Oliere S, Tumilasci V, Hernandez E, Wilkinson P, Lin R, Bell J, Hiscott J. Bax-dependent mitochondrial membrane permeabilization enhances IRF3-mediated innate immune response during VSV infection. Virology. 2007;365:20–33. doi: 10.1016/j.virol.2007.03.011. [DOI] [PubMed] [Google Scholar]
- 151.Chattopadhyay S, Marques JT, Yamashita M, Peters KL, Smith K, Desai A, Williams BR, Sen GC. Viral apoptosis is induced by IRF-3-mediated activation of Bax. EMBO J. 2010;29:1762–1773. doi: 10.1038/emboj.2010.50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 152.Lei Y, Moore CB, Liesman RM, O'Connor BP, Bergstralh DT, Chen ZJ, Pickles RJ, Ting JP. MAVS-mediated apoptosis and its inhibition by viral proteins. PLoS ONE. 2009;4:e5466. doi: 10.1371/journal.pone.0005466. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 153.Scott I, Norris KL. The mitochondrial antiviral signaling protein, MAVS, is cleaved during apoptosis. Biochem Biophys Res Commun. 2008;375:101–106. doi: 10.1016/j.bbrc.2008.07.147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 154.Zamanian-Daryoush M, Mogensen TH, DiDonato JA, Williams BR. NF-kappaB activation by double-stranded-RNA-activated protein kinase (PKR) is mediated through NF-kappaB-inducing kinase and IkappaB kinase. Mol Cell Biol. 2000;20:1278–1290. doi: 10.1128/mcb.20.4.1278-1290.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 155.Sato S, Sugiyama M, Yamamoto M, Watanabe Y, Kawai T, Takeda K, Akira S. Toll/IL-1 receptor domain-containing adaptor inducing IFN-beta (TRIF) associates with TNF receptor-associated factor 6 and TANK-binding kinase 1, and activates two distinct transcription factors, NF-kappa B and IFN-regulatory factor-3, in the Toll-like receptor signaling. J Immunol. 2003;171:4304–4310. doi: 10.4049/jimmunol.171.8.4304. [DOI] [PubMed] [Google Scholar]
- 156.Kumar A, Haque J, Lacoste J, Hiscott J, Williams BR. Double-stranded RNA-dependent protein kinase activates transcription factor NF-kappa B by phosphorylating I kappa B. Proc Natl Acad Sci U S A. 1994;91:6288–6292. doi: 10.1073/pnas.91.14.6288. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 157.Unterholzner L, Bowie AG. The interplay between viruses and innate immune signaling: Recent insights and therapeutic opportunities. Biochem Pharmacol. 2008;75:589–602. doi: 10.1016/j.bcp.2007.07.043. [DOI] [PubMed] [Google Scholar]
- 158.Weber F, Wagner V, Rasmussen SB, Hartmann R, Paludan SR. Double-stranded RNA is produced by positive-strand RNA viruses and DNA viruses but not in detectable amounts by negative-strand RNA viruses. J Virol. 2006;80:5059–5064. doi: 10.1128/JVI.80.10.5059-5064.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 159.Gale M, Jr, Katze MG. Molecular mechanisms of interferon resistance mediated by viral-directed inhibition of PKR, the interferon-induced protein kinase. Pharmacol Ther. 1998;78:29–46. doi: 10.1016/s0163-7258(97)00165-4. [DOI] [PubMed] [Google Scholar]
- 160.Feng Z, Cerveny M, Yan Z, He B. The VP35 protein of Ebola virus inhibits the antiviral effect mediated by double-stranded RNA-dependent protein kinase PKR. J Virol. 2007;81:182–192. doi: 10.1128/JVI.01006-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 161.Schümann M, Gantke T, Mühlberger E. Ebola virus VP35 antagonizes PKR activity through its C-terminal interferon inhibitory domain. J Virol. 2009;83:8993–8997. doi: 10.1128/JVI.00523-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 162.Zhang P, Jacobs BL, Samuel CE. Loss of protein kinase PKR expression in human HeLa cells complements the vaccinia virus E3L deletion mutant phenotype by restoration of viral protein synthesis. J Virol. 2008;82:840–848. doi: 10.1128/JVI.01891-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 163.Gale M, Jr, Kwieciszewski B, Dossett M, Nakao H, Katze MG. Antiapoptotic and oncogenic potentials of hepatitis C virus are linked to interferon resistance by viral repression of the PKR protein kinase. J Virol. 1999;73:6506–6516. doi: 10.1128/jvi.73.8.6506-6516.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 164.Garcia MA, Guerra S, Gil J, Jimenez V, Esteban M. Anti-apoptotic and oncogenic properties of the dsRNA-binding protein of vaccinia virus, E3L. Oncogene. 2002;21:8379–8387. doi: 10.1038/sj.onc.1206036. [DOI] [PubMed] [Google Scholar]
- 165.Francois S, El Benna J, Dang PM, Pedruzzi E, Gougerot-Pocidalo MA, Elbim C. Inhibition of neutrophil apoptosis by TLR agonists in whole blood: Involvement of the phosphoinositide 3-kinase/Akt and NF-kappaB signaling pathways, leading to increased levels of Mcl-1, A1, and phosphorylated Bad. J Immunol. 2005;174:3633–3642. doi: 10.4049/jimmunol.174.6.3633. [DOI] [PubMed] [Google Scholar]
- 166.Aliprantis AO, Yang RB, Mark MR, Suggett S, Devaux B, Radolf JD, Klimpel GR, Godowski P, Zychlinsky A. Cell activation and apoptosis by bacterial lipoproteins through toll-like receptor-2. Science. 1999;285:736–739. doi: 10.1126/science.285.5428.736. [DOI] [PubMed] [Google Scholar]
- 167.Okumura A, Pitha PM, Yoshimura A, Harty RN. Interaction between Ebola virus glycoprotein and host toll-like receptor 4 leads to induction of proinflammatory cytokines and SOCS1. J Virol. 2010;84:27–33. doi: 10.1128/JVI.01462-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 168.Jin H, Yan Z, Prabhakar BS, Feng Z, Ma Y, Verpooten D, Ganesh B, He B. The VP35 protein of Ebola virus impairs dendritic cell maturation induced by virus and lipopolysaccharide. J Gen Virol. 2010;91:352–361. doi: 10.1099/vir.0.017343-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 169.Chang TH, Kubota T, Matsuoka M, Jones S, Bradfute SB, Bray M, Ozato K. Ebola Zaire virus blocks type I interferon production by exploiting the host SUMO modification machinery. PLoS Pathog. 2009;5:e1000493. doi: 10.1371/journal.ppat.1000493. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 170.Leung LW, Park MS, Martinez O, Valmas C, Lopez CB, Basler CF. Ebolavirus VP35 suppresses IFN production from conventional but not plasmacytoid dendritic cells. Immunol Cell Biol. 2011 doi: 10.1038/icb.2010.169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 171.Yang ZY, Duckers HJ, Sullivan NJ, Sanchez A, Nabel EG, Nabel GJ. Identification of the Ebola virus glycoprotein as the main viral determinant of vascular cell cytotoxicity and injury. Nat Med. 2000;6:886–889. doi: 10.1038/78645. [DOI] [PubMed] [Google Scholar]
- 172.Chan SY, Ma MC, Goldsmith MA. Differential induction of cellular detachment by envelope glycoproteins of Marburg and Ebola (Zaire) viruses. J Gen Virol. 2000;81:2155–2159. doi: 10.1099/0022-1317-81-9-2155. [DOI] [PubMed] [Google Scholar]
- 173.Volchkov VE, Volchkova VA, Mühlberger E, Kolesnikova LV, Weik M, Dolnik O, Klenk HD. Recovery of infectious Ebola virus from complementary DNA: RNA editing of the GP gene and viral cytotoxicity. Science. 2001;291:1965–1969. doi: 10.1126/science.1057269. [DOI] [PubMed] [Google Scholar]
- 174.Simmons G, Wool-Lewis RJ, Baribaud F, Netter RC, Bates P. Ebola virus glycoproteins induce global surface protein down-modulation and loss of cell adherence. J Virol. 2002;76:2518–2528. doi: 10.1128/jvi.76.5.2518-2528.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 175.Ray RB, Basu A, Steele R, Beyene A, McHowat J, Meyer K, Ghosh AK, Ray R. Ebola virus glycoprotein-mediated anoikis of primary human cardiac microvascular endothelial cells. Virology. 2004;321:181–188. doi: 10.1016/j.virol.2003.12.014. [DOI] [PubMed] [Google Scholar]
- 176.Sullivan NJ, Peterson M, Yang ZY, Kong WP, Duckers H, Nabel E, Nabel GJ. Ebola virus glycoprotein toxicity is mediated by a dynamin-dependent protein-trafficking pathway. J Virol. 2005;79:547–553. doi: 10.1128/JVI.79.1.547-553.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 177.Han Z, Licata JM, Paragas J, Harty RN. Permeabilization of the plasma membrane by Ebola virus GP2. Virus Genes. 2007;34:273–281. doi: 10.1007/s11262-006-0009-4. [DOI] [PubMed] [Google Scholar]
- 178.Francica JR, Matukonis MK, Bates P. Requirements for cell rounding and surface protein down-regulation by Ebola virus glycoprotein. Virology. 2009;383:237–247. doi: 10.1016/j.virol.2008.10.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 179.Zampieri CA, Fortin JF, Nolan GP, Nabel GJ. The ERK Mitogen-Activated Protein Kinase Pathway Contributes to Ebola Glycoprotein-Induced Cytotoxicity. J Virol. 2006 doi: 10.1128/JVI.01586-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 180.Martinez O, Valmas C, Basler CF. Ebola virus-like particle-induced activation of NF-kappaB and Erk signaling in human dendritic cells requires the glycoprotein mucin domain. Virology. 2007;364:342–354. doi: 10.1016/j.virol.2007.03.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 181.Bhattacharyya S, Hope TJ. Full-length Ebola glycoprotein accumulates in the endoplasmic reticulum. Virol J. 2011;8:11. doi: 10.1186/1743-422X-8-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 182.Geisbert TW, Young HA, Jahrling PB, Davis KJ, Kagan E, Hensley LE. Mechanisms underlying coagulation abnormalities in ebola hemorrhagic fever: Overexpression of tissue factor in primate monocytes/macrophages is a key event. J Infect Dis. 2003;188:1618–1629. doi: 10.1086/379724. [DOI] [PubMed] [Google Scholar]
- 183.Aleksandrowicz P, Wolf K, Falzarano D, Feldmann H, Seebach J, Schnittler H. Viral haemorrhagic fever and vascular alterations. Hamostaseologie. 2008;28:77–84. [PubMed] [Google Scholar]
- 184.Wool-Lewis RJ, Bates P. Characterization of Ebola virus entry by using pseudotyped viruses: Identification of receptor-deficient cell lines. J Virol. 1998;72:3155–3160. doi: 10.1128/jvi.72.4.3155-3160.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 185.Yang Z, Delgado R, Xu L, Todd RF, Nabel EG, Sanchez A, Nabel GJ. Distinct cellular interactions of secreted and transmembrane Ebola virus glycoproteins. Science. 1998;279:1034–1037. doi: 10.1126/science.279.5353.1034. [DOI] [PubMed] [Google Scholar]
- 186.Reed DS, Hensley LE, Geisbert JB, Jahrling PB, Geisbert TW. Depletion of peripheral blood T lymphocytes and NK cells during the course of ebola hemorrhagic Fever in cynomolgus macaques. Viral Immunol. 2004;17:390–400. doi: 10.1089/vim.2004.17.390. [DOI] [PubMed] [Google Scholar]
- 187.Bradfute SB, Braun DR, Shamblin JD, Geisbert JB, Paragas J, Garrison A, Hensley LE, Geisbert TW. Lymphocyte death in a mouse model of Ebola virus infection. J Infect Dis. 2007;196:S296–S304. doi: 10.1086/520602. [DOI] [PubMed] [Google Scholar]
- 188.Wauquier N, Becquart P, Padilla C, Baize S, Leroy EM. Human fatal zaire ebola virus infection is associated with an aberrant innate immunity and with massive lymphocyte apoptosis. PLoS Negl Trop Dis. 2010;4:e837. doi: 10.1371/journal.pntd.0000837. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 189.Zarozinski CC, McNally JM, Lohman BL, Daniels KA, Welsh RM. Bystander sensitization to activation-induced cell death as a mechanism of virus-induced immune suppression. J Virol. 2000;74:3650–3658. doi: 10.1128/jvi.74.8.3650-3658.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 190.Finkel TH, Tudor-Williams G, Banda NK, Cotton MF, Curiel T, Monks C, Baba TW, Ruprecht RM, Kupfer A. Apoptosis occurs predominantly in bystander cells and not in productively infected cells of HIV- and SIV-infected lymph nodes. Nat Med. 1995;1:129–134. doi: 10.1038/nm0295-129. [DOI] [PubMed] [Google Scholar]
- 191.Inoue Y, Yasukawa M, Fujita S. Induction of T-cell apoptosis by human herpesvirus 6. J Virol. 1997;71:3751–3759. doi: 10.1128/jvi.71.5.3751-3759.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 192.Yates NL, Yammani RD, Alexander-Miller MA. Dose-dependent lymphocyte apoptosis following respiratory infection with Vaccinia virus. Virus Res. 2008;137:198–205. doi: 10.1016/j.virusres.2008.07.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 193.Hilleman MR. Strategies and mechanisms for host and pathogen survival in acute and persistent viral infections. Proc Natl Acad Sci U S A. 2004;101:14560–14566. doi: 10.1073/pnas.0404758101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 194.Gougeon ML. Apoptosis as an HIV strategy to escape immune attack. Nat Rev Immunol. 2003;3:392–404. doi: 10.1038/nri1087. [DOI] [PubMed] [Google Scholar]
- 195.Hengartner MO. The biochemistry of apoptosis. Nature. 2000;407:770–776. doi: 10.1038/35037710. [DOI] [PubMed] [Google Scholar]
- 196.Jekle A, Keppler OT, De Clercq E, Schols D, Weinstein M, Goldsmith MA. In vivo evolution of human immunodeficiency virus type 1 toward increased pathogenicity through CXCR4-mediated killing of uninfected CD4 T cells. J Virol. 2003;77:5846–5854. doi: 10.1128/JVI.77.10.5846-5854.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 197.Schindler M, Munch J, Kutsch O, Li H, Santiago ML, Bibollet-Ruche F, Muller-Trutwin MC, Novembre FJ, Peeters M, Courgnaud V, Bailes E, Roques P, Sodora DL, Silvestri G, Sharp PM, Hahn BH, Kirchhoff F. Nef-mediated suppression of T cell activation was lost in a lentiviral lineage that gave rise to HIV-1. Cell. 2006;125:1055–1067. doi: 10.1016/j.cell.2006.04.033. [DOI] [PubMed] [Google Scholar]
- 198.Westendorp MO, Frank R, Ochsenbauer C, Stricker K, Dhein J, Walczak H, Debatin KM, Krammer PH. Sensitization of T cells to CD95-mediated apoptosis by HIV-1 Tat and gp120. Nature. 1995;375:497–500. doi: 10.1038/375497a0. [DOI] [PubMed] [Google Scholar]
- 199.Lenassi M, Cagney G, Liao M, Vaupotic T, Bartholomeeusen K, Cheng Y, Krogan NJ, Plemenitas A, Peterlin BM. HIV Nef is secreted in exosomes and triggers apoptosis in bystander CD4+ T cells. Traffic. 2010;11:110–122. doi: 10.1111/j.1600-0854.2009.01006.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 200.Gandhi RT, Chen BK, Straus SE, Dale JK, Lenardo MJ, Baltimore D. HIV-1 directly kills CD4+ T cells by a Fas-independent mechanism. J Exp Med. 1998;187:1113–1122. doi: 10.1084/jem.187.7.1113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 201.Herbeuval JP, Grivel JC, Boasso A, Hardy AW, Chougnet C, Dolan MJ, Yagita H, Lifson JD, Shearer GM. CD4+ T-cell death induced by infectious and noninfectious HIV-1: role of type 1 interferon-dependent, TRAIL/DR5-mediated apoptosis. Blood. 2005;106:3524–3531. doi: 10.1182/blood-2005-03-1243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 202.Doitsh G, Cavrois M, Lassen KG, Zepeda O, Yang Z, Santiago ML, Hebbeler AM, Greene WC. Abortive HIV infection mediates CD4 T cell depletion and inflammation in human lymphoid tissue. Cell. 2010;143:789–801. doi: 10.1016/j.cell.2010.11.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 203.Hevey M, Negley D, Pushko P, Smith J, Schmaljohn A. Marburg virus vaccines based upon alphavirus replicons protect guinea pigs and nonhuman primates. Virology. 1998;251:28–37. doi: 10.1006/viro.1998.9367. [DOI] [PubMed] [Google Scholar]
- 204.Ignatyev GM. Immune response to filovirus infections. Curr Top Microbiol Immunol. 1999;235:205–217. doi: 10.1007/978-3-642-59949-1_11. [DOI] [PubMed] [Google Scholar]
- 205.Leroy EM, Baize S, Volchkov VE, Fisher-Hoch SP, Georges-Courbot MC, Lansoud-Soukate J, Capron M, Debre P, McCormick JB, Georges AJ. Human asymptomatic Ebola infection and strong inflammatory response. Lancet. 2000;355:2210–2215. doi: 10.1016/s0140-6736(00)02405-3. [DOI] [PubMed] [Google Scholar]
- 206.Hensley LE, Young HA, Jahrling PB, Geisbert TW. Proinflammatory response during Ebola virus infection of primate models: Possible involvement of the tumor necrosis factor receptor superfamily. Immunol Lett. 2002;80:169–179. doi: 10.1016/s0165-2478(01)00327-3. [DOI] [PubMed] [Google Scholar]
- 207.Park CG, Lee SY, Kandala G, Choi Y. A novel gene product that couples TCR signaling to Fas(CD95) expression in activation-induced cell death. Immunity. 1996;4:583–591. doi: 10.1016/s1074-7613(00)80484-7. [DOI] [PubMed] [Google Scholar]
- 208.Bosio CM, Aman MJ, Grogan C, Hogan R, Ruthel G, Negley D, Mohamadzadeh M, Bavari S, Schmaljohn A. Ebola and Marburg viruses replicate in monocyte-derived dendritic cells without inducing the production of cytokines and full maturation. J Infect Dis. 2003;188:1630–1638. doi: 10.1086/379199. [DOI] [PubMed] [Google Scholar]
- 209.Mahanty S, Hutchinson K, Agarwal S, McRae M, Rollin PE, Pulendran B. Cutting edge: Impairment of dendritic cells and adaptive immunity by Ebola and Lassa viruses. J Immunol. 2003;170:2797–2801. doi: 10.4049/jimmunol.170.6.2797. [DOI] [PubMed] [Google Scholar]
- 210.Mohamadzadeh M. Potential factors induced by filoviruses that lead to immune supression. Curr Mol Med. 2009;9:174–185. doi: 10.2174/156652409787581628. [DOI] [PubMed] [Google Scholar]
- 211.Fife BT, Bluestone JA. Control of peripheral T-cell tolerance and autoimmunity via the CTLA-4 and PD-1 pathways. Immunol Rev. 2008;224:166–182. doi: 10.1111/j.1600-065X.2008.00662.x. [DOI] [PubMed] [Google Scholar]
- 212.Dong H, Strome SE, Salomao DR, Tamura H, Hirano F, Flies DB, Roche PC, Lu J, Zhu G, Tamada K, Lennon VA, Celis E, Chen L. Tumor-associated B7-H1 promotes T-cell apoptosis: a potential mechanism of immune evasion. Nat Med. 2002;8:793–800. doi: 10.1038/nm730. [DOI] [PubMed] [Google Scholar]
- 213.Freeman GJ, Long AJ, Iwai Y, Bourque K, Chernova T, Nishimura H, Fitz LJ, Malenkovich N, Okazaki T, Byrne MC, et al. Engagement of the PD-1 immunoinhibitory receptor by a novel B7 family member leads to negative regulation of lymphocyte activation. J Exp Med. 2000;192:1027–1034. doi: 10.1084/jem.192.7.1027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 214.Badovinac VP, Harty JT. Programming, demarcating, and manipulating CD8+ T-cell memory. Immunol Rev. 2006;211:67–80. doi: 10.1111/j.0105-2896.2006.00384.x. [DOI] [PubMed] [Google Scholar]
- 215.Volchkov VE, Blinov VM, Netesov SV. The envelope glycoprotein of Ebola virus contains an immunosuppressive- like domain similar to oncogenic retroviruses. FEBS Lett. 1992;305:181–184. doi: 10.1016/0014-5793(92)80662-z. [DOI] [PubMed] [Google Scholar]
- 216.Bukreyev A, Volchkov VE, Blinov VM, Netesov SV. The GP-protein of Marburg virus contains the region similar to the ‘immunosuppressive domain’ of oncogenic retrovirus P15E proteins. FEBS Lett. 1993;323:183–187. doi: 10.1016/0014-5793(93)81476-g. [DOI] [PubMed] [Google Scholar]
- 217.Yaddanapudi K, Palacios G, Towner JS, Chen I, Sariol CA, Nichol ST, Lipkin WI. Implication of a retrovirus-like glycoprotein peptide in the immunopathogenesis of Ebola and Marburg viruses. Faseb J. 2006;20:2519–2530. doi: 10.1096/fj.06-6151com. [DOI] [PubMed] [Google Scholar]