Abstract
We describe the clinical and radiological long-term outcomes of 77 primary total hip replacements in 69 patients using the fully hydroxyapatite-coated JRI (Furlong) total hip replacement. The total cases followed up were 77 hips, performed at a mean duration of 11 years and 2 months. Twelve hips could not be followed up for various reasons, which are discussed in the results section. The mean Harris hip score was 89. Seventeen acetabular cups were revised for aseptic loosening. Only one femoral stem was revised, for fracture. By Engh’s criteria there were a further two unstable cups with no symptoms, and all femoral stems were stable. Kaplan–Meier survivorship analysis revealed a survival of 98.8% for the femoral stem, 78.7% for the acetabular cup, and a combined survival of 77.8% for both components. Our findings suggest that the JRI (Furlong) hip gives a durable femoral stem implant fixation, whereas the prosthesis–bone interface achieved with the acetabular component is questionable.
Résumé
Le but de cette étude est d’évaluer les résultats à long terme d’une prothèse de type FURLONG revêtue d’hydroxy-apatite. Pour cela, les auteurs ont étudié le devenir clinique et radiologique de 77 prothèses totales de hanche implantées chez 69 patients en utilisant la prothèse de type Furlong recouverte d’hydroxy-apatite. Le temps d’implantation moyen a été de 11 ans et deux mois. Douze hanches n’ont pu être revues. Le score moyen de Harris a été de 89. 17 cupules acétabulaires ont été révisées pour décellement aseptique, une seule queue a été révisée pour fracture. Selon les critères de Engh, nous avons mis en évidence deux cupules instables, sans symptomatologie clinique. La courbe de survie selon la méthode de Kaplan Meier a montré une survie de 98,8% pour la pièce fémorale, 78,7% pour la cupule acétabulaire et 77,8% pour les deux composants. Notre étude nous permet de penser que la prothèse JRI de Furlong donne des résultats durables notamment en ce qui concerne les pièces fémorales. On peut par contre se poser quelques questions sur l’implant acétabulaire.
Introduction
Despite the success of total hip replacement in the elderly population, concerns remain about whether cemented or uncemented components are most appropriate in younger patients to limit the rate of aseptic loosening. Uncemented total hip replacements were introduced to address the problem, but some three decades later, there is limited information on their survival rates. More recently, hydroxyapatite (HA) coating has been introduced to encourage good bone growth, but the literature has so far reported mixed results. The osteoconductive role of HA coating on metallic implants is well established. Postmortem studies of retrieved total hip arthroplasty have shown direct contact between bone and implant [7].
The JRI HA-coated (Furlong) hip was introduced in 1986, and the results for the femoral component have been excellent to date. However, the results for the acetabular component have not been reported in the literature. This paper presents our results with this hip and in particular addresses the problems encountered with the acetabular component. The objective of this study was to evaluate the long-term outcome of a fully HA-coated total hip arthroplasty.
Patients and methods
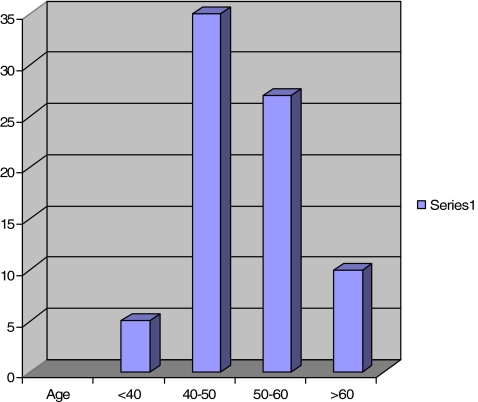
Between March 1987 and February 1996, the JRI HA total hip replacement was implanted in 89 patients between the ages of 27 and 68 years (mean age 51; Fig. 1). Nearly 50% were less than 50 years old at the time of operation. Most operations were performed by the senior author. A standard technique via a Hardinge approach was used. The bilateral cases were performed 6 months apart. All patients were regularly followed up in the clinic, all 77 hips were seen in a special clinic for this study.
Fig. 1.
Age distribution
All cases had a JRI fully HA-coated femoral stem (Furlong) with the modular stainless steel head (size 28 in all cases, acetabulum of 28 inner diameter), being either a JRI HA-coated screw fit or a CSF type (press fit with screws inserted). Out of 77 cups, six were the CSF type and the rest were the screw-fit type. All patients were mobilised partial weight bearing, the following day and were allowed to bear full weight at 6 weeks. A regular yearly review was performed in the clinic by an independent observer.
Harris hip score, data regarding further surgery, and current symptoms were obtained. X-rays were taken and details recorded using Gruen zones. Engh's criteria [5] were used to assess the stability of both components. Kaplan–Meier survivorship analysis was performed, with the end point being revision for any cause. Confidence intervals were determined using a modified Rothman equation [3].
Results
Eighty-nine operations were performed, of these 77 hips were available for review. There were four deaths and three patients lost to follow-up, and four patients had moved from the area. There were eight bilateral cases. Forty-six hip replacements were implanted in females and 31 in males, with slightly more right hips than left.
The preoperative diagnosis was mainly osteoarthritis, with four cases of dysplastic hip (5.2%), six cases of secondary osteoarthritis due to childhood conditions (7.8%), and two cases of rheumatoid arthritis (2.6%).
The mean Harris score was 89, with a range of 44–100. Only seven patients were in the “excellent” category (90–100), 38 in “good” (80–90), 20 in “average” (60–80), and the remaining 12 in “poor,” having scores less than 60.
Normal postoperative problems occurred: deep vein thrombosis in four patients, pulmonary embolism in two, superficial infection in three, and recurrent dislocations in three. In particular, nine patients had anterior knee pain after surgery, all of which settled within 1 year. There was one case of femoral stem fracture. Radiology of the femoral component showed no radiolucent lines. There was a sclerotic reaction at the tip of the femoral component in all cases, and in 67 of these it was of measurable depth (4–26 mm). There was no correlation between the amount of calcar resorption and the depth of the osteoblastic tip.
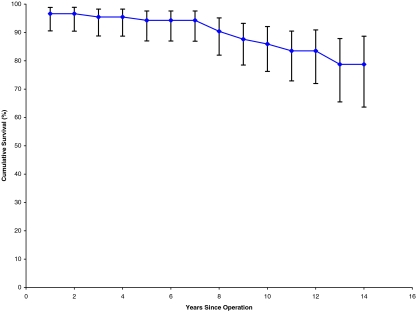
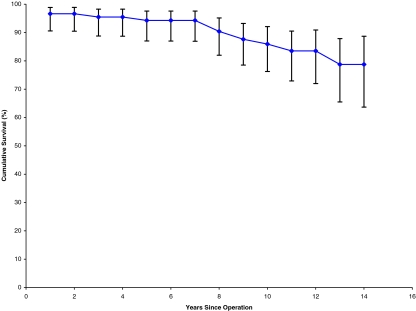
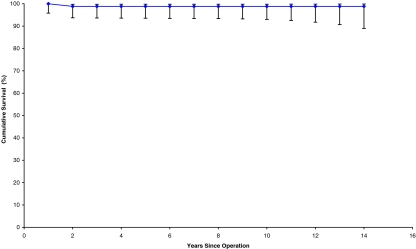
Seventeen acetabular cups were revised. The mean time to revision was 6 years and 7 months, of which the earliest was 2 months for recurrent dislocation following migration of the cup. The longest time to revision of the cup was 12 years. All of these cups were revised for aseptic loosening. We encountered no infection in our series. Out of the 17 revised cups, 16 were the threaded type and one was the CSF type. Furthermore, two cups showed evidence of aseptic loosening (osteolysis in ≥2 DeLee and Charnley zones) but had no symptoms; hence, we decided to keep them under observation until they become symptomatic, when to a Charnley cup is planned. Kaplan–Meier survivorship analysis revealed a cumulative survival of 77.8% for both components (Fig. 2), 78.7% for the acetabular cup (Table 1, Fig. 3), and 98.8% for the femoral stem (Fig. 4, Table 2).
Fig. 2.
Survival curve for both
Table 1.
Cup survival: 78.73% cumulative survival for the cup alone
| Years since operation | Number at start | Failure | Withdrawn | Number at risk | Effective number at risk | Annual failure rate (%) | Annual success rate (%) | Cumulative survival rate (%) | 95% lower bound | 95% upper bound |
|---|---|---|---|---|---|---|---|---|---|---|
| 0–1 | 89 | 3 | 0 | 89 | 89 | 0.0337 | 0.9663 | 0.9663 | 0.9055 | 0.9885 |
| 1–2 | 86 | 0 | 2 | 85 | 86.95 | 0.0000 | 1.0000 | 0.9663 | 0.9045 | 0.9886 |
| 2–3 | 84 | 1 | 2 | 83 | 85.59 | 0.0120 | 0.9880 | 0.9547 | 0.8878 | 0.9825 |
| 3–4 | 81 | 0 | 2 | 80 | 84.12 | 0.0000 | 1.0000 | 0.9547 | 0.8870 | 0.9826 |
| 4–5 | 79 | 1 | 0 | 79 | 83.05 | 0.0127 | 0.9873 | 0.9426 | 0.8703 | 0.9757 |
| 5–6 | 78 | 0 | 1 | 77.5 | 82.07 | 0.0000 | 1.0000 | 0.9426 | 0.8697 | 0.9758 |
| 6–7 | 77 | 0 | 3 | 75.5 | 81.06 | 0.0000 | 1.0000 | 0.9426 | 0.8692 | 0.9759 |
| 7–8 | 74 | 3 | 1 | 73.5 | 80.03 | 0.0408 | 0.9592 | 0.9041 | 0.8199 | 0.9513 |
| 8–9 | 70 | 2 | 10 | 65 | 78.03 | 0.0308 | 0.9692 | 0.8763 | 0.7851 | 0.9321 |
| 9–10 | 58 | 1 | 13 | 51.5 | 74.20 | 0.0194 | 0.9806 | 0.8593 | 0.7624 | 0.9207 |
| 10–11 | 44 | 1 | 17 | 35.5 | 67.51 | 0.0282 | 0.9718 | 0.8351 | 0.7290 | 0.9050 |
| 11–12 | 26 | 0 | 5 | 23.5 | 58.40 | 0.0000 | 1.0000 | 0.8351 | 0.7199 | 0.9089 |
| 12–13 | 21 | 1 | 7 | 17.5 | 49.50 | 0.0571 | 0.9429 | 0.7873 | 0.6549 | 0.8784 |
| 13–14 | 13 | 0 | 5 | 10.5 | 39.12 | 0.0000 | 1.0000 | 0.7873 | 0.6366 | 0.8867 |
| 14–15 | 8 | 1 | 4 | 6 | 28.60 | 0.1667 | 0.8333 | 0.6561 | 0.4731 | 0.8021 |
| 15–16 | 3 | 0 | 3 | 1.5 | 13.43 | 0.0000 | 1.0000 | 0.6561 | 0.3947 | 0.8481 |
Fig. 3.
Cup survival curve
Fig. 4.
Stem survival curve
Table 2.
Femoral stem survival: 98.83% cumulative survival for the stem alone
| Years since operation | Number at start | Failure | Withdrawn | Number at risk | Effective number at risk | Annual failure rate (%) | Annual success rate (%) | Cumulative survival rate (%) | 95% lower bound | 95% upper bound |
|---|---|---|---|---|---|---|---|---|---|---|
| 0–1 | 89 | 0 | 3 | 87.5 | 87.50 | 0.0000 | 1.0000 | 1.0000 | 0.9579 | 1.0000 |
| 1–2 | 86 | 1 | 1 | 85.5 | 86.49 | 0.0117 | 0.9883 | 0.9883 | 0.9372 | 0.9979 |
| 2–3 | 84 | 0 | 3 | 82.5 | 85.12 | 0.0000 | 1.0000 | 0.9883 | 0.9365 | 0.9979 |
| 3–4 | 81 | 0 | 2 | 80 | 83.78 | 0.0000 | 1.0000 | 0.9883 | 0.9358 | 0.9980 |
| 4–5 | 79 | 0 | 1 | 78.5 | 82.67 | 0.0000 | 1.0000 | 0.9883 | 0.9353 | 0.9980 |
| 5–6 | 78 | 0 | 1 | 77.5 | 81.76 | 0.0000 | 1.0000 | 0.9883 | 0.9348 | 0.9980 |
| 6–7 | 77 | 0 | 3 | 75.5 | 80.80 | 0.0000 | 1.0000 | 0.9883 | 0.9343 | 0.9980 |
| 7–8 | 74 | 0 | 4 | 72 | 79.58 | 0.0000 | 1.0000 | 0.9883 | 0.9336 | 0.9980 |
| 8–9 | 70 | 0 | 12 | 64 | 77.49 | 0.0000 | 1.0000 | 0.9883 | 0.9324 | 0.9981 |
| 9–10 | 58 | 0 | 14 | 51 | 73.66 | 0.0000 | 1.0000 | 0.9883 | 0.9301 | 0.9981 |
| 10–11 | 44 | 0 | 18 | 35 | 66.94 | 0.0000 | 1.0000 | 0.9883 | 0.9253 | 0.9983 |
| 11–12 | 26 | 0 | 5 | 23.5 | 58.00 | 0.0000 | 1.0000 | 0.9883 | 0.9175 | 0.9984 |
| 12–13 | 21 | 0 | 8 | 17 | 48.93 | 0.0000 | 1.0000 | 0.9883 | 0.9069 | 0.9986 |
| 13–14 | 13 | 0 | 5 | 10.5 | 38.79 | 0.0000 | 1.0000 | 0.9883 | 0.8897 | 0.9989 |
| 14–15 | 8 | 0 | 5 | 5.5 | 27.64 | 0.0000 | 1.0000 | 0.9883 | 0.8583 | 0.9992 |
| 15–16 | 3 | 0 | 3 | 1.5 | 13.23 | 0.0000 | 1.0000 | 0.9883 | 0.7573 | 0.9996 |
Discussion
This study confirms the encouraging reports already in the literature of the high success rate for the femoral component of the JRI HA total hip replacement and of HA-coated femoral stems in general. The single failure in this series was a fractured femoral stem, which has already been reported in the literature [14] (Figs. 5, 6).
Fig. 5.
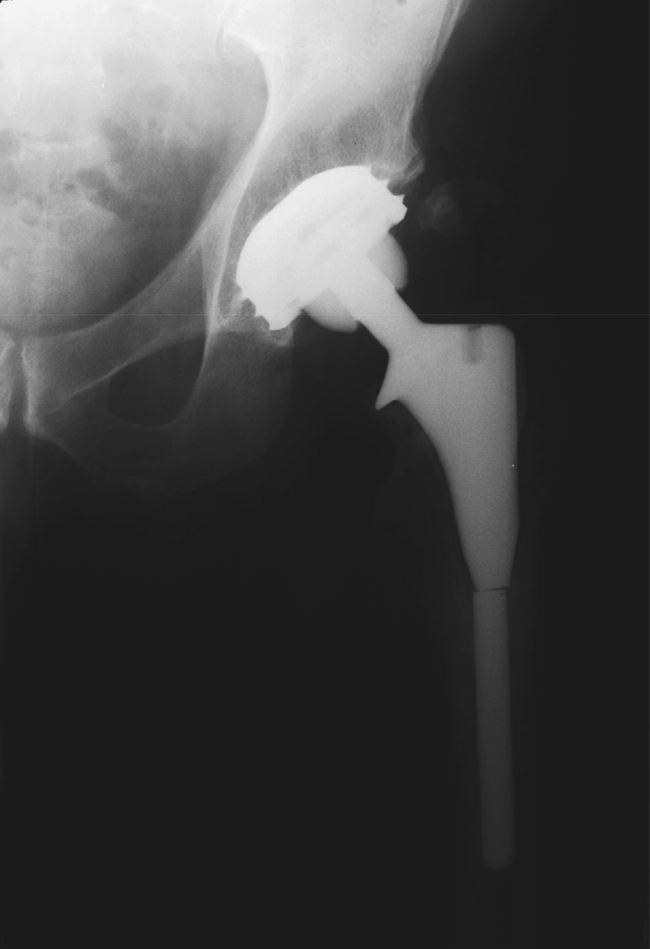
Stem fracture
Fig. 6.
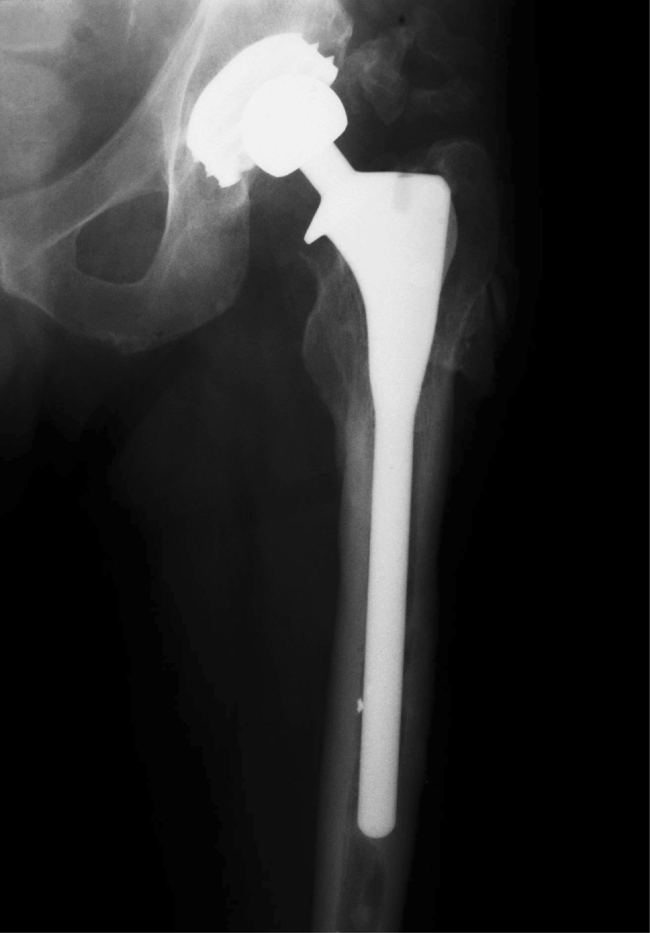
Stem revision
However, the results for the acetabular component are of more concern. Threaded acetabular cups have been used for many decades with known poor results, both with and without porous coating. It was anticipated that the addition of HA to the surface would improve survival rates, but although initial stability is obtained, it appears that secondary ingrowth of the bone into the HA does not always occur, causing midterm loosening, migration of the cup, or both.
Similar problems have occurred with press-fit cups with initial stability and then failure of secondary ingrowth of bone into the HA, causing loosening. The literature has mixed reports of the success rate of HA-coated acetabular components: a 26.2% failure rate for both components with a press-fit Osteonic cup [2], 5% cup loosening at 7 years [13], and 38% failure rate [10]. The Norwegian Arthroplasty Register [8] also noted an inferior survival rate for HA-coated acetabular cups compared with the cemented Charnley cup.
All of these studies lead us to ask what the rationale is behind the use of HA in total hip replacement. Hydroxyapatite coating was introduced in response to the failure to achieve reproducible bone ingrowths or ongrowths clinically with porous–coated implants; HA coating was added to the prosthesis in total hip replacement to achieve osteointegration. Soballe et al. [16] reported that HA coating could stimulate bone to bridge up to 2-mm gaps in canine studies. Hofmann et al. [9] confirmed the osteoconductive and bidirectional bone remodelling properties of HA but did not substantiate the claims of accelerated bone apposition rates, increased bone apposition, and gap closure [16]. This was confirmed by the postmortem total hip replacement retrieval study of Bloebaum et al. [1]. For the HA coating to stimulate osteoconduction, part of it must dissolve and release molecules containing calcium and phosphate to stimulate osteoblasts [6]. This in turn could weaken the HA implant bond strength. This theory is supported by the study of Shen et al. [15], which showed delamination of the HA from a smooth rod in a weight-bearing canine model. A similar finding was observed by David et al. [3] in a sheep model. There are many postmortem retrieval studies of HA-coated hip arthroplasty, but all of these have reported on the femoral component [7]. The exact mechanism of failure of osteointegration of the HA-coated cup remains unclear. Soballe et al. [16] have also shown that applying the HA coating to a smooth-surfaced implant increases the risk of delamination of the HA coating compared with a porous surface. Any micromovement between the implant and bone promotes formation of a fibrous layer and increases the resorption of HA. If there is poor press fit or misalignment of the cup, the micromovements may increase, leading to delamination of the HA coating, formation of a fibrous layer, and early failure. As far as the femoral stem is concerned, if there is any misalignment, a minor gap between the bone and prosthesis can be accommodated by the cylindrical femoral canal, which can resist torsional forces, unlike the hemispherical acetabulum, with little resistance to torsional forces [7].
The other question we asked in our study concerned the role of polyethylene wear. Although the radiological appearance was suggestive of polyethylene wear (Fig. 7), the retrieved cups (Figs. 8, 9) did not support this. The typical appearance was complete delamination of the HA coating with a fairly well-preserved polyethylene lining.
Fig. 7.
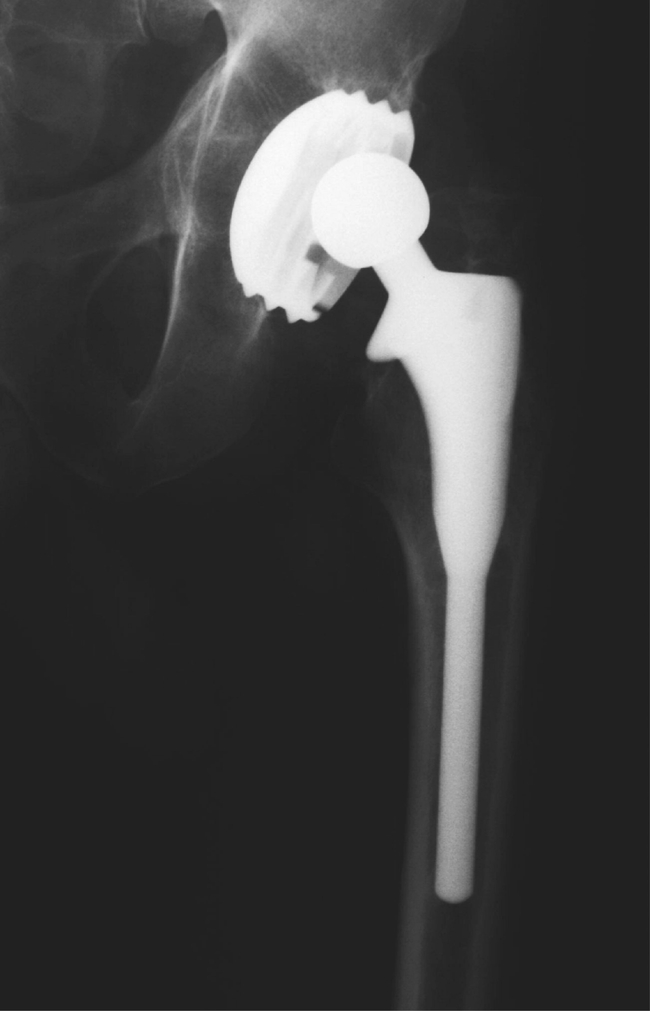
Cup showing appearance of polyethylene wear
Fig. 8.
Cup showing complete delamination
Fig. 9.
Another view of cup in Fig. 8
In contrast, the femoral stem results are excellent. Rahbek et al. [12] demonstrated in a dog study that HA-coated femoral implants formed a protective barrier that prevented the intrusion of articular wear debris into the bone implant surface. It has been postulated that because of its biocompatibility and the potential for circumferential apposition, HA-coated implants may prevent metal and polyethylene wear debris from migrating distally thereby minimising the loss of endosteal bone. Donnelly et al. [4] have proposed that HA generates spot-welding of bone to the prosthesis and that this layer in turn may impede the access of polyethylene debris to the interface. The only case revised in our series was because of metal stem fatigue fracture [14]. This is a rare complication, and if we exclude this case, the survival of femoral stems would be 100%. There was no aseptic loosening or periprosthetic fracture observed in our series, and there was no impending or potential loose stem in the radiographs. Our results are comparable to those reported by McNally et al. [11], which showed a survival rate of 99% for the femoral stem of the same prosthesis.
In summary, this paper supports the increasing evidence of high success rates for HA-coated femoral components, a fact reflected in the first annual report of the British Joint Register that showed the JRI femoral stem to be the third most frequently implanted femoral component and the most common uncemented femoral component used. However, the continued use of uncemented HA acetabular components must be questioned in view of the increasing evidence of poor midterm results.
References
- 1.Bloebaum RD, Bachus KN, Rubman MH, Dorr LD. Post-mortem comparative analysis of titanium and hydroxyapatite porous coated femoral implants retrieved from the same patient. J Arthroplasty. 1993;8:203–211. doi: 10.1016/s0883-5403(09)80014-4. [DOI] [PubMed] [Google Scholar]
- 2.Capello WN, D’Antonio JA, Manley MT, Feiberg JR. Hydroxy-apatite in total hip arthroplasty: clinical results and critical issues. Clin Orthop. 1998;355:200–211. doi: 10.1097/00003086-199810000-00021. [DOI] [PubMed] [Google Scholar]
- 3.David A, Eitenmuller J, Muher G, et al. Mechanical and histological evaluation of hydroxyapatite-coated, titanium-coated and grit-blasted surfaces under weight-bearing surfaces. Arch Orthop Trauma Surg. 1995;114:112–118. doi: 10.1007/BF00422838. [DOI] [PubMed] [Google Scholar]
- 4.Donnelly WJ, Kobayashi A, Freeman MA, et al. Radiological and survival comparison of four methods of fixation of proximal femoral stem. J Bone Joint Surg [Br] 1997;79:351–360. doi: 10.1302/0301-620X.79B3.7060. [DOI] [PubMed] [Google Scholar]
- 5.Engh CA, Bobyn JD, Glassman AH. Porous-coated hip replacement; the factors governing bone ingrowth, stress shielding and clinical results. J Bone Joint Surg [Br] 1987; 79:45–55. doi: 10.1302/0301-620X.69B1.3818732. [DOI] [PubMed] [Google Scholar]
- 6.Jaffe WL, Scott DF. Jaffe WL, Scott DF (1996) Current concept review: total hip arthroplasty with hydroxyapatite–coated prosthesis. J Bone Joint Surg. 1996; 78:1918–1934. doi: 10.2106/00004623-199612000-00018. [DOI] [PubMed] [Google Scholar]
- 7.Furlong RJ, Osborn JF. Fixation of hip prostheses by hydroxyapatite ceramic coating. J Bone Joint Surg. 1991;73:741–745. doi: 10.1302/0301-620X.73B5.1654336. [DOI] [PubMed] [Google Scholar]
- 8.Havelin LI, Espehaug B, Engesaeter LB. Havelin LI, Espehaug B, Engesaeter LB (2002) The performance of two hydroxyapatite-coated acetabular cups compared with Charnley cup. J Bone Joint Surg [Br] 2002;84:839–844. doi: 10.1302/0301-620X.84B6.12492. [DOI] [PubMed] [Google Scholar]
- 9.Hofmann AA, Bachus KN. Comparative study of human cancellous bone remodelling to titanium and hydroxyapatite coated implants. J Arthroplasty. 1993;8:157–161. doi: 10.1016/S0883-5403(06)80056-2. [DOI] [PubMed] [Google Scholar]
- 10.Kuo-An L, Wun-Jer S, Chyun-Hsiang C, Chyun-Yu Y, et al. Failure of hydroxyapatite-coated acetabular cups. Ten-year follow-up of 85 Landos Atoll. J Bone Joint Surg [Br] 2002;84:641–646. doi: 10.1302/0301-620X.84B5.12384. [DOI] [PubMed] [Google Scholar]
- 11.McNally SA, Shepperd JAN, Mann CV, Walczak JP. The results at nine to twelve years of the use of a hydroxyapatite-coated femoral stem. J Bone Joint Surg [Br] 2000;82:378–382. doi: 10.1302/0301-620X.82B3.10114. [DOI] [PubMed] [Google Scholar]
- 12.Murray DW, Carr AJ, Bulstrode C. Survival analysis of joint replacements. JBJS (Br) 1993;75-B:697–704. doi: 10.1302/0301-620X.75B5.8376423. [DOI] [PubMed] [Google Scholar]
- 13.Rahbek O, Overgaard S, Soballe S, Bunger C. Polyethylene wear, osteolysis and acetabular loosening with an HA-coated prosthesis; a follow up of 94 consecutive arthroplasties. J Bone Joint Surg [Br] 1996;81:582–589. doi: 10.1302/0301-620x.81b4.8715. [DOI] [PubMed] [Google Scholar]
- 14.Rokkum M, Brandt M, Bye K, et al. Fracture of fully hydroxyapatite-coated titanium femoral stem of a total hip replacement—a report of 3 cases. Acta Ortho Scand. 1999;75(6):768–771. doi: 10.1080/00016470410004175. [DOI] [PubMed] [Google Scholar]
- 15.Shen WJ, Chung KC, Wang GJ, McLaughlin RE. Mechanical failure of hydroxyapatite and polysulfone-coated titanium rods in a weight bearing canine model. J Arthroplasty. 1992;7:43–49. doi: 10.1016/0883-5403(92)90031-K. [DOI] [PubMed] [Google Scholar]
- 16.Soballe K, Hansen ES, Brockstedt-Rasmussen H, et al. Bone graft incorporation around titanium alloy and hydroxyapatite-coated implants in dogs. Clin Orthop. 1991;272:255–258. [PubMed] [Google Scholar]