Abstract
The giant cell tumour of bone (GCT) is a locally aggressive intraosseous neoplasm of obscure biological behaviour. Although well defined in clinical, radiological and histological terms, detailed information on its biological development is still relatively incomplete. The tumoral tissue consists of three cell types – the neoplastic giant cell tumour stromal cells (GCTSC), representing the proliferative fraction, secondarily recruited mononuclear histiocytic cells (MNHC) and multinuclear giant cells (MNGC). These cellular components interact together with factors that have a role in regulating osteoclast function in normal bone tissue (e.g. RANK, RANKL, OPG, M-CSF). Recent publications suggest that the neoplastic stromal cells express differentiation features of mesenchymal stem cells. Further research of the pathogenesis of GCT as well as the complex interactions of its cellular populations may provide the knowledge necessary for developing approaches for a biological-based therapy of this neoplasm.
Résumé
Les tumeurs à cellules géantes (GCT) sont des tumeurs localement agressives de type néoplasme intra osseux don't les mécanismes biologiques restent relativement obscurs. Cependant si ces tumeurs sont bien définies sur le plan clinique, radiologique et histologique, les détails marquants de leur développement biologique restent inconnus. Le tissu tumoral consiste en un stroma de tumeurs à cellules géantes (GCTSC) qui représente la partie proliférative de la tumeur avec adjonction secondaire de cellules de type histogitaire mononuclées (MNHC) et de cellules géantes (MNGC). Ces composants cellulaires interagissent avec un certain nombre de facteurs régulant l’action ostéoclastique du tissu osseux normal (e.g. RANK, RANKL, OPG, M-CSF). De récentes publications permettent de penser que ce stroma cellulaire de type néoplasique exprime une différenciation de cellules mésenchymateuses. De prochaines recherches sur la pathogénèse des tumeurs à cellules géantes ainsi que sur les interactions complexes des différentes populations cellulaires devraient permettre d’approcher un traitement médical pour ce type de néoplasme.
Introduction
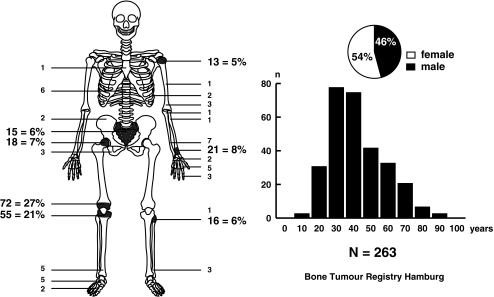
The giant cell tumour (GCT) of bone is a clinico-pathological tumour entity that is identifiable on the basis of relatively typical radiological findings and characteristic histological features. The tumours occur predominantly in the meta-epiphyseal regions of the long bones. Other manifestations – for example, in the sacrum – are considerably rarer. The tumours generally appear on an X-ray film as expansile osteolytic defects, leading eventually to significant local bone destruction. GCTs occur predominantly after skeletal maturity (Fig. 1).
Fig. 1.
Distribution of giant cell tumours (GCTs) in the Bone Tumour Registry Hamburg according to localisation, age and sex. Note the epiphyseal localisation of most tumours at the ends of the long bones
Histology and biological behaviour
It has long been known that multinucleated giant cells (whence the name) constitute only part of the tumour’s cellular composition. The GCT is also made up of mononuclear precursor cells to the giant cells. Mononuclear histiocytic cells (MNHC) and multinucleated giant cells (MNGC) express the CD68-antigen, thus explaining how MNHC and MNGC are considered to belong to the monocytic-histiocytic system. It is widely accepted that MNHC and MNGC of the GCT are recruited secondarily and do not constitute the actual neoplastic cell population. The proliferatively active neoplastic tumour cells, which are also described as giant cell tumour stroma cells (GCTSC) and constitute varying proportions of the tumoral tissue (Fig. 2), do not belong to the monocytic-histiocytic system. Due to the complex histological composition of GCT, differential diagnosis is required to exclude the diagnosis of other lesions also containing giant cells, such as the variants of aneurysmal bone cysts, fibrous metaphyseal defects, chrondroblastoma, brown tumour in hyperparathyroidism and giant cell-rich variants of osteosarcoma. In addition to the histological findings, precise details regarding age, localisation and X-ray findings within the framework of GCT differential diagnosis are vital.
Fig 2.
a Typical X-ray of a giant cell tumour in the distal femur situated in the epi- and metaphysis (growth plates are closed), b typical histology with multinuclear giant cells (MNGC, arrows) between numerous mononuclear cells without nuclear atypia (H&E stain, original magnification 200×), c a portion of the mononuclear cells (MNHC, arrows) reacts with an antibody against the histiocytic marker CD68 (original magnification 400×), d in this part of the tumour the MNHC are fused to the MNGC, the CD68-negative cells represent the neoplastic stromal cells (GCTSC, arrows, original magnification 400×)
Attempts have been made to classify GCT histological findings into a grading system [8]. However, it has not been possible to provide any definite prognostic significance in terms of clinical course [28, 55], and the grading system is not generally accepted. DNA cytometry has led to contradictory results, and most publications decline to accept its prognostic significance [26, 34, 44]. GCTs are seen as tumours with an undefined biological behavior or even as low-grade malignant tumours because their clinical course is not sufficiently predictable on the basis of histological findings. Pulmonary metastases may occur in addition to local bone destruction [4, 10, 22, 54]. In rare instances, a GCT may transform into a giant cell-rich sarcoma [7, 27, 32, 35, 42].
Cytogenetical features
Although the GCT is very well defined in clinical, radiological and histological terms, the properties of its biological development still need to be precisely defined. The tumours are generally discovered at a relatively clinically advanced stage and, unlike epithelial tumours, there is no known pre-neoplastic lesion. The cellular composition of the GCT does not have a comparable non-neoplastic tissue structure in the skeleton. For some years attempts have been made to characterise the GCTSC in terms of its cytogenetics and cell biology. Telomeric associations are typical in GCT, and these associations lead to the fusion of the telomeric ends of different chromosomes resulting, in some cases, in a clonal character within a tumour [46–49]. Structural changes due to the presence of different chromosomes also occur; cytogenetic aberrations have been found, but to date there has been no discovery of any uniform structural or numeric chromosomal aberrations for GCT [3, 6, 30, 37, 45, 48], although it is commonly believed that there are links between telomeric associations and the induction of structural chromosomal aberrations [5, 29, 45].
Functional connections between the neoplastic and non-neoplastic cells in GCTs
Initial progress has been made in identifying the biological qualities of GCTSC, revealing that GCTSC and MNHC/MNGC, as previously established, interact functionally with various factors [receptor activator of nuclear factor-κB(RANK) and of RANK ligand (RANKL), and osteoprotegerin ligand (OPGL)] which also act in regulating osteoclast function in normal bone tissue [1,2, 50, 58]. MNGC express RANK as well as osteoclasts in normal bone tissue. In contrast, RANKL is expressed by the neoplastic GCTSC promoting the fusion of MNHC to MNGC, with macrophage colony-stimulating factor (M-CSF) acting as a co-factor [1, 2, 15]. Cell culture experiments have revealed that giant cell formation takes place even in the absence of any direct cell contact between GCTSC and MNHC [36]. In contrast, all three cell types of the GCT express the antagonistic ligand OPG [15]. MNHC, which is required for the formation of MNGC, are derived from CD14- and CD34-positive (both chemokine receptors) precursor cells, which also express the chemokine receptor CXCR4. The known factor SDF1 (CXCR4-ligand) from normal osteoclastogenesis is required for chemoattraction of these cells. SDF1 (stromal-cell derived factor 1) expression in GCTSC has been proven and is believed to have an antiapoptotic effect as well [25]. The functional links in the recruitment of MNHC by GCTSC for the formation of MNGC clearly represent a broad-based principle on account of the fact that this principle also applies to the significantly rarer extra-skeletal variants of GCTs of soft tissue and other soft tissue neoplasms containing giant cells [24]. This also has relevance for other co-factors, such as TGFß1/ß2 and their receptors [21, 53].
In addition to these factors that enable the GCTSC to condition the micro-milieu for both the chemoattraction of MNHC and the formation of MNGC, there are also local autocrin/paracrin mechanisms that perform a role in maintaining the specific GCT phenotype. This has been described in terms of a RANKL expression by the MNGC component itself [19, 43], which clearly demonstrates that recruitment by RANKL-expressive GCTSC is not exclusively passive [31]. The implantation of GCTSC into SCID mice has not resulted in the inevitable induction of an osteoclastogenesis [17]. At present question is discussed of whether there is an as yet unknown common precursor cell for all of the three currently known cellular components of the GCT in which the osteoclastic phenotype occurs on an autocrin/paracrin level as a result of an aberrant RANKL expression, or whether the RANKL constitutes a downstream effector of an as yet unknown super-ordinate molecule forming part of a regulatory mechanism (but not that of the initiating signal) [31].
Differentiation level of the neoplastic stromal cells
The ability of the GCTSC to express RANKL suggests that these cells have biological qualities similar to those of osteoblasts. This supposition is supported by the gene expression of additional markers of an early osteoblastic differentiation (e.g. collagen I, bone sialoprotein, and osteonectin) and the ability to differentiate osteoblasts (Cbfa-1, osterix, osteocalcin) under cell culture conditions [16, 33,36]. A hypothesis is based on this fact that the neoplastic cells of the giant cell tumour (GCTSC) are derived histogenetically from osteoblastic cells. Evidence of FGF-R3 in the GCTSC and the ability of these cells for osteoblastic differentiation under stimulation from retinoic acid indicate that the GCTSC are located at a lower mesenchymal differentiation level [41]. It has also been revealed that the GCTSC show differentiation features of mesenchymal stem cells in the form of CD105 (SH2) and CD73 (SH3, SH4) markers, in addition to expressing markers of early osteoblastic differentiation (Thy 1.1, Stro1) [56]. In confirming the level of differentiation of GCTSC at the level of the mesenchymal stem cells, it is particularly important to have experimental confirmation of their ability to differentiate to chondrocytic and adipocytic cells under cell culture conditions [56].
Additional pathogenetic factors in GCT
The over-expression of interleukin 6 (IL6) in GCT has been seen as a further autocrin/paracrin factor connected with the formation of MNGC [13, 39]. Unlike non-neoplastic mesenchymal stem cells, gene expression analyses have proven that cJUN is activated in GCTSC and that this activation is considered to be the cause of IL6 over-expression [14, 57]. Moreover, the cJUN over-expression may also be involved in changes to the regulation of extra-cellular matrix proteins, may inhibit osteoblastic or chondroblastic differentiation of GCTSC and also perform a role as proto-oncogene in the oncogenesis of GCT [57]. The over-expressed tumour suppressor gene NME2, which occurs with variable frequency in GCT, is another gene that may be involved in the pathogenesis. The NME2 gene product is a known activating transcription factor for c-myc [38, 57]. IL6 also performs a role in regulating the resorption of giant cells in both GCT and other bone lesions containing giant cells. Depending on the dose, resorption can be reduced using IL6-neutralizing antibodies [39, 40]. Cell cycle-regulating proteins are also a focal point of interest in the search for the pathogenetic factors of GCT. The pathogenetic significance of these proteins in the development of other neoplasms has been an area of discussion for considerable time [52, 60]. An over-expression of the cell cycle regulating protein cyclin D1 was established in GCT and in the central giant cell granuloma of the jaw [20]. Of particular note in this study is the fact that there was an over-expression of cyclinD1-mRNA and an increase in the cyclinD1 protein, particularly in the nuclei of the MNGC. This was not the case in the mononuclear cells because of an increased nuclear accumulation influenced by p21 over-expression. Although cyclinD1 initiates the transfer of a cell into the cell cycle, this protein clearly works in concert with p21 and – depending on nuclear retention – proliferation can be inhibited, which concurs with the differentiation to proliferatively inactive giant cells [18].
The expression profile of the different cytokines and enzymes that play a significant part in the degradation of the extra-cellular matrix may be of significant importance in the future for assessing the biological behavior of GCT [59]. In addition to IL6, higher levels of the urokinase-type plasminogen activator (u-PA), its receptor (u-PAR) and its inhibitor (PAI-1) have been found in GCT and/or their pulmonary metastases [13]. There may also be links between the expression of matrix metallo-proteinases in GCT and biological behaviour because the expression of MMP-9 can sometimes occur and be maintained by means of an auto-stimulation mechanism in conjunction with interleukin-1ß [23].
Therapeutic approaches
At present the therapy of choice is curettage or sometimes resection of the tumour-bearing part of bone, depending on local spread. The osteoclastic character of MNGC in GCT is the basis for the therapeutic use of bisphosphonates to inhibit tumour-induced osteolysis. Bisphosphonate-induced apoptosis of MNGC has already been shown under cell culture conditions [11, 12] in which the dose-dependent effects of treatment with zolendronate were far greater than those with pamidronate [9]. There are currently no reports available on the clinical application of bisphosphonates in GCT as carried out under conditions of a clinical study. Discussions are currently being held into whether gene expression within the framework of the RANK/OPGL system may provide approaches for a specific therapy for GCT [51].
References
- 1.Atkins GJ, Haynes DR, Graves SE, Evdokiou A, Hay S, Bouralexis S, Findlay DM. Expression of osteoclast differentiation signals by stromal elements of giant cell tumors. J Bone Miner Res. 2000;15:640–649. doi: 10.1359/jbmr.2000.15.4.640. [DOI] [PubMed] [Google Scholar]
- 2.Atkins GJ, Bouralexis S, Haynes DR, Graves SE, Geary SM, Evdokiou A, Zannettino AC, Hay S, Findlay DM. Osteoprotegerin inhibits osteoclast formation and bone resorbing activity in giant cell tumors of bone. Bone. 2001;28:370–377. doi: 10.1016/S8756-3282(01)00404-5. [DOI] [PubMed] [Google Scholar]
- 3.Bardi G, Pandis N, Mandahl N, Heim S, Sfikas K, Willén H, Panagiotopoulos G, Rydholm A, Mitelman F. Chromosomal abnormalities in giant cell tumors of bone. Cancer Genet Cytogenet. 1991;57:161–167. doi: 10.1016/0165-4608(91)90147-M. [DOI] [PubMed] [Google Scholar]
- 4.Bertoni F, Present D, Sudanese A, Baldini N, Bacchini P, Campanacci M. Giant-cell tumor of bone with pulmonary metastases. Six case reports and a review of the literature. Clin Orthop Relat Res. 1988;237:275–285. [PubMed] [Google Scholar]
- 5.Bridge JA, Neff JR, Bhatia PS, Sanger WG, Murphy MD. Cytogenetic findings and biologic behavior of giant cell tumors of bone. Cancer. 1990;65:2697–2703. doi: 10.1002/1097-0142(19900615)65:12<2697::AID-CNCR2820651217>3.0.CO;2-3. [DOI] [PubMed] [Google Scholar]
- 6.Bridge JA, Neff JR, Mouron BJ. Giant cell tumor of bone. Chromosomal analysis of 48 specimens and review of the literature. Cancer Genet Cytogenet. 1992;58:2–13. doi: 10.1016/0165-4608(92)90125-R. [DOI] [PubMed] [Google Scholar]
- 7.Brien EW, Mirra JM, Kessler S, Suen M, Ho JKS, Yang WT. Benign giant cell tumor of bone with osteosarcomatous transformation (“dedifferentiated” primary malignant GCT): Report of two cases. Skeletal Radiol. 1997;26:246–255. doi: 10.1007/s002560050230. [DOI] [PubMed] [Google Scholar]
- 8.Campanacci M. Giant-cell tumor and chondrosarcoma: grading, treatment and results. Recent Results Cancer Res. 1994;999:257–261. doi: 10.1007/978-3-642-80997-2_22. [DOI] [PubMed] [Google Scholar]
- 9.Chang SS, Suratwala SJ, Jung KM, Doppelt JD, Zhang HZ, Blaine TA, Kim TW, Winchester RJ, Lee FY. Bisphosphonates may reduce recurrence in giant cell tumor by inducing apoptosis. Clin Orthop Relat Res. 2004;426:103–109. doi: 10.1097/01.blo.0000141372.54456.80. [DOI] [PubMed] [Google Scholar]
- 10.Cheng JC, Johnston JO. Giant cell tumor of bone-Prognosis and treatment of pulmonary metastases. Clin Orthop. 1997;338:205–214. doi: 10.1097/00003086-199705000-00027. [DOI] [PubMed] [Google Scholar]
- 11.Cheng YY, Huang L, Kumta SM, Lee KM, Lai FM, Tam JS. Cytochemical and ultrastructural changes in the osteoclast-like giant cells of giant cell tumor of bone following bisphosphonate administration. Ultrastruct Pathol. 2003;27:385–391. [PubMed] [Google Scholar]
- 12.Cheng YY, Huang L, Lee KM, Xu JK, Zheng MH, Kumta SM. Bisphosphonates induce apoptosis of stromal tumor cells in giant cell tumor of bone. Calcif Tissue Int. 2004;75:71–77. doi: 10.1007/s00223-004-0120-2. [DOI] [PubMed] [Google Scholar]
- 13.Gamberi G, Benassi MS, Ragazzini P, Pazzaglia L, Ponticelli F, Ferrari C, Balladelli A, Mercuri M, Gigli M, Bertoni F, Picci P. Proteases and interleukin-6 gene analysis in 92 giant cell tumors of bone. Ann Oncol. 2004;15:498–503. doi: 10.1093/annonc/mdh091. [DOI] [PubMed] [Google Scholar]
- 14.Guenther R, Krenn V, Morawietz L, Dankof A, Melcher I, Schaser KD, Kasper HU, Kuban RJ, Ungethum U, Sers C. Giant cell tumors of the bone: molecular profiling and expression analysis of Ephrin A1 receptor, Claudin 7, CD52, FGFR3 and AMFR. Pathol Res Pract. 2005;201:649–663. doi: 10.1016/j.prp.2005.07.005. [DOI] [PubMed] [Google Scholar]
- 15.Huang L, Xu J, Wood DJ, Zheng MH. Gene expression of osteoprotegerin ligand, osteoprotegerin, and receptor activator of NF-kappaB in giant cell tumor of bone: possible involvement in tumor cell-induced osteoclast-like cell formation. Am J Pathol. 2000;156:761–767. doi: 10.1016/s0002-9440(10)64942-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Huang L, Teng XY, Cheng YY, Lee KM, Kumta SM. Expression of preosteoblast markers and Cbfa-1 and Osterix gene transcripts in stromal tumour cells of giant cell tumour of bone. Bone. 2004;34:393–401. doi: 10.1016/j.bone.2003.10.013. [DOI] [PubMed] [Google Scholar]
- 17.James IE, Dodds RA, Olivera DL, Nuttall ME, Gowen M. Human osteoclastoma-derived stromal cells: correlation of the ability to form mineralized nodules in vitro with formation of bone in vivo. J Bone Miner Res. 1996;11:1453–1460. doi: 10.1002/jbmr.5650111012. [DOI] [PubMed] [Google Scholar]
- 18.Kandel R, Li SQ, Bell R, Wunder J, Ferguson P, Kauzman A, Diehl JA, Werier J. Cyclin D1 and p21 is elevated in the giant cells of giant cell tumors. J Orthop Res. 2006;24:428–437. doi: 10.1002/jor.20036. [DOI] [PubMed] [Google Scholar]
- 19.Kartsogiannis V, Zhou H, Horwood NJ, Thomas RJ, Hards DK, Quinn JM, Niforas P, Ng KW, Martin TJ, Gillespie MT. Localization of RANKL (receptor activator of NF kappa B ligand) mRNA and protein in skeletal and extraskeletal tissues. Bone. 1999;25:525–534. doi: 10.1016/S8756-3282(99)00214-8. [DOI] [PubMed] [Google Scholar]
- 20.Kauzman A, Li SQ, Bradley G, Bell RS, Wunder JS, Kandel R. Central giant cell granuloma of the jaws: assessment of cell cycle proteins. J Oral Pathol Med. 2004;33:170–176. doi: 10.1111/j.0904-2512.2004.00101.x. [DOI] [PubMed] [Google Scholar]
- 21.Kawaguchi K, Oda Y, Saito T, Yamamoto H, Takahira T, Tamiya S, Iwamoto Y, Tsuneyoshi M. Decreased expression of transforming growth factor-beta II receptor is associated with that of p27KIP1 in giant cell tumor of bone: a possible link between transforming growth factor-beta and cell cycle-related protein. Hum Pathol. 2004;35:61–68. doi: 10.1016/j.humpath.2003.07.004. [DOI] [PubMed] [Google Scholar]
- 22.Kay RM, Eckardt JJ, Seeger LL, Mirra JM, Hak DJ. Pulmonary metastasis of benign giant cell tumor of bone. Six histologically confirmed cases, including one of spontaneous regression. Clin Orthop Relat Res. 1994;302:219–230. [PubMed] [Google Scholar]
- 23.Kumta SM, Huang L, Cheng YY, Chow LT, Lee KM, Zheng MH. Expression of VEGF and MMP-9 in giant cell tumor of bone and other osteolytic lesions. Life Sci. 2003;73:1427–1436. doi: 10.1016/S0024-3205(03)00434-X. [DOI] [PubMed] [Google Scholar]
- 24.Lau YS, Sabokbar A, Gibbons CL, Giele H, Athanasou N. Phenotypic and molecular studies of giant-cell tumors of bone and soft tissue. Hum Pathol. 2005;36:945–954. doi: 10.1016/j.humpath.2005.07.005. [DOI] [PubMed] [Google Scholar]
- 25.Liao TS, Yurgelun MB, Chang SS, Zhang HZ, Murakami K, Blaine TA, Parisien MV, Kim W, Winchester RJ, Lee FY. Recruitment of osteoclast precursors by stromal cell derived factor-1 (SDF-1) in giant cell tumor of bone. J Orthop Res. 2005;23:203–209. doi: 10.1016/j.orthres.2004.06.018. [DOI] [PubMed] [Google Scholar]
- 26.Mankin HJ, Connor JF, Schiller AL, Perlmutter N, Alho A, McGuire M. Grading of bone tumors by analysis of nuclear DNA content using flow cytometry. J Bone Joint Surg Am. 1985;67:404–413. [PubMed] [Google Scholar]
- 27.Marui T, Yamamoto T, Yoshihara H, Kurosaka M, Mizuno K, Akamatsu T. De novo malignant transformation of giant cell tumor of bone. Skeletal Radiol. 2001;30:104–108. doi: 10.1007/s002560000305. [DOI] [PubMed] [Google Scholar]
- 28.Masui F, Ushigome S, Fujii K. Giant cell tumor of bone: a clinicopathologic study of prognostic factors. Pathol Int. 1998;48:723–729. doi: 10.1111/j.1440-1827.1998.tb03973.x. [DOI] [PubMed] [Google Scholar]
- 29.McComb EN, Johansson SL, Neff JR, Nelson M, Bridge JA. Chromosomal anomalies exclusive of telomeric associations in giant cell tumor of bone. Cancer Genet Cytogenet. 1996;88:163–166. doi: 10.1016/0165-4608(95)00362-2. [DOI] [PubMed] [Google Scholar]
- 30.Montero O, Salle MT, Guevara R, Olivera P, Maldonado V, Melendez-Zajgla J. Cytogenetic analysis of the mononuclear cell component of giant cell tumors of bone. Cancer Genet Cytogenet. 2003;146:170–172. doi: 10.1016/S0165-4608(03)00153-5. [DOI] [PubMed] [Google Scholar]
- 31.Morgan T, Atkins GJ, Trivett MK, Johnson SA, Kansara M, Schlicht SL, Slavin JL, Simmons P, Dickinson I, Powell G, Choong PF, Holloway AJ, Thomas DM. Molecular profiling of giant cell tumor of bone and the osteoclastic localization of ligand for receptor activator of nuclear factor {kappa}B. Am J Pathol. 2005;167:117–128. doi: 10.1016/s0002-9440(10)62959-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Mori Y, Tsuchiya H, Karita M, Nonomura A, Nojima T, Tomita K (2000) Malignant transformation of a giant cell tumor 25 years after initial treatment. Clin Orthop 185–191 [DOI] [PubMed]
- 33.Murata A, Fujita T, Kawahara N, Tsuchiya H, Tomita K. Osteoblast lineage properties in giant cell tumors of bone. J Orthop Sci. 2005;10:581–588. doi: 10.1007/s00776-005-0946-0. [DOI] [PubMed] [Google Scholar]
- 34.Murata H, Kusuzaki K, Takeshita H, Hirata M, Hashiguchi S, Ashihara T, Hirasawa Y. Cytofluorometric DNA ploidy analysis in giant cell tumor of bone: histologic and prognostic value. Cancer Lett. 1999;136:223–229. doi: 10.1016/S0304-3835(98)00325-5. [DOI] [PubMed] [Google Scholar]
- 35.Nascimento AG, Huvos AG, Marcove RC. Primary malignant giant cell tumor of bone: a study of eight cases and review of the literature. Cancer. 1979;44:1393–1402. doi: 10.1002/1097-0142(197910)44:4<1393::AID-CNCR2820440433>3.0.CO;2-Z. [DOI] [PubMed] [Google Scholar]
- 36.Nishimura M, Yuasa K, Mori K, Miyamoto N, Ito M, Tsurudome M, Nishio M, Kawano M, Komada H, Uchida A, Ito Y. Cytological properties of stromal cells derived from giant cell tumor of bone (GCTSC) which can induce osteoclast formation of human blood monocytes without cell to cell contact. J Orthop Res. 2005;23:979–987. doi: 10.1016/j.orthres.2005.01.004. [DOI] [PubMed] [Google Scholar]
- 37.Noguera R, Llombart-Bosch A, Lopez-Gines C, Carda C, Fernandes CI. Giant-cell tumor of bone, stage II, displaying translocation t(12;19)(q13;q13) Virchows Arch A Pathol Anat Histopathol. 1989;415:377–382. doi: 10.1007/BF00718640. [DOI] [PubMed] [Google Scholar]
- 38.Oda Y, Walter H, Radig K, Rose I, Neumann W, Roessner A. Immunohistochemical analysis of nm23 protein expression in malignant bone tumors. J Cancer Res Clin Oncol. 1995;121:667–673. doi: 10.1007/BF01218525. [DOI] [PubMed] [Google Scholar]
- 39.Ohsaki Y, Takahashi S, Scarcez T, Demulder A, Nishihara T, Williams R, Roodman GD. Evidence for an autocrine/paracrine role for interleukin-6 in bone resorption by giant cells from giant cell tumors of bone. Endocrinology. 1992;131:2229–2234. doi: 10.1210/en.131.5.2229. [DOI] [PubMed] [Google Scholar]
- 40.Riminucci M, Kuznetsov SA, Cherman N, Corsi A, Bianco P, Gehron RP. Osteoclastogenesis in fibrous dysplasia of bone: in situ and in vitro analysis of IL-6 expression. Bone. 2003;33:434–442. doi: 10.1016/S8756-3282(03)00064-4. [DOI] [PubMed] [Google Scholar]
- 41.Robinson D, Segal M, Nevo Z. Giant cell tumor of bone the role of fibroblast growth factor 3 positive mesenchymal stem cells in its pathogenesis. Pathobiology. 2002;70:333–342. doi: 10.1159/000071273. [DOI] [PubMed] [Google Scholar]
- 42.Rock MG, Sim FH, Unni KK, Witrak GA, Frassica FJ, Schray MF, Beabout JW, Dahlin DC. Secondary malignant giant-cell tumor of bone clinicopathological assessment of nineteen patients. J Bone Joint Surg Am. 1986;68:1073–1079. [PubMed] [Google Scholar]
- 43.Roux S, Amazit L, Meduri G, Guiochon-Mantel A, Milgrom E, Mariette X. RANK (receptor activator of nuclear factor kappa B) and RANK ligand are expressed in giant cell tumors of bone. Am J Clin Pathol. 2002;117:210–216. doi: 10.1309/BPET-F2PE-P2BD-J3P3. [DOI] [PubMed] [Google Scholar]
- 44.Sara AS, Ayala AG, Naggar A, Ro JY, Raymond AK, Murray JA. Giant cell tumor of bone a clinicopathologic and DNA flow cytometric analysis. Cancer. 1990;66:2186–2190. doi: 10.1002/1097-0142(19901115)66:10<2186::AID-CNCR2820661024>3.0.CO;2-Y. [DOI] [PubMed] [Google Scholar]
- 45.Sawyer JR, Goosen LS, Binz RL, Swanson CM, Nicholas RW. Evidence for telomeric fusions as a mechanism for recurring structural aberrations of chromosome 11 in giant cell tumor of bone. Cancer Genet Cytogenet. 2005;159:32–36. doi: 10.1016/j.cancergencyto.2004.09.001. [DOI] [PubMed] [Google Scholar]
- 46.Schwartz HS, Butler MG, Jenkins RB, Miller DA, Moses HL. Telomeric associations and consistent growth factor overexpression detected in giant cell tumor of bone. Cancer Genet Cytogenet. 1991;56:263–276. doi: 10.1016/0165-4608(91)90179-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Schwartz HS, Dahir GA, Butler MG. Telomere reduction in giant cell tumor of bone and with aging. Cancer Genet Cytogenet. 1993;71:132–138. doi: 10.1016/0165-4608(93)90018-H. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Schwartz HS, Jenkins RB, Dahl R, Dewald GW. Cytogenetic analyses in giant-cell tumors of bone. Clin Orthop. 1989;240:250–260. [PubMed] [Google Scholar]
- 49.Schwartz HS, Juliao SF, Sciadini MF, Miller LK, Butler MG. Telomerase activity and oncogenesis in giant cell tumor of bone. Cancer. 1995;75:1094–1099. doi: 10.1002/1097-0142(19950301)75:5<1094::AID-CNCR2820750507>3.0.CO;2-B. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Simonet WS, Lacey DL, Dunstan CR, Kelley M, Chang MS, Luthy R, Nguyen HQ, Wooden S, Bennett L, Boone T, Shimamoto G, DeRose M, Elliott R, Colombero A, Tan HL, Trail G, Sullivan J, Davy E, Bucay N, Renshaw-Gegg L, Hughes TM, Hill D, Pattison W, Campbell P, Sander S, Van G, Tarpley J, Derby P, Lee R, Boyle WJ. Osteoprotegerin: a novel secreted protein involved in the regulation of bone density. Cell. 1997;89:309–319. doi: 10.1016/S0092-8674(00)80209-3. [DOI] [PubMed] [Google Scholar]
- 51.Skubitz KM, Cheng EY, Clohisy DR, Thompson RC, Skubitz AP. Gene expression in giant-cell tumors. J Lab Clin Med. 2004;144:193–200. doi: 10.1016/j.lab.2004.06.005. [DOI] [PubMed] [Google Scholar]
- 52.Sunters A, McCluskey J, Grigoriadis AE. Control of cell cycle gene expression in bone development and during c-Fos-induced osteosarcoma formation. Dev Genet. 1998;22:386–397. doi: 10.1002/(SICI)1520-6408(1998)22:4<386::AID-DVG8>3.0.CO;2-2. [DOI] [PubMed] [Google Scholar]
- 53.Teot LA, O'Keefe RJ, Rosier RN, O'Connell JX, Fox EJ, Hicks DG. Extraosseous primary and recurrent giant cell tumors: Transforming growth factor-b1 and-b2 expression may explain metaplastic bone formation. Hum Pathol. 1996;27:625–632. doi: 10.1016/S0046-8177(96)90389-5. [DOI] [PubMed] [Google Scholar]
- 54.Tubbs WS, Brown LR, Beabout JW, Rock MG, Unni KK. Benign giant-cell tumor of bone with pulmonary metastases: clinical findings and radiologic appearance of metastases in 13 cases. AJR Am J Roentgenol. 1992;158:331–334. doi: 10.2214/ajr.158.2.1729794. [DOI] [PubMed] [Google Scholar]
- 55.Turcotte RE, Wunder JS, Isler MH, Bell RS, Schachar N, Masri BA, Moreau G, Davis AM. Giant cell tumor of long bone: a Canadian Sarcoma Group study. Clin Orthop. 2002;397:248–258. doi: 10.1097/00003086-200204000-00029. [DOI] [PubMed] [Google Scholar]
- 56.Wülling M, Delling G, Kaiser E. The origin of the neoplastic stromal cell in giant cell tumor of bone. Hum Pathol. 2003;34:983–993. doi: 10.1053/S0046-8177(03)00413-1. [DOI] [PubMed] [Google Scholar]
- 57.Wülling M, Delling G, Kaiser E. Differential gene expression in stromal cells of human giant cell tumor of bone. Virchows Arch. 2004;445:621–630. doi: 10.1007/s00428-004-1113-2. [DOI] [PubMed] [Google Scholar]
- 58.Yasuda H, Shima N, Nakagawa N, Yamaguchi K, Kinosaki M, Mochizuki S, Tomoyasu A, Yano K, Goto M, Murakami A, Tsuda E, Morinaga T, Higashio K, Udagawa N, Takahashi N, Suda T. Osteoclast differentiation factor is a ligand for osteoprotegerin/osteoclastogenesis-inhibitory factor and is identical to TRANCE/RANKL. Proc Natl Acad Sci USA. 1998;95:3597–3602. doi: 10.1073/pnas.95.7.3597. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Zheng MH, Fan Y, Panicker A, Smith A, Robertson T, Wysocki S, Robbins P, Papadimitriou JM, Wood DJ. Detection of mRNAs for urokinase-type plasminogen activator, its receptor, and type 1 inhibitor in giant cell tumors of bone with in situ hybridization. Am J Pathol. 1995;147:1559–1566. [PMC free article] [PubMed] [Google Scholar]
- 60.Zhu XL, Hartwick W, Rohan T, Kandel R. Cyclin D1 gene amplification and protein expression in benign breast disease and breast carcinoma. Mod Pathol. 1998;11:1082–1088. [PubMed] [Google Scholar]