Abstract
The purpose of this study was to evaluate the femoral component rotation in a small subset of patients who had developed arthrofibrosis after mobile-bearing total knee arthroplasty (TKA). Arthrofibrosis was defined as flexion less than 90° or a flexion contracture greater than 10° following TKA. From a consecutive cohort of 3,058 mobile-bearing TKAs, 49 (1.6%) patients were diagnosed as having arthrofibrosis, of which 38 (86%) could be recruited for clinical assessment. Femoral rotation of a control group of 38 asymptomatic TKA patients matched for age, gender, and body mass index was also evaluated. The surgical epicondylar axis was compared with the posterior condylar axis for the femoral prosthesis. Femoral components in the arthrofibrosis group were significantly internally rotated by a mean of 4.7° (SD 2.2°, range 10° internal to 1° external). In the control group, the femoral component had a mean 0.3° internal rotation (SD 2.3°, range 4° internal to 6° external). Following mobile-bearing TKA, there is a significant correlation between internal femoral component rotation and chronic arthrofibrosis.
Résumé
Le propos de cette étude est d’évaluer la rotation du composant fémoral des sujets ayant développé une raideur du genou après prothèse à plateau mobile. La raideur du genou se définit comme une flexion inférieure à 90° avec un défaut d’extension de 10°. Sur une cohorte consécutive de 3 058 prothèses du genou à plateau mobile 1,6% ont présenté une raideur. 86% de ces patients ont pu être évalués cliniquement. La rotation du composant fémoral, outre le contrôle de 38 patients évalués, de même que l’âge, le sexe, le BMI. L’axe épicondylien a été comparé à l’axe du condyle postérieur, les composants fémoraux chez les sujets présentant une raideur du genou étaient, de manière significative, une rotation interne en moyenne de 4,7° (10° de rotation interne à 1° de rotation externe). Dans le groupe contrôle, le composant fémoral ne présentait que 0,3° de rotation interne (4° interne, 6° externe). Il existe donc une corrélation significative chez ces patients après prothèse à plateau mobile du genou entre la raideur du genou et la rotation du composant fémoral.
Introduction
Arthrofibrosis results in stiffness and functional impairment following total knee arthroplasty (TKA). The prevalence of arthrofibrosis in TKA ranges from 1.3% to 6.3% [8]. The specific aetiology of arthrofibrosis has not been clearly identified, but significant factors include patient predisposition, intraoperative surgical errors, and postoperative surgical complications. Published reports cite mechanical factors, low-grade infection, heterotopic ossification, postoperative haematoma formation, complex regional pain syndrome, metal allergies, and villonodular synovitis as being related to the pathogenesis [8]. Numerous clinical studies have shown that postoperative range of motion is statistically related to preoperative range of motion and that a preoperative flexion contracture may be an important predictor of postoperative flexion [9, 14]. Common symptoms in patients who develop arthrofibrosis in TKA are reduced range of motion, swelling, and pain.
In general, surgical causes include failure to remove osteophytes or to release a contracted posterior capsule, failure to correctly balance the ligaments of the joint, failure to create symmetrical flexion and extension gaps that are equally matched, and overstuffing the joint with implants that are too large or that impinge on soft tissues [8]. Malrotation of the femoral or tibial components have been shown to be a source of chronic pain with a potential for the patient to develop arthrofibrosis. Specifically for the femoral component, malrotation may lead to patellar instability, ligamentous instability, and disturbed functional joint kinematics [5, 6, 10, 15, 17, 23].
This study retrospectively evaluated a large cohort of patients who had undergone a mobile-bearing TKA with the Low Contact Stress (LCS) prosthesis (Depuy, Warsaw, IN, USA) and postoperatively developed chronic arthrofibrosis. Kinematic studies have shown that tibial rotation following mobile total knee arthroplasty is variable and unpredictable [3]. With the mobile-bearing prosthesis, tibial component rotation is unconstrained such that precise alignment of the tibial tray is not a crucial factor for kinematic performance. We then focused on the radiographic assessment of the femoral component rotation, which was determined using helical computed tomography (CT). Earlier studies have shown the precision with which femoral rotation can be measured with CT [3–6]. The radiographic results of the arthrofibrosis group were then compared with those of a control group of patients who had undergone an identical surgical technique using the same prosthesis but who had satisfactory outcomes.
Materials and methods
A consecutive series of 3,058 mobile-bearing TKAs performed at one centre from 1988 until 1999 were retrospectively evaluated for the occurrence of arthrofibrosis. Inclusion criteria were reduced range of motion with less than 90º of maximal flexion or a flexion contracture greater than 10°. Exclusion criteria were any diagnoses of an associated intrinsic cause such as infection, heterotopic ossification, complex regional pain syndrome, or pigmented villonodular synovitis. Of the entire cohort, 49 (1.6%) patients had arthrofibrosis. The prosthesis used in this study was the LCS prosthesis (Depuy), which was originally designed in 1977 and features an articular geometry that offers very high conformity from extension to 40° of flexion. All patients in this study had a cruciate sacrificing technique, and the implant used was the rotating platform design, which has a kidney-shaped tibial insert with a central cone projection for articulation with the metal tibial tray.
The surgical procedure was standardised for all cases with initial ligament balancing followed by resection of the proximal tibia such that the surface was made perpendicular to the mechanical axis of the tibia in the coronal plane and parallel to the original tibial joint surface in the sagittal plane [7] (Fig. 1). The flexion gap was prepared using an anterior cortical reference and careful spacing to create a symmetrical and balanced flexion space (Fig 2). The distal femur was then resected to create a femorotibial alignment of 5–7° in the coronal plane. The femoral component design requires a 15° sloped cut of the distal femoral surface in the sagittal plane. The patella was resurfaced with a mobile-bearing metal-backed patella prosthesis or was left unresurfaced based on the quality of the remaining cartilage.
Fig. 1.
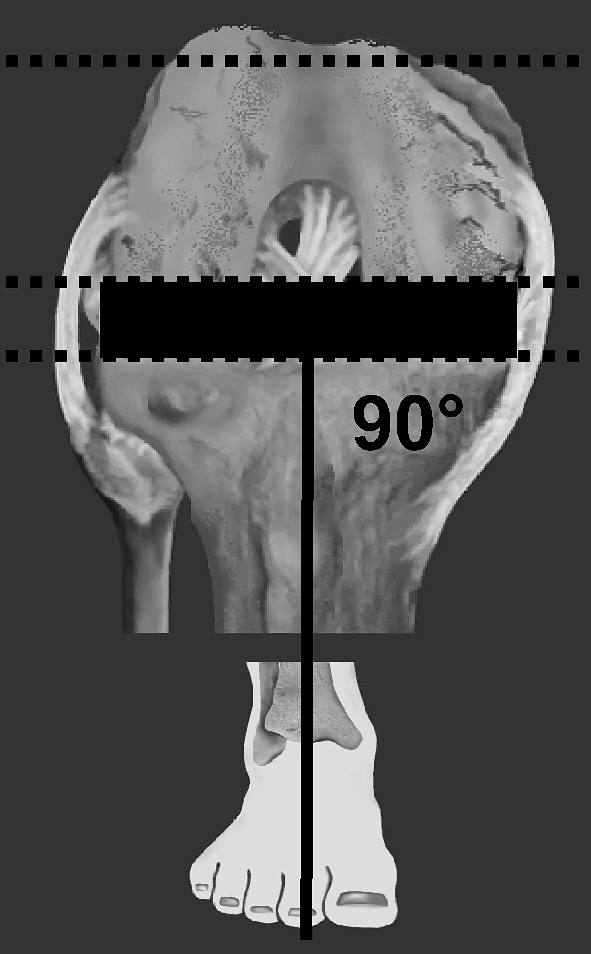
Surgical technique resects the proximal tibial surface perpendicular to the mechanical axis of the tibia and resects the posterior condyles to create a rectangular flexion space
Fig. 2.
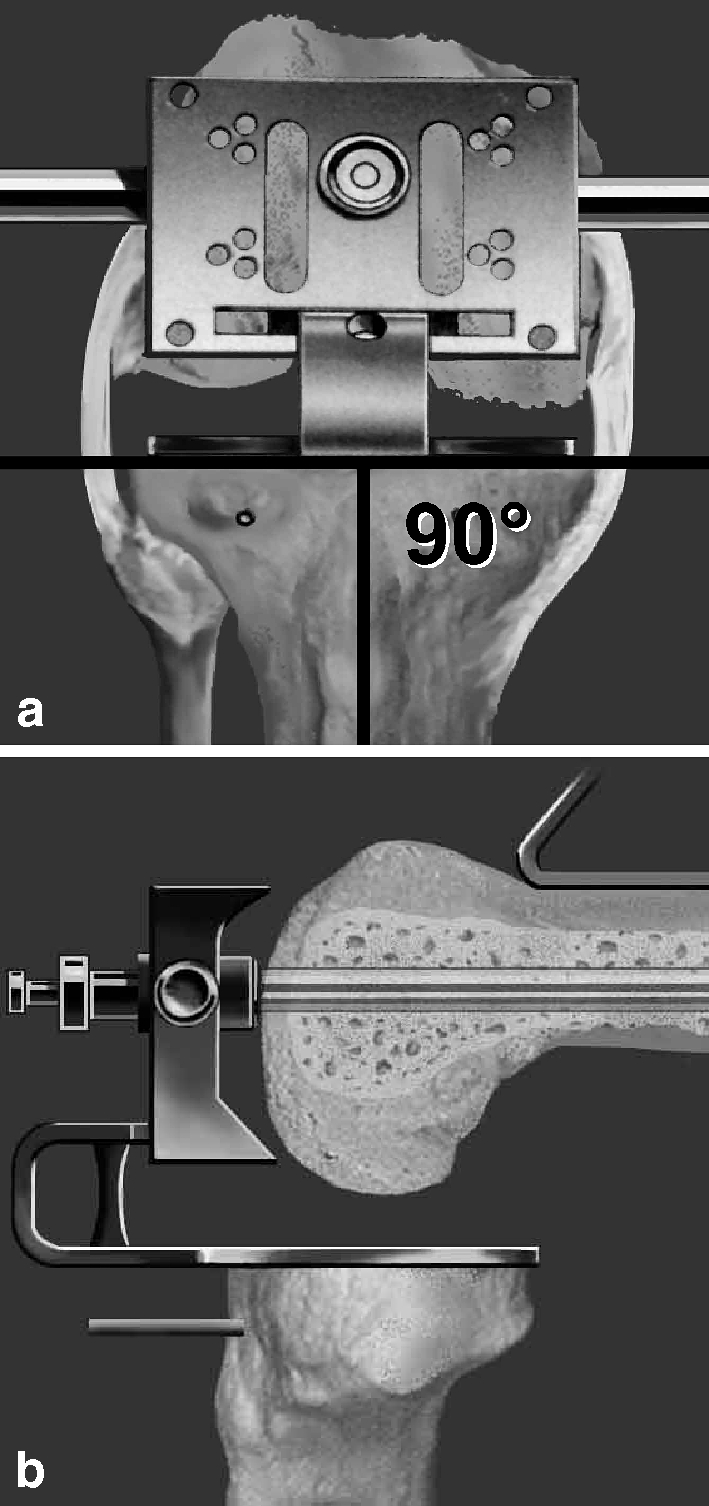
a Distal femoral posterior condylar resection is done using an intramedullary fixed resection block that is tensed with a spacer. b Sagittal plane view demonstrates position of the resection block with anterior cortical reference
Arthrofibrosis group
Of the identified group of 49 patients, five patients died and six were lost to follow-up, leaving 38 patients (86%; 15 male and 23 female) for evaluation. The mean age was 65 years (range 49–76). For patients who developed arthrofibrosis, the mean preoperative range of motion was 109º (range 75–125º). Four patients had a preoperative flexion contracture that ranged from 5º to 15º. The mean body mass index was 28.3 (range 19–43). From preoperative radiographic evaluation, the mean mechanical axis alignment was 7.5º of varus alignment with a range of 35º of varus alignment to 25º of valgus alignment. The preoperative diagnosis was ostheoarthritis in 30, rheumatoid arthritis in two, posttraumatic arthritis in three, and postpolio syndrome in three.
Control group
A control group of 38 patients, 16 male and 22 female, who had undergone the LCS mobile-bearing rotating platform TKA with the same technique and done by the same two senior surgeons were matched for age, gender, and body habitus, but they had satisfactory clinical outcomes of at least 80 points (range 80–100) on the modified HSS score. Mean age was 67 years (range 54–77). The mean preoperative range of motion in the control group was 116º (range 95–130º). The mean body mass index was 29.6 (range 20–45). Osteoarthritis was the preoperative diagnosis in 36 patients, and two patients had rheumatoid arthritis. In both the arthrofibrosis and the control group, nine patients had had prior arthroscopic knee meniscus surgery.
CT scan
Postoperative CT scans were done to determine femoral component rotation. A helical CT scanner (Siemens, Somatom, Erlangen, Germany) was used. The patient was placed supine on the CT scanning table with the involved extremity in full extension with the extremity adjusted to allow the scans to be perpendicular to the mechanical axis of the knee. Scans 2 mm in thickness were taken through the transepicondylar axis. In all patients, femoral component rotational alignment was calculated from computed tomograms evaluated by independent radiologists. Femoral component rotational alignment was determined using a single CT image through the femoral epicondyles. The surgical transepicondylar axis was a line drawn between the spike of the lateral epicondyle and the sulcus of the medial epicondyle [6] (Fig. 3). The prosthetic posterior condylar line connects the medial and lateral prosthetic posterior condylar surfaces. The angle subtended by these two lines, the prosthetic posterior condylar angle, was measured using software components. These values were then normalised for gender [3, 4]. From the studies of Berger et al. [6], the mean native rotation values for the posterior condylar angle are 0.3º (±1.2º) internal rotation for females and 3.5º (±1.2º) internal rotation for males, relative to the surgical epicondylar axis [7].
Fig. 3.
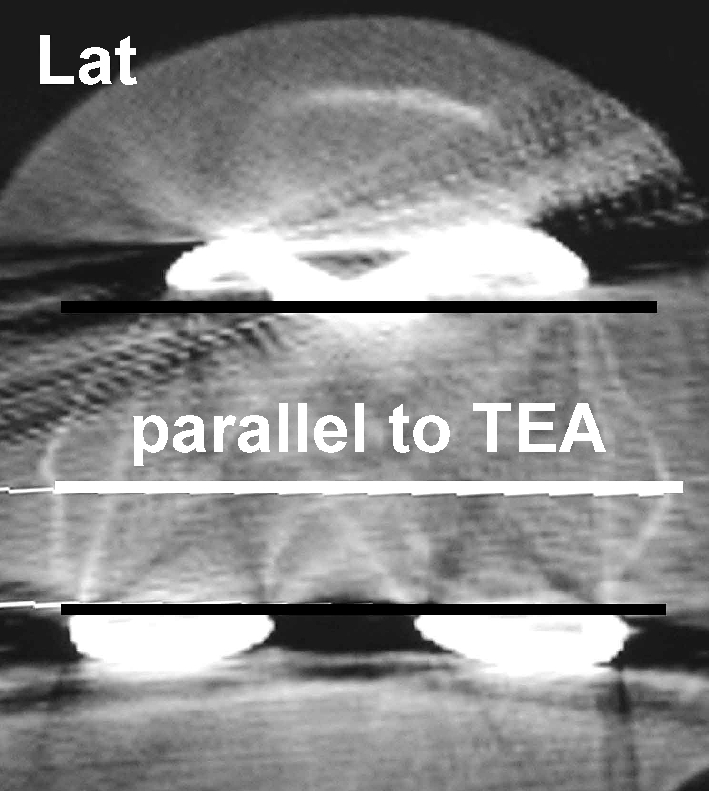
Transverse computed tomogram through the surgical transepicondylar axis allows calculation of the posterior condylar angle of the femoral total knee prosthesis
Statistical analyses
All data were analysed by an independent statistician. The distribution of angles in each group showed a normal distribution (one-sample Kolmogorov-Smirnov test). Differences between groups for the parameter of femoral rotation were examined using the unpaired Student's t-test.
Results
Clinical results
Thirty-eight cases with arthrofibrosis following mobile-bearing TKA were compared with a matched control of 38 TKAs using an identical prosthesis and surgical technique. The arthrofibrosis group had an average modified HSS score of 58 postoperatively, with a range of 12–85. The postoperative range of motion was a mean 63° with a range of 20–80°. All 38 arthrofibrosis cases underwent one or more manipulations under anesthesia (mean 1.9), 26 (71%) underwent open debridement, 15 (40%) had arthroscopic debridement, six (16%) were revised for bearing exchange, four (11%) were revised for tibial component revision, three (8%) underwent resurfacing of a primarily unresurfaced patella, three (8%) underwent repositioning of the tibial tubercle, and one (3%) had the patella removed. None of the patients had undergone femoral or tibial component revision (Table 1.) In the control group, all patients had excellent or good clinical results according to a modified HSS score, with a mean of 92.5 points and range of 80–100 points. Range of motion was a mean 115° flexion with a range of 100–135°. No patient had a significant flexion contracture.
Table 1.
Procedures done to improve range of motion in the arthrofibrosis group
| Manipulations (mean 1.9, range 1–3) | 100% |
| Open debridement | 71% |
| Arthroscopic debridement | 40% |
| Bearing exchange | 16% |
| Tibial component revision | 11% |
| Secondary patella resurfacing | 8% |
| Alteration of tibial tubercle | 8% |
| Removal of patella component | 3% |
| Oversizing | 3% |
CT results
Femoral component alignment in the arthrofibrosis group was significantly internally rotated to the surgical transepicondylar axis, with a mean posterior condylar angle of 4.7º ranging from 10º internal rotation to 1º external rotation. Standard deviation in the arthrofibrosis group was 2.3 and standard error 0.5. Gender normalisation showed no significant differences. Femoral alignment in the control group had a mean posterior condylar angle nearly parallel to the surgical transepicondylar axis at 0.3° of internal rotation, ranging from 6º external rotation to 4º internal rotation. Standard deviation was 2.2 and standard error 0.4. Gender normalisation showed no significant differences. The difference of femoral rotational alignment in both groups was highly significant (p<0.00001).
Discussion
Arthrofibrosis is defined as periarticular fibrosis that limits range of motion by forming bands of scar tissue between the quadriceps mechanism and the distal femur. Some patients may be predisposed to this condition or may develop it as a response to the surgical trauma and postoperative rehabilitation. For TKA, errors in surgical technique may lead to kinematics that create exaggerated mechanical stresses on soft tissues sufficient to induce fibrous metaplasia. Numerous authors have cited specific surgical problems that may cause pain and lead to scar tissue formation, such as ligamentous imbalance, component malposition, and joint line elevation.
Rotational alignment of the femoral component is essential for stability of the knee in flexion. In a cadaver study, Anouchi et al. [1] investigated the influence of 5° internal and 5° external femoral component malrotation on femorotibial laxity without axial load or quadriceps force. In full extension, they reported the same stability for internally and externally rotated femoral components. In general, varus laxity increased with increasing knee flexion and was largest at 90° with internal malrotation of the femoral component [1]. Romero et al. [22] performed a similar study but added significant load-bearing forces to the quadriceps mechanism, finding that in extension and 90° flexion, neither external nor internal femoral malrotation of 6° had a significant effect on the stability of the joint. However, at 60° knee flexion, varus laxity was significantly larger with a femoral component in 6° internal rotation compared with a component in neutral rotation. Varus laxity was not increased significantly with a femoral component in 3° internal malrotation [22].
In TKA, femoral component rotation must be physiological, and malalignment may cause chronic anterior pain, patellar instability, and decreased range of motion [3, 5, 21]. The transepicondylar axis, the anterior-posterior axis of Whiteside, and the posterior condylar axis are commonly used for a measured femoral resection in rotational positioning of the femoral component in TKA [2, 11, 18, 20, 24]. However, recent studies have noted the clinical difficulty of achieving flexion space symmetry in all cases [12, 16, 25]. The problem is the inability to deal with anomalies such as lateral femoral condylar hypoplasia or severe angular deformities such as proximal tibia vara with a varus joint line [13]. In these patients, ligamentous imbalance commonly occurs and can lead to chronic instability. Fehring [11] compared the Insall tensioning method with the measured resection method of distal femur resection using a fixed 3° posterior condylar reference guide, finding that the measured resection technique resulted in rotational errors of greater than 3° and flexion space asymmetry in 45% of knees. Olcott and Scott [19] found that flexion space symmetry within 3° of that desired was created with 90% using the transepicondylar axis, 83% using the anterior-posterior axis of Whiteside, and 70% using a posterior condylar reference.
The original designers of the LCS mobile-bearing TKA chose a tibia-cut-first surgical technique that is a variation of the ligament tensing method as originally described by Insall. That method, which was used in the current study, requires a tensioner to adjust the amount of femoral component rotation to create a symmetrical flexion space. Several studies have shown the tibia-cut-first technique to be optimal for creating a balanced flexion space, avoiding mobile-bearing complications from flexion instability [7]. Katz et al. [16] found the tensed ligament method to be the most accurate and reproducible for creating balanced gaps compared with measured resection methods. The tensed ligament method produced a mean 1.9±2.3° external rotation to the posterior condylar axis [16]. Griffin et al. [12] performed an in vivo measurement of the flexion and extension gaps in 84 total knee procedures after using the tensed ligament method for bone resection. They found that none of the knees had any gaps measuring more than 3 mm either medial to lateral or flexion to extension [12]. Winemaker [25] used a method similar to the current study but with a precise tensor. He found that the resultant femoral rotation from balanced ligament tension was similar to methods using femoral landmarks with overall femoral external rotation of 4.8±3.2° [25].
Combined internal rotation of both the femoral and tibial components has been shown to cause significant patellar complications and to be associated with chronic anterior knee pain following TKA. Berger et al. [5] found that combined component rotation of 1–17° of internal rotation could be associated with patellar complications ranging from lateral subluxation to patellar implant failure. Similarly, Barrack et al. [3] reported total knee patients with chronic postoperative anterior knee pain who had an average of 4.7° combined component internal rotation compared with the control group, who had 2.0° of external rotation. Our series identified an average of 4.7° of femoral component internal rotation for patients who developed arthrofibrosis compared with the control group, who had an average of 0.3° of internal rotation.
We believe that chronic arthrofibrosis may reflect an overrelease of the medial ligaments of the knee, which is known to occur after cases with severe varus deformity and contracture. Krackow et al. [17] found that a medial soft tissue released for adequate ligament balancing in extension may increase the flexion gap unpredictably, often creating very large medial gaps in flexion. We would suggest that for the tibia-cut-first method, an abnormal amount of femoral component internal rotation may be needed to balance the large medial gap and create a well-balanced knee in flexion.
Based on the study’s results of this study, we believe that increased femoral component internal rotation is one important factor leading to the development of arthrofibrosis after mobile-bearing TKA. Future studies should consider such multiplanar problems as abnormal femoral and tibial component rotation.
References
- 1.Anouchi YS, Whiteside LA, Kaiser AD, Milliano MT. The effects of axial rotational alignment of the femoral component on knee stability and patellar tracking in total knee arthroplasty demonstrated on autopsy specimens. Clin Orthop. 1983;287:170–177. [PubMed] [Google Scholar]
- 2.Arima J, Whiteside LA, McCarthy DS, White SE. Femoral rotational alignment, based on the anteroposterior axis, in total knee arthroplasty in a valgus knee. A technical note. J Bone Joint Surg Am. 1995;77:1331–1334. doi: 10.2106/00004623-199509000-00006. [DOI] [PubMed] [Google Scholar]
- 3.Barrack RL, Schrader T, Bertot AJ, Wolfe MW, Myers L. Component rotation and anterior knee pain after total knee arthroplasty. Clin Orthop. 2001;392:46–55. doi: 10.1097/00003086-200111000-00006. [DOI] [PubMed] [Google Scholar]
- 4.Berger RA, Crossett LS (1998) Determining the rotation of the femoral and tibial components in total knee arthroplasty: a computed tomography technique. Oper Tech Orthop 8:128–133
- 5.Berger RA, Crossett LS, Jacobs JJ, Rubash HE. Rotation causing patellofemoral complications after total knee arthroplasty. Clin Orthop. 1998;356:144–153. doi: 10.1097/00003086-199811000-00021. [DOI] [PubMed] [Google Scholar]
- 6.Berger RA, Rubash HE, Seel MJ, Thompson WH, Crossett LS. Determining the rotational alignment of the femoral component in total knee arthroplasty using the epicondylar axis. Clin Orthop. 1993;286:40–47. [PubMed] [Google Scholar]
- 7.Boldt JG, Stiehl JB, Thuemler P. Femoral rotation based on tibial axis. In: Hamelynck KJ, Stiehl JB, editors. LCS mobile bearing knee arthroplasty. Berlin Heidelberge New York: Springer; 2002. pp. 175–182. [Google Scholar]
- 8.Bong MR, Cesare PE. Stiffness after total knee arthroplasty. J Am Acad Orthop Surg. 2004;12:164–171. doi: 10.5435/00124635-200405000-00004. [DOI] [PubMed] [Google Scholar]
- 9.Dennis DA, Komistek RD, Stiehl JB, Walker SA, Dennis KN. Range of motion following total knee arthroplasty: the effect of implant design and weight-bearing conditions. J Arthroplasty. 1998;13:748–752. doi: 10.1016/S0883-5403(98)90025-0. [DOI] [PubMed] [Google Scholar]
- 10.Eckhoff DG, Metzger RG, Vandewalle MV. Malrotation associated with implant alignment technique in total knee arthroplasty. Clin Orthop. 1995;321:28–31. [PubMed] [Google Scholar]
- 11.Fehring TK. Rotational malalignment of the femoral component in total knee arthroplasty. Clin Orthop. 2000;380:72–79. doi: 10.1097/00003086-200011000-00010. [DOI] [PubMed] [Google Scholar]
- 12.Griffin FM, Insall JN, Scuderi GR. Accuracy of soft tissue balancing in total knee arthroplasty. J Arthroplasty. 2000;15:970–973. doi: 10.1054/arth.2000.6503. [DOI] [PubMed] [Google Scholar]
- 13.Griffin FM, Insall JN, Scuderi GR. The posterior condylar angle in osteoarthritic knees. J Arthroplasty. 1998;13:812–815. doi: 10.1016/S0883-5403(98)90036-5. [DOI] [PubMed] [Google Scholar]
- 14.Harvey IA, Barry K, Kirby SP, Johnson R, Elloy MA. Factors affecting the range of movement of total knee arthroplasty. J Bone Joint Surg. 1993;75:950–955. doi: 10.1302/0301-620X.75B6.8245090. [DOI] [PubMed] [Google Scholar]
- 15.Healy WL, Wasilewski SA, Takei R, et al. Patellofemoral complications following total knee arthroplasty: correlation with implant design and patient risk factors. J Arthroplasty. 1995;10:197–201. doi: 10.1016/S0883-5403(05)80127-5. [DOI] [PubMed] [Google Scholar]
- 16.Katz MA, Beck TD, Silber JS, Seldes RM, Lotke PA. Determining femoral rotational alignment in total knee arthroplasty: reliability of techniques. J Arthroplasty. 2001;16:301–305. doi: 10.1054/arth.2001.21456. [DOI] [PubMed] [Google Scholar]
- 17.Krackow AK, Mihalko WM. The effect of medial release on flexion and extension gaps in cadaveric knees. Am J Knee Surg. 1999;12:222–228. [PubMed] [Google Scholar]
- 18.Olcott CW, Scott RD. The Ranawat Award. Femoral component rotation during total knee arthroplasty. Clin Orthop. 1999;367:39–42. doi: 10.1097/00003086-199910000-00005. [DOI] [PubMed] [Google Scholar]
- 19.Olcott CW, Scott RD. A comparison of 4 intraoperative methods to determine femoral component rotation during total knee arthroplasty. J Arthroplasty. 2000;15:22–26. doi: 10.1016/S0883-5403(00)91051-9. [DOI] [PubMed] [Google Scholar]
- 20.Poilvache PL, Insall JN, Scuderi GR, Font-Rodriguez DE. Rotational landmarks and sizing of the distal femur in total knee arthroplasty. Clin Orthop. 1996;331:35–46. doi: 10.1097/00003086-199610000-00006. [DOI] [PubMed] [Google Scholar]
- 21.Rhoads DD, Noble PC, Reuben JD, Mahoney OM, Tullos HS. The effect of femoral component position on patellar tracking after total knee arthroplasty. Clin Orthop. 1990;260:43–51. [PubMed] [Google Scholar]
- 22.Romero J, Duronio JF, Sohrabi A, Alexander N, MacWilliams BA, Jones LC, Hungerford DS. Varus and valgus flexion laxity of total knee alignment methods in loaded cadaveric knees. Clin Orthop. 2002;394:243–253. doi: 10.1097/00003086-200201000-00029. [DOI] [PubMed] [Google Scholar]
- 23.Stiehl JB. Patellar instability in total knee arthroplasty. J Knee Surg. 2003;16:229–235. [PubMed] [Google Scholar]
- 24.Whiteside LA, Arima J. The anteroposterior axis for femoral rotational alignment in valgus total knee arthroplasty. Clin Orthop. 1995;321:168–172. [PubMed] [Google Scholar]
- 25.Winemaker MJ. Perfect balance in total knee arthroplasty. The elusive compromise. J Arthroplasty. 2001;17:2–10. doi: 10.1054/arth.2002.29321. [DOI] [PubMed] [Google Scholar]


