Abstract
Aseptic loosening is well known following endoprosthetic replacement (EPR) using cemented intramedullary stems (CISs). The Compress® (CPS) implant uses a novel spring system, achieving immediate, high compression fixation that induces bone hypertrophy and avoids stress shielding. We compared 26 oncologic distal femoral CPS patients treated at the University of California, San Francisco (UCSF, USA) with 26 matched CIS patients from the Royal Orthopaedic Hospital, Birmingham (ROH, UK). The predominant diagnosis was osteosarcoma. Each centre had only one device-related prosthetic failure. In the short term these results show CPS to be safe and effective. We await longer follow-up to assess the ongoing potential for prosthetic failure.
Résumé
Le descellement aseptique est une des complications bien connues des prothèses cimentées sur le plan fémoral. L’implant Compress® (CPS) qui permet une fixation en compression et qui entraîne une hypertrophie osseuse et évite le stress-shielding est une alternative. Nous avons comparé 26 patients traités pour des problèmes oncologiques avec une prothèse utilisant le nouveau système (CPS) à l’Université de Californie (UCSF, USA) à 26 patients traités par cimentation intra médullaire d’une pièce fémorale traitée au Royal Orthopaedic Hospital de Birmingham (ROH, UK). Le diagnostic le plus fréquent a été l’ostéosarcome. Chaque centre n’a eu qu’un seul échec prothétique. Bien que le suivi ait été court, ces résultats montrent que l’implant CPS à compression est une technique satisfaisante qui, néanmoins, va nécessiter une surveillance à plus long terme afin d’étudier la survenue éventuelle d’échecs.
Background
The distal femur is the most common site for primary bone tumours [8]. In the past 30 years there have been great advances in chemotherapy, biomechanical engineering and surgical techniques, making limb salvage the standard treatment as opposed to amputation. Techniques used for limb salvage include endoprosthetic replacement with a custom-built or modular endoprosthesis, allograft or alloprosthetic replacement, or resection with arthrodesis. The advantages and disadvantages of various techniques have been the focus of much study and debate [4, 7, 15, 22, 28, 39, 41]. Endoprosthetic replacement (EPR) is initially reliable, allows rapid restoration of function, and has excellent cosmetic appearance and emotional acceptance; good-to-excellent functional results are achievable. However, EPRs are associated with mid- to long-term problems of infection, mechanical breakage and, especially, aseptic loosening, which ultimately leads to failure [35].
EPR with custom-built cemented intramedullary stem (CIS), such as the Stanmore massive prosthetic replacement (Department of Biomedical Engineering at Stanmore, UK), has been the treatment of choice at the Royal Orthopaedic Hospital, Birmingham, for over 20 years. The devices are commonly implanted in the distal femur, proximal femur and proximal tibia for primary oncological, metastatic and failed conventional prosthetic indications. Refined surgical techniques have led to a low complication rate with long follow-up periods [14, 31, 35–37]. Comparable results with similar cemented [10, 12, 17, 25, 33, 34] and non-cemented [3, 26] implants have been reported from many international centers, establishing conventional stemmed technology as the current standard of care for such indications.
Compress® (CPS) technology (Biomet, Warsaw, Ind., USA) has been developed to attempt to eliminate the problem of aseptic loosening associated with massive endoprosthetic reconstruction. Following tumour resection, the device is secured to the bone without cement, instead using a stacked set of Belleville washers that function as a spring, producing ongoing, compressive force [2, 6]. The fixation thus achieved results in bone hypertrophy, avoiding stress shielding and sealing the medullary canal from particulate debris. The possibility of particle-induced osteolysis and aseptic loosening, which has replaced infection as the principal mode of failure of CISs [1, 17], is thus theoretically minimised by the CPS device.
Because of CPS’ lack of reliance on stems and cement, its versatility in treating even the shortest of residual metaphyseal segments, and its ease of revision, it is hoped that the technology will meet or exceed the prosthetic longevity established by CIS systems over the intermediate to long term. In fact, little has been published regarding the survival of CISs over the short term (less than 5 years). The resultant lack of reference material has presented difficulties for clinical studies seeking to compare new technologies (CPS) to an accepted ‘gold standard’ technology (CIS), for which rigorous early ‘standards’ of success have not been widely published. Our aim was to assess the safety and effectiveness of the CPS and CIS implants immediately after surgery, by direct comparison of the experience with similar patient populations at two orthopaedic oncology centres.
Methods and patients
Patients
All distal femoral CPS patients treated consecutively at the University of California, San Francisco (UCSF) over a 3-year period by one surgeon between January 1, 2000 and December 31, 2002 were included in the review. These patients were part of a prospective, multicentre, Food and Drug Administration (FDA), substantive study (IDE #G990287) of CPS usage for primary and revision distal femoral oncological indications; the study was approved by the UCSF Committee on Human Research (H10712-18027-04 and H10712-17076-04). This group was matched to 26 patients from the Royal Orthopaedic Hospital, Birmingham (ROH) who had received their first distal femoral CIS in the same 3-year period under the care of three surgeons. The selection pool for the ROH group was composed of all patients who had undergone their first distal femoral CIS EPR between these dates. They were then matched to the UCSF group by age (within 3 years) and gender, and the authors were blind to the outcome measures during the matching process. Patients with metastatic disease were excluded from both groups due to FDA restrictions placed upon the UCSF group.
Methods
The data on the CPS patients were taken from the prospectively collected UCSF Orthopaedic Oncology Service databases. Data for the matched control CIS patients was taken from the Orthopaedic Oncology Database at the Royal Orthopaedic Hospital Oncology Service (ROHOS). In cases where there was incomplete information, reference was made to the original patient records. All individual data were made anonymous for purposes of analysis. Ethics committee approval was obtained from the West Midlands Local Research Ethics Committee for use of the ROH database, and Committee for Human Research approval was obtained for use of the UCSF database, as noted above. Records were analysed with respect to patient age, gender, diagnosis, affected side, length of follow-up, overall survival, operative complications, prosthetic survival, and cause of failure.
Statistical methods
The main outcome measure was revision of the first EPR, and data on amputation and death were analysed. Demographic details, including the distribution of age and gender, are described. Differences between groups were assessed with the paired t-test. Overall survival was calculated by Kaplan–Meier survival curves [19], and the impact of prognostic factors was assessed with the log-rank test and Cox regression analysis [5]. Significance was set at P<0.05. Prosthesis survival time was taken from the date of the first EPR to the date of first revision or amputation, or to the last documented time alive if no complication had occurred. Overall survival time was calculated from the time of diagnosis when the significance of the tumour was investigated, and the end point was taken as time of death or the last documented time the patient was known to be alive. Analysis was carried out with SPSS software (SPSS Institute, version 10.0).
The endoprostheses
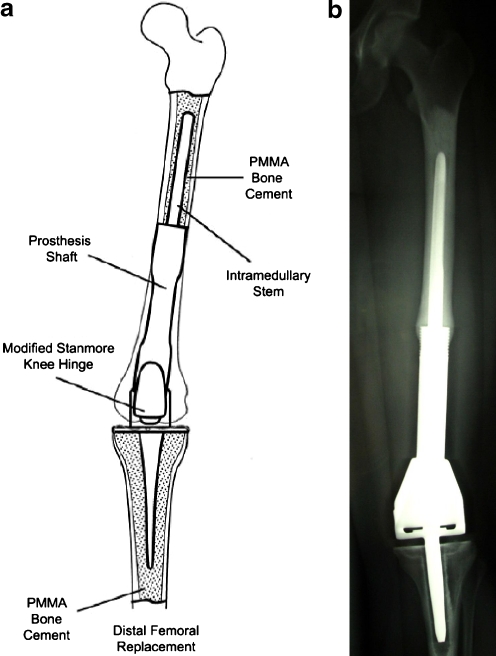
The implants used at the ROH were custom designed by the Department of Biomedical Engineering at Stanmore, UK. The shaft and intramedullary stem are made from titanium alloy, and when indicated, the stem was shaped to follow the natural curvature of the bone; a Stanmore fully constrained knee hinge made from cast cobalt–chromium–molybdenum was used (Fig. 1a) [31].
Fig. 1.
a Schematic diagram of CIS implant demonstrating cemented femoral and tibial stems, prosthetic shaft, and Stanmore hinged total knee replacement. b Anteroposterior radiograph demonstrating CIS device implanted into femoral shaft (PMMA polymethylmethacrylate)
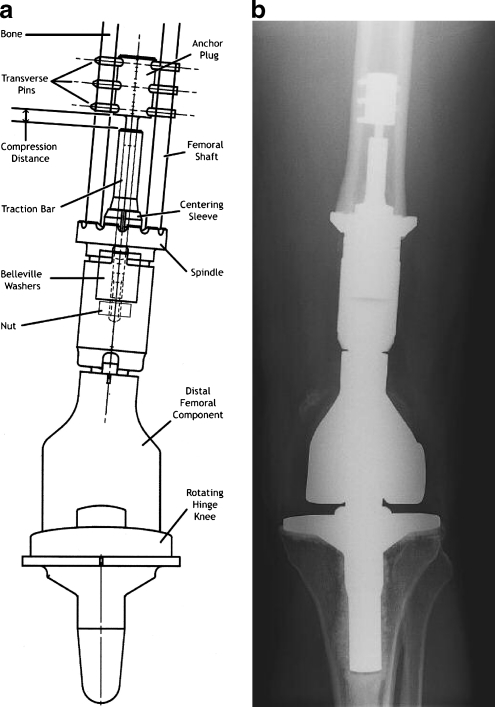
The CPS also attaches a standard tumour prosthesis to the host bone. It has a porous, coated, titanium surface with a conical section mounted transversely to the anatomical axis of the femur. To maximise contact the osteotomy surface is precision milled to fit against the shoulder of the implant. Belleville washers are loaded over the intramedullary traction bar. These circular washers have a curved cross-section, and when compressed by a nut tightened over the traction bar, they act like a spring and generate a linear force proportional to the amount of their deformation (Fig. 2a). The resulting compression provides immediate fixation of the implant to bone. With time, the compressive force results in bone hypertrophy at the prosthetic interface, according to Wolff’s law [6]. The stability of the bone–prosthesis interaction thus obviates the complications of osteolysis secondary to stress shielding or particle-induced debris.
Fig. 2.
a Schematic diagram of CPS implant demonstrating anchor plug, transverse pins, traction bar, compression distance, centering sleeve, spindle, Belleville washers, nut, distal femoral component, and rotating hinge total knee replacement. b Anteroposterior radiograph demonstrating CPS device implanted into femoral shaft
Surgical techniques
In both centres the patient’s condition is fully investigated and staged using computed tomography, bone isotope scans and magnetic resonance imaging where appropriate. At both centres standard techniques for tumour resection are used, with the principle of obtaining a wide surgical margin [11].
In all of the ROH cases the intramedullary stem was fixed with polymethylmethacrylate (PMMA) bone cement (Fig. 1b). More detailed descriptions of the design of the Stanmore massive prosthesis replacement and the operating procedure have been previously published [31].
The CPS uses a shorter intramedullary system, which is implanted under pre-stress and thus not cemented. The cortical portion of the host bone is fixed proximally by five smooth transverse pins placed through an anchor plug connected to a traction bar, over which the bone interface spindle is secured with a set of stacked Belleville washers placed within the taper of the device. The washers are fabricated to provide 400 lb, 600 lb, or 800 lb of fixation force, depending upon the thickness of cortical bone at the prosthetic interface. Anti-rotational pins can be placed through the spindle to increase torsional stability at the bone–implant interface. Biomet Orthopaedic Oncology Salvage System distal femoral and rotating hinge knee components are then fitted to the CPS (Fig. 2b).
The ROH patients are allowed partial weight bearing after 48 h, after which activity level is gradually increased. The CPS patients are maintained non-weight bearing status for 6 weeks and then allowed to increase weight bearing by 25% of body weight per week. CPS patients are instructed to avoid activities that cause undue torque for the first 3 months.
Results
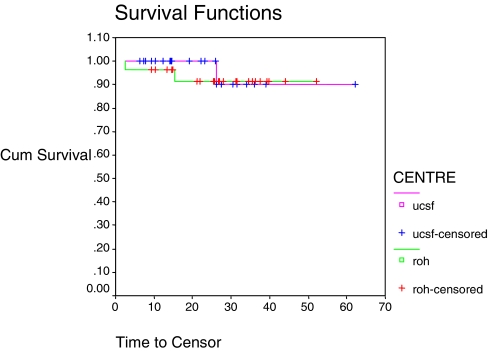
The mean age of the 52 patients was 24.6 years (UCSF 24.9 years, ROH 24.3 years), with an age range of 7–59 years at UCSF and 8–60 years at the ROH. There were no patients between the ages of 27–48 years in either group. The age distribution showed a peak in the second decade and again at ages 45–60 years. There were no significant differences between age, gender, side of tumour or time to follow-up between the two groups. The predominant diagnosis in the 52 cases was osteosarcoma (UCSF 65%, ROH 69%), followed by revision in the UCSF group (15.4%) and chondrosarcoma in the ROH group (7.8%). Miscellaneous diagnoses (one case each) included leiomyosarcoma, synovial sarcoma, malignant fibrous histiocytoma, spindle cell sarcoma, and multiple myeloma. Average follow-up period for CPS patients was 2.2 years and, for CIS patients, 1.8 years (P=0.2). Overall survival rate after a mean period of 2.0 years was 94%, with one death in the UCSF group (3.8%) and two deaths in the ROH group (7.7%) (P=0.702, Table 1, Fig. 3).
Table 1.
Outcome measures (NS)
| Complication | Total (n=52) | UCSF (n=26) | ROH (n=26) | Log rank P value | |||
|---|---|---|---|---|---|---|---|
| Number | % | Number | % | Number | % | (Significant/not significant) | |
| Alive and no problems with prosthesis | 41 | 78.8 | 22 | 84.6 | 19 | 73.1 | 0.292 (NS) |
| Revision: device-related | 2 | 3.8 | 1 | 3.9 | 1 | 3.9 | 0.742 (NS) |
| Revision: infection | 1 | 1.9 | 0 | 0 | 1 | 3.9 | 0.329 (NS) |
| Revision: Bone fracture | 1 | 1.9 | 1 | 3.9 | 0 | 0 | 0.434 (NS) |
| Amputation | 3 | 5.8 | 1 | 3.9 | 2 | 7.6 | 0.686 (NS) |
| Infection: no revision or amputation | 1 | 1.9 | 0 | 0 | 1 | 3.9 | 0.338 (NS) |
| Dead | 3 | 5.8 | 1 | 3.9 | 2 | 7.7 | 0.702 (NS) |
| Total | 52 | 100 | 26 | 100 | 26 | 100 | |
| Total revisions (all cause) | 4 | 7.6 | 2 | 7.6 | 2 | 7.6 | 0.782 (NS) |
Fig. 3.
Overall patient survival and centre, P=0.702
Complications and outcomes
Revision
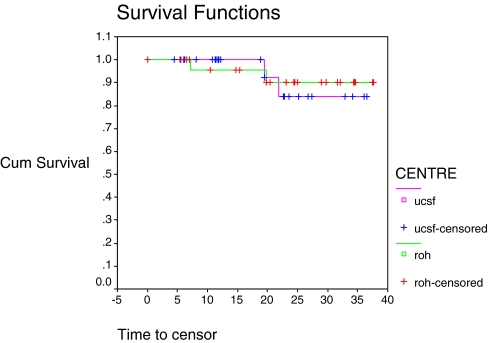
In each group there was one device-related failure leading to revision and one non-device-related revision. Thus, for both groups, the device-related revision rate was 1/26 (3.8%), which was not significantly different between the two groups (P=0.592, Table 2). Age (<30 years or >30 years) was not a significant factor for revision (P=0.353), nor was gender (P=0.323, Table 2). In the 52 patients there were thus four all-cause revision procedures overall (two mechanical failures, one infection related, one fracture related), giving a total revision rate of 2/26 (7.7%) in each group, which was not significantly different between groups (P=0.782, Fig. 4).
Table 2.
Factor analysis–Cox regression
| Factor | Device-related revision (n=2) | All-cause revision (n=4) | ||||
|---|---|---|---|---|---|---|
| Exp B | Confidence limits | P | Exp B | Confidence limits | P | |
| Centre | 0.466 | 0.03–7.59 | 0.592 | 0.751 | 0.10–5.59 | 0.780 |
| Gender | 0.000 | 0.00 | 0.985 | 2.915 | 0.31–27.80 | 0.353 |
| Age >40 years | 0.229 | 0.014–3.69 | 0.298 | 0.269 | 0.02–3.64 | 0.323 |
Fig. 4.
All-cause revision (two device-related, two non-device-related), split by centre, P=0.782
The device-related failure in the ROH group was caused by a fractured tibial component 19.8 months after the first operation, with subsequent amputation after a further 13 days. With the CPS device the compression distance decreased to zero, indicating loss of fixation and impending failure; the device was revised successfully 21.9 months after the first operation.
In the ROH group the non-device-related failure was due to infection 7.2 months after the primary procedure. The non-device-related failure in the UCSF group was due to fractured bone above the implant following a fall 19.4 months after a successful and uneventful implantation procedure.
The two revised CPS devices both survive at 5.8 and 2.7 months, and the remaining revised CIS survives at 5.3 months.
Amputation
In all 52 patients there was a total of three amputations, giving an overall rate of 5.8%. There was one amputation at UCSF, with a resultant 96% maintenance rate of limb salvage, compared with two amputations at ROH, yielding a limb salvage rate of 92.3%, showing no significant difference (P=0.686, Table 1). The amputation in the UCSF group was 13.3 months after the index procedure (infective cause), and the two ROH cases were after 1.3 months (infective cause) and 20.2 months (fractured device, as discussed).
Infection
In the 52 patients there were three infections, giving an overall infection rate of 5.8%. There was one deep infection (3.8%) in the UCSF group, which led to amputation, and two deep infections in the ROH group (7.7%), one of which led to revision and one of which to amputation. Infection was a significant factor for amputation in all 52 patients (P<0.0001).
Discussion
The aim of our study was to establish whether the CPS implant is a safe and effective device in the short term by comparing it with a cohort of case-matched CIS EPRs. The matching process produced groups that had very comparable demographic characteristics in terms of age, gender and follow-up period. The mean age of the 52 patients was 24.6 years, which is comparable to the mean age of patients from other similar studies [1, 9, 10, 12, 17, 20, 21, 24–27, 30, 32–34, 38].
Neither group showed evidence of aseptic loosening, but each group had one device-related failure. This comparable device-related failure rate of 3.8% at 2 years is reassuring for the potential safety and efficacy of the CPS device, but it is not necessarily surprising or definitive. Although these results certainly must be understood to be preliminary in nature, they must also be assessed in the context of what is known about early survivorship of massive endoprostheses. There is simply very little extant in the peer-reviewed literature regarding 2-year results. Comparable short-term studies include that of Shih et al., which reported on the Kinematic rotating hinge prosthesis in a group of 16 patients with an average follow-up of 2.3 years. A device-related revision rate of 6% (one fractured component with no aseptic loosening) was noted, similar to our findings [34]. Freedman and Eckardt reported a similarly small series of nine patients, with an average group follow-up of 17 months, who also received a Howmedica modular endoprosthesis, one of which was revised for aseptic loosening (11%), and a second for infection (combined =22%) [12].
Slightly longer rates of interval follow-up were reported by Mitermayer et al. in a study of 251 uncemented Kotz modular implants over 15 years; an 8.4% revision rate due to aseptic loosening, after a mean of 36 months, was described [26]. Unwin et al. reported in 1996 the long-term follow-up of patients receiving 1,001 custom made Stanmore implants in the lower extremities, 493 of which were in the distal femur. The overall rate of revision for aseptic loosening in the distal femoral implants was 9.9% at an average of 4 years, and they indicated that the risk of revision due to aseptic loosening within the first 3 years was very low in all groups [35]. The prostheses survival curve fell steadily after approximately 3 years, and so we may expect to see the same process with the cohort of ROH patients reported here, as they received the same implant and are currently at an average of 1.8 years after follow-up. Finally, Kawai et al. reported a group of 40 patients with malignant distal femoral tumours reconstructed with a Biomet cemented prosthesis, for which an aseptic loosening rate of 27.5% occurred after a mean of 4.25 years; this was their most common mode of failure [21]. Most other studies of massive oncological endoprostheses report survivorship values for 5 years of longer [1, 3, 10, 30, 32, 38, 40].
The UCSF limb salvage rate was 96% and that for ROH was 92.3%, which is without statistical difference. Kawai et al. reported comparable rates at 93% at 3 years and 90% at 5 and 10 years [21]. The Kinematic group from the Shih study of 16 patients showed no amputation at 2.3 years, but their early Walldius group showed four amputations at a rate of 8.8%, which is still comparable [34].
Infection is reported in our study and continues to be an acknowledged complication of orthopaedic oncological procedures. There are many factors that are difficult to control, including lengthy open procedures and immunocompromised status secondary to chemotherapy. Infection was related to amputation for all 52 patients (P=0.0001, Fig. 4), as one might expect. Malawer and Chou reported an 11% infection rate at an average of 24 months [25], and Shih et al. showed a 10% rate [34], both comparable with our results of 5.8% for both groups. Maintenance of limb salvage by re-implantation after a deep infection has occurred is exceedingly difficult, with amputation being a frequent ultimate outcome [13, 16, 18, 23].
In summary, none of the endpoint factors used to compare CPS and CIS outcomes was significantly different. In the short term, distal femoral endoprosthetic survival is similar when CPS patients are compared with those receiving the ‘gold standard’ CIS device. Furthermore, compared with those in the available literature, the CPS implants are doing extremely well at 2 years. These results suggest that the CPS implant is safe and effective when compared with conventional stems; this must be regarded as a preliminary conclusion but one which is also supported by the results of a multi-institutional prospective cohort study that resulted in FDA clearance for the distal femoral CPS device in December 2003 [29]. The authors hope that the current study serves to provide a baseline for what can be expected in the short term for oncological endoprosthetic survival, since such benchmarks are often sought by clinical investigators and regulatory agencies evaluating new technologies. We eagerly await longer follow-up to assess the ongoing risks of device-related failure and aseptic loosening in both CPS and CIS groups.
Contributor Information
A. A. Bhangu, Email: aneelbhangu@yahoo.co.uk
M. J. Kramer, Email: kramerm@orthosurg.ucsf.edu
R. J. Grimer, Email: Rob.grimer@roh.nhs.uk
R. J. O’Donnell, Phone: +1-415-8853800, FAX: +1-415-8853802, Email: odonnelr@orthosurg.ucsf.edu
References
- 1.Bickels J, Wittig JC, Kollender Y, Henshaw RM, Kellar-Graney KL, Meller I, Malawer M. Distal femoral resection with endoprosthetic reconstruction: a long-term followup study. Clin Orthop Relat Res. 2002;400:225–235. doi: 10.1097/00003086-200207000-00028. [DOI] [PubMed] [Google Scholar]
- 2.Bini SA, Johnston JO, Martin DL. Compliant prestress fixation in tumor prostheses: interface retrieval data. Orthopedics. 2000;23:707–712. doi: 10.3928/0147-7447-20000701-18. [DOI] [PubMed] [Google Scholar]
- 3.Blunn GW, Briggs TWR, Cannon SR, Walker PS, Unwin PS, Culligan S, Cobb JP. Cementless fixation for primary segmental bone tumor endoprostheses. Clin Orthop Relat Res. 2000;372:223–230. doi: 10.1097/00003086-200003000-00024. [DOI] [PubMed] [Google Scholar]
- 4.Cannon CP, Zeegen E, Eckardt JJ. Techniques in endoprosthetic reconstruction. Oper Tech Orthop. 2005;14:225–235. doi: 10.1053/j.oto.2004.10.003. [DOI] [Google Scholar]
- 5.Cox DR. Regression models and life tables. J R Stat Soc. 1972;34:187–220. [Google Scholar]
- 6.Cristofolini L, Bini SA, Toni A. In vitro testing of a novel limb salvage prosthesis for the distal femur. Clin Biomech. 1998;13:608–615. doi: 10.1016/S0268-0033(98)00024-2. [DOI] [PubMed] [Google Scholar]
- 7.Damron TA. Endoprosthetic replacement following limb-sparing resection for bone sarcoma. Semin Surg Oncol. 1997;13:3–10. doi: 10.1002/(SICI)1098-2388(199701/02)13:1<3::AID-SSU2>3.0.CO;2-F. [DOI] [PubMed] [Google Scholar]
- 8.Dorfman HD, Czerniak B. Bone tumors. St. Louis: Mosby; 1998. [Google Scholar]
- 9.Eckardt JJ, Eilber FR, Dorey FJ, Mirra JM. The UCLA experience in limb salvage surgery for malignant tumors. Orthopedics. 1985;8:612–621. doi: 10.3928/0147-7447-19850501-15. [DOI] [PubMed] [Google Scholar]
- 10.Eckardt JJ, Eilber FR, Rosen G, Mirra JM, Dorey FJ, Ward WG, Kabo JM. Endoprosthetic replacement for stage IIB osteosarcoma. Clin Orthop Relat Res. 1991;270:202–213. [PubMed] [Google Scholar]
- 11.Enneking WF, Spanier SS, Goodman MA. A system for the surgical staging of musculoskeletal sarcoma. Clin Orthop Relat Res. 1980;153:106–120. [PubMed] [Google Scholar]
- 12.Freedman EL, Eckardt JJ. A modular endoprosthetic system for tumor and non-tumor reconstruction: preliminary experience. Orthopedics. 1997;20:27–36. doi: 10.3928/0147-7447-19970101-06. [DOI] [PubMed] [Google Scholar]
- 13.Grimer RJ, Belthur M, Chandrasekar C, Carter SR, Tillman RM. Two-stage revision for infected endoprostheses used in tumor surgery. Clin Orthop Relat Res. 2002;395:193–203. doi: 10.1097/00003086-200202000-00022. [DOI] [PubMed] [Google Scholar]
- 14.Grimer RJ, Carter SR, Tillman RM, Sneath RS, Walker PS, Unwin PS, Shewell PC. Endoprosthetic replacement of the proximal tibia. J Bone Joint Surg Br. 1999;81:488–494. doi: 10.1302/0301-620X.81B3.9234. [DOI] [PubMed] [Google Scholar]
- 15.Henshaw RM, Bickels J, Malawer MM. Modular endoprosthetic reconstruction for lower extremity skeletal defects: oncologic and reconstructive indications. Semin Arthroplasty. 1999;10:180–187. [Google Scholar]
- 16.Holzer G, Windhager R, Kotz R. One-stage revision surgery for infected megaprostheses. J Bone Joint Surg Br. 1997;79:31–35. doi: 10.1302/0301-620X.79B1.7139. [DOI] [PubMed] [Google Scholar]
- 17.Horowitz SM, Glasser DB, Lane JM, Healey JH. Prosthetic and extremity survivorship after limb salvage for sarcoma: how long do the reconstructions last? Clin Orthop Relat Res. 1993;293:280–286. [PubMed] [Google Scholar]
- 18.Jeys LM, Grimer RJ, Carter SR, Tillman RM. Periprosthetic infection in patients treated for an orthopaedic oncologic condition. J Bone Joint Surg Am. 2005;87:842–849. doi: 10.2106/JBJS.C.01222. [DOI] [PubMed] [Google Scholar]
- 19.Kaplan EL, Meier P (1958) Nonparametric observations from incomplete observations J Am Stat Assoc 53
- 20.Kawai A, Healey JH, Boland PJ, Athanasian EA, Jeon D-G. A rotating hinge knee replacement for malignant tumors of the femur and tibia. J Arthroplasty. 1999;14:187–196. doi: 10.1016/S0883-5403(99)90124-9. [DOI] [PubMed] [Google Scholar]
- 21.Kawai A, Muschler GF, Lane JM, Otis JC, Healey JH. Prosthetic knee replacement after resection of a malignant tumor of the distal part of the femur. J Bone Joint Surg Am. 1998;80:636–647. doi: 10.1302/0301-620X.80B4.8216. [DOI] [PubMed] [Google Scholar]
- 22.Lackman RD. Musculoskeletal oncology. In: Vaccaro AR, editor. Orthopaedic knowledge update: 8. Rosemont, IL: American Academy of Orthopaedic Surgeons; 2005. pp. 197–215. [Google Scholar]
- 23.Lee SH, Oh JH, Lee KS, Yoo KH, Kim HS. Infection after prosthetic reconstruction in limb salvage surgery. Int Orthop. 2002;26:179–184. doi: 10.1007/s00264-001-0328-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Malawer M. Surgical technique and results of limb sparing surgery for high grade bone sarcomas of the knee and shoulder. Orthopedics. 1985;8:597–607. doi: 10.3928/0147-7447-19850501-14. [DOI] [PubMed] [Google Scholar]
- 25.Malawer MM, Chou LB. Prosthetic survival and clinical results with use of large-segment replacements in the treatment of high-grade bone sarcomas. J Bone Joint Surg Am. 1995;77:1154–1165. doi: 10.2106/00004623-199508000-00003. [DOI] [PubMed] [Google Scholar]
- 26.Mitermayer F, Windhager R, Dominkus M, Krepler P, Schwameis E, Sluga M, Kotz R, Strasser G. Revision of the Kotz type of tumour endoprosthesis for the lower limb. J Bone Joint Surg Br. 2002;84:401–406. doi: 10.1302/0301-620X.84B3.12204. [DOI] [PubMed] [Google Scholar]
- 27.Muschler GF, Levine MJ, Ihara K, Otis JC, Lane JM, Burstein AH, Healey JH. A custom distal femoral prosthesis for reconstruction of large defects following wide excision for sarcoma: results and prognostic factors. Orthopedics. 1995;18:527–538. doi: 10.3928/0147-7447-19950601-04. [DOI] [PubMed] [Google Scholar]
- 28.Nichter LS, Menendez LR. Reconstructive considerations for limb salvage surgery. Orthop Clin North Am. 1993;24:511–521. [PubMed] [Google Scholar]
- 29.O’Donnell RJ, Johnston JO, Berrey BH Jr, Boland PJ, Scarborough MT, Yasko AW, Lin PP, Mallin BA, Morris CD, Weber KL, Lewis VO, Marco RAW, Randall RL, Healey JH (2003) Preliminary report of the multi-institutional ComPreSs distal femoral study. 12th international symposium on limb salvage, Rio de Janiero, Brazil, September 15–17 2003
- 30.Plötz W, Rechl H, Burkgkart R, Messmer C, Schelter R, Hipp E, Gradinger R. Limb salvage with tumor endoprostheses for malignant tumors of the knee. Clin Orthop Relat Res. 2002;405:207–215. doi: 10.1097/00003086-200212000-00027. [DOI] [PubMed] [Google Scholar]
- 31.Roberts P, Chan D, Grimer RJ, Sneath RS, Scales JT. Prosthetic replacement of the distal femur for primary bone tumours. J Bone Joint Surg Br. 1991;73:762–769. doi: 10.1302/0301-620X.73B5.1894662. [DOI] [PubMed] [Google Scholar]
- 32.Safran MR, Kody MH, Namba RS, Larson KR, Kabo JM, Dorey FJ, Eilber FR, Eckardt JJ. 151 endoprosthetic reconstructions for patients with primary tumors involving bone. Contemp Orthop. 1994;29:15–25. [PubMed] [Google Scholar]
- 33.Shih L-Y, Chen T-S, Lo W-H. Limb salvage surgery for locally aggressive and malignant bone tumors. J Surg Oncol. 1993;53:154–160. doi: 10.1002/jso.2930530305. [DOI] [PubMed] [Google Scholar]
- 34.Shih L-Y, Sim FH, Pritchard DJ, Rock MG, Chao EYS. Segmental total knee arthroplasty after distal femoral resection for tumor. Clin Orthop Relat Res. 1993;292:269–281. [PubMed] [Google Scholar]
- 35.Unwin PS, Cannon SR, Grimer RJ, Kemp HBS, Sneath RS, Walker PS. Aseptic loosening in cemented custom-made prosthetic replacements for bone tumours of the lower limb. J Bone Joint Surg Br. 1996;78:5–13. [PubMed] [Google Scholar]
- 36.Unwin PS, Cobb JP, Walker PS. Distal femoral arthroplasty using custom-made prostheses. J Arthroplasty. 1993;8:259–268. doi: 10.1016/s0883-5403(06)80087-2. [DOI] [PubMed] [Google Scholar]
- 37.Unwin PS, Cobb JP, Walker PS. Distal femoral arthroplasty using custom-made prostheses: the first 218 cases. J Arthroplasty. 1993;8:259–268. doi: 10.1016/S0883-5403(06)80087-2. [DOI] [PubMed] [Google Scholar]
- 38.Ward WG, Eckardt JJ, Johnston-Jones KS, Eilber FR, Namba R, Dorey FJ, Mirra J, Kabo JM. Five to ten year results of custom endoprosthetic replacement for tumors of the distal femur. In: Brown KLB, editor. Complications of limb salvage: prevention, management and outcomes. Montreal: International Society of Limb Salvage; 1991. pp. 483–491. [Google Scholar]
- 39.Ward WG, Sr, Dorey F, Kelly C, Kabo JM, Wirganowicz PZ, Eckardt JJ. Lessons from massive tumor endoprostheses: implications for future tumor and total joint endoprostheses. Semin Arthroplasty. 1999;10:124–132. [Google Scholar]
- 40.Yasko AW, Lin PP, Weber KL. Survivorship of segmental prosthetic arthroplasty for limb salvage following bone sarcoma resections. Sarcoma. 2001;5:61–62. [Google Scholar]
- 41.Yasko AW, Reece GP, Gillis TA, Pollock RE. Limb-salvage strategies to optimize quality of life: the MD Anderson Cancer Center experience. CA Cancer J Clin. 1997;47:226–238. doi: 10.3322/canjclin.47.4.226. [DOI] [PubMed] [Google Scholar]