Abstract
Background
During infections, polymorphonuclear neutrophilic granulocytes (PMN) are mobilized from their bone marrow stores, travel with blood to the affected tissue, and kill invading microbes there. The signal(s) from the inflammatory site to the marrow are unknown, even though a number of humoral factors that can mobilize PMN, are well known. We have employed a standardized, non-infectious human model to elucidate relevant PMN mobilizers. Well-trained athletes performed a 60-min strenuous strength workout of leg muscles. Blood samples were drawn before, during and just after exercise, and then repeatedly during the following day. Cortisol, GH, ACTH, complement factors, high-sensitive CRP (muCRP), IL-6, G-CSF, IL-8 (CXCL8) and MIP-1β (CCL4) were measured in blood samples. PMN chemotaxins in test plasma was assessed with a micropore membrane technique.
Results
About 5 hr after the workout, blood granulocytosis peaked to about 150% of baseline. Plasma levels of GH increased significantly 30 min into and 5 min after the exercise, but no increase was recorded for the other hormones. No significant correlation was found between concentrations of stress hormones and the subjects' later occurring PMN increases above their individual baselines. Plasma G-CSF increased significantly – but within the normal range – 65 min after the workout. IL-6 increased very slightly within the normal range, and the chemokines IL-8 and MIP-1β did not increase consistently. However, we found a significant increase of hitherto non-identified PMN-chemotactic activity in plasma 35, 50, and 60 min after the exercise. No systemic complement activation was detected, and (mu)CRP was within the reference range at rest, 5 h and 23 h after the exercise. After endurance exercise, similar findings were made, except for a cortisol response, especially from non-elite runners.
Conclusion
Apparently, a multitude of humoral factors can – directly or indirectly – mobilize PMN from marrow to blood; some of the factors are, others are not known to be, chemotactic. Under different conditions, different selections of these mobilizers may be used. In the late granulocytosis after heavy, long-lasting exercise a number of factors thought capable of mimicking the granulocytosis of infectious diseases were apparently irrelevant.
Keywords: neutrophil granulocytes, stress hormones, chemotaxis, cytokines, chemokines, CRP, complement system
Background
Leukocytosis can result from increased cell traffic (mobilization) from bone marrow to blood, demargination from the blood vessel walls (e.g. after intense physical exercise), and decreased exit to tissues. We still do not have a thorough understanding of the release of blood cells from the bone marrow [1]. It has been suggested that similar or the same factors that control cell recruitment into inflamed tissues also regulate the mobilization from the bone marrow [1]. However, exceptions have been found; for example, leukocyte mobilization depends on adhesive interactions distinct from those formed during diapedesis of polymorphonuclear, neutrophilic granulocytes (PMN) to inflamed tissues [2].
PMN play a pivotal role in the defense against infections, especially bacterial infections. A large reserve of bone marrow PMN exists, which can be mobilized during infections or other types of inflammation. In the blood, about half the PMN population is freely circulating and in dynamic equilibrium with so-called marginated PMN. These latter PMN are found in the lungs, liver, spleen and bone marrow – adherent to or at least in contact with the endothelium of small blood vessels. PMN, as well as the other types of leukocytes, can be rapidly released (demarginated) by for example adrenaline or physical exercise [3].
Numerous studies have established that intensive endurance exercise (70–85% VO2max) induces a biphasic perturbation of the circulating leukocyte count [4]. Immediately post exercise, total leukocytes increase 50–100%, comprising all leukocyte types. Within 30 minutes of recovery, the lymphocyte count starts to decline to 30–60% below baseline levels, remaining low for 3 to 6 hours. However, if the exercise has been moderate, e.g. around 50% of VO2max, the lymphocyte count does not decline in the recovery period [4,5]. In contrast, the concentration of PMN, after a transient decrease the first hour after exercise, increases as cells are released from the bone marrow reserve, and stay high for several hours [6,7]. A similar pattern of leukocyte concentration changes can be seen after hard strength exercise [8].
Most studies of the immune response to exercise have concerned endurance exercise, while strength exercise has received relatively little attention. In one of the few latter reports, Nieman et al. [8] showed that a single bout of strength exercise, i.e. leg squat exercise to muscular failure, can result in increased immune cell levels in blood, similar to those observed with intense endurance exercise, despite only a moderate hormonal response. Furthermore, in a previous study, we have shown that strength exercise performed by strength-trained athletes induced PMN accumulation in damaged muscles [9]. However, the magnitude of the leukocytosis is apparently related to the intensity and duration of exercise and seems to be most pronounced after strenuous endurance exercise [10].
In the present study we have explored possible mechanisms behind the late occurring granulocytosis after an exhaustive run (preliminary experiments) and after a single bout of heavy strength exercise (main study), for the following reasons: (i) this granulocytosis may be a model of the granulocytosis of inflammatory diseases, (ii) hormones and other factors are known to mobilize PMN, but their – or other, hitherto unknown or unsuspected factors' – roles have not been fully characterized in a physiological setting [3]. Animal experiments suggest that signals from inflamed tissues are humoral and not nervous [11]. Some of these humoral factors are adrenaline (epinephrine), noradrenaline (norepinephrine), growth hormone (GH) and cortisol [4,12], as well as plasma granulocyte colony-stimulating factor (G-CSF) and interleukin-6 (IL-6) [7]. We figured that comparisons between the time profiles of blood concentrations of leukocytes on the one hand and suggested signal substances on the other might reveal which signals be the important mobilizers in either or both endurance and strength exercises. In particular, we hypothesized that correlations between increased plasma concentrations of putative regulators and the subsequent PMN response to exercise might give important cues.
Results
Endurance exercise study
Baseline values for all leukocytes and for sub-types neutrophils, lymphocytes and monocytes were all within the normal range for both athletes and controls, but the neutrophil concentration was significantly higher for the athletes than for the controls (4.8 ± 0.8 vs. 3.6 ± 0.9 · 109/l).
Both experimental groups had increased total leukocyte counts after the 1.0–1.5 h run, statistically significant only for the control group just after the exercise. There was also a difference between the groups after 3 h (p < 0.01), with the control group having the greater rise in leukocytes, averages compared with baseline values being 211% and 131%, respectively (Table 1). Rather than the early leukocytosis, due to demargination of leukocytes, the aim of this study was the late leukocytosis of exercise – as reflected by the 3-hour values – and its regulation.
Table 1.
Blood leukocyte subset responses to the 1–1.5 hour long run
| Blood sampling | Subjects | Total leukocytes. | Neutrophil Granuloc. | Band neutrophils | Lymphoc. | Monocytes |
| Before | Athletes1 | 7.1 ± 1.1 | 4.8 ± 0.8 | 0.06 ± 0.05 | 1.3 ± 0.5 | 0.6 ± 0.2 |
| Control2 | 6.0 ± 1.2 | 3.6 ± 0.9# | 0.09 ± 0.05 | 1.2 ± 0.3 | 0.7 ± 0.3 | |
| Just after | Athletes | 8.5 ± 1.6 | 5.7 ± 1.0 | 0.09 ± 0.06 | 1.7 ± 0.8 | 0.8 ± 0.3 |
| Control | 9.9 ± 3.8* | 7.1 ± 2.8* | 0.21 ± 0.20 | 1.4 ± 0.7 | 0.8 ± 0.4 | |
| 3 h after | Athletes | 9.2 ± 1.7* | 6.7 ± 1.3* | 0.15 ± 0.09 | 1.3 ± 0.4 | 0.8 ± 0.2 |
| Control | 12.2 ± 2.8* | 8.7 ± 2.3* | 0.63 ± 0.2*# | 1.3 ± 0.2 | 1.2 ± 0.3*# |
The values given are means ± SD in 109 cells/l. * = different from Pre, p < 0.05, # = difference between groups, p < 0.05; 1n = 7, 2n = 8.
The 3-hour neutrophil blood concentration was significantly raised (p < 0.05), the increase again being larger among the controls than among the athletes (258% vs. 142% of baselines; p < 0.01). Band neutrophils had then increased to 700% of baseline (p < 0.01) for the controls and 250% of baseline (p = 0.055) for the athletes, with a significant difference (p < 0.01) between the two groups (Table 1). Monocyte concentration also increased to 171% of baseline 3 h after the run in the control group (p < 0.05), but not detectably for the athletes. No significant changes in the concentration of lymphocytes were measured shortly or 3 h after the run.
Blood cortisol increased during the run to an average of 167% of baseline for the controls, 139% for the athletes, the rise being statistically significant (p < 0.05) only for the former. Three hours after the run the cortisol concentrations in both groups had fallen below baseline levels (70% of baseline for both groups taken together; Table 2).
Table 2.
Changes in hormones and inflammatory mediators before, just after and 3 h after completion of the 1–1.5 hour long run
| Blood sampling | Subjects | Cortisol (nmol.l-1) | GH (mIU.l-1) | IL-1β (pg. ml-1) | G-CSF (pg.ml-1) | CRP (mg.l-1) |
| Before | Athletes1 | 290 ± 98 | 4.5 ± 10.3 | 6.5 ± 2.2 | 118 ± 39 | 0.7 ± 1.9 |
| Control2 | 470 ± 221 | 1.5 ± 2.2 | 7.4 ± 1.4 | 103 ± 29 | 2.6 ± 2.7 | |
| Just after | Athletes | 395 ± 192 | 16.9 ± 7.0* | 6.1 ± 1.6 | 120 ± 31 | 1.4 ± 3.8 |
| Control | 663 ± 132* | 35.7 ± 14.0** | 7.3 ± 2.0 | 119 ± 51 | 5.1 ± 3.6 | |
| 3 h after | Athletes | 186 ± 153 | 3.1 ± 6.4 | 6.9 ± 2.2 | 125 ± 45 | 0.0 ± 0.0 |
| Control | 366 ± 258 | 2.5 ± 5.7 | 8.3 ± 3.0 | 100 ± 23 | 4.9 ± 2.2* |
Values are means ± SD. * = p < 0.05; ** = p < 0.01, compared with baseline group value. 1n = 7 2n = 8
Both groups had a significant growth hormone (GH) increase immediately after running (from 4.5 ± 10.3 mIU.l-1 at baseline to 16.9 ± 7.0 mIU.l-1 for the athletes and from 1.5 ± 2.2 mIU.l-1 to 37.5 ± 14.0 mIU.l-1 for the controls; Table 2), the levels declining to approximately resting values 3 h later. Immediately after running, the control group had a significantly higher GH level than the athletes (p = 0.02). IL-1β and G-CSF did not vary detectably, nor were there any significant differences between the groups (Table 2). At rest, CRP values were low (Table 2), indicating absence of inflammations and infections (reference values: < 10 mg.l-1). The controls had an increase – though within the reference range – both immediately and 3 h after the exercise burst, only the latter being statistically significant (p < 0.05), however. The athletes' values were apparently unchanged from before to 0 and 3 h after the run, and in the last blood samples significantly lower than in the controls (p < 0.01).
The strength exercise study
At the first test workout (TW1) the subjects lifted (mean ± SE) a total weight of 4495 ± 211 kg and at TW2 a total of 4922 ± 270 kg (warm up excluded).
Baseline values of total blood leukocytes (5–6 · 10 9/l), neutrophils (3–4 · 10 9/l), lymphocytes (~2 · 10 9/l) and mixed cells (i.e. monocytes, eosinophils and basophils) (~0.5 · 10 9/l) for both groups before TW1 and TW2 were all within normal ranges. There was no significant difference between the groups. In general, the blood leukocyte responses for both groups at TW1 and TW2 (after 2 weeks of heavy strength training for the heavy training (HT) group) were similar, but with a few exceptions: A significant reduction in lymphocytes 0–5 h and an increase in mixed cells 5 h after exercise, as detailed below.
Total leukocytes in both groups were increased 25–43% compared with baseline 30 min into TW1 and TW2, and 18–20% 5 hr after the exercise bouts (p < 0.05). The concentrations were not significantly different from pre-exercise levels 5–65 min and 23 h after the bouts. Moreover, there were no significant differences between TW1 and TW2 concerning the total leukocyte time profiles.
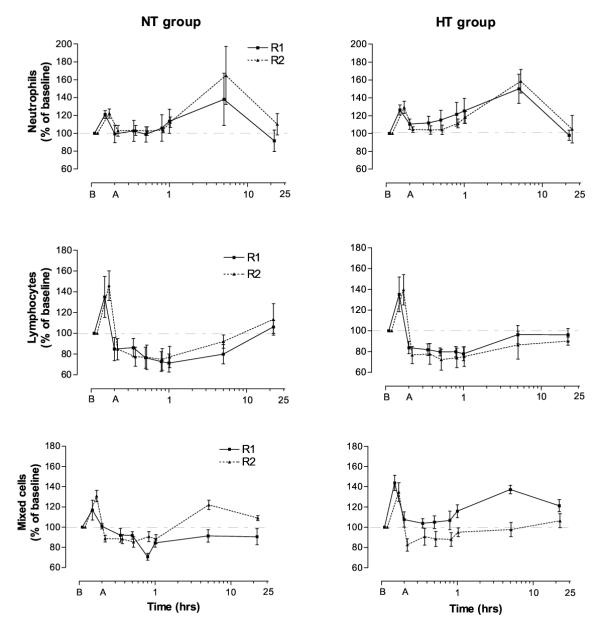
Neutrophil concentrations in both groups (for TW1 and TW2) were increased 25–26% compared with baseline 30 min into the exercise bouts. The concentrations in general rapidly approached baseline after the end of the exercise, except for the HT group which showed a significant neutrophilia (p < 0.05) 5–65 min after TW2. Blood neutrophils then increased 48–63% above baseline in both groups, for TW1 and TW2 5 h after the exercise, before returning to pre-exercise levels at 23 h. No significant differences in neutrophil counts between TW1 and TW2 were observed, nor between the HT and normal training (NT) groups (Fig. 1).
Figure 1.
Exercise leukocytosis. Blood leukocyte subset responses to single bouts of strength exercise before (R1 = TW1) and after (R2 = TW2) a 2-week period of high volume strength training for the HT (heavy training) and NT (normal training) groups. Values are means ± SE. Note the logarithmic time axes; time B = before and A = 5 min after exercise.
Lymphocytes in both groups (for TW1 and TW2), after the expected rise during exercise, fell 15–27% below pre-exercise levels 5–65 min after the bout (p < 0.01; Fig. 1). After two weeks of heavy training the HT group showed an increased lymphocytopenia (p = 0.01 – 0.03) 0–5 h after TW2, as compared with TW1. For TW2, blood lymphocytes in the HT group were still below pre-exercise values at 23 h (p = 0.03; Fig. 1).
Mixed cell concentration for both groups also increased 30 min into the workouts (p = 0.01 – 0.04; non-significant for the NT group in TW1). The mixed cell concentration ran a variable course after exercise (Fig. 1). Five hours after TW1 the mixed cell levels had increased 37% above baseline (p = 0.001) for the HT group, which was significantly higher than after the heavy training period (TW2) (p = 0.008; Fig. 1). In further experiments, we observed a 47% increase in monocytes 5 h after a similar test workout for the NT group by using differential counting (p < 0.01; n = 6).
Plasma levels of GH for both the NT and HT groups increased significantly 30 min into and 5 min after TW1 and TW2 (Table 3). In contrast to the increased cortisol levels observed after the endurance exercise, blood cortisol in general tended to be reduced to below pre-exercise levels 30 min into and 5 min after the strength workouts (Table 3). However, when the normal diurnal rhythm of reduced secretion of cortisol throughout the day was taken into account, the reduction was probably not due to the exercise per se. Plasma levels of ACTH were apparently unchanged 30 min into and 5 min after strength workouts, compared with pre-exercise values (Table 3). However, after the heavy training we observed higher ACTH levels during TW2 than TW1, 30 min after start of exercise (p < 0.05; Table 3).
Table 3.
Acute hormonal responses to single bouts of 6 RM strength exercise
| Blood sampling | Subjects | Cortisol (nmol.l-1) | GH (mIU.l-1) | ACTH (ng.l-1) |
| HT group | ||||
| Pre (08.00 h) | TW1 | 513 ± 48 | 0.6 ± 0.2 | 45.7 ± 10.2 |
| TW2 | 527 ± 34 | 0.6 ± 0.2 | 46.2 ± 10.5 | |
| NT group | ||||
| TW1 | 441 ± 56 | 0.4 ± 0.1 | 42.6 ± 10.7 | |
| TW2 | 484 ± 39 | 0.5 ± 0.1 | 42.0 ± 7.0 | |
| HT group | ||||
| Mid (09.30 h) | TW1 | 428 ± 42 | 10.8 ± 5.1* | 41.6 ± 6.2 |
| TW2 | 492 ± 35 | 8.0 ± 3.8* | 55.1 ± 7.4# | |
| NT group | ||||
| TW1 | 383 ± 45 | 1.5 ± 0.6 | 50.7 ± 12.0 | |
| TW2 | 381 ± 26* | 11.5 ± 3.5*# | 50.6 ± 15.1 | |
| HT group | ||||
| 5 m post (10.05 h) | TW1 | 420 ± 32* | 7.4 ± 1.8* | 36.8 ± 4.5 |
| TW2 | 473 ± 47 | 7.4 ± 2.9* | 35.8 ± 4.3 | |
| NT group | ||||
| TW1 | 410 ± 45 | 9.3 ± 3.2* | 42.3 ± 7.5 | |
| TW2 | 318 ± 30* | 12.0 ± 1.3* | 38.3 ± 7.4 |
TW1 (before) and TW2 (after) a 2-week period of high volume strength training for the HT group and normal strength training (NT group). * = different from baseline, p < 0.05, # = different from TW1, p < 0.05. Values are means ± SE.
A positive correlation was observed between individual increases (% of baseline) in GH during exercise and neutrophils 5 min after exercise (Table 4). An inconsistent and statistically non-significant negative correlation was found between the individual changes (% of baseline) in cortisol immediately after exercise and lymphocyte levels 65 min later (TW1, r = -0.506; p = 0.054; TW2, r = - 0.147; p = 0.573) (Table 4). No correlations could be found associating individual hormone levels to the later occurring neutrophilia (Table 4).
Table 4.
Correlations between individual serum levels of stress hormones and leukocyte deviations (% changes from baseline)
| Hormone | Leukocyte | Time after exerc.(h) (hormones/leukoc.) | Correlation coeff. (r) | P value |
| Cortisol | Neutrophils | 0/0 – TW1 | -0.49 | 0.06 |
| 0/0 – TW2 | -0.32 | 0.21 | ||
| Lymphocytes | 0/1 – TW1 | -0.51 | 0.05 | |
| Mixed cells | 0/5 – TW1 | -0.31 | 0.26 | |
| 0/5 – TW2 | 0.31 | 0.25 | ||
| GH | Neutrophils | During/0 – TW1 | 0.56 | 0.025 |
| During/0 – TW2 | 0.76 | 0.001 |
The table shows correlations for the HT and NT groups taken together at various times after TW1 and TW2. All other correlations (e.g. cortisol at 5 min vs. lymphocytes 65 min after, r = -0.15; p = 0.57) were <0.3 or >-0.3.
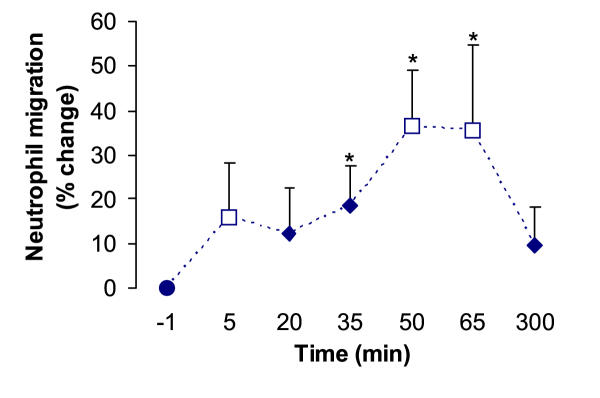
Blood neutrophil migration increased when exposed to chemotactic substances in blood (i.e. 20 % plasma) drawn 35, 50 and 65 min after strength exercise, in comparison with pre-exercise plasma (p < 0.001; Fig. 2). The migration was not significantly different from pre-exercise levels at 5 min, 20 min or 5 h after the bouts.
Figure 2.
Neutrophil migration stimulated by pre- and post-exercise plasma. The responses are shown as percentage change from chemotaxis obtained with pre-exercise plasma in two strength exercise experiments, TW1 (open squares, n = 9) and TW2 (filled diamonds, n = 10). Six migration chambers were used to assay chemotaxis in each batch of plasma. Values are means ± SE. * P < 0.05.
Positive correlations between individual changes in chemotactic activity (peak neutrophil migration) and blood neutrophil concentrations (% change from baseline) 5 h after exercise were found after both TW1 (r = 0.630, p = 0.13, n = 7 subjects) and TW2 (r = 0.655, p = 0.056, n = 9 subjects). The failing statistical significance in these experiments may have been due to the few subjects tested (type II statistical error).
Plasma levels of IL-6 increased slightly – but within the normal range – for all six subjects at 5 and 35 min after exercise and for five of the six subjects at 50 and 65 min. Measured as optical density of the ELISA assay, they increased 25–30% above baseline levels, but the changes in absolute values were small and ranged from 0.72 to1.04 pg. ml-1. Plasma levels of G-CSF increased significantly from 11.4 ± 2.7 pg.ml-1 at baseline to 17.2 ± 4.6 pg. ml-1 65 min after the workout (p < 0.05). The increases were again within the normal range, occurred in five of six subjects, and were not significantly different from baseline at any other time point. In contrast to the endurance exercise study, G-CSF levels were here measured with high sensitivity ELISA kits. We found no consistent increases in IL-8 or MIP-1β plasma concentrations. Positive, but statistically non-significant, correlations were observed between blood neutrophil concentrations 5 h after exercise and the increase in G-CSF (r = 0.55, p > 0.26, n = 6) and IL-6 (r = 0.71, p = 0.11, n = 6).
The CRP values were below the upper limit for the reference values at rest, 5 h and 23 h after a bout of heavy strength exercise (TW1), except for one subject's values (that were elevated at all time points, probably due to a slight infection the days before the exercise).
Complement activation was assessed by measuring C1inhibitor-C1rs complexes, C4bc, C3bBbP, C3bc and TCC to detect activation of the classical, alternative, final common and terminal pathways, respectively. We found no evidence of systemic complement activation being involved in the mobilization of neutrophils from the bone marrow, hours after heavy strength exercise.
Discussion
In our endurance experiments both athletes and non-trained controls showed the well-known, late occurring leukocytosis, due to granulocytosis with release of band neutrophils, but the response was larger among the untrained runners. The latter also had a slight monocytosis 3 h after the test run. The same pattern was seen after the strength workout, where both blood granulocytes and monocytes at 5 hr had increased to about 150% of baseline concentration, before returning to pre-exercise levels at 23 h. The increase in the percentage of immature band (nonsegmented) neutrophils (a shift to the left), in connection with the delayed onset neutrophilia, provides evidence for the release of neutrophils from the bone marrow.
In the present study we failed to find a statistically significant relationship between the delayed-onset neutrophilia and any of the possible mediators measured. However, we discovered a significant increase of (hitherto non-identified) granulocyte chemotaxins in plasma 30–60 min after the strength exercise bout. The individual increases in this chemotactic activity (assessed as peak neutrophil migration) correlated positively with the late neutrophilia 5 h after both strength workouts. The borderline statistical significance in these experiments may have been due to the number of subjects tested (type II statistical error); more experiments have to be done. Moreover, we shall try to identify the chemotaxin(s), since none of the mediators discussed below are prime suspects as relevant chemotactic factors in our assay.
We found that plasma G-CSF increased significantly – but within the normal range – 65 min after the workout. IL-6 increased very slightly within the normal range, whereas the chemokines IL-8 (CXCL8) and MIP-1β (CCL4) did not increase consistently – nor did IL-1β after the endurance exercise. Possibly, synergism between G-CSF and IL-6 (and enhanced (nor)adrenaline levels during the exercise) may explain at least part of the PMN mobilization and monocytosis. Infusions of G-CSF [13], IL-6 [14] and epinephrine [15] have all been reported to increase blood levels of granulocytes and monocytes. Furthermore, IL-6 can prime hematopoietic [16] and hemic cells [17] for a strengthened response to other cytokines. Our findings are also in accordance with other studies, showing that IL-6 treatment mobilizes neutrophils from the marginated pool into the circulation after 2–6 h [18]. In addition, animal experiments have shown that both G-CSF [19] and IL-6 [18] plays a role in the marrow release of neutrophil lineage cells and in shortening the neutrophil transit time through the bone marrow.
In a recent study, Yamada et al. [7] found a significant increase in plasma G-CSF immediately after a maximal exercise on a treadmill, which correlated positively, not only with the neutrophil counts, but also with the stab cell (band-nucleated granulocyte) counts 1 h post exercise. In addition, the increased levels of plasma IL-6 1 h post exercise correlated positively with the neutrophil counts 2 h post exercise. Our respective positive, but non-significant, correlations between blood neutrophil concentration 5 h after exercise and the slight increase in G-CSF and IL-6 are in line with these results.
Others have shown that strenuous endurance exercise provokes increased levels of several cytokines in the blood, in particular IL-1β, IL-6, IL-8, IL-10, TNF-α and MIP-1β [20]. Also, intensive eccentric strength exercise led to increased circulating levels of several cytokines (e.g. IL-6, IL-10 and M-CSF), but the response seemed smaller and occurred later after the workout, compared with strenuous endurance exercise [21]. The cytokine response therefore seems to be dependent on both duration and nature of the activity [22].
Cytokines are produced by a wide variety of cell types, exposed to a multitude of stimuli. The sources and mechanisms of systemic cytokine release after exercise are at present largely unknown. A possible exception is the IL-6 production by exercised and thereby slightly damaged muscle [20]. Adrenaline infusion also raises blood IL-6 levels [23]. Non-specific injury, as occurring during major abdominal surgery, also raises blood levels of cytokines like IL-6 and G-CSF [24].
Blood levels of cortisol and GH generally increase in response to both prolonged, intensive endurance exercise and heavy strength exercise – and more after endurance than strength exercise [8]. Cortisol has been shown to mobilize neutrophils from the bone marrow to the circulation in some studies [3], while others have indicated that corticosteroid-induced neutrophilia occurs primarily by demargination of cells from the blood vessel walls, with a minor contribution from the bone marrow [25]. Injected growth hormone, resulting in blood concentrations similar to those recorded during and after exercise, induced granulocytosis, but without affecting the blood mononuclear cells [26].
We found that plasma levels of GH were elevated midway during and 5 min after the strength exercise, while no increases were recorded for the other stress hormones, cortisol and ACTH. The GH increase during exercise correlated significantly with the blood neutrophil concentration 5 min after exercise, but no other correlations were found between each individual's hormone concentrations and their neutrophil increases above baseline value.
Conflicting results have been reported concerning cortisol's role for the late neutrophilia. Some authors claimed that there is no positive correlation between blood concentration of cortisol and the degree of second-phase granulocytosis of exercise [27,28], while others did find a positive correlation [29]. Our results from the strength exercise are in line with the former view; our results from the endurance run with the latter. In conclusion, neither cortisol alone, nor growth hormone, could explain both the delayed onset neutrophilia and the monocytosis, found after our two kinds of exercise.
It has previously been demonstrated in animal experiments that intravenously administered complement proteins (e.g. C5a, C3) can mobilize PMN from bone marrow to blood [2,30,31]. Furthermore, a significant elevation of C5a has been reported after a marathon race, presumably due to tissue damage activating the complement system [32]. High-intensity cycling also allegedly activated the complement system [33]. It should be noted, however, that care has to be taken to avoid activation during preparation of the plasma sample for analysis. On the other hand, other studies found no changes or only small changes in blood levels of complement proteins after aerobic exercise in trained and untrained persons [34,35]. We found no evidence for increased complement activation after the bout of strength exercise, measuring activation products at several points in the cascade reactions. However, this cannot totally rule out the possibility that the exercise induces local complement activation in exerted muscle, contributing to the local inflammatory response.
CRP has been claimed capable of increasing production of inflammatory cytokines from monocytes [36]. We found a rapid blood increase in CRP, in the runners. This was surprising, has to our knowledge not been reported earlier, and was probably caused by release of preformed protein from the liver. The finding must be validated in further studies. In the strength exercise study the micro-CRP (μCRP) was within the reference range at rest, 5 h and 23 h after the strength workout. Long-lasting, strenuous exercise has led to CRP increases – 16 h after a marathon run [32].
The present study showed that a 2-week period of excessive strength exercise did not alter the resting immune system – as judged by blood leukocyte levels. In general, also the exercise-induced leukocytosis was similar, before and after the training period, except for small deviations, like the HT group showing a slightly increased lymphocytopenia after the second test workout. This is in accordance with other studies suggesting that regularly performed, normal resistance exercise does not alter either the resting immune system or the exercise-induced leukocytosis [37]. Moreover, trained orienteerers had no detectably lower baseline concentrations of blood granulocytes than untrained controls. This is in contrast to findings by others [38,39]. Moreover, we found a more marked acute leukocytosis, late granulocytosis and monocytosis in non-athletes (controls) than in the well-trained subjects (possibly explainable by increases in blood cortisol and growth hormone that were larger among the non-athletes than among the orienteerers). Again, this is in contrast to the findings by others [38,40]. We suggest that differences concerning mode of exercise, in particular with respect to its duration and intensity, together with the timing of blood sampling, can explain at least some of the discrepancies mentioned above.
Conclusions
We found no convincing relationship between the late mobilization of neutrophils and any of the suggested signal substances measured, even though "the jury is still out" concerning G-CSF and IL-6. Different mechanisms, or different selections of mobilizers, may be involved in the delayed-onset neutrophilia in response to strength exercise, endurance exercise, and infections, since the (patho)physiological stress mechanisms differ considerably between these inflammation models. The most important finding of the present work may be (i) that an impressive granulocytosis may be provoked without detectable presence in blood of factors generally thought to be mobilizers of the bone marrow PMN store and (ii) the detection of chemotactic activity of unexplained origin in the participants' plasma 30–60 minutes after exercise.
Methods
Subjects
Endurance exercise protocol
Seven athletes (3 females and 4 males, age (mean ± SD) 27 ± 4 yr) from the national orienteering team and eight controls (3 females and 5 males, age 23 ± 1 yr), representing the normal population, were selected for the preliminary study. The controls had not been engaged in hard endurance training more than twice a week.
Strength exercise protocol
Seventeen male students participated in the definitive study. All subjects had performed recreational strength training for at least two years. They were randomly divided into a heavy training group (HT, age 26.4 ± 4.3 yr, n = 10) and a normal training group (NT, age 25.4 ± 3.5 yr, n = 7) after a period of 4 weeks when all of them had performed normal strength training.
The experiment complied with the current national laws, and the Regional Ethics Committee of Norway approved the protocol. Written informed consent was secured from all the participants.
Experimental designs
In the endurance exercise study, subjects ran for 1–1.5 hours. They were told to run to exhaustion and not to take breaks. Blood samples were taken before, immediately after and 3 h after the exercise bout. For practical reasons, the test runs could not be arranged at the same time of the day in both groups of participants, so the first blood sample was drawn at about Hr 11.00 for the control group and Hr 17.00 for the athletes.
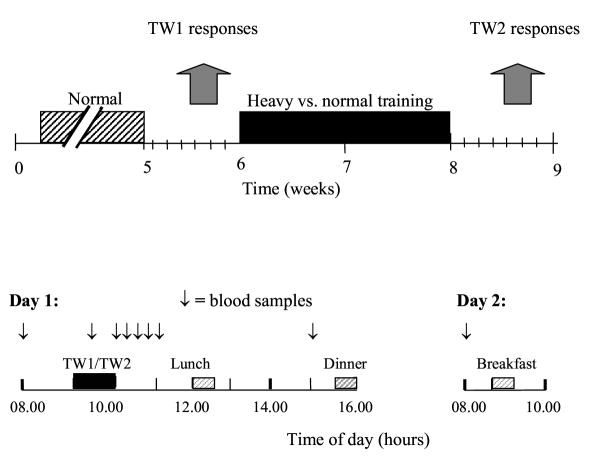
As detailed previously [41], all subjects in the strength exercise study had their first test workout after the 4 weeks of normal strength training and the second test workout after an additional 2-week period with heavy training of leg extensors for the HT group only (Fig. 1). The standardised test workouts (TW) were performed on the 4th day after both the normal (TW1) and the heavy training period (TW2).
Test workout
The strength workout consisted of squats, front-squats, and knee extensions. The subjects performed 3 sets of 6 repetitions with a load that could be lifted for a maximum of 6 repetitions (100% of 6 RM) in all three exercises (same relative intensity in TW1 and TW2). Warm-up was accomplished as detailed previously [41]. Rest between sets and exercises were 3 min, except for 8 min between squats and front squats in order to draw a blood sample. The workouts lasted 62 min. Meals were served at the same time of the day during both standardised strength workout trials. The subjects ate the same type and amount of food before and after each trial.
Blood sampling
In the endurance exercise study, blood samples (one 9-ml EDTA-tube and two 9-ml vacutainers for serum analyses) were collected just before, immediately after and 3 h after the exercise bout.
In the strength exercise study, blood samples were collected 30 minutes before the heavy strength exercise, 25 min into the workout, and 5, 20, 35, 50, 65 min and 5 and 23 h after the workout (Fig. 3). Blood was drawn from an antecubital vein into one 5-ml heparin, one 9-ml serum and two 3-ml EDTA vacutainers. Heparin and EDTA tubes were set on ice and within 30 min centrifuged at 1000 g for 10 min at 4°C. Some EDTA plasma samples for analysis of cytokines and chemokines were centrifuged once more to get rid of platelet microvesicles, 11000 g for 5 min. Serum tubes stood 30–45 min in room temperature before centrifugation. Plasma and serum were stored at -20°C until analysis.
Figure 3.
Training and test session time lines of the strength exercise study. Experimental design (upper panel): The acute haematological and immune responses to single bouts of 6 RM strength exercise was measured before (TW1) and after (TW2) a 2-week period of high volume strength training for the HT group and normal strength training for the NT group. Lower panel illustrates timing of blood samples and meals in TW1 and TW2.
Analysis of haematological and immune variables
White blood cell (WBC) counts in the endurance exercise study were performed with a coulter counter. Differential counts of 200 WBC were done on a May-Grünwald/Giemsa stained blood smear from each sample, so that absolute numbers of the white blood cell subtypes could be calculated. C-Reactive Protein (CRP) was in this preliminary study measured with "Nycocard" (Nycomed Pharma A/S, Oslo, Norway), where the amount of CRP was estimated from a colour scale. In the strength exercise study the CRP was measured by means of a high sensitivity immunoturbidimetric assay (Tina-quant®) on the Roche® Hitachi 917 automated clinical chemistry analyzer. Internal quality controls at 2.5 and 13.9 mg· l-1 had an analytical CV (day-to-day variation) of 2.5 and 3.0%, respectively (courtesy of Dr. Lars Mørkrid, Rikshospitalet, Oslo). The effect of haemoconcentration, taking place during the exercise, was adjusted for by correcting all values according to baseline hematokrit (Hct) values [i.e. reported blood cell count = (measured cell no. · resting Hct)/measured Hct].
Total and differential WBC counts in the strength exercise study were performed with a Sysmex K-1000 (TOA Medical Electronics Co., Ltd., Kobe, Japan). Coefficient of variation (CV) for replicate determination was <4% for neutrophils and lymphocytes. Changes in plasma volumes were calculated from changes in the plasma total protein (TP) concentration. TP in plasma was measured with a Kodak Ektachem DT60 analyser (Eastman Kodak Company, NY, USA), CV<5%.
Hormones
Cortisol and growth hormone were analysed in serum samples, while ACTH was analysed in EDTA plasma samples. All measurements were performed at Hormone Laboratory, Aker University Hospital, Oslo, by commercially available kits, as described in detail by Raastad et al. [41]. The coefficients of intra-assay (CVintra) and inter-assay (CVinter) variation were 4% and 8% for cortisol, 10% and 13% for GH, and 7% and 8% for ACTH.
Complement factors
Enzyme immunoassays (EIA) were used for analysing complement factors in EDTA plasma. Activation of the classical complement pathway was quantified with EIAs detecting C1rs-C1inhibitor complexes (C1rs-C1inh) and C4bc, the latter also being indicative of the lectin pathway. Both methods have been described in detail elsewhere [42,43], and the antibodies used were a kind gift from Professor C.E. Hack, Amsterdam, The Netherlands. Activation of the alternative pathway was detected by quantifying the alternative convertase C3bBbP in an EIA described previously [44]. Activation of the final common pathway was recorded with an EIA using the monoclonal antibody bH6, specific for a neo-epitope exposed in C3b, iC3b and C3c, as previously described [45]. Activation of the terminal pathway was quantified with an EIA using the monoclonal antibody aE11 specific for C9 incorporated in the terminal sC5b-9 complex (TCC), as described previously [46].
Cytokine and chemokine analysis
IL-1β and G-CSF in serum collected in the preliminary study were immuno-assayed with Quantikine™ ELISA-kits, according to the instructions from the manufacturer (R&D System, Oxon, UK). Serum IL-6, G-CSF, IL-8 and MIP-1β concentrations were in the strength exercise study immuno-assayed for six subjects at TW1. A high sensitivity kit was used for analysing G-CSF.
Leukocyte chemotaxis
Leukocyte chemotactic migration was assessed with a micropore membrane technique described by Grimstad & Benestad [47]. Heparinized (20 IU.ml-1) blood was drawn from healthy laboratory personnel and separated according to a method originally described by Boyum [48], slightly modified. Briefly, leukocytes were isolated after erythrocyte aggregation and sedimentation with hydroxyethyl starch (HES) (Fresenius AG, Bad Homburg, Germany) and centrifugation of the leukocyte supernatant at 400 g for 10 min. The leukocytes were washed once and resuspended to a final cell concentration of 7 · 109/l in medium. The migration assembly is an acrylic chamber with a 140-μm thick micropore membrane (Sartorius SM 11324) with an average pore diameter of 5 μm as floor, immersed in a retrieval compartment. The cells were inoculated into the acrylic chamber, and the leukocytes that had migrated to the retrieval compartment after 2 h, in the presence of different chemoattractants or other modulators of migration, were brought into suspension by vigorous shaking and counted with a coulter counter. More than 97% of the migrated leukocytes during 2 h of incubation have been shown to be neutrophils [46]. In the present experiments we assayed chemotaxins present in 20% v/v test plasma.
Statistics
In the pre-study, arithmetic mean values with their standard deviations (SD) are used to characterize dispersion of the data. The Mann-Whitney test was applied to analyse the difference between two groups of data (two-sided test; statistical significance level 0.05). In the strength exercise study paired t-tests and when indicated Wilcoxon Signed-Ranks test were used to identify exercise induced hormonal, leukocyte, cytokine and chemotactic responses to the two test workouts. To compensate for inter-experimental variability, the non-parametric Wilcoxon-van Elteren [49] test was used to calculate the significance of differences between test and control groups of several experiments taken together. This procedure allows us to divide the sample into blocks and then combine the information from each of the blocks to arrive at a probability for the entire set of data. This test becomes identical to Wilcoxon Signed-Ranks test with n = 1+1 in all groups. We also examined selected bivariate relationships using a Pearson Product moment correlation coefficient. Tabulated data are given as means ± SE. Statistical significance was set at p ≤ 0.05.
Authors' contributions
BAR performed the chemotaxis experiments, part of the strength exercise experiment, and wrote the manuscript; TR and JH performed the strength exercise experiments; KTL supervised the complement analyses; KB and AK performed the endurance exercise experiments; EMS supervised the chemotaxis experiments and designed one of the graphs; HBB conceived of parts of the study, and participated in its design, coordination, analysis and writing. All authors read and approved the final manuscript.
Acknowledgments
Acknowledgements
Support to HBB from the Norwegian Cancer Society, the Research Council of Norway, and Anders Jahre's Foundation for the Promotion of Science is gratefully acknowledged. We also thank Inger Strøm-Gundersen for excellent technical assistance and Lars Mørkrid for the high sensitivity CRP analyses. Grethe Bergseth, Dorte Christensen and Hilde Fure assisted in performing the complement analyses.
Contributor Information
Bjørn Audun Risøy, Email: bjornaudun@hotmail.com.
Truls Raastad, Email: Truls.Raastad@nih.no.
Jostein Hallén, Email: Jostein.Hallen@nih.no.
Knut T Lappegård, Email: Knut.Lappegard@Nordlandssykehuset.no.
Kjersti Bæverfjord, Email: kjersti.baverfjord@norgespost.no.
Astrid Kravdal, Email: astrid.kravdal@sensewave.com.
Else Marie Siebke, Email: e.m.siebke@basalmed.uio.no.
Haakon B Benestad, Email: h.b.benestad@basalmed.uio.no.
References
- Opdenakker G, Fibbe WE, Van Damme J. The molecular basis of leukocytosis. Immunol Today. 1998;19:182–89. doi: 10.1016/S0167-5699(97)01243-7. [DOI] [PubMed] [Google Scholar]
- Jagels MA, Chambers JD, Arfors KE, Hugli TE. C5a- and tumor necrosis factor-alpha-induced leukocytosis occurs independently of beta 2 integrins and L-selectin: differential effects on neutrophil adhesion molecule expression in vivo. Blood. 1995;85:2900–909. [PubMed] [Google Scholar]
- Benestad HB, Laerum OD. The neutrophilic granulocyte. Curr Top Pathol. 1989;79:7–36. doi: 10.1007/978-3-642-73855-5_2. [DOI] [PubMed] [Google Scholar]
- Pedersen BK, Rohde T, Ostrowski K. Recovery of the immune system after exercise. Acta Physiol Scand. 1998;162:325–32. doi: 10.1046/j.1365-201X.1998.0325e.x. [DOI] [PubMed] [Google Scholar]
- Pedersen BK. Influence of physical activity on the cellular immune system: Mechanisms of action. Int J Sports Med. 1991. pp. 23–29. [DOI] [PubMed]
- Suzuki K, Totsuka M, Nakaji S, Yamada M, Kudoh S, Liu Q, Sugawara K, Yamaya K, Sato K. Endurance exercise causes interaction among stress hormones, cytokines, neutrophil dynamics, and muscle damage. J Appl Physiol. 1999;87:1360–67. doi: 10.1152/jappl.1999.87.4.1360. [DOI] [PubMed] [Google Scholar]
- Yamada M, Suzuki K, Kudo S, Totsuka M, Nakaji S, Sugawara K. Raised plasma G-CSF and IL-6 after exercise may play a role in neutrophil mobilization into the circulation. J Appl Pysiol. 2002;92:1789–94. doi: 10.1152/japplphysiol.00629.2001. [DOI] [PubMed] [Google Scholar]
- Nieman DC, Henson DA, Sampson CS, Herring JL, Suttles J, Conley M, Stone HM, Butterworth DE, Davis JM. The acute immune response to exhaustive resistance exercise. Int J Sports Med. 1995;16:322–328. doi: 10.1055/s-2007-973013. [DOI] [PubMed] [Google Scholar]
- Raastad T, Risøy BA, Benestad HB, Fjeld JG, Hallén J. Temporal relationship between leukocyte accumulation in muscles and halted recovery 10–20 h after strength exercise. J Appl Physiol. 2003;285:2503–2509. doi: 10.1152/japplphysiol.01064.2002. [DOI] [PubMed] [Google Scholar]
- Natale VM, Brenner IK, Moldoveanu AI, Vasiliou P, Shek P, Shephard RJ. Effects of three different types of exercise on blood leukocyte count during and following exercise. Sao Paulo Med J. 2003;121:9–14. doi: 10.1590/S1516-31802003000100003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Benestad HB, Strom-Gundersen I, Iversen OP, Haug E, Nja A. No neuronal regulation of murine bone marrow function. Blood. 1998;91:1280–7. [PubMed] [Google Scholar]
- Pedersen BK, Toft AD. Effects of exercise on lymphocytes and cytokines. Br J Sports Med. 2000;34:246–51. doi: 10.1136/bjsm.34.4.246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ulich TR, del Castillo J, McNiece IK, Yin SM, Irwin B, Busser K, Guo KZ. Acute and subacute hematologic effects of multi-colony stimulating factor in combination with granulocyte colony-stimulating factor in vivo. Blood. 1990;75:48–53. [PubMed] [Google Scholar]
- Asano S, Okano A, Ozawa K, Nakahata T, Ishibashi T, Koike K, Kimura H, Tanioka Y, Shibuya A, Hirano T. In vivo effects of recombinant human interleukin-6 in primates: stimulated production of platelets. Blood. 1990;75:1602–5. [PubMed] [Google Scholar]
- Richardson RP, Rhyne CD, Fong Y, Hesse DG, Tracey KJ, Marano MA, Lowry SF, Antonacci AC, Calvano SE. Peripheral blood leukocyte kinetics following in vivo lipopolysaccharide (LPS) administration to normal human subjects. Influence of elicited hormones and cytokines. Ann Surg. 1989;210:239–45. doi: 10.1097/00000658-198908000-00018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ikebuchi K, Wong GG, Clark SC, Ihle JN, Hirai Y, Ogawa M. Interleukin 6 enhancement of interleukin 3-dependent proliferation of multipotential hemopoietic progenitors. Proc Natl Acad Sci USA. 1987;84:9035–9. doi: 10.1073/pnas.84.24.9035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kharazmi A, Nielsen H, Rechnitzer C, Bendtzen K. Interleukin 6 primes human neutrophil and monocyte oxidative burst response. Immunol Lett. 1989;21:177–84. doi: 10.1016/0165-2478(89)90056-4. [DOI] [PubMed] [Google Scholar]
- Suwa T, Hogg JC, English D, van Eeden SF. Interleukin-6 induces demargination of intravascular neutrophils and shortens their transit in marrow. Am J Physiol Heart Circ Physiol. 2000;279:2954–60. doi: 10.1152/ajpheart.2000.279.6.H2954. [DOI] [PubMed] [Google Scholar]
- Price TH, Chatta GS, Dale DC. Effect of recombinant granulocyte colony-stimulating factor on neutrophil kinetics in normal young and elderly humans. Blood. 1996;88:335–40. [PubMed] [Google Scholar]
- Pedersen BK, Steensberg A, Fischer C, Keller C, Ostrowski K, Schjerling P. Exercise and cytokines with particular focus an muscle-derived IL-6. Exerc Immunol Rev. 2001;7:18–31. [PubMed] [Google Scholar]
- Smith LL, Anwar A, Fragen M, Rananto C, Johnson R, Holbert D. Cytokines and cell adhesion molecules associated with high-intensity eccentric exercise. Eur J Appl Physiol. 2000;82:61–67. doi: 10.1007/s004210050652. [DOI] [PubMed] [Google Scholar]
- Suzuki K, Nakaji S, Yamada M, Liu Q, Kurakake S, Okamura N, Kumae T, Umeda T, Sugawara K. Impact of a competitive marathon race on systemic cytokine and neutrophil responses. Med Sci Sports Exerc. 2003;35:348–55. doi: 10.1249/01.MSS.0000048861.57899.04. [DOI] [PubMed] [Google Scholar]
- Sondergaard SR, Ostrowski K, Ullum H, Pedersen BK. Changes in plasma concentrations of interleukin-6 and interleukin-1 receptor antagonists in response to adrenaline infusion in humans. Eur J Appl Physiol. 2000;83:95–8. doi: 10.1007/s004210000257. [DOI] [PubMed] [Google Scholar]
- Kato M, Suzuki H, Murakami M, Akama M, Matsukawa S, Hashimoto Y. Elevated plasma levels of interleukin-6, interleukin-8, and granulocyte colony-stimulating factor during and after major abdominal surgery. J Clin Anesth. 1997;9:293–8. doi: 10.1016/S0952-8180(97)00006-8. [DOI] [PubMed] [Google Scholar]
- Nakagawa M, Terashima T, D'yachkova Y, Bondy GP, Hogg JC, van Eeden SF. Glucocorticoid-induced granulocytosis – Contribution of marrow release and demargination of intravascular granulocytes. Circulation. 1998;98:2307–13. doi: 10.1161/01.cir.98.21.2307. [DOI] [PubMed] [Google Scholar]
- Kappel M, Hansen MB, Diamant M, Jorgensen JO, Gyhrs A, Pedersen BK. Effects of an acute bolus growth hormone infusion on the human immune system. Horm Metab Res. 1993;25:579–85. doi: 10.1055/s-2007-1002181. [DOI] [PubMed] [Google Scholar]
- Hansen JB, Wilsgard L, Østerud B. Biphasic changes in leukocytes induced by strenuous exercise. Eur J Appl Physiol. 1991;62:157–61. doi: 10.1007/BF00643735. [DOI] [PubMed] [Google Scholar]
- Shinkai S, Watanabe S, Asai H, Shek PN. Cortisol response to exercise and post-exercise suppression of blood lymphocyte subset counts. Int J Sports Med. 1996;17:597–603. doi: 10.1055/s-2007-972901. [DOI] [PubMed] [Google Scholar]
- McCarthy DA, Macdonald I, Grant M, Marbut M, Watling M, Nicholson S, Deeks JJ, Wade AJ, Perry JD. Studies of the immediate and delayed leukocytosis elicted by brief (30-min) strenuous exercise. Eur J Appl Physiol. 1992;64:513–517. doi: 10.1007/BF00843760. [DOI] [PubMed] [Google Scholar]
- Kubo H, Graham L, Doyle NA, Quinlan WM, Hogg JC, Doerschuk CM. Complement fragment-induced release of neutrophils from bone marrow and sequestration within pulmonary capillaries in rabbits. Blood. 1998;92:283–90. [PubMed] [Google Scholar]
- Saito H, Lai J, Rogers R, Doerschuk CM. Mechanical properties of rat bone marrow and circulating neutrophils and their responses to inflammatory mediators. Blood. 2002;99:2207–13. doi: 10.1182/blood.V99.6.2207. [DOI] [PubMed] [Google Scholar]
- Castell LM, Poortmans JR, Leclercq R, Brasseur M, Duchateau J, Newsholme EA. Some aspects of the acute phase response after a marathon race, and the effects of glutamine supplementation. Eur J Appl Physiol. 1997;75:47–53. doi: 10.1007/s004210050125. [DOI] [PubMed] [Google Scholar]
- Camus G, Duchateau J, Deby-Dupont G, Pincemail J, Deby C, Juchmes-Ferir A, Feron F, Lamy M. Anaphylatoxin C5a production during short-term submaximal dynamic exercise in man. Int J Sports Med. 1994;15:32–35. doi: 10.1055/s-2007-1021016. [DOI] [PubMed] [Google Scholar]
- Nieman DC, Tan SA, Lee JW, Berk LS. Complement and immunoglobulin levels in athletes and sedentary controls. Int J Sports Med. 1989;10:124–28. doi: 10.1055/s-2007-1024887. [DOI] [PubMed] [Google Scholar]
- Wolach B, Eliakim A, Gavrieli R, Kodesh E, Yarom Y, Schlesinger M. Aspects of leukocyte function and the complement system following aerobic exercise in young female gymnasts. Scand J Med Sci Sports. 1998;8:91–97. doi: 10.1111/j.1600-0838.1998.tb00174.x. [DOI] [PubMed] [Google Scholar]
- Ballou SP, Lozanski G. Induction of inflammatory cytokine release from cultured human monocytes by C-reactive protein. Cytokine. 1992;4:361–8. doi: 10.1016/1043-4666(92)90079-7. [DOI] [PubMed] [Google Scholar]
- Simonson SR. The immune response to resistance exercise. J Strength Cond Res. 2001;15:378–84. [PubMed] [Google Scholar]
- Ferry A, Picard F, Duvallet A, Weill B, Rieu M. Changes in blood leukocyte populations induced by acute maximal and chronic submaximal exercise. Eur J Appl Physiol. 1990;59:435–42. doi: 10.1007/BF02388625. [DOI] [PubMed] [Google Scholar]
- Nieman DC, Buckley KS, Henson DA, Warren BJ, Suttles J, Ahle JC, Simandle S, Fagoaga OR, Nehlsen-Cannarella SL. Immune function in marathon runners versus sedentary controls. Med Sci Sports Exerc. 1995;27:986–92. doi: 10.1249/00005768-199507000-00006. [DOI] [PubMed] [Google Scholar]
- Ndon JA, Snyder AC, Foster C, Wehrenberg WB. Effects of chronic intense exercise training on the leukocyte response to acute exercise. Int J Sports Med. 1992;13:176–82. doi: 10.1055/s-2007-1021252. [DOI] [PubMed] [Google Scholar]
- Raastad T, Glomsheller T, Bjoro T, Hallen J. Recovery of skeletal muscle contractility and hormonal responses to strength exercise after two weeks of high-volume strength training. Scand J Med Sci Sports. 2003;13:159–68. doi: 10.1034/j.1600-0838.2003.00297.x. [DOI] [PubMed] [Google Scholar]
- Fure H, Nielsen EW, Hack CE, Mollnes TE. A neoepitope-based enzyme immunoassay for quantification of C1-inhibitor in complex with C1r and C1s. Scand J Immunol. 1997;46:553–7. doi: 10.1046/j.1365-3083.1997.d01-168.x. [DOI] [PubMed] [Google Scholar]
- Wolbink GJ, Bollen J, Baars JW, ten Berge RJ, Swaak AJ, Paardekooper J, Hack CE. Application of a monoclonal antibody against a neoepitope on activated C4 in an ELISA for the quantification of complement activation via the classical pathway. J Immunol Methods. 1993;163:67–76. doi: 10.1016/0022-1759(93)90240-8. [DOI] [PubMed] [Google Scholar]
- Mollnes TE, Brekke O-L, Fung M, Bergseth G, Christiansen D, Fure H, Videm V, Lappegård KT, Köhl J, Lambris JD. Essential role of the C5a receptor in E. Coli-induced oxidative burst revealed by a novel lepirudin based human whole blood model of inflammation. Blood. 2002;100:1869–1877. [PubMed] [Google Scholar]
- Garred P, Mollnes TE, Lea T. Quantification in enzyme-linked immunosorbent assay of a C3 neoepitope expressed on activated human complement factor C3. Scand J Immunol. 1988;27:329–35. doi: 10.1111/j.1365-3083.1988.tb02354.x. [DOI] [PubMed] [Google Scholar]
- Mollnes TE. Analysis of in vivo complement activation, In: Herzenberg LA, Weir DM, Herzenberg L, Blackwell C, editor. in : Weir's Handbook of Experimental Immunology. Boston, Blackwell Science; 1997. pp. 78.1–78.8. [Google Scholar]
- Grimstad IA, Benestad HB. A new assay for leukocyte chemotaxis using cell retrieval, electronic particle counting and flow cytometry. J Immunol Methods. 1982;49:215–33. doi: 10.1016/0022-1759(82)90279-4. [DOI] [PubMed] [Google Scholar]
- Boyum A. Separation of leukocytes from blood and bone marrow. Scand J Clin Lab Invest. 1968;21:77–89. [PubMed] [Google Scholar]
- Van Elteren P. On the combination of independent two sample tests of Wilcoxon. Bull Inst Int Stat. 1960;37:351. [Google Scholar]