Abstract
Heparin-induced thrombocytopenia (HIT) is caused by antibodies that recognize complexes between platelet factor 4 (PF4) and heparin or glycosaminoglycan side chains. These antibodies can lead to a limb- and life-threatening prothrombotic state. We now show that HIT antibodies are able to inhibit generation of activated protein C (aPC) by thrombin/thrombomodulin (IIa/TM) in the presence of PF4. Tetrameric PF4 potentiates aPC generation by formation of complexes with chondroitin sulfate (CS) on TM. Formation of these complexes occurs at a specific molar ratio of PF4 to glycosaminoglycan. This observation and the finding that the effect of heparin on aPC generation depends on the concentration of PF4 suggest similarity between PF4/CS complexes and those that bind HIT antibodies. HIT antibodies reduced the ability of PF4 to augment aPC formation. Cationic protamine sulfate, which forms similar complexes with heparin, also enhanced aPC generation, but its activity was not blocked by HIT antibodies. Our studies provide evidence that complexes formed between PF4 and TM's CS may play a physiologic role in potentiating aPC generation. Recognition of these complexes by HIT antibodies reverses the PF4-dependent enhancement in aPC generation and may contribute to the prothrombotic nature of HIT.
Introduction
Many of the biologic effects of platelet factor 4 (PF4) result from its ability to bind to cell-surface glycosaminoglycans (GAGs) and other negatively charged molecules.1 GAGs bind with high affinity to an equatorial band of positively charged residues on the surface of the PF4 homotetramer.2 Using PF4 mutant K50E, we have previously shown that interfering with tetramer formation between PF4 dimers results in a marked loss in affinity for GAGs.3 Tetrameric PF4 bound to negatively charged molecules, such as heparin, forms large complexes at a specific molar ratio that dissociate in the presence of excess of either PF4 or the negatively charged molecule.3,4 At least 2 populations of PF4/heparin complexes were observed depending on the PF4 to heparin molar ratio.3 The ultralarge (> 670 kDa) complexes formed at 1:1 ratio are stable and have been visualized using rotary shadowed electron microscopy.3 They are also the colloidal complexes at neutralizing molar ratios of PF4 and heparin.4 Similar large, colloidal complexes form between heparin or GAGs and other small positively charged proteins, including protamine sulfate (PRT),5 supporting an electrostatic basis for this interaction. These PF4/heparin complexes are an antigenic target in heparin-induced thrombocytopenia (HIT), and each complex is capable of binding multiple HIT-like monoclonal antibodies KKO.3 The observation that these complexes form only over a narrow range of PF4 to heparin ratio probably explains why binding of HIT antibodies and KKO to PF4/heparin mixture follows a bell-shaped curve that depends on the molar ratio of PF4 and heparin.3,6 KKO and patients' HIT antibodies also recognize PF4 bound to surface GAGs on platelets7 and monocytes,8 following a similar bell-shaped curve with maximal binding observed at an exogenous PF4 concentration of 1.6μM. Others have shown similar results for surface GAGs on neutrophils.9
Antibodies present in patients with HIT can lead to limb- and life-threatening thrombosis. The basis for the prothrombotic state associated with thrombocytopenia is paradoxical and not well understood. In addition to activation of platelets, HIT antibodies deposit on monocytes and endothelial cells, which induces expression of procoagulant tissue factor,8,10,11 but other possible effects on the coagulation system have received little study. In this paper, we investigate whether HIT antibodies perturb the interaction of PF4 with thrombomodulin (TM) and thereby affect PF4's function in regulating activated protein C (aPC) formation. PF4 has previously been shown to increase generation of aPC by thrombin (IIa)/TM both in vitro and after infusion of PF4 in vivo.12,13 Binding studies using surface plasmon resonance14 confirmed a strong interaction between PF4 and Gla domain of PC as well as PF4 and TM containing the GAG moiety chondroitin sulfate (CS). Both Gla domain of PC and CS side chain of TM were necessary for PF4 to increase aPC generation. We have shown the physiologic relevance of these findings in that PF4 released from platelets in mice enhanced aPC generation in a model of IIa infusion and can protect against lipopolysaccharide (LPS)–induced endotoxemia.15 We now show that PF4/TM interaction involves similar PF4/GAG complexes to those formed in HIT, demonstrating an example of a physiologic role for such complexes. Further, we show that HIT antibodies block the capacity of this PF4/TM complex to generate aPC, thereby identifying a novel prothrombotic pathway in HIT.
Methods
Reagents
Chromogenic substrate S2366 was from Chromogenix/diaPharma, recombinant hirudin from Calbiochem, and PRT from American Pharmaceutical Partners. All other reagents, including unfractionated high molecular weight porcine heparin, sodium salt (specific activity 196 U/mg), mouse thrombin (IIa), human IIa, heparinase III, or chondroitinase (ABC) were from Sigma-Aldrich. Cell culture materials were from Invitrogen. Purified KKO, a monoclonal antibody specific to PF4/GAG complexes,16 was a gift from Dr G. Arepally, Duke University. Control mouse IgG was purchased from Innovative Research.
Purification of human PF4, wild-type (WT), PF4K50E, and PF4T38Q mutants was performed as described.17 Recombinant protein was isolated from bacterial lysate supernatant by affinity chromatography using a HiTrap Heparin HP column (GE Healthcare Bio-Sciences). Proteins were purified further by fast protein liquid chromatography using a Resource RPC column (GE Healthcare). Protein purity was assessed by Coomassie Blue staining of samples analyzed by 15% SDS-PAGE (Invitrogen). Proteins studied had negligible endotoxin content (< 1 EU per 1 μg of protein) as measured by the limulus amebocyte lysate assay (Lonza Walkersville).
Soluble human TM was purified from TM-expressing HEK293 cells by anion-exchange chromatography and affinity chromatography on IIa-Sepharose.18 The nonglycosylated (low molecular weight) forms were separated from glycosylated forms (high molecular weight) by high performance anion exchange chromatography as described to yield CS-free human TM (TM-CS).19 The presence of both forms of TM was validated by Western blot. Rabbit TM was purchased from Hematologic Technologies. Human protein C was isolated from plasma as described20 and further purified by immunoaffinity chromatography using the Ca2+ dependent monoclonal antibody HPC4.21
HIT antibodies were isolated from plasma of 4 adult patients (patients 1-4) who had been treated with unfractionated heparin for 6, 6, 2, and 7 days, respectively. HIT was diagnosed based on a drop-in platelet count of > 50% from baseline values and a strongly positive PF4 ELISA test result confirmed by showing that the test became negative when excess unfractionated heparin (100 units/mL) was added to the reaction mixture.22 All patients also had a strongly positive serotonin release assay test result.23
Total IgG was separated on a protein G column (Invitrogen) as described by the manufacturer. Control human antibody (hIgG) was isolated the same way from plasma of a healthy volunteer. Patient IgG specific to PF4/GAG complex was then isolated using beads with PF4 bound to heparin immobilized on diamino-dipropylamine agarose (Pierce Chemical), as previously described,24 followed by repurification using protein G column. The purity of the HIT IgG was evaluated using silver staining following SDS-PAGE on a 10% (weight/volume) and showed absence of both heparin and PF4 in the sample (data not shown). Specific binding of antibodies to PF4/heparin complexes was checked by ELISA. Human samples were obtained with Institutional Review Board permission.
In vitro aPC assay
Generation of aPC was assayed in 96-well plates as described previously.12 Briefly, rabbit or soluble human TM or TM-CS was mixed with various amounts of PF4 (or PRT) and/or heparin for 10 minutes in assay buffer (final concentration, 20mM Tris, 100mM NaCl, 1mM CaCl2, 0.1% BSA, pH 7.5). PC was added for an additional 10 minutes followed by addition of IIa at a final concentration of either 0.2 or 2nM and 15 minutes of incubation. All incubations were done at 37°C. Final concentrations of TM and PC were 0.5nM and 500nM, respectively.
HEK-K293 cells stably expressing human TM on their surface were kindly supplied by Dr L. O. Mosnier (Scripps Research Institute, La Jolla, CA). To measure aPC formation, K293 or TM/K293 cells were allowed to adhere overnight onto 24-well plates (5 × 105 cells/well) in DMEM/F12 media containing 10% FBS as described earlier.25 Cells were washed twice with DMEM/F12 media followed by Dulbecco PBS. In some experiments, cells were pretreated with 1 U/mL of heparinase III, chondroitinase ABC, or both for 1 hour at 37°C followed by Dulbecco PBS wash. Various concentrations of PF4 or PRT were incubated with cells for 10 minutes at 37°C followed by the addition of PC and IIa, as described above. In some experiments, KKO or control IgG was added for 15 minutes before the addition of PC and IIa. After incubation for 45 minutes, aliquots of the reaction mixture were transferred to 96-well plates and quenched by the addition of 1mM EDTA and 100nM hirudin. Generation of aPC was measured after the addition of 0.5mM S2366 and determination of the initial rate of chromogenic substrate cleavage from absorbance measurements at 405 nm on a Thermomax Microtiter Vmax plate reader (Molecular Devices).
Human aortic endothelial cells (HAECs) were purchased (Lonza Walkersville) and cultured in the presence of Clonetics EGM-2 media supplemented with growth factors and containing 2% FBS. HAECs were also allowed to adhere overnight onto 24-well plate cells (1 × 105 cells/well) in EGM-2 media and washed twice with media lacking FBS or heparin. Cells were then incubated without or with 2.5 U/mL chondroitinase ABC in Tris-sodium acetate buffer (0.02M Tris, 0.05M NaOAc, pH 8.0) or buffer alone, followed by 2 times wash with Dulbecco PBS. aPC generation assay was then performed in the presence of 100nM PC as described above. We used the cells cultured at the lowest passage possible (fifth and sixth).
Mice models studied
PF4 knockout (PF4KO) mice were generated previously and characterized by us.26 Endothelial-targeted deletion of the biosynthetic enzyme N-acetylglucosamine N-deacetylase-N-sulfotransferase-1 (Ndst1) was created using the floxed Ndst1f/f27 and endothelial-specific Cre recombinase Tie2Cre+ mice28 as previously described.29 Those animals have Ndst1 inactivated also in leukocytes.27 Male Ndst1f/f/Tie2Cre+ mice were bred with Ndst1f/f female mice, resulting in both the endothelial Ndst1-deficient Ndst1f/f/Tie2Cre+ mice and Ndst1f/f/Tie2Cre− littermate controls. Endothelial cells of Ndst1f/f/Tie2Cre+ mice have reduced N-sulfated heparan sulfate (HS) to 15% compared with 45% in WT mice.27 These knockdown mice were termed HSKD mice. HSKD on PF4KO background were used in our studies. All mice lines had been previously backcrossed > 10 times onto C57Bl6 background, and studies focused on comparison with littermate controls. Mice studied were 8-12 weeks of age. All experiments were approved by The Children's Hospital of Philadelphia's Institutional Animal Care and Use Committee.
In vivo aPC assay
Generation of aPC in vivo was assayed in plasma of mice injected intravenously with IIa as described.15 Mouse IIa at 80 U/kg was injected into the jugular vein over 2 minutes concurrent with 2-5 mg/kg of PF4 or PF4K50E or 2 mg/kg of PRT. After 10 minutes, blood was drawn into sodium citrate/benzamidine (final concentration, 5mM/50mM, respectively), centrifuged for 10 minutes at 4500g at 4°C, and plasma was frozen. Plasma aPC levels were subsequently measured by capture ELISA30 using antimouse aPC antibody (kindly supplied by Dr C.T. Esmon, University of Oklahoma) and chromogenic substrate Spectrozyme PCa (American Diagnostica).
Statistical analysis
Differences between groups were compared using a 2-tailed Student t test. Statistical analyses were performed using Microsoft Excel (Microsoft). Differences were considered significant when P values were < .05.
Results
Role of tetrameric PF4 and TM CS side chains in aPC generation in solution
Previous studies have shown that PF4, at concentrations of up to 3μM, increased aPC generation in the presence of TM both in solution and on endothelial cells.12,13 We performed additional studies over the wider range of PF4 concentrations (up to 25μM) that is within the estimated range seen after platelet activation at the concentration effective in many biologic responses.31,32 In the presence of soluble TM, we now show that PF4 enhances aPC generation with a bell-shaped response peaking at 1-2.5μM at both low (0.2nM) and high (2.0nM) concentrations of IIa (Figure 1A and B, respectively). Peak rates of aPC formation were approximately 4-fold greater than those in the absence of PF4. PF4K50E mutant that can dimerize but fails to form tetramers,2 did not enhance aPC generation at 0.2nM IIa (Figure 1A), and yielded only a modest enhancing effect at 2nM IIa (Figure 1B). This result is consistent with the need for PF4 to form tetramers to enhance aPC generation by TM.
Figure 1.
PF4 stimulation of aPC generation in-solution by IIa/TM complex. aPC generation was monitored using the chromogenic substrate S2366. Reaction mixtures contained IIa at either 0.2nM (A) or 2nM (B). Increasing concentrations of PF4 (WT) or PF4K50E mutant that can dimerize but fail to form tetramers were used in the presence of TM that either contains (TM) or lacks CS side chain (TM-CS). Each curve represents the mean ± SD of 4 or 5 experiments, each performed in duplicate. *P < .01 vs no added PF4.
Cells express 2 native species of TM: a higher molecular weight form containing a single CS side chain that varies in length and a lower molecular weight form lacking CS (TM-CS).33 CS side chain is attached to the TM GAG domain (Ser/Thr-rich domain) and can be cleaved by chondroitinase ABC.34 Thrombin binds with high affinity to the fifth and sixth epidermal growth factor-like domains of TM,35 but direct interaction between thrombin and CS has also been demonstrated.36 Therefore, we compared the effect of PF4 on aPC generation in the presence of TM that either contains or lacks CS side chain. In the absence of PF4 and the presence of a low concentration of IIa (0.2nM), there was a 60% decrease of aPC generation when TM-CS was used (Figure 1A), whereas aPC generation at 2nM IIa was identical for TM and TM-CS (Figure 1B). In keeping with previous findings,12 there was no increase in aPC generation with increasing concentrations of PF4 using TM-CS at both low and high concentrations of IIa (Figure 1).
Thus, both the capacity of PF4 to form tetramers and the presence of CS on TM are required to enhance aPC formation in vitro. The bell-shaped response of aPC formation raises the possibility that formation of large complexes between tetrameric PF4 and TM CS-side chain is optimal at a specific molar ratio of PF4 to CS, as we reported previously for PF4 interaction with heparin and cell surface GAGs.3,7 The concentration of PF4 needed to maximize aPC generation is nearly identical to the amount of PF4 necessary to optimize formation of PF4/GAG complexes that are recognized by HIT antibody on platelets and monocytes.7,8
Role of tetrameric PF4 and TM CS side chains in aPC generation on TM-expressing cells
We next asked whether PF4 enhances aPC generation on cell surfaces expressing TM and whether this enhancement involves additional cell surface GAGs. PF4 enhanced aPC generation by IIa on TM-expressing cells followed a similar bell-shaped curve as in solution, with a peak activity seen at approximately 3μM of PF4 (Figure 2A). PF4-mediated stimulation on cells was dependent on the presence of both IIa and TM. At optimal PF4 concentrations, aPC generation was enhanced 1.5-fold on TM-expressing cells compared with 4-fold using soluble TM (Figures 2A and 1A, respectively). This difference is probably because only a subpopulation of the cell-associated TM contains CS.33
Figure 2.
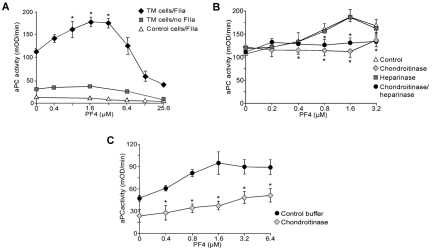
PF4 stimulation of aPC generation on the surface of TM-expressing cells. (A) aPC generation studies using TM-expressing or control K293 cells. Cells were incubated with increased concentration of PF4 followed by addition of PC (500nM) in the absence or presence of 2nM IIa. Data are mean ± SD of 3 experiments, each performed in duplicate. *P < .05 vs no added PF4. (B) Generation of aPC as in panel A, either untreated or treated with 1 U/mL of heparinase, chondroitinase, or both enzymes. Data are mean ± SD of 3 experiments, each performed in duplicate. *P < .02 vs control. (C) Generation of aPC using primary HAECs treated with either 2.5 U/mL of chondroitinase or buffer. Data are mean ± SD of 2 experiments performed on cells on passage P5 and P6, each done twice. *P < .01 vs buffer.
Endothelial cell surfaces bear HS and CS that have a biologic role in capturing chemokines.37 Thus, we examined the effect of PF4 on the generation of aPC in the presence of TM expressed on K293 cells pretreated with chondroitinase, which removes CS, and/or heparinase, which cleaves HS38 (Figure 2B). Exposure of TM-K293 cells to chondroitinase abolished PF4's stimulatory effect on aPC generation, whereas treatment with heparinase alone had no effect. The combination of the 2 enzymes had the same effect as chondroitinase alone. These studies are consistent with the CS, presumably on the GAG domain of TM, as being critical for PF4-mediated stimulation of aPC generation and suggest no contribution from cell-surface heparan sulfate.
Further we examined the PF4's effect on the generation of aPC by IIa in the presence of TM expressed on primary endothelial cells. We have chosen HAECs as they were reported to have the highest (up to 34%) fraction of TM modified with CS.39,40 PF4 increased the levels of aPC generated on the surface of those cells (Figure 2C), similarly as it was shown earlier for endothelial cells derived from human umbilical vein or human dermal microvasculature.13 Treatment of HAECs with chondroitinase inhibited this increase. Thus, we attributed these observations to the fraction of TM containing CS on these human endothelial cells.
aPC generation in vivo
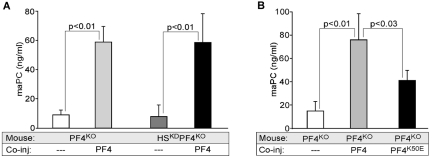
To further explore whether surface HS present on the endothelium was involved in augmenting aPC formation by PF4 effect, we carried out in vivo studies using HSKD mice that have reduced sulfation of endothelial cell surface HS.27 These HSKD mice were on a murine PF4KO background to limit available PF4 to that which was administered exogenously. After infusion of 80 U/kg of murine IIa in the presence of 5 mg/kg of human PF4 (Figure 3A), aPC generation was increased to the same extent in PF4KO and in PF4KO/HSKD mice. PF4K50E at the same concentration did not enhance aPC formation to the same extent as WT PF4 (Figure 3B). These in vivo results provide further support for a CS-specific role rather than additional surface HS in the enhancement of aPC generation by PF4. As in the in vitro studies, infusion of PF4K50E with its impaired ability to form tetramers was less effective than infused WT PF4 in amplifying aPC formation.
Figure 3.
PF4 stimulation of aPC in vivo in mice plasma after injection of IIa. Mouse aPC measured in plasma after coinjection of IIa (80 U/kg) and PF4 (5 mg/kg). (A) Control and HSKD mice on PF4KO background (n = 5-10 animals per arm). (B) PF4KO mice as in panel A but coinjected with either WT PF4 or PF4K50E (both at 5 mg/kg). Levels of n = 5 to 10 animals per group. Data are mean ± SD.
aPC generation in the presence of PRT
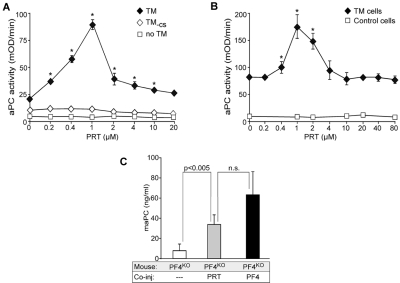
The fact that the effect of PF4 on aPC generation was optimal at the same concentration needed to form large PF4/GAG complexes on cell surfaces led us to ask whether PRT, another positively charged small molecule that also forms large complexes with GAGs,5 can enhance aPC generation by a similar mechanism. Slungaard and Key already noted that PRT can increase aPC generation.12 Addition of PRT increased aPC formation in the presence of IIa following a similar bell-shape pattern as PF4, both in the presence of TM in solution and on TM-expressing K239 cells (Figure 4A and B, respectively). Similarly to PF4, PRT in the presence of TM-CS did not affect aPC generation (Figure 4A). PRT also augmented aPC formation in vivo in the mouse IIa infusion model (Figure 4C). Thus, PRT, which can substitute for PF4 in the formation of large complexes with GAGs, enhances aPC generation following a similar bell-shaped curve of activation, providing additional support for complex development between PF4 with CS on TM at a specific molar ratio.
Figure 4.
PRT stimulation of aPC generation in vitro and in vivo. (A) Generation of aPC as in Figure 1, but with increasing concentrations of PRT. Data are ± SD of 3 experiments, each performed in duplicate. *P < .01 vs no PRT. (B) Generation of aPC as in Figure 2 with TM-expressing or control K293 cells, but with increasing concentrations of PRT. Data are mean ± SD of 3 experiments, each performed in duplicate. *P < .02 vs no PRT. (C) Generation of aPC as in Figure 3 with PF4KO mice, but with PRT or PF4 infused (n = 4 or 5 animals per arm). Data are mean ± SD. n.s. indicates not significant.
Effect of heparin on PF4- and PRT-dependent aPC generation
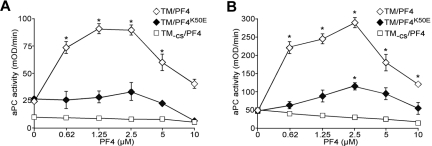
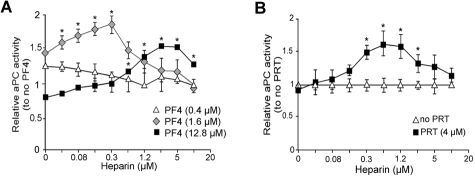
Heparin has a higher affinity for PF4 than other GAGs and efficiently competes for binding to PF4.7,16 We examined the effect of heparin on the PF4-mediated stimulation of aPC generation on TM-K293 cells (Figure 5A). At a low PF4 concentration (0.4μM), low-dose heparin rapidly abolished increased aPC generation. At a near-optimal concentration of PF4 (1.6μM), low-dose heparin increased PF4 stimulation of aPC generation until the heparin concentration reached 0.3μM. Further increases in heparin concentration decreased aPC formation. At a high, above peak, concentration of PF4 (12.8μM), addition of heparin increased aPC generation until the heparin concentration exceeded 5μM. Thus, the biphasic effect of heparin on aPC activity is seen in the presence of higher than optimal PF4 concentrations. At lower PF4 concentrations, heparin decreases aPC generation in a dose-dependent manner. Similar outcomes were seen when PRT was used to enhance aPC generation (Figure 5B), suggesting that both PF4 and PRT affect aPC generation by the analogous mechanisms. This result suggests that the effect of heparin on aPC generation in vivo will depend on the level of free PF4 and perhaps on the presence of other small, positively charged proteins, such as histones, recently described to be present in severe sepsis.41
Figure 5.
Effect of heparin on aPC generation on TM-K293 cells. (A) Effect of heparin on aPC generation on TM-K293 cells in the presence of 3 different concentrations of PF4: below (0.4μM), near (1.6μM), or above (12.8μM) maximal aPC stimulation activity. Data are mean ± SD of 3 or 4 experiments, each performed in duplicate. *P < .01 vs no heparin. (B) Various amounts of heparin were added to TM cells in the presence of 4μM PRT, a concentration above peak aPC stimulation. Graphs represent the mean ± SD of 3 experiments, each performed in duplicate. *P < .01 vs no heparin. Heparin activity was 196 U/mg, which corresponds to 3 U/mL for 1μM of heparin in the assay mix.
Effect of HIT antibodies on the PF4/TM complexes and aPC generation
Our studies are consistent with PF4 stimulating aPC generation by forming large complexes with CS moieties on TM molecules. Similar PF4 complexes with heparin and/or surface GAGs are antigenic in HIT.3 Antibodies formed in patients with HIT and the HIT-like murine monoclonal antibody KKO16,42 bind to the PF4/GAG complexes in solution and on cell surfaces depending on PF4/GAG ratio. Heparin shifts the bell-shaped curve of surface PF4/GAG HIT antigenicity, such that higher PF4 concentrations are required to mediate binding of the same amount of HIT antibody.7
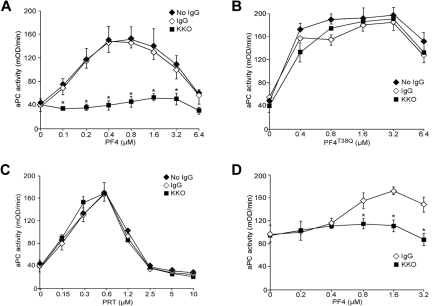
We therefore tested whether PF4/TM complexes are also recognized by these antibodies. Using soluble TM, we found that KKO specifically inhibited PF4's ability to enhance aPC generation (Figure 6A). Partial activity was observed at the concentration of 100 μg/mL and 200 μg/mL (data not shown), and 400 μg/mL completely blocked aPC generation. Thus, we have used 400 μg/mL (3μM) as concentration to use in subsequent studies. In contrast, a previously described mutant PF4T38Q, which forms complexes with GAGs that are not recognized by KKO or a subgroup of HIT antibodies,42 was able to form complexes with TM and potentiate aPC generation, but this potentiation was not inhibited by KKO (Figure 6B). PRT complexes with heparin or GAGs are also not recognized by KKO.5 Consistent with this observation, KKO did not have any effect when PF4 was replaced with PRT in this aPC assay (Figure 6C). Finally, inhibition of aPC generation on the surface of TM-K293 cells was also inhibited by KKO (Figure 6D).
Figure 6.
Effect of KKO on PF4 enhancement of aPC formation. (A) aPC generation study as in Figure 1B, but with 400 μg/mL of KKO or control isotype IgG present. Experiments were done 4 times in duplicate. Data are mean ± SD. *P < .05 vs control IgG. (B) Same as in panel A, but PF4T38Q was used (n = 3, each done in duplicate). (C) Same as in panel A, but PRT at increasing concentration was used (n = 3, each done in duplicate). Data are mean ± SD of 4 experiments. (D) aPC generation study as in Figure 2A, but with 400 μg/mL of KKO or control isotype IgG present. Experiments were done 3 times in duplicate. Data are mean ± SD. *P < .05 vs control IgG.
Throughout the study, we used TM-expressing cells that express a low (0.4:1) endothelial cell protein C receptor (EPCR)/TM ratio.43 We also performed the experiments using cells expressing higher (8.4:1) EPCR/TM ratio43 and observed that similar biphasic response of aPC generation with increased concentrations of PF4 and KKO was able to inhibit PF4 effect (supplemental Figure 1, available on the Blood Web site; see the Supplemental Materials link at the top of the online article).
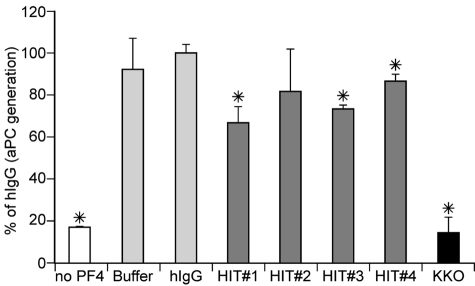
Although KKO behaves in many ways like HIT antibodies, we extended our studies using PF4/heparin affinity purified IgG isolated from 4 persons that had developed HIT by both clinical and laboratory criteria compared with a control hIgG (Figure 7). Antibodies from 3 of 4 patients significantly inhibited the increase in aPC generation in the presence of PF4 (P < .03 compared with hIgG for each), providing further additional evidence that HIT-like PF4/GAG complexes are involved in the PF4-mediated enhancement of aPC formation and endogenous antithrombotic mechanisms.
Figure 7.
Effect of HIT patients on PF4 enhancement of aPC formation. aPC generation was performed as in Figure 1B, but in the absence or presence of a single concentration of PF4 (3.2μM) and 400 μg/mL of either control hIgG, HIT anti-PF4/heparin IgG antibodies from patients 1 to 4 or KKO. Experiments were done 3 times. Data are mean plus or minus SD. *P < .03 vs control hIgG.
Discussion
Our studies show that PF4-mediated acceleration of aPC activity via CS bound to TM exhibits the same bell-shape profile as seen for the formation of HIT antigenic complexes of PF4 with heparin in solution or with GAGs on cell surfaces.7,8 Heparin competition for the binding of PF4 to TM's CS can modulate PF4-dependent enhancement of aPC formation by altering the effective concentration of PF4. This is similar to heparin effect on binding of HIT antibody to platelets and monocytes7,8 and depends on PF4 and heparin relative concentrations. Enhanced aPC generation, like HIT antigen formation, is also dependent on tetramerization of PF4. HIT antibodies preferentially recognize tetrameric PF4 bound to heparin or cellular GAGs when PF4/GAG ratio allows for the formation of large complexes.3 Our findings that HIT antibodies block PF4's effect further support the conclusion that large complexes formed between PF4 and TM's CS are involved in the ability of PF4 to modulate aPC activity. PRT, also known to form large complexes with negatively charged molecules,5 similarly augment aPC generation. However, KKO does not recognize PRT/GAG complexes5 and does not block PRT potentiation of aPC generation. The mechanism by which formation of large complexes between TM's CS and PF4 increases TM's cofactor activity toward aPC generation will require additional study. In part, it might involve complex stability or TM orientation, but other possibilities, including approximation of TM and PC molecules through CS/PF4 complexes, can be envisioned.
We have proposed that the capacity of PF4 to form large complexes with anionic domains on cell surface proteins may have an important role in hemostasis based on a murine FeCl3 carotid artery injury model that platelet-released PF4 is needed for optimal thrombus formation in vivo.26 Mice with either excess or deficient levels of PF4 were characterized by a decrease in thrombus formation. In PF4null mice, infusion of increasing concentrations of PF4 improved thrombus formation following a bell-shaped curve. Correspondingly, infusion of heparin corrected the thrombus formation in mice with an excess of PF4. Besides its role in enhancing aPC generation, PF4 has been shown to affect multiple steps in hemostasis. This includes neutralizing heparin-dependent enhancement of the inhibition of thrombin inhibition by antithrombin and the potentiation of platelet aggregation.32 If these multiple effects of PF4 in hemostasis/thrombosis are dependent on PF4/GAG complex formation, then HIT antibodies may affect many of these pathways in addition to its blocking PF4's interaction with TM. Whether the ability of HIT antibodies to block PF4's enhancement of aPC generation contributes to the prothrombotic state seen in patients with HIT will need further study. Infusion of exogenous aPC or the aforementioned approaches to enhance endogenous aPC activity using small cationic molecules merits study in an animal model of HIT.
Heparinization is the standard of care in patients with disseminated intravascular coagulation. We show that heparin can improve aPC generation when the level of PF4 is above what is optimal for its complex formation with CS on TM, but it has the opposite effect when PF4 level is low. We have previously shown that there is an individual variability in PF4 content per platelet in the general population.44 Whether PF4 levels in patients with disseminated intravascular coagulation before or on heparin can predict efficacy of aPC generation and clinical outcome can be tested. Our studies also suggest that PF4 or other small positively charged molecules, such as PRT, might be useful to enhance aPC generation in patients, but only in situations where there is not already excessive circulating PF4 and/or other small positively charged molecules, such as histones.41
In conclusion, we provide evidence that enhancement of aPC by PF4 through its interaction with TM requires formation of large complexes between PF4 and the CS side chain of TM at a specific molar ratio. This model also explains how heparin affects aPC generation in the presence of PF4. Large PF4/TM CS complexes are similar to those involved in HIT. HIT antibodies appear to bind to the complexes and block, to a variable extent, the enhanced aPC generation. Other small positively charged molecules, such as PRT, can substitute for PF4 in this process, but HIT antibody does not affect PRT-enhanced aPC generation. We think that these studies provide one example of how PF4/GAG “HIT-like” complexes can have a physiologic role, offer insights into the potential usefulness of heparin, PF4, and PRT in disseminated intravascular coagulation, and provide a novel pathway by which HIT antibodies might be prothrombotic.
Supplementary Material
Acknowledgments
The authors thank Dr Charles Esmon and Garry L. Farrell for reagents for mouse aPC ELISA, Dr Rodney M. Camire for the supply of human protein C, Dr Laurent O. Mosnier for TM/K293 cells, and Mia Theroux and Dr Tibor Turoczi for assistance in mouse breeding and experiments.
This work was supported by the American Heart Association grants (2251065, M.A.K.; and 0735277N, L.R.) and National Institutes of Health grants (PO1HL57345, J.D.E.; HL13629, R.H.A.; and PO1HL40387 and RO1HL84006, M.P.).
Footnotes
The online version of this article contains a data supplement.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Authorship
Contribution: M.A.K. was the primary investigator who designed, supervised, and carried out the described studies, analyzed and interpreted data, and wrote the manuscript; S.K. contributed to the design and interpretation of the aPC study, provided the TM-CS, and helped with manuscript revision; L.R. and D.B.C. assisted in KKO assays and interpretation; L.Z. performed purification of WT and mutant PF4s; V.H. purified and evaluated antigen-specific HIT antibodies; K.A. performed in vivo studies; J.D.E. created and supplied HSKD mice and assisted in analysis of data obtained with the use of those mice; D.W.B. and R.H.A. supplied HIT patient plasma and clinical data, assisted with assay interpretation, and revised the manuscript; and M.P. provided overall direction, helped with designing and analysis of the studies, and contributed to manuscript preparation.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Correspondence: M. Anna Kowalska, Children's Hospital of Philadelphia, 3615 Civic Center Blvd, Philadelphia, PA 19104; e-mail: kowalska@email.chop.edu.
References
- 1.Poncz M, Rauova L, Cines DB. The role of surface PF4: glycosaminoglycan complexes in the pathogenesis of heparin-induced thrombocytopenia (HIT). Pathophysiol Haemost Thromb. 2006;35:46–49. doi: 10.1159/000093543. [DOI] [PubMed] [Google Scholar]
- 2.Zhang X, Chen L, Bancroft DP, Lai CK, Maione TE. Crystal structure of recombinant human platelet factor 4. Biochemistry. 1994;33:8361–8366. doi: 10.1021/bi00193a025. [DOI] [PubMed] [Google Scholar]
- 3.Rauova L, Poncz M, McKenzie SE, et al. Ultralarge complexes of PF4 and heparin are central to the pathogenesis of heparin-induced thrombocytopenia. Blood. 2005;105(1):131–138. doi: 10.1182/blood-2004-04-1544. [DOI] [PubMed] [Google Scholar]
- 4.Suvarna S, Espinasse B, Qi R, et al. Determinants of PF4/heparin immunogenicity. Blood. 2007;110(13):4253–4260. doi: 10.1182/blood-2007-08-105098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Chudasama SL, Espinasse B, Hwang F, et al. Heparin modifies the immunogenicity of positively-charged proteins. Blood. 2010;116(26):6046–6053. doi: 10.1182/blood-2010-06-292938. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Amiral J, Pouplard C, Vissac AM, Walenga JM, Jeske W, Gruel Y. Affinity purification of heparin-dependent antibodies to platelet factor 4 developed in heparin-induced thrombocytopenia: biological characteristics and effects on platelet activation. Br J Haematol. 2000;109(2):336–341. doi: 10.1046/j.1365-2141.2000.02034.x. [DOI] [PubMed] [Google Scholar]
- 7.Rauova L, Zhai L, Kowalska MA, Arepally GM, Cines DB, Poncz M. Role of platelet surface PF4 antigenic complexes in heparin-induced thrombocytopenia pathogenesis: diagnostic and therapeutic implications. Blood. 2006;107(6):2346–2353. doi: 10.1182/blood-2005-08-3122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Rauova L, Hirsch JD, Greene TK, et al. Monocyte-bound PF4 in the pathogenesis of heparin-induced thrombocytopenia. Blood. 2010;116(23):5021–5031. doi: 10.1182/blood-2010-03-276964. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Xiao Z, Visentin GP, Dayananda KM, Neelamegham S. Immune complexes formed following the binding of anti-platelet factor 4 (CXCL4) antibodies to CXCL4 stimulate human neutrophil activation and cell adhesion. Blood. 2008;112(4):1091–1100. doi: 10.1182/blood-2008-04-153288. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Cines DB, Tomaski A, Tannenbaum S. Immune endothelial-cell injury in heparin-associated thrombocytopenia. N Engl J Med. 1987;316(10):581–589. doi: 10.1056/NEJM198703053161004. [DOI] [PubMed] [Google Scholar]
- 11.Arepally G, Cines DB. Pathogenesis of heparin-induced thrombocytopenia and thrombosis. Autoimmun Rev. 2002;1(3):125–132. doi: 10.1016/s1568-9972(02)00031-9. [DOI] [PubMed] [Google Scholar]
- 12.Slungaard A, Key NS. Platelet factor 4 stimulates thrombomodulin protein C-activating cofactor activity: a structure-function analysis. J Biol Chem. 1994;269(41):25549–25556. [PubMed] [Google Scholar]
- 13.Slungaard A, Fernandez JA, Griffin JH, et al. Platelet factor 4 enhances generation of activated protein C in vitro and in vivo. Blood. 2003;102(1):146–151. doi: 10.1182/blood-2002-11-3529. [DOI] [PubMed] [Google Scholar]
- 14.Dudek AZ, Pennell CA, Decker TD, Young TA, Key NS, Slungaard A. Platelet factor 4 binds to glycanated forms of thrombomodulin and to protein C: a potential mechanism for enhancing generation of activated protein C. J Biol Chem. 1997;272(50):31785–31792. doi: 10.1074/jbc.272.50.31785. [DOI] [PubMed] [Google Scholar]
- 15.Kowalska MA, Mahmud SA, Lambert MP, Poncz M, Slungaard A. Endogenous platelet factor 4 stimulates activated protein C generation in vivo and improves survival after thrombin or lipopolysaccharide challenge. Blood. 2007;110(6):1903–1905. doi: 10.1182/blood-2007-03-081901. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Arepally GM, Kamei S, Park KS, et al. Characterization of a murine monoclonal antibody that mimics heparin-induced thrombocytopenia antibodies. Blood. 2000;95(5):1533–1540. [PubMed] [Google Scholar]
- 17.Park KS, Rifat S, Eck H, Adachi K, Surrey S, Poncz M. Biologic and biochemic properties of recombinant platelet factor 4 demonstrate identity with the native protein. Blood. 1990;75(6):1290–1295. [PubMed] [Google Scholar]
- 18.Lu G, Chhum S, Krishnaswamy S. The affinity of protein C for the thrombin.thrombomodulin complex is determined in a primary way by active site-dependent interactions. J Biol Chem. 2005;280(15):15471–15478. doi: 10.1074/jbc.M500881200. [DOI] [PubMed] [Google Scholar]
- 19.Parkinson JF, Grinnell BW, Moore RE, Hoskins J, Vlahos CJ, Bang NU. Stable expression of a secretable deletion mutant of recombinant human thrombomodulin in mammalian cells. J Biol Chem. 1990;265(21):12602–12610. [PubMed] [Google Scholar]
- 20.Baugh RJ, Krishnaswamy S. Role of the activation peptide domain in human factor X activation by the extrinsic Xase complex. J Biol Chem. 1996;271(27):16126–16134. doi: 10.1074/jbc.271.27.16126. [DOI] [PubMed] [Google Scholar]
- 21.Stearns DJ, Kurosawa S, Sims PJ, Esmon NL, Esmon CT. The interaction of a Ca2+-dependent monoclonal antibody with the protein C activation peptide region: evidence for obligatory Ca2+ binding to both antigen and antibody. J Biol Chem. 1988;263(2):826–832. [PubMed] [Google Scholar]
- 22.Whitlatch NL, Kong DF, Metjian AD, Arepally GM, Ortel TL. Validation of the high-dose heparin confirmatory step for the diagnosis of heparin-induced thrombocytopenia. Blood. 2010;116(10):1761–1766. doi: 10.1182/blood-2010-01-262659. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Sheridan D, Carter C, Kelton JG. A diagnostic test for heparin-induced thrombocytopenia. Blood. 1986;67(1):27–30. [PubMed] [Google Scholar]
- 24.Suh JS, Aster RH, Visentin GP. Antibodies from patients with heparin-induced thrombocytopenia/thrombosis recognize different epitopes on heparin: platelet factor 4. Blood. 1998;91(3):916–922. [PubMed] [Google Scholar]
- 25.Feistritzer C, Schuepbach RA, Mosnier LO, et al. Protective signaling by activated protein C is mechanistically linked to protein C activation on endothelial cells. J Biol Chem. 2006;281(29):20077–20084. doi: 10.1074/jbc.M600506200. [DOI] [PubMed] [Google Scholar]
- 26.Eslin DE, Zhang C, Samuels KJ, et al. Transgenic mice studies demonstrate a role for platelet factor 4 in thrombosis: dissociation between anticoagulant and antithrombotic effect of heparin. Blood. 2004;104(10):3173–3180. doi: 10.1182/blood-2003-11-3994. [DOI] [PubMed] [Google Scholar]
- 27.Wang L, Fuster M, Sriramarao P, Esko JD. Endothelial heparan sulfate deficiency impairs L-selectin- and chemokine-mediated neutrophil trafficking during inflammatory responses. Nat Immunol. 2005;6(9):902–910. doi: 10.1038/ni1233. [DOI] [PubMed] [Google Scholar]
- 28.Kisanuki YY, Hammer RE, Miyazaki J, Williams SC, Richardson JA, Yanagisawa M. Tie2-Cre transgenic mice: a new model for endothelial cell-lineage analysis in vivo. Dev Biol. 2001;230(2):230–242. doi: 10.1006/dbio.2000.0106. [DOI] [PubMed] [Google Scholar]
- 29.Grobe K, Ledin J, Ringvall M, et al. Heparan sulfate and development: differential roles of the N-acetylglucosamine N-deacetylase/N-sulfotransferase isozymes. Biochim Biophys Acta. 2002;1573(3):209–215. doi: 10.1016/s0304-4165(02)00386-0. [DOI] [PubMed] [Google Scholar]
- 30.Li W, Zheng X, Gu J, et al. Overexpressing endothelial cell protein C receptor alters the hemostatic balance and protects mice from endotoxin. J Thromb Haemost. 2005;3(7):1351–1359. doi: 10.1111/j.1538-7836.2005.01385.x. [DOI] [PubMed] [Google Scholar]
- 31.Brandt E, Ludwig A, Petersen F, Flad HD. Platelet-derived CXC chemokines: old players in new games. Immunol Rev. 2000;177:204–216. doi: 10.1034/j.1600-065x.2000.17705.x. [DOI] [PubMed] [Google Scholar]
- 32.Lambert MP, Sachais BS, Kowalska MA. Chemokines and thrombogenicity. Thromb Haemost. 2007;97(5):722–729. doi: 10.1160/th07-01-0046. [DOI] [PubMed] [Google Scholar]
- 33.Gerlitz B, Hassell T, Vlahos CJ, Parkinson JF, Bang NU, Grinnell BW. Identification of the predominant glycosaminoglycan-attachment site in soluble recombinant human thrombomodulin: potential regulation of functionality by glycosyltransferase competition for serine474. Biochem J. 1993;295(1):131–140. doi: 10.1042/bj2950131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Wen DZ, Dittman WA, Ye RD, Deaven LL, Majerus PW, Sadler JE. Human thrombomodulin: complete cDNA sequence and chromosome localization of the gene. Biochemistry. 1987;26(14):4350–4357. doi: 10.1021/bi00388a025. [DOI] [PubMed] [Google Scholar]
- 35.Nagashima M, Lundh E, Leonard JC, Morser J, Parkinson JF. Alanine-scanning mutagenesis of the epidermal growth factor-like domains of human thrombomodulin identifies critical residues for its cofactor activity. J Biol Chem. 1993;268(4):2888–2892. [PubMed] [Google Scholar]
- 36.Ye J, Esmon CT, Johnson AE. The chondroitin sulfate moiety of thrombomodulin binds a second molecule of thrombin. J Biol Chem. 1993;268(4):2373–2379. [PubMed] [Google Scholar]
- 37.Wang D, Sai J, Richmond A. Cell surface heparan sulfate participates in CXCL1-induced signaling. Biochemistry. 2003;42(4):1071–1077. doi: 10.1021/bi026425a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Ernst S, Langer R, Cooney CL, Sasisekharan R. Enzymatic degradation of glycosaminoglycans. Crit Rev Biochem Mol Biol. 1995;30(5):387–444. doi: 10.3109/10409239509083490. [DOI] [PubMed] [Google Scholar]
- 39.Lin JH, McLean K, Morser J, et al. Modulation of glycosaminoglycan addition in naturally expressed and recombinant human thrombomodulin. J Biol Chem. 1994;269(40):25021–25030. [PubMed] [Google Scholar]
- 40.Sadler JE. Thrombomodulin structure and function. Thromb Haemost. 1997;78(1):392–395. [PubMed] [Google Scholar]
- 41.Xu J, Zhang X, Pelayo R, et al. Extracellular histones are major mediators of death in sepsis. Nat Med. 2009;15(11):1318–1321. doi: 10.1038/nm.2053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Ziporen L, Li ZQ, Park KS, et al. Defining an antigenic epitope on platelet factor 4 associated with heparin-induced thrombocytopenia. Blood. 1998;92(9):3250–3259. [PubMed] [Google Scholar]
- 43.Liaw PC, Mather T, Oganesyan N, Ferrell GL, Esmon CT. Identification of the protein C/activated protein C binding sites on the endothelial cell protein C receptor: implications for a novel mode of ligand recognition by a major histocompatibility complex class 1-type receptor. J Biol Chem. 2001;276(11):8364–8370. doi: 10.1074/jbc.M010572200. [DOI] [PubMed] [Google Scholar]
- 44.Lambert MP, Rauova L, Bailey M, Sola-Visner MC, Kowalska MA, Poncz M. Platelet factor 4 is a negative autocrine in vivo regulator of megakaryopoiesis: clinical and therapeutic implications. Blood. 2007;110(4):1153–1160. doi: 10.1182/blood-2007-01-067116. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.