Abstract
The aim of this retrospective study was to compare and assess the effect of bone grafting and cementing techniques – two common applications used in the treatment of subchondral giant cell tumours of bone (GCTs) – on the development of degenerative changes in the weight-bearing joints of the lower extremity. Eighty patients were included in this follow-up study, 44 of whom underwent curettage followed by bone grafting, and 36 who had curettage followed by cementation. At the 24-month post-operative examination, significantly less degenerative change was found in patients with bone cement than in those with bone grafting. At the 50-month and later (range: 50–148 months) post-operative examination, however, no significant differences were found between the two groups, indicating that there was a significant acceleration of degenerative changes in the cemented group after the 24-month follow-up.
Résumé
Le but de cette étude rétrospective sur 80 patients était d’apprécier l’effet des greffes osseuses et du cimentage - utilisés dans le traitement des tumeurs à cellules géantes sous chondrales - sur le développement des lésions dégénératives des articulations portantes du membre inférieur. Pour 44 patients le curetage était suivi d’une greffe osseuse et pour 36 d’un cimentage. A un recul de 24 mois il y avait moins de modifications dégénératives chez les patients avec cimentage que chez ceux avec greffe osseuse. A 50 mois et plus (de 50 à 148 mois) il n’y avait aucune différence entre les deux groupes. Cela signifie qu’il y a une accélération des modifications dégénératives dans le groupe cimenté après 24 mois.
Introduction
Curettage followed by bone cement plugging is a currently accepted surgical treatment of bone tumours that is applied mainly to large cystic lesions, such as giant cell tumours, aneurysmal bone cysts, chondroblastomas, localised in the proximity of the greater joints [13, 17]. The risk of subchondral cement causing damage to the cartilage and subsequently degenerative arthritis has been cited in the literature, but remains unproven [4, 8, 9, 12, 22]. Articular degeneration with associated biomechanical changes after treatment with cement has been noted in the weight-bearing area in animal studies, whereas other studies have demonstrated the superior ability of subchondral autogenous bone grafts to restore the subchondral osseus anatomy to its normal state [8, 9, 12, 22].
The aim of this retrospective study was to compare and assess the effect of the bone grafting and cementing techniques used in the treatment of subchondral giant cell tumours of bone (GCT) of bone on the development of degenerative changes in the weight-bearing joints of the lower extremity.
Materials and methods
Between 1970 and 2006, 199 patients with giant cell tumours of bone were treated surgically in the Orthopaedic Clinic of Semmelweis University, Budapest, Hungary. The study cohort comprised patients with subchondral GCTs treated by curettage of the proximal femur, distal femur, proximal tibia and distal tibia.
Of the 199 patients, 129 had GCTs in the weight-bearing bones of the lower extremity, and 70 in other locations (upper extremity, pelvis, etc.). A subchondral tumour was present in 109 patients (84%). Phenol was used as adjuvant therapy in 85 patients. Seventeen (20%) of the patients treated with phenol showed local recurrence of the tumour, while 16 (36%) of those not treated with phenol showed recurrence.
To eliminate factors which can result in secondary osteoarthrosis, 49 patients were excluded from the study for various reasons: pathological fracture (seven patients), septic complications (three patients), local recurrences (33 patients), non-subchondral localisation (20 patients) or patients with a follow-up of less than 50 months (30 patients). Some of these factors occurred in combination.
When possible, a pneumatic tourniquet was used during the procedure to decrease local bleeding. Extensive curettage was performed. Maximal care was taken to spare the subchondral bone. Bone cement or cancellous bone graft from a bone bank was packed into the cavity of the curetted defect.
Post-operative complications were recorded. All patients had a routine follow-up with a physical examination and radiographs of the involved limb every 3 months for 2 years, then every 6 months for 3 years, and then annually thereafter. The radiographs were reviewed and compared with previous images. Patient documentation was reviewed in all cases to monitor signs of degeneration of a given joint over time. The most recent follow-up data were compared with the 24-month and 50-month follow-up data. The area of affected subchondral bone was measured on anteroposterior and lateral radiographs. The subchondral bone was defined as affected when the distance between it and the tumour was less than 3 mm [5]. The GCTs were staged according to the system described by Campanacci et al. [3]: stage 1 lesions were intraosseus, stage 2 lesions were intraosseus lesions with cortical thinning and stage 3 lesions extended extraosseously. The size of the subchondral involvement of the tumour was assessed according to the method published by Chen et al. [5] with the percentage of affected subchondral bone calculated as the ratio of the affected length to the total length of the compartment’s subchondral bone.
Degenerative status was defined on the basis of radiological changes, decreased range of motion and pain. Signs of degenerative changes in radiographs were documented by the senior author (MS).
Functional evaluation was done according to the Musculoskeletal Tumor Society (MSTS) system developed by Enneking [7]. This system evaluates seven parameters, most of which are clinical, including pain, range of motion, stability of the joint, strength, deformity, general functional activity and emotional acceptance of the procedure by the patient. Health status was assessed by the Short Form-36 questionnaire, which is a validated generic tool for evaluating eight health concepts: physical functioning, role limitations because of physical problems, bodily pain, general health, vitality, social functioning, role limitations because of emotional problems, and mental health [21]. Each health profile is scored on a 100-point scale, with a higher score indicating a lesser disability.
The cumulative arthrosis-free survival time of patients was calculated by the Kaplan-Meier method and evaluated by the Log-Rank test. The difference in the mean rank between the two groups was evaluated by the Chi-square test. The association between the final functional rating score and filling material (bone grafting versus cementing) was analysed with a one-way ANOVA test.
Results
Eighty patients participated in our retrospective study, with an average follow-up time of 84 months (range: 50–148 months). The mean age of the patients was 32.4 years (range: 14–69 years). The proximal femur was affected in five patients, distal femur in 45 patients, the proximal tibia in 19 patients and the distal tibia in 11 patients. There were three patients with Stage 1 lesions, 45 patients with Stage 2 lesions and 32 patients with Stage 3 lesions. Average subchondral involvement by the tumour was 27.5% (range: 15–89%) according to the method of Chen et al. [5]. The Campanacci staging did not show a significant correlation with degenerative changes at any of the time points in the follow-up (p>0.05). However, significant differences were found between tumour groups involving more or less than 50% of the subchondral bone, as measured by the method of Chen et al. [5], in the period between 50 and 114 months post-operative (p<0.05).
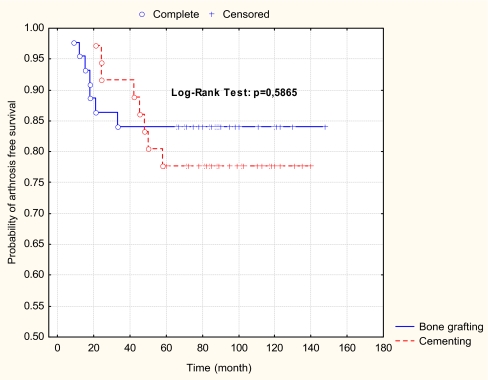
Curettage and bone grafting was performed in 44 patients, while 36 patients underwent cementation after curettage. At the 24-month follow-up, degenerative joint changes were found in six (13.6%) patients of the bone grafting group and in three (8.3%) patients of the bone cemented group. At the 50-month follow-up, seven (15.9%) patients treated with bone grafting and seven (19.4%) patients with cementing showed degenerative signs in the joints, which represents one new patient with degenerative signs among those treated with bone grafting and four new patients with degenerative signs among those treated with cementing. After the first post-operative examination at 24 months, significantly less degenerative changes were found in patients with bone cementing than in those with bone grafting. At the 50-month follow-up and later (range: 50–148 months), however, no significant differences were found between the two groups. These results show that after 24 months there was a significant acceleration of degenerative changes in the cemented group. The initial difference between the two groups equalised over years (Fig. 1).
Fig. 1.
Probability of arthrosis-free survival
Seventy-one patients were evaluated at the last follow-up on the basis of the Musculoskeletal Tumor Society MSTS 1987 score and the Short-Form-36 (SF-36) survey. Based on the data of the medium-term follow-up, neither the function measured with the MSTS 1987 score (p>0.05) nor the quality of life assessed by the SF-36 differed between the two groups (p>0.05) (Table 1).
Table 1.
Quality of life and functional assessment scores for giant cell tumour patients treated by curettage and cementing versus bone grafting
| Tests (number of patients) | Functions assessed | Assessment score: | |
|---|---|---|---|
| Curettage | |||
| Bone grafting | Cementing | ||
| Short Form-36 (bone grafting, 41; cementing, 30) | Physical function | 75 | 80 |
| Role physical | 77 | 79 | |
| Bodily pain | 74 | 80 | |
| General health | 81 | 80 | |
| Vitality | 74 | 72 | |
| Social | 92 | 86 | |
| Role emotional | 86 | 81 | |
| Mental health | 81 | 79 | |
| MSTS (bone grafting, 41; cementing, 30) | Pain | 4.41 | 4.37 |
| Range of motion | 4.51 | 4.61 | |
| Strength | 4.33 | 4.28 | |
| Stability | 4.83 | 4.79 | |
| Deformity | 4.77 | 4.71 | |
| Function | 4.21 | 4.32 | |
| Emotional Acceptance | 4.65 | 4.61 | |
Discussion
In the patients included in our study, 56% of all GCTs had reached the subchondral region (within 3 mm), but 84% subchondral involvement was seen in the lower extremity localisation. Because of frequent subchondral involvement, most authors favour an intralesional curettage that preserves the joint function. Wide resection is associated with a much better local tumour control but impairs limb function by sacrificing the joint [6, 14].
Subchondral bone has a limited role in the nutrition of the cartilage, but it is very important as a shock absorber. Radin and colleagues, who applied a repeated compressive load to the knee joint of a rabbit to create a minimal fracture to subchondral bone tissue, observed callus formation and increased trabecular bone formation during the healing process that led to stiffening of the subchondral bone tissue and consequently, to degeneration of the knee joint cartilage [18, 19].
In focusing on the effect of the two grafting methods on the development of the degenerative change, it is important to exclude all other possible causative factors. As such, the exclusion criteria consisted of patients with pathological fracture, septic complications and local tumour recurrence. The first two factors are intrinsically capable of directly damaging the subchondral bone and the articular cartilage while in the case of recurrence, the subchondral bone and the articular cartilage may be damaged during repeated surgery. It is an accepted fact that even short-term functional results are inferior in patients having curettage following pathological fracture than in those without pathological fracture [6, 20].
Polymethylmethacrylate (PMMA) has the added benefit that the heat released during polymerisation kills any remaining tumour cells left behind after curettage in the subchondral bone [1, 12]. Further advantages of using PMMA over bone grafting are immediate mechanical support and the prevention of cartilage rarefaction and of the collapse and fracture of the subchondral bone [8, 12]. The immediate stabilisation obtained with PMMA allows for early physical therapy without the need for bracing which enables an early return to maximal function. Radiographic follow-up of the affected site is easy since recurrence or secondary changes are visible at an early stage and are relatively simple to localise. In the event of a recurrence, curettage and bone cement plugging can be repeated [13, 17]. Despite these advantages, the benefit of PMMA has been questioned in recent publications [2, 16, 20]. The disadvantages of PMMA are that it is non-biodegradable and lacks the capability to integrate biologically into the surrounding host bone [22]. Welch et al. described a sclerotic rim, created by increased new trabecular bone formation, separating the cement from the surrounding bone and subchondral bone layer [22]. This sclerotic rim could decrease the shock-absorbing capacity of the subchondral bone layer. When PMMA is used in subchondral defects, thermal necrosis of the subchondral plate and articular cartilage can be of concern [8, 12].
Bone grafting is typically used as a defect filler, and neither autograft nor allograft provides optimal mechanical support for subchondral defects [8, 11]. Animal model experiments have proved that the subchondral strength of a defect filled with tamped spongious bone is only slightly greater than that of the empty defect [9, 11]. Consequently, there is a high probability of collapse and fracture of the subchondral bone, and of articular cartilage damage. After complete remodelling of the bone, however, the full functionality of the subchondral bone is regained. According to animal study data and our results, there is an increased risk of degenerative change during the first 2 years following surgery in joints of weight-bearing bones treated with bone grafting [22].
Defects of the subchondral bone lamella created during the intensive curettage seem to promote the development of osteoarthrosis [9, 22]. Defects emerging this way and subsequently filled either with bone graft or bone cement actively participate in the degenerative process.
The results of our study revealed that there was a clear relationship between the size of the subchondral bone involvement and secondary degenerative changes in the joints. This provides us with a suitable diagnostic tool for assessing the risk of subsequent osteoarthritis.
In 1987, at the Limb Salvage in Musculo-skeletal Oncology Symposium, the effectiveness of PMMA as an adjuvant in local tumour control was addressed. In the five series of patients presented, the local recurrence rate was 15%; this was significantly lower than the rate of 40–50% obtained using traditional curettage procedures. The rate of osteoarthrosis in the joints treated by cementing in these series was 12% – but the follow-up period in these patients was short [7]. Our results suggest that an increased incidence of arthrosis can be expected in the bone grafting group within the first two post-operative years, after which no further degenerative changes will occur. Conversely, the number of arthrosis cases in the group treated with cement was low during the first two post-operative years, increasing subsequently: new arthrosis cases developed at an incidence rate higher than that in the bone grafting group.
It is worth noting, however, that despite the high rate of arthrosis, the functions and life quality parameters assessed by the MSTS score and SF-26 survey, respectively, were quite good in both groups.
Several authors have reported removal of the bone cement once the risk of recurrence has diminished in order to avoid the joint being damaged by the cement [17]. We consider this method a half-way solution, since repeated surgery may cause intra-operative damage to the remaining subchondral bone and the articular cartilage. Furthermore, such a procedure can be considered as a postponement of the basic problem since the articular cartilage and the remaining subchondral bone are left without effective and stabile support for an additional long time following the bone grafting. A significant number of patients are reluctant to undergo treatment involving multiple operations.
An alternative possibility is the interposition of autologous bone graft between the cartilage or subchondral bone layer and the cement in an attempt to prevent joint degeneration [15]. Compared to bone grafting there is immediate full support, and the situation supports the formation of a new subchondral layer. However, concerns have arisen about the remaining bone cement and the lack of a adjuvant heating effect in the subchondral area.
In the light of our results, the optimal filling material should provide an immediate mechanical support, should be osteoconductive and osteoinductive and should have the capacity to be resorbed and replaced by host bone in the long-term without jeopardising the mechanical integrity of the bone. The ideal solution would be the use of bioactive ceramics, which fulfill all these criteria [10].
References
- 1.Bini SA, Gill K, Johnston JO. Giant cell tumor of bone. Curettage and cement reconstruction. Clin Ortop. 1995;321:245–250. [PubMed] [Google Scholar]
- 2.Blackley HR, Wunder JS, Davis AM, White LM, Kandel R, Bell RS. Treatment of giant-cell tumors of long bones with curettage and bone-grafting. J Bone Jt Surg Am. 1999;81:811–820. doi: 10.2106/00004623-199906000-00008. [DOI] [PubMed] [Google Scholar]
- 3.Campanacci M. Giant cell tumor and chondrosarcoma. Grading, treatment and results. Cancer Res. 1976;54:257–261. doi: 10.1007/978-3-642-80997-2_22. [DOI] [PubMed] [Google Scholar]
- 4.Campanacci M, Giunti A, Olmi R. Giant-cell tumours of bone. A study of 209 cases with long term follow-up. Ital J Orthop Traumatol. 1975;1:249–277. [Google Scholar]
- 5.Chen TH, Su YP, Chen WM. Giant cell tumors of the knee: subchondral bone integrity affects the outcome. Int Orthop. 2005;29:30–34. doi: 10.1007/s00264-004-0613-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Dreinhofer KE, Rydholm A, Bauer HC, Kreicbergs A. Giant-cell tumours with fractue at diagnosis. J Bone Jt Surg Br. 1995;77:189–193. [PubMed] [Google Scholar]
- 7.Enneking WF. Modification of the system for functional evaluation of surgical management of musculoskeletal tumors. In: Enneking WF, editor. Limb salvage in musculoskeletal oncology. New York: Churchill Livingstone; 1987. pp. 626–639. [Google Scholar]
- 8.Frassica FJ, Gorski JP, Pritchard DJ, Sim FH, Chao EYS. A comparative analysis of subchondral replacement with polymethylmethacrylate or autologous bone graft in dogs. Clin Orthop Rel Res. 1993;293:339–378. [PubMed] [Google Scholar]
- 9.Hisatome T, Yasunaga Y, Ikuta Y, Fujimoto Y. Effects on articular cartilage of subchondral replacement with polymethylmethacrylate and calcium phosphate cement. J Biomed Mater Res. 2002;59:490–498. doi: 10.1002/jbm.1263. [DOI] [PubMed] [Google Scholar]
- 10.Hollinger JO, Battistone GC. Biodegradable bone repair materials. Synthetic polymers and ceramics. Clin Ortop. 1986;207:290–305. [PubMed] [Google Scholar]
- 11.Hopp SG, Dahners LE, Gilbert JA. A study of the mechanical strength of long bone defects treated with various autograft substitutes. An experimental investigation in the rabbit. J Orthop Res. 1989;7:579–584. doi: 10.1002/jor.1100070416. [DOI] [PubMed] [Google Scholar]
- 12.Johnston JO. Treatment of a giant cell tumor by aggressive curettage and packing with bone cement. In: Enneking WF, editor. Limb salvage in musculoskeletal oncology. New York: Churchill Livingstone; 1987. pp. 512–515. [Google Scholar]
- 13.Labs K, Perka C, Schmidt RG. Treatment of stages 2 and 3 giant-cell tumor. Arch Orthop Trauma Surg. 2001;121:83–86. doi: 10.1007/s004020000158. [DOI] [PubMed] [Google Scholar]
- 14.Liu HS, Wang JW. Treatment of giant cell tumor of bone: a comparison of local curettage and wide resection. Chang Keng I Hsuey. 1998;21:37–43. [PubMed] [Google Scholar]
- 15.Malawer M, Bickels J, Meller I, Buch RG, Henshaw RM, Kollender Y. Cryosurgery in the treatment of giant cell tumor. Clin Ortop. 1999;359:176–188. doi: 10.1097/00003086-199902000-00019. [DOI] [PubMed] [Google Scholar]
- 16.McGough RL, Rutledge J, Lewis V, Lin PP, Yasko AW. Impact severity of local recurrence in giant cell tumor of bone. Clin Orthop Rel Res. 2005;438:116–122. doi: 10.1097/01.blo.0000180055.76969.08. [DOI] [PubMed] [Google Scholar]
- 17.Ozaki T, Hillmann A, Lindner N, Winkelmann W. Cementation of primary aneurysmal bone cysts. Clin Orthop Rel Res. 1997;337:240–248. doi: 10.1097/00003086-199704000-00026. [DOI] [PubMed] [Google Scholar]
- 18.Radin EL, Martin RB, Burr DB, Caterson B, Boyd RD, Goodwin C. Effects of mechanical loading on the tissues of the rabbit knee. J Orthop Res. 1984;2:221–234. doi: 10.1002/jor.1100020303. [DOI] [PubMed] [Google Scholar]
- 19.Radin EL, Rose RM. Role of subchondral bone in the initiation and progression of cartilage damage. Clin Orthop. 1986;213:34–40. [PubMed] [Google Scholar]
- 20.Turcotte RE, Wunder JS, Isler MH, Bell RS, Schachar N, Masri BA, Moreau G, Davis AM. Giant cell tumor of long bone: a Canadian Sarcoma Group study. Clin Orthop Rel Res. 2002;397:248–258. doi: 10.1097/00003086-200204000-00029. [DOI] [PubMed] [Google Scholar]
- 21.Ware JE, Jr, Sherbourne CD. The MOS 36-item short form health survey (SF-36) Med Care. 1992;30:473–483. doi: 10.1097/00005650-199206000-00002. [DOI] [PubMed] [Google Scholar]
- 22.Welch RD, Berry BH, Crawford K, Zhang H, Zobitz M, Bronson D, Krishnan S. Subchondral defects in caprine femora augmented with in situ setting hydroxyapatite cement, polymethylmethacrylate, or autogenous bone graft: biomechanical and histomorhological analysis after two-years. J Orthop Res. 2002;20:464–472. doi: 10.1016/S0736-0266(01)00124-3. [DOI] [PubMed] [Google Scholar]