Abstract
Free light chains (FLCs) are the most commonly detected paraproteins in chronic lymphocytic leukemia (CLL). We examined the types of FLC abnormalities and prognostic utility of the FLC assay compared with standard prognostic biomarkers in a prospective cohort of 339 patients with newly diagnosed CLL. Three types of FLC abnormalities were identified: monoclonal elevated FLC (elevated κ and/or λ with abnormal FLC ratio), polyclonal elevated FLC (elevated κ and/or λ with normal FLC ratio), and ratio-only FLC abnormality (normal range κ and λ with abnormal FLC ratio). One hundred sixty-five patients (49%) had a FLC abnormality with approximately equal distribution among monoclonal elevation, polyclonal elevation, and ratio-only abnormality. All FLC abnormalities were associated with poor time to first treatment: monoclonal FLC (hazard ratio [HR], 4.99; 95% confidence interval [CI], 2.94-8.48), polyclonal FLC (HR, 2.40; 95% CI, 1.24-4.64), ratio-only FLC (HR, 2.57; 95% CI, 1.40-4.69). Monoclonal FLC and polyclonal FLC were associated with poor overall survival compared with patients with normal FLC. Results remained significant after adjusting for Rai stage. The FLC assay is a simple, widely available clinical test with similar prognostic utility as routinely used prognostic biomarkers for CLL. Among persons with FLC abnormalities, the type of abnormality affects prognostic significance.
Introduction
A clonal lymphoid cell can differentiate to the point where it secretes paraproteins. This can occur in a variety of lymphoid conditions, including multiple myeloma, monoclonal gammapathy of undetermined significance, and lymphoplasmacytic lymphoma (Waldenström macroglobulinemia). Paraproteins are usually measured with assays that detect intact antibodies. However, assays are now available that can measure free light chains (FLCs, κ and λ) in serum.1 Quantitation of the serum FLC paraprotein has been shown to have prognostic value in monoclonal gammapathy of undetermined significance,2 multiple myeloma,3 solitary plasmacytoma,4 and light chain amyloidosis.5 In addition to clonal FLC elevations, polyclonal increases in serum FLCs have been identified in a number of patient populations with malignant and nonmalignant diseases. These polyclonal increases in serum FLCs are not associated with an abnormal FLC ratio and may be indicative of renal dysfunction,6 older age,7 autoimmune disease,8 chronic inflammation,9 or general immune stimulation.
FLCs are also the most commonly detected paraproteins in chronic lymphocytic leukemia (CLL).10–13 Our initial report on the prevalence of FLC abnormalities detected with the FLC assay in CLL samples from the Mayo Clinic Lymphoma Specialized Program of Research Excellence (SPORE) serum bank reported a monoclonal protein in 44% of 18 patients with CLL with 39% detected by FLC and 11% detected by protein electrophoresis and immunofixation (IF).14 Notably, in 6 of the 8 patients with CLL with a paraprotein, the M protein was only detectable by the FLC method.
Two subsequent retrospective studies, including both treated and untreated patients, reported abnormal FLC ratios in 30%-40% of patients with CLL.15,16 In both those series, patients with an abnormal FLC ratio had shorter time to treatment and overall survival (OS), findings that persisted on multivariable analysis adjusting for immunoglobulin heavy chain variable region gene (IGHV) mutation status, β-2 microglobulin (B2M) levels, and ζ-associated protein 70 (ZAP-70) expression.15 However, neither study used the FLC ratio to distinguish monoclonal from polyclonal elevations of FLC.
The FLC assay is clinically available, simple, and relatively inexpensive; thus, it has the potential to be a valuable prognostic biomarker for patients with CLL. Here, we report the first study on the prognostic implications of analysis of serum FLC in a prospectively enrolled cohort of newly diagnosed, untreated patients with CLL. The analysis is the first to examine prevalence and outcome association for both monoclonal and polyclonal FLC abnormalities and to perform comparative analysis assessing the prognostic value of the FLC assay relative to that of the other well-established prognostic biomarkers for CLL.
Methods
Patients with CLL seen within 9 months of diagnosis were prospectively enrolled in the University of Iowa/Mayo Clinic SPORE Molecular Epidemiology Resource (MER) from September 2002 through February 2008. Enrollment to the SPORE MER is offered to consecutive patients with newly diagnosed CLL who were evaluated at Mayo Clinic Rochester or the University of Iowa within 9 months of diagnosis, were age 18 years and older, and were a US resident at diagnosis. The SPORE MER collects clinical, pathologic, demographic, and epidemiologic data along with blood and tumor samples from the patients at enrollment. Patients are systematically followed every 6 months for the first 3 years and then annually thereafter. This study was reviewed and approved by the Human Subjects Institutional Review Board at the Mayo Clinic and the University of Iowa, and written informed consent was obtained from all participants in accordance with the Declaration of Helsinki. The clinical characteristics of this cohort have been previously reported.17 All leukemia cell immunophenotype and pathology analyses were reviewed to confirm each case met the criteria for a diagnosis of CLL. All patients either had an absolute lymphocyte count > 5.0 × 109/L and fulfilled the 1996 criteria for CLL18 or fulfilled the World Health Organization criteria for the small lymphocytic lymphoma variant of CLL.19 Baseline clinical, laboratory, and treatment data were abstracted from medical records with the use of a standardized protocol. Prognostic testing (IGHV gene mutation analysis, ZAP-70 status, CD38 status, CD49d status, and cytogenetic abnormalities assessed by interphase FISH testing) was performed as part of clinical or research studies with the use of methods previously described by our group.20–22 Because treatment of lymphoid malignancies decreases the serum FLC,23 analyses were limited to the subset of patients with research serum draws collected before any treatment.
The Freelite assay (The Binding Site Ltd) was used to determine serum κ and λ FLC concentrations on stored research serum samples. All assays were performed at the Mayo Clinic Clinical Immunology Laboratory with the use of reagent sets provided courtesy of The Binding Site. Mayo Clinic reference ranges were used to define the normal range for κ and λ light chain levels and the FLC ratio.7 Elevated FLC was defined as a κ or λ level above the reference range (κ > 19.4 mg/L, λ > 26.3 mg/L). An elevated FLC was considered monoclonal if the FLC κ/λ ratio was outside of the normal range of 0.26-1.65 for patients with normal creatinine and 0.37-3.1 for patients with elevated creatinine.6 All elevated FLC levels with normal κ/λ ratios were considered polyclonal. Patients with normal FLC levels but an abnormal κ/λ ratio were considered to have ratio-only FLC abnormalities.
Time to first treatment (TTFT) was defined as the time from diagnosis to disease progression requiring treatment. OS was defined as time from diagnosis to death because of any cause. Patients without an event were censored at time of last known follow-up. Chi-square tests and the Fisher exact test, when appropriate, were used to assess the association of FLC with clinical, prognostic, and demographic factors. Cox proportional hazards models and Kaplan-Meier curves were used to assess associations of FLC abnormalities with time-to-event outcomes. Cox models were performed unadjusted as well as adjusted for Rai stage. The prognostic capability of biomarkers was assessed with time-dependent receiver operator curves (ROCs)24; the area under the curve (AUC) with bootstrap confidence intervals was used to assess the significance of differences. Standard CLL biomarkers were assessed as used clinically or previously published for prognostication.20–22,25 Lack of a sufficient number of events precluded multivariable modeling of FLC with the full set of other CLL prognostic markers. Thus, to explore the independent prognostic value of the FLC assay while accounting for known risk from these markers, we created a 1 degree of freedom risk score to use as an adjustment variable in multivariable models. The following risk factors were used as covariates: age, sex, Rai stage, CD38, ZAP-70, IGHV, CD49d, and FISH. The risk score was generated by the linear combination of the factor variables multiplied by their regression coefficients (ie, ×β) from Cox models for OS and TTFT. All statistical analyses were performed with SAS Version 9.1.3 (SAS Institute) and R Version 2.11.0 (R Project).
Results
We studied 339 patients with newly diagnosed CLL who had pretreatment serum collected for research purposes. Median age at diagnosis was 63 years (range, 37-91 years), and 67% of patients were men. At a median follow-up of 47 months (range, 1-92 months), 89 patients (26%) had been treated and 34 patients (10%) had died. Median κ, λ, and total (κ + λ) light chain levels were 1.3 mg/dL (range, 0.02-73.2 mg/dL), 1.2 mg/dL (range, 0.01-17.4 mg/dL), and 2.6 mg/dL (range, 0.4-74.4 mg/dL), respectively. Collectively, 163 patients (48%) had a FLC abnormality. Among the 109 patients with an elevated FLC level, 57 (52%) had an abnormal FLC ratio (monoclonal) and 52 (48%) had a normal FLC ratio (polyclonal). An additional 54 patients (17%) patients had an abnormal FLC ratio without an elevation of either κ or λ light chain (ratio-only abnormality). Among the 111 patients with an abnormal FLC ratio, 83 (75%) had excess κ. Paraproteins were also evaluated by serum protein electrophoresis (SPEP) with IF as part of routine clinical management in 243 of these patients (71.6%). Of these, only 54 patients (22%) had a detectable abnormality on SPEP/IF, with 40 clonal and 14 polyclonal abnormalities. Although 37 of 243 patients (15%) had a paraprotein abnormality detected by both the FLC and SPEP/IF assays, 85 of 243 patients (35%) had abnormalities detected only by FLC analysis, and 17 of 243 patients (7%) had abnormalities detected only by SPEP/IF.
Compared with patients with normal FLC, patients with monoclonal elevated FLC were more likely to have elevated creatinine levels; to have a higher absolute lymphocyte count; to be κ light chain restricted; to have CLL cells that were CD38+, CD49d+, ZAP-70+, and IGHV unmutated; and to have high-risk FISH and higher serum B2M values (Table 1). Patients with polyclonal elevated FLC were older; had higher creatinine levels, PS, and B2M levels; and more high-risk FISH abnormalities than patients with normal FLC. Patients with ratio-only FLC abnormalities were more likely to be κ light chain restricted and were more often CD49d+. Patients with all types of FLC abnormalities had higher Rai stage than patients with normal FLC.
Table 1.
Clinical characteristics by FLC abnormality in 339 patients
| Normal FLC (n = 176; 51%) | Polyclonal FLC elevation (n = 52; 15%) | Monoclonal FLC elevation (n = 57; 17%) | Ratio-only FLC abnormality (n = 54; 17%) | |
|---|---|---|---|---|
| Median κ, mg/dL (range) | 1.0 (0.2-1.9) | 2.5 (1.1-5.6) | 3.0 (0.2-73.2) | 1.2 (0.02-1.9) |
| Median λ, mg/dL (range) | 1.1 (0.2-2.4) | 2.5 (1.0-7.0) | 1.1 (0.2-17.4) | 0.8 (0.01-2.1) |
| Median κ + λ, mg/dL (range) | 2.3 (0.4-4.1) | 5.0 (3.1-11.7) | 4.9 (2.4-74.4) | 1.7 (0.7-2.9) |
| Age > 60 y, n (%) | 101 (58) | 41 (80)† | 39 (67) | 28 (50) |
| Male, n (%) | 113 (64) | 36 (69) | 44 (77) | 33 (61) |
| κ tumor light chain, n (%) | 76 (52) | 21 (53) | 38 (79)‡ | 34 (69)* |
| PS > 0, n (%) | 14 (8) | 13 (25)† | 8 (14) | 2 (4) |
| Elevated creatinine level, n (%) | 11 (6) | 13 (25)‡ | 7 (12) | 1 (2) |
| Rai stage | ||||
| 0, n (%) | 103 (59) | 23 (45)* | 17 (30)§ | 25 (46)* |
| 1, n (%) | 69 (39) | 23 (45) | 27 (47) | 23 (43) |
| 2-4, n (%) | 4 (2) | 5 (10) | 13 (23) | 6 (11) |
| ALC | ||||
| < 20 × 109/L, n (%) | 143 (82) | 40 (77) | 28 (49)§ | 37 (70)* |
| 20-50 × 109/L, n (%) | 25 (14) | 9 (17) | 15 (26) | 9 (17) |
| > 50 × 109/L, n (%) | 7 (4) | 3 (6) | 14 (25) | 7 (13) |
| B2M level | ||||
| < ULN, n (%) | 36 (23) | 3 (7)§ | 1 (2)§ | 14 (27) |
| 1-2 times ULN, n (%) | 115 (72) | 19 (42) | 29 (57) | 34 (67) |
| > 2 times ULN, n (%) | 8 (5) | 23 (51) | 21 (42) | 3 (6) |
| CD38+, n (%) | 27 (18) | 13 (30) | 24 (49)§ | 14 (29) |
| CD49d+, n (%) | 15 (19) | 7 (28) | 18 (49)‡ | 11 (41)* |
| ZAP-70+, n (%) | 31 (22) | 8 (20) | 23 (49)‡ | 14 (32) |
| IGHV unmutated, n (%) | 29 (24) | 13 (37) | 26 (55)§ | 15 (33) |
| High-risk FISH, n (%) | 8 (6) | 8 (22)† | 10 (22)‡ | 5 (11) |
PS indicates Eastern Cooperative Oncology Group performance score; ALC, absolute lymphocyte count; ULN, upper limit of normal; and IGHV unmutated includes VH-321 for risk assessment, High-risk FISH, 17p−, 11q−.
P < .05 compared with the normal FLC group.
P < .01 compared with the normal FLC group.
P < .001 compared with the normal FLC group.
P < .0001 when compared with the normal FLC group.
FLC and tumor cell light chain restriction
In patients with both an elevated FLC and an abnormal FLC ratio (eg, patients with monoclonal FLC elevations) the dominant serum FLC matched the CLL B-cell light chain restriction in 96% of cases. Among patients with an abnormal FLC ratio but no elevation in absolute FLC level (eg, patients with ratio-only abnormalities), the dominant light chain in the serum matched the CLL B-cell light chain restriction in 92% of cases, suggesting this abnormality was disease related in most patients. In patients with increased FLC level but a normal ratio (eg, patients with polyclonal elevation), the more abundant serum FLC matched the CLL B-cell light chain restriction in 75% of cases.
FLC and clinical outcome
FLC abnormalities were associated with both TTFT (Figure 1A) and OS (Figure 1B). All FLC abnormalities were associated with shorter time to treatment (monoclonal FLC: hazard ratio [HR], 5.57, 95% confidence interval [CI], 3.29-9.44; polyclonal FLC: HR, 2.12; 95% CI, 1.08-4.17; and ratio-only FLC: HR, 2.58; 95% CI, 1.51-5.03). For OS, shorter survival was observed for patients with monoclonal and polyclonal FLC elevations relative to patients with normal FLC results (monoclonal FLC: HR, 5.38; 95% CI, 2.23-12.98; polyclonal FLC: HR, 5.37; 95% CI, 2.19-13.17). Ratio-only FLC abnormalities were not associated with inferior OS (HR, 0.38; 95% CI, 0.05-3.03). Stage-adjusted HRs and HRs for patients with Rai stage 0 are shown in Table 2. In addition, we assessed the prognostic value of FLC in a multivariable Cox model after adjusting for a risk score accounting for age, sex, Rai stage, CD38, ZAP-70, IGHV, CD49d, and FISH. Monoclonal FLC remained a strong predictor of TTFT (HR, 2.97; 95% CI, 1.75-5.06; P < .001) in the adjusted model. Elevated FLC (either monoclonal or polyclonal) remained prognostic for OS (HR, 2.25; 95% CI, 1.04-4.86; P = .039) in the adjusted OS model. Cause of death was reviewed in all cases; the most common cause of death in the monoclonal FLC group was progressive CLL, whereas most deaths in the polyclonal FLC group were because of other causes (data not shown).
Figure 1.
TTFT and OS by FLC abnormality. (A) TTFT by FLC abnormality. (B) OS by FLC abnormality.
Table 2.
Cox proportional hazards model for association of FLC abnormalities with clinical outcome: unadjusted in all patients, adjusted for Rai stage in all patients, and unadjusted in patients with Rai stage 0
| All patients, unadjusted |
All patients, adjusted for Rai stage |
Rai stage 0 only |
|||||||
|---|---|---|---|---|---|---|---|---|---|
| HR | 95% CI | P | HR | 95% CI | P | HR | 95% CI | P | |
| TTFT | |||||||||
| Normal FLC | 1.00 | Ref | — | 1.00 | Ref | — | 1.00 | Ref | — |
| Monoclonal FLC elevation | 5.57 | 3.29-9.44 | < .001 | 4.23 | 2.47-7.25 | < .001 | 7.49 | 1.77-31.6 | .0062 |
| Polyclonal FLC elevation | 2.12 | 1.08-4.17 | .029 | 1.52 | 1.06-4.12 | .25 | 2.73 | 0.46-16.7 | .27 |
| Ratio-only FLC abnormality | 2.75 | 1.51-5.03 | .0010 | 2.19 | 1.20-4.02 | .011 | 9.08 | 2.27-36.4 | .0018 |
| OS | |||||||||
| Normal FLC | 1.00 | Ref | — | 1.00 | Ref | — | 1.00 | Ref | — |
| Monoclonal FLC elevation | 5.38 | 2.23-12.98 | .001 | 4.89 | 2.00-11.92 | .001 | 10.50 | 2.49-44.26 | .0014 |
| Polyclonal FLC elevation | 5.37 | 2.19-13.17 | .001 | 4.76 | 1.90-11.89 | .001 | 5.00 | 1.00-24.92 | .050 |
| Ratio-only FLC abnormality | 0.38 | 0.05-3.03 | .36 | 0.34 | 0.04-2.67 | .30 | NA | ||
Ref indicates reference group; and NA, not applicable because of limited number of patients or events in the subgroup.
Prognostic utility of FLC relative to other prognostic biomarkers
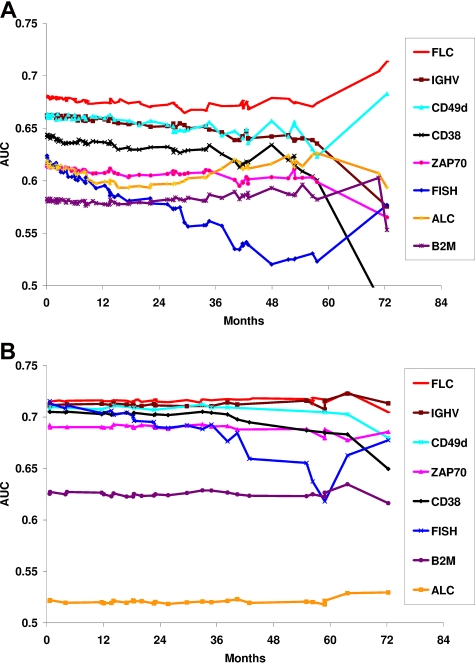
Finally, time-dependent ROCs were used to assess the prognostic utility of FLC relative to other CLL prognostic biomarkers. On the basis of the observed outcome results (Figure 1), the FLC grouping used for ROC analysis were as follows: normal FLC versus polyclonal or ratio-only FLC versus monoclonal FLC for TTFT and normal or ratio-only FLC versus monoclonal or polyclonal FLC for OS. The prognostic ability of FLC compared favorably with the established CLL prognostic parameters, with AUCs > 0.67 for TFTT and > 0.70 for OS (Figure 2).
Figure 2.
Comparison of prognostic biomarkers and OS by time-dependent AUC for TTFT. (A) Comparison of prognostic biomarkers by time-dependent AUC for TTFT. An AUC of 0.5 signifies no prognostic utility; AUC of 1.0 signifies a perfect predictor. The FLC assay has comparable-to-superior AUC to other CLL prognostic biomarkers. (B) Comparison of prognostic biomarkers for OS by time-dependent AUC.
Discussion
This is the first prospective study reporting the prognostic value of the FLC assay in a cohort of patients with newly diagnosed CLL. Approximately one-half of the patients with CLL had FLC abnormalities, and these abnormalities were strongly associated with clinical outcome. Notably, among patients with FLC abnormalities, the type of abnormality determines its prognostic implications. Our study is the first to perform detailed assessment of the clinical implications of the various types of FLC abnormalities (eg, clonal vs polyclonal abnormalities) and to compare the prognostic value of the FLC assay, as measured by ROC analysis, with other CLL biomarkers. We show that serum FLC measurements compare favorably with other CLL prognostic biomarkers. This is an important finding because the FLC assay is a robust assay that is simple to perform, widely available, has a well-established interlaboratory reference range,7 and is relatively inexpensive.
The 50% of patients with CLL with FLC abnormalities were distributed into 3 approximately equal-sized groups comprising patients with monoclonal elevations (an elevated FLC with abnormal ratio), polyclonal elevations (elevated FLC without abnormal FLC ratio), and ratio-only abnormalities (abnormal ratio without elevated FLC). Each of these groups appears to have distinct clinical characteristics and outcomes.
An elevated FLC with an abnormal κ/λ ratio is probably caused by secretion of clonal FLC from the tumor cells. This proposed mechanism is supported by 96% concordance between the light chain restriction of the CLL cell and the predominant serum light chain in these patients. This group of patients with monoclonal FLC elevations appears to have a higher prevalence of high-risk biologic characteristics (CD38, CD49d, ZAP-70, IGHV, FISH) and to have the poorest prognosis as measured by TTFT and OS, suggesting that increased light chain secretion is associated with more aggressive CLL cell biology. Although the majority of patients with monoclonal FLC elevations (71%) in our series were Rai stage I or higher, patients with Rai stage 0 with monoclonal FLC elevations also had a shorter time to treatment. Cause of death for deceased patients in this group was primarily because of progressive CLL, further supporting the hypothesis of an aggressive CLL cell biology.
Patients with polyclonal FLC elevations tended to be older, have higher PS, and have elevated creatinine levels, suggesting multiple possible causes for the FLC abnormality and indicating that this finding could be a marker of host “fitness.”26 Prevalence of high-risk CLL biologic characteristics were similar to patients without FLC abnormalities, except for high-risk FISH. Cause of death in these patients was primarily because of non-CLL causes, again supporting an association of elevated polyclonal FLC with poor host fitness. However, because polyclonal FLC elevations were associated not only with shorter OS but also modestly associated with a shorter TTFT, it is also possible that polyclonal FLC may nonetheless be a marker of poorer disease-specific outcomes.
In patients with an abnormal FLC ratio and nonelevated levels (eg ratio-only abnormalities), there was concordance of the predominant serum light chain and CLL light chain restriction in 92% of cases, suggesting that the abnormal FLC ratio was because of CLL cell light chain secretion. However, compared with patients with monoclonal elevated FLC, patients with ratio-only abnormalities were more similar to patients with normal FLC assay in terms of Rai stage and other prognostic factors. As a group, ratio-only FLC is associated with shorter TTFT. Although OS is similar to patients with normal FLC in our study, this observation should be interpreted with caution until longer follow-up is available.
Strengths of our study include its prospective design, inclusion of only patients with newly diagnosed disease all of whom provided samples before receiving any treatment, systematic follow-up, use of a single reference laboratory for FLC assays, and the ability to correlate the FLC measurements to multiple other CLL prognostic biomarkers. To the best of our knowledge, this study is also the largest to evaluate the prognostic importance of FLC in CLL and the first to assess how the type of FLC abnormality relates to outcome. The main limitation of this study is the relatively short follow-up and limited number of events for a disease that has a relatively indolent course.
In summary, ∼ 50% of patients with CLL have serum FLC abnormalities when evaluated by routine, clinically available FLC assays. When serum FLC abnormalities are detected, it is important to distinguish the type of FLC abnormality (monoclonal elevation, polyclonal elevation, ratio-only abnormality) because these subtypes have distinct clinical implications. FLC abnormalities are associated with inferior TTFT and OS, and the prognostic value of the FLC assay appears equivalent or superior to that of other routinely used CLL biomarkers. Further research into the role of serum FLC as a prognostic marker and the biologic characteristics related to FLC secretion by CLL B cells is warranted.
Acknowledgments
This work was supported by the National Institutes of Health (CA97274 [University of Iowa/Mayo Clinic Lymphoma SPORE], CA113408) and the Henry J. Predolin Foundation.
Footnotes
Presented in part at the 52nd annual meeting of the American Society of Hematology, Orlando, FL, December 5, 2010.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Authorship
Contribution: M.J.M., J.R.C., T.E.W., and T.D.S. provided the conception and design; J.C.R. and T.E.W. provided the financial support; J.R.C., B.K.L., C.S.Z., T.G.C., C.A.H., N.E.K., T.E.W., and T.D.S. provided the study materials or patients; M.J.M., J.R.C., J.A.K., C.A., K.G.R., C.A.H., S.L.S., T.E.W., and T.D.S. provided the collection and assembly of data; M.J.M., J.R.C., J.A.K., C.A., K.G.R., C.S.Z., T.E.W., and T.D.S. provided the data analysis and interpretation; M.J.M. and T.D.S. were responsible for the manuscript writing; and J.R.C. provided administrative support. All authors approved and contributed to the final manuscript.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Correspondence: Matthew J. Maurer, Department of Health Sciences Research, Mayo Clinic, 200 First St SW, Rochester, MN 55905; e-mail: maurer.matthew@mayo.edu.
References
- 1.Bradwell AR, Carr-Smith HD, Mead GP, et al. Highly sensitive, automated immunoassay for immunoglobulin free light chains in serum and urine. Clin Chem. 2001;47(4):673–680. [PubMed] [Google Scholar]
- 2.Rajkumar SV, Kyle RA, Therneau TM, et al. Serum free light chain ratio is an independent risk factor for progression in monoclonal gammopathy of undetermined significance. Blood. 2005;106(3):812–817. doi: 10.1182/blood-2005-03-1038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Dispenzieri A, Kyle RA, Katzmann JA, et al. Immunoglobulin free light chain ratio is an independent risk factor for progression of smoldering (asymptomatic) multiple myeloma. Blood. 2008;111(2):785–789. doi: 10.1182/blood-2007-08-108357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Dingli D, Kyle RA, Rajkumar SV, et al. Immunoglobulin free light chains and solitary plasmacytoma of bone. Blood. 2006;108(6):1979–1983. doi: 10.1182/blood-2006-04-015784. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Kumar S, Dispenzieri A, Katzmann JA, et al. Serum immunoglobulin free light-chain measurement in primary amyloidosis: prognostic value and correlations with clinical features. Blood. 2010;116(24):5126–5129. doi: 10.1182/blood-2010-06-290668. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Hutchison CA, Harding S, Hewins P, et al. Quantitative assessment of serum and urinary polyclonal free light chains in patients with chronic kidney disease. Clin J Am Soc Nephrol. 2008;3(6):1684–1690. doi: 10.2215/CJN.02290508. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Katzmann JA, Clark RJ, Abraham RS, et al. Serum reference intervals and diagnostic ranges for free {kappa} and free {lambda} immunoglobulin light chains: relative sensitivity for detection of monoclonal light chains. Clin Chem. 2002;48(9):1437–1444. [PubMed] [Google Scholar]
- 8.Gottenberg J-E, Aucouturier F, Goetz J, et al. Serum immunoglobulin free light chain assessment in rheumatoid arthritis and primary Sjögren’s syndrome. Ann Rheum Dis. 2007;66(1):23–27. doi: 10.1136/ard.2006.052159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.van der Heijden M, Kraneveld A, Redegeld F. Free immunoglobulin light chains as target in the treatment of chronic inflammatory diseases. Eur J Pharmacol. 2006;533(1–3):319–326. doi: 10.1016/j.ejphar.2005.12.065. [DOI] [PubMed] [Google Scholar]
- 10.Maino VC, Kurnick JT, Kubo RT, Grey HM. Mitogen activation of human chronic lymphatic leukemia cells. J Immunol. 1977;118(3):742–748. [PubMed] [Google Scholar]
- 11.Gordon J, Howlett AR, Smith JL. Free light chain synthesis by neoplastic cells in chronic lymphocytic leukaemia and non-Hodkgin’s lymphoma. Immunology. 1978;34(3):397–404. [PMC free article] [PubMed] [Google Scholar]
- 12.Hannam-Harris A, Gordon J, Smith J. Immunoglobulin synthesis by neoplastic B lymphocytes: free light chain synthesis as a marker of B cell differentiation. J Immunol. 1980;125(5):2177–2181. [PubMed] [Google Scholar]
- 13.Deegan M, Abraham J, Sawdyk M, Van Slyck E. High incidence of monoclonal proteins in the serum and urine of chronic lymphocytic leukemia patients. Blood. 1984;64(6):1207–1211. [PubMed] [Google Scholar]
- 14.Martin W, Abraham R, Shanafelt T, et al. Serum-free light chain–a new biomarker for patients with B-cell non-Hodgkin lymphoma and chronic lymphocytic leukemia. Transl Res. 2007;149(4):231–235. doi: 10.1016/j.trsl.2006.11.001. [DOI] [PubMed] [Google Scholar]
- 15.Pratt G, Harding S, Holder R, et al. Abnormal serum free light chain ratios are associated with poor survival and may reflect biological subgroups in patients with chronic lymphocytic leukaemia. Br J Haematol. 2009;144(2):217–222. doi: 10.1111/j.1365-2141.2008.07456.x. [DOI] [PubMed] [Google Scholar]
- 16.Yegin ZA, Özkurt ZN, Yağcı M. Free light chain: a novel predictor of adverse outcome in chronic lymphocytic leukemia. Eur J Haematol. 2010;84(5):406–411. doi: 10.1111/j.1600-0609.2010.01412.x. [DOI] [PubMed] [Google Scholar]
- 17.Shanafelt TD, Drake MT, Maurer MJ, et al. Vitamin D insufficiency and prognosis in chronic lymphocytic leukemia. Blood. 2011;117(5):1492–1498. doi: 10.1182/blood-2010-07-295683. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Cheson B, Bennett J, Grever M, et al. National Cancer Institute-sponsored Working Group guidelines for chronic lymphocytic leukemia: revised guidelines for diagnosis and treatment. Blood. 1996;87(12):4990–4997. [PubMed] [Google Scholar]
- 19.Harris NL, Jaffe ES, Diebold J, et al. World Health Organization classification of neoplastic diseases of the hematopoietic and lymphoid tissues: report of the Clinical Advisory Committee meeting–Airlie House, Virginia, November 1997. J Clin Oncol. 1999;17(12):3835–3849. doi: 10.1200/JCO.1999.17.12.3835. [DOI] [PubMed] [Google Scholar]
- 20.Jelinek DF, Tschumper RC, Geyer SM, et al. Analysis of clonal B-cell CD38 and immunoglobulin variable region sequence status in relation to clinical outcome for B-chronic lymphocytic leukaemia. Br J Haematol. 2001;115(4):854–861. doi: 10.1046/j.1365-2141.2001.03149.x. [DOI] [PubMed] [Google Scholar]
- 21.Dewald GW, Brockman SR, Paternoster SF, et al. Chromosome anomalies detected by interphase fluorescence in situ hybridization: correlation with significant biological features of B-cell chronic lymphocytic leukaemia. Br J Haematol. 2003;121(2):287–295. doi: 10.1046/j.1365-2141.2003.04265.x. [DOI] [PubMed] [Google Scholar]
- 22.Shanafelt TD, Witzig TE, Fink SR, et al. Prospective evaluation of clonal evolution during long-term follow-up of patients with untreated early-stage chronic lymphocytic leukemia. J Clin Oncol. 2006;24(28):4634–4641. doi: 10.1200/JCO.2006.06.9492. [DOI] [PubMed] [Google Scholar]
- 23.Maurer MJ, Micallef INM, Cerhan JR, et al. Elevated serum free light chains are associated with event-free and overall survival in two independent cohorts of patients with diffuse large B-cell lymphoma. J Clin Oncol. 2011;29(12):1620–1626. doi: 10.1200/JCO.2010.29.4413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Heagerty PJ, Zheng Y. Survival model predictive accuracy and ROC curves. Biometrics. 2005;61(1):92–105. doi: 10.1111/j.0006-341X.2005.030814.x. [DOI] [PubMed] [Google Scholar]
- 25.Shanafelt TD, Jenkins G, Call TG, et al. Validation of a new prognostic index for patients with chronic lymphocytic leukemia. Cancer. 2009;115(2):363–372. doi: 10.1002/cncr.24004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Dispenzieri A, Kyle R, Katzmann JA, et al. Non-clonal serum immunoglobulin free light chains (FLC) as markers of overall survival [abstract]. Blood. 2010;116(21) Abstract 3893. [Google Scholar]