Abstract
Thrombomodulin (TM) is a predominantly endothelial transmembrane glycoprotein that modulates hemostatic function through a domain that controls thrombin-mediated proteolysis and an N-terminal lectin-like domain that controls inflammatory processes. To test the hypothesis that TM is a determinant of malignancy and dissect the importance of these functional domains in cancer biology, metastatic potential was evaluated in TMPro mice expressing a mutant form of TM with reduced thrombin affinity and TMLeD mice lacking the N-terminal lectin-like domain. Studies of TMPro mice revealed that TM is a powerful determinant of hematogenous metastasis. TMPro mice exhibited a strongly prometastatic phenotype relative to control mice that was found to result from increased survival of tumor cells newly localized to the lung rather than any alteration in tumor growth. The impact of the TMPro mutation on metastasis was dependent on both tumor cell-associated tissue factor and thrombin procoagulant function. In contrast, expression of a mutant form of TM lacking the lectin-like domain had no significant impact on metastasis. These studies directly demonstrate for the first time that TM-mediated regulation of tumor cell-driven procoagulant function strongly influences metastatic potential and suggest that endothelial cell-associated modulators of hemostasis may represent novel therapeutic targets in limiting tumor dissemination.
Introduction
Detailed studies of the role of hemostatic factors in cancer biology have established that metastasis is strongly dependent on a cooperative interplay between tumor cell-associated procoagulant function and circulating hemostatic system components.1,2 However, the significance of endothelial cell-associated regulators of coagulation to cancer progression remains largely unexplored. Thrombomodulin (TM) is a predominantly endothelial cell-associated transmembrane glycoprotein that serves as a high affinity receptor for thrombin as well as other ligands through distinct extracellular domains.3–6 TM engagement of thrombin results in a profound restriction in thrombin-mediated cleavage of prothrombotic substrates, including fibrinogen, factor V, factor VIII, factor XI, factor XIII, and platelet-associated protease activated receptors (PARs), while enhancing the proteolytic activation of the anticoagulant/anti-inflammatory protease zymogen, protein C, and the carboxypeptidase zymogen, thrombin-activatable fibrinolysis inhibitor (TAFI).5,6 TM also controls multiple biologic processes through thrombin-independent mechanisms that are, at least in part, mediated by the N-terminal lectin-like domain, including complement activation, sequestration of inflammatory mediators, apoptosis, inflammatory cell migration, cytokine production and cell signaling events.3,7–11 The broad biologic significance of TM is underscored by studies establishing the early developmental failure of TM-deficient embryos and pronounced derangements in inflammatory processes and vascular integrity in adult mice expressing mutant forms of TM.3–5,12–14
TM has been implicated in cancer biology through several studies showing that TM expression within tumor tissue correlates with a better prognosis for multiple cancers.12,15–17 Other studies have suggested that modifying TM expression by tumor cells can alter important aspects of the transformed phenotype, including tumor cell migration in vitro and tumor cell proliferation.18–21 An influence of nonnative, exogenous TM on cancer biology has also been reported in studies showing that administration of soluble TM limits metastasis in mice.22 The concept that TM may be significant in cancer biology is also compatible with indirect studies focusing on activated protein C (aPC) and the endothelial protein C receptor (EPCR). However, no direct data are available regarding the contribution of native, membrane-associated TM within normal tissues to the malignant phenotype, the precise functional features of the endothelial TM that influence cancer biology, and the potential significance of TM as a therapeutic target in malignancy. In the absence of direct data, native TM could be of little significance or could influence malignancy either positively or negatively through hemostatic and/or inflammatory processes linked to either modulation of thrombin function or the lectin-like domain.3,8,9,11,23
To test the hypothesis that native TM is a fundamental determinant of malignancy, comparative analyses of experimental and spontaneous metastasis were performed in mice homozygous for a TMGlu387Pro substitution (TMPro), mice expressing normal levels of a mutant TM lacking the lectin-like domain (TMLeD), and wild-type (WT) control animals. The TMGlu387Pro substitution is known to result in a pronounced reduction in thrombin binding affinity and a 3 orders-of-magnitude diminution in protein C activation.14 Deletion of the TM lectin-like domain results in no apparent alterations in hemostatic function or generation of activated protein C (aPC), but leads to a pro-inflammatory phenotype in the context of several experimental challenges.8,9,11 Despite these biochemical alterations, neither unchallenged TMPro nor TMLeD mice spontaneously develop overt pathologies, and thus survive well into adulthood. Detailed studies of multiple distinct transplantable murine tumor models revealed that TM is an exceptionally powerful modifier of metastatic potential, demonstrating for the first time that nontumor cell derived TM is a major determinant of tumor dissemination. TMPro expression had no impact on primary tumor growth, but resulted in a phenotype that very strongly favored the development of hematogenous pulmonary metastases. The prometastatic phenotype of TMPro mice was driven by mechanisms that were highly dependent on tumor cell-associated tissue factor, circulating prothrombin, as well as at least one downstream thrombin target, platelets. In contrast, mice expressing TMLeD developed metastases comparable with control animals, consistent with the conclusion that TM is mechanistically coupled to metastasis primarily through mechanisms involving the regulation of thrombin.
Methods
Transgenic mice and statistical analyses
Both TMPro and TMLeD mice have been previously described.8,14 All studies were performed using 7- to 8-week-old sex-matched cohorts of C57BL/6-inbred TMPro or TMLeD mice paired with C57Bl/6-inbred WT mice derived from the same breeding colony as the experimental animals. The study protocols were approved by the Cincinnati Children's Hospital Research Foundation Institutional Animal Care and Use Committee in accordance with the guidelines of the National Institutes of Health. All P values were determined with a Mann-Whitney U test unless otherwise indicated.
Metastasis assays and histologic analyses
Green fluorescent protein (GFP)–expressing Lewis lung carcinoma cells (LLCGFP), B16 melanoma cells, as well as the tissue factor-expressing (TF+) and tissue factor-deficient (TFO) fibrosarcoma cells used in these studies have all been previously described.2,24–26 The culture and inoculation of tumor cells into mice for experimental and spontaneous metastasis assays followed previously established protocols as indicated within each experiment.2,24,25,27 Tumor and lung tissues were fixed in formalin before processing for H&E and fibrin(ogen) immunostaining essentially as previously described.2,25 Mitotic indices within tumor tissues were established by analyzing ∼ 3000 viable tumor cells from 4 representative H&E-stained sections of tumor tissue harvested from each genotype.28
Analyses of the fate of circulating tumor cells
LLCGFP cells were radiolabeled with the thymidine analog 5-[125I]iodo-2′-deoxyuridine as previously described.2,25 Greater than 99% of the radiolabel within the tumor cells was incorporated into macromolecules based on TCA precipitation. Organs from mice injected with radiolabeled tumor cells were harvested at specified time points and washed in 70% ethanol for 3 days to remove any free isotope. Radioisotope levels in the input inoculums and mouse organs were measured with a Cobra Gamma Counter (Packard).
Antisense oligonucleotide-mediated prothrombin depletion, recombinant hirudin treatment and antibody-mediated platelet depletion
Hepatic prothrombin synthesis was suppressed using a 20-mer ASO “gapmer”29 complementary to a portion of the 3′ noncoding region of the fII mRNA. Cohorts of TMPro and control mice were injected subcutaneously with 50 mg/kg ASO (5′-ATTCCATAGTGTAGGTCCTT-3′) in 200 μL of sterile PBS or a control 20-mer (5′-CCTTCCCTGAAGGTTCCTCC-3′) in parallel on a weekly basis for a total of 4 weeks before tumor cell inoculation. As described previously, these oligonucleotides were formulated with a phosphorothiolate backbone and 2′-O-methoxymethyl groups on the first and last 5 nucleotides.29 Western blot analyses of plasma prothrombin levels in fII ASO treated mice were performed as previously described.30 Recombinant hirudin (Lepirudin; Baxter) was used to directly inhibit thrombin. Here, TMPro mice were IP injected with 20 mg/kg hirudin or saline carrier 25 minutes before intravenous tumor cell injection followed by a second 10 mg/kg hirudin or saline carrier injection 3 hours after tumor cell inoculation. For platelet-depletion experiments, cohorts of TMPro and control mice were injected intraperitoneally with a single dose of 1 mg/kg of a hamster-derived IgG3κ antiplatelet antibody (1B5) or nonimmune total hamster IgG control antibodies (Innovative Research) as previously described 24 hours before tumor cell inoculation.31
Results
TMPro mice exhibit a profoundly increased metastatic phenotype
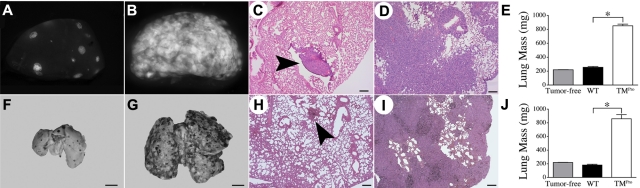
To directly test the hypothesis that host-derived thrombomodulin is an important determinant of metastatic potential, cohorts of WT mice (n = 10) and TMPro mice (n = 8) were intravenously injected with 3 × 105 Lewis lung carcinoma cells engineered to express green fluorescent protein to simplify detection of tumor foci in vivo (LLCGFP). Within 13 days of tumor cell injection, all of the control mice were active and overtly healthy, whereas all of the TMPro mice became distinctly lethargic. After sacrifice, gross examination of the lungs harvested from WT mice revealed a median of 42 surface pulmonary metastases per animal (range 6-90; Figure 1A). In sharp contrast, the lungs harvested from TMPro mice had confluent metastases that were too numerous to count (Figure 1B) and were clearly enlarged. Lungs harvested from WT mice injected with LLCGFP tumor cells were not significantly different in weight than lungs collected from control mice that were not injected with tumor cells. However, the lungs harvested from TMPro mice were 4-fold heavier than lungs collected from WT mice, a difference presumably accounted for by genotype-dependent differences in tumor burden (Figure 1E). This conclusion was confirmed by histologic analyses that revealed scattered small foci of tumor tissue within the lungs harvested from WT mice, while the lungs harvested from TMPro mice were nearly completely effaced by tumor tissue (Figure 1C-D). This dramatic increase in metastatic potential conferred by the TMGlu387Pro substitution was not limited solely to LLC cells. Injection of 5 × 104 B16-BL6 melanoma cells into TMPro mice (n = 6) resulted in almost complete replacement of the lungs with tumor after 15 days (Figure 1G). This is in striking contrast to WT mice (n = 6) injected in parallel, where a median of 50 readily discernable metastatic foci could be detected on gross examination (range 24-92; Figure 1F). In line with these findings, histologic analyses of lungs harvested from B16-challenged TMPro mice revealed near complete effacement of normal lung tissue with tumor (compare Figure 1H and I) and were significantly heavier than those harvested from WT mice (Figure 1J).
Figure 1.
TMPro mice exhibit a profoundly prometastatic phenotype relative to WT mice. Gross appearance of representative single lung lobes harvested from a WT (A) and TMPro mouse (B) 13 days after IV injection of 3 × 105 LLCGFP cells viewed under a fluorescence stereoscope. Note the overwhelming amount of GFP+ tumor tissue in the TMPro lung lobe in panel B relative to the WT animal in panel A. H&E stained sections of lung tissue revealed small, discreet metastatic foci (arrowhead) in lungs from WT mice (C), whereas lungs harvested from TMPro mice were largely effaced by tumor tissue (D). (E) Consistent with the apparent increase in tumor burden, the weight of lung tissue harvested from LLCGFP-challenged TMPro mice was significantly greater than that of lungs harvested from LLCGFP-challenged WT mice, or lungs from tumor-free animals. Similar findings were observed in cohorts of WT and TMPro mice intravenously injected in parallel with B16 melanoma cells. Shown are representative whole lungs from WT (F) and TMPro mice (G) as well as H&E-stained sections of lung tissue from WT (H) and TMPro mice (I). (J) Also paralleling results with LLC cells, the weight of lungs harvested from B16-challenged TMPro mice was significantly greater than that of lungs harvested from B16-challeged WT mice or tumor-free animals. Size bars represent 200 μm (C-D,H-I) or 250 mm (F-G). Data represents mean and SEM, *P < .005.
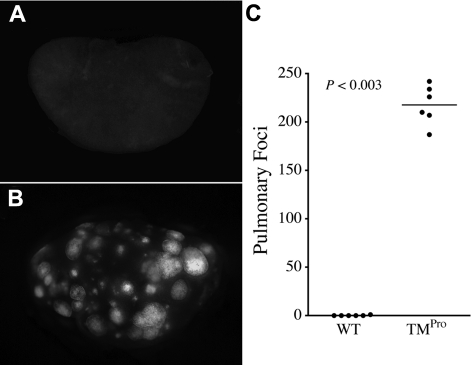
The relative increase in metastatic potential conferred by the TMPro mutation was even more apparent in analyses in which TMPro and WT mice were intravenously injected with 10-fold fewer LLCGFP cells (3 × 104 cells/mouse). This tumor cell dose resulted in essentially no metastases in WT mice, whereas TMPro mice challenged in parallel uniformly developed hundreds of pulmonary metastases (Figure 2). These results suggest that the imposition of the TMGlu387Pro substitution significantly increases the probability that a given circulating tumor cell will successfully form a metastatic focus.
Figure 2.
TMPro expression results in a large metastatic burden even after injection of a relatively low tumor cell burden. Gross appearance of lung lobes harvested from WT (A) and TMPro mice (B) 13 days after intravenous injection of 3 × 104 LLCGFP cells. (C) This modest tumor cell inoculum resulted in essentially no pulmonary metastases in WT mice, whereas TMPro mice developed hundreds of metastatic foci. Horizontal bars represent medians.
TMPro expression promotes spontaneous metastasis
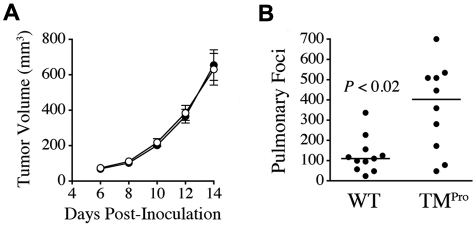
To determine whether TMPro expression influenced the growth of established tumors, 5 × 105 LLCGFP cells were injected into the dorsal subcutis of TMPro and WT mice in parallel (n = 9/group). Palpable tumors were present in mice of both genotypes within 5 days of inoculation and tumor growth rates were indistinguishable between genotypes based on estimation of tumor volume by serial calipation. Tumor mass 12 days after inoculation was similar in TMPro mice (median 400 mg, range 260-670 mg) and control mice (median 410 mg, range 250-740 mg; P = .8). Microscopic analysis of tumor tissue demonstrated dense masses of highly vascularized anaplastic cells with numerous mitoses and focal areas of necrosis that did not differ based on genotype (supplemental Figure 1, available on the Blood Web site; see the Supplemental Materials link at the top of the online article). Consistent with the overall similarity in tumor growth rates, the imposition of TMPro had no significant impact on tumor cell proliferation based on quantitative analyses of mitotic indices in H&E-stained tumor sections harvested from control mice and TMPro animals (mitotic indices of 2.0 ± 0.3% and 1.81 ± 0.26%, respectively, P = not significant). Complementary microscopic analyses of fibrin(ogen) deposition within tumor tissue in WT and TMPro mice indicated that fibrin(ogen) was scant in tumors harvested from animals of both genotypes, qualitatively similar and primarily peritumoral or associated with small areas of focal necrosis (supplemental Figure 1).
The fact that TMPro mice did not exhibit alterations in the growth rate of established tumors provided the opportunity to analyze the effect of the TMGlu387Pro substitution on the more complex process of spontaneous metastasis. Here, LLCGFP cells were injected into the dorsal subcutis of 10 TMPro and 11 WT mice in parallel, and the primary tumors resected after 14 days following an established protocol.27 Again, tumor growth rates were indistinguishable between genotypes (Figure 3A). As expected with this model, metastases were found in the lungs, liver, and regional lymph nodes.25,27 Regional lymphatic metastases of similar size were seen in 8 of 11 WT mice and 7 of 10 TMPro mice (P > .5, Fisher exact test), indicating that TMPro expression had no significant impact on lymphatic metastasis. Liver metastases were too few in either genotype to make meaningful comparisons. In contrast, spontaneous hematogenous metastases to the lungs were significantly increased in the TMPro mice relative to control animals (Figure 3B), paralleling what was observed in experi-mental metastasis assays. This experiment was done twice with similar results.
Figure 3.
The TMPro variant is a significant determinant of spontaneous metastasis, but not tumor growth. (A) Serial calipation after transplantation of LLCGFP cells into the dorsal subcutis of WT (●, n = 11) and TMPro mice (○, n = 10) revealed no genotype-dependent difference in tumor volume at any time point. Data represent means with SEM. (B) Quantitative analysis of spontaneous pulmonary metastases enumerated 14 days after primary tumor resection.
The TMGlu387Pro substitution supports the survival of tumor cells newly localized to the lung
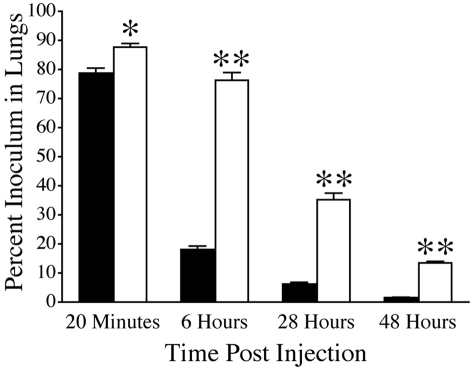
The fact that TMPro mice exhibited increased metastatic potential, but no significant alterations relative to control mice in the growth of established tumors, suggests that TMPro expression more effectively supports events involved in the early establishment of metastatic foci. To better define the role of TM on these early events, we used an established 5-[125I]iodo-2′-deoxyuridine cell labeling technique as a means of tracking tumor cell fate.2,24,25 This label has been shown to remain stable in living tumor cells but is rapidly cleared from dead tumor cells and excreted from the animal.32 Cohorts of TMPro and WT mice were intravenously injected with 1 × 105 radiolabeled LLCGFP cells. Blood, liver, spleen, kidney, heart and lung were harvested from individual cohorts 20 minutes, 6 hours, 28 hours, and 48 hours after inoculation and the amount of radiolabel in each organ measured. Twenty minutes after tumor cell injection the majority of the tumor cell inoculum was present in the lungs of both genotypes, whereas negligible amounts of radiolabel were present in the other organs analyzed regardless of genotype. The apparent pulmonary tumor cell burden was statistically greater in TMPro mice compared with WT mice at this early time point, but the difference was incremental (Figure 4). However, within 6 hours of injection there was a profound genotype-dependent difference in the apparent pulmonary tumor cell load. At this time point < 20% of the initial inoculum remained in lungs harvested from WT mice, whereas ∼ 75% of the initial inoculum was still present in lungs from TMPro mice (Figure 4). The relative difference in the number of apparent tumor cells remaining in the lung increased to almost 10-fold by 48 hours postinjection. Scant radiolabel was detectable in the other organs analyzed at these later time points regardless of genotype. These data suggest that the expression of TMPro promotes metastasis by supporting the early survival of tumor cells newly localized to the lung.
Figure 4.
TMPro expression is a determinant of the sustained adhesion and/or early survival of tumor cells newly localized to the lung. Quantitative analyses of residual radiolabel within the lungs of WT (solid bars) and TMPro mice (open bars) after IV injection of 125I-labeled LLCGFP cells. Cohorts harvested at each time point consisted of 5-6 mice of each genotype. Data represents mean and SEM (*P < .05, **P < .005).
The influence of TM on metastasis is primarily dependent on tumor cell–associated procoagulant function, prothrombin, and platelets
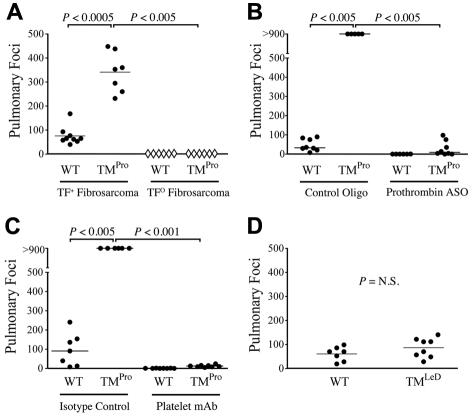
TM is a multifunctional receptor that could influence metastasis independently of hemostatic function. To determine whether the prometastatic phenotype conferred by the TMPro mutation is dependent on tumor cell-associated tissue factor (TF) expression, TMPro and control mice were intravenously challenged with previously described fibrosarcoma cells genetically incapable of TF expression (TFO), or the same fibrosarcoma cells in which TF expression was restored using a murine TF expression vector (TF+).2 As expected based on our previous findings using LLC and B16 cells, TMPro mice injected with 3 × 105 TF+ cells developed significantly more pulmonary metastases than WT mice challenged in parallel (Figure 5A). In contrast, TFO cells were essentially incapable of forming metastases in either WT or TMPro mice, even when injected with 1 × 106 cells/mouse (Figure 5A). These experiments were done twice with similar outcomes. These results indicate that the prometastatic phenotype observed in TMPro mice is strongly dependent on tumor cell-associated TF expression.
Figure 5.
TM influences metastasis primarily via regulation of thrombin function. (A) Quantification of pulmonary foci in WT and TMPro mice 21 days after IV injection of 3 × 105 TF-expressing fibrosarcoma cells (●) or 1 × 106 TF-deficient fibrosarcoma cells (♢). Note that TF-deficient cells were rarely successful in forming metastases, regardless of mouse genotype, even at doses significantly greater than those used to evaluate TF-expressing cells. (B) Diminution of prothrombin levels with a prothrombin specific ASO significantly diminished the number of pulmonary metastases formed 10 days after IV injection of 3 × 105 LLCGFP cells in both WT and TMPro mice. (C) Similarly, reduction of circulating platelets with a platelet-specific antibody resulted in a near complete abrogation of pulmonary metastases formed after IV injection of LLCGFP cells in both WT and TMPro mice. (D) TMLeD mice developed a similar number of pulmonary metastases compared with WT mice after intravenous injection of 3.5 × 104 B16 melanoma cells.
To define the role of circulating prothrombin in the prometastatic phenotype conferred by TMPro expression, cohorts of TMPro and control mice were treated weekly with a prothrombin-specific antisense oligonucleotide (ASO) “gapmer”29 that specifically suppresses hepatic prothrombin expression, or a biologically-irrelevant control oligonucleotide (see “Antisense oligonucleotide-mediated prothrombin depletion, recombinant hirudin treatment, and antibody-mediated platelet depletion” for details). Four weeks after initial ASO treatment, when prothrombin levels were < 5% of normal based on plasma immunoassay (supplemental Figure 2) and the PT and aPTT are prolonged ∼ 3 fold, both groups were challenged with 3 × 105 LLCGFP cells. As expected, TMPro mice treated with the control oligonucleotide, and thus carrying normal prothrombin levels, developed dramatically more pulmonary metastases than WT mice treated with the control oligonucleotide and also carrying normal prothrombin levels. However, in mice carrying very low levels of prothrombin as a consequence of prothrombin-specific ASO-treatment, very few pulmonary metastases were observed in either TMPro or WT mice (Figure 5B). These findings indicate that imposing a pronounced reduction in circulating prothrombin effectively supersedes the prometastatic phenotype conferred by TMPro expression. These data are compatible with the general concept that uncontrolled local thrombin activity at the tumor cell-endothelial cell interface in TMPro mice drives the profoundly prometastatic phenotype observed in these animals. Consistent with this view, the strongly prometastatic phenotype of TMPro mice was also largely eliminated by pretreatment of these animals with the direct thrombin inhibitor, hirudin. TMPro mice treated with saline carrier (n = 7) developed a mean of 288 ± 21 metastatic foci 13 days after IV injection with 3 × 104 LLCGFP cells, whereas hirudin treated TMPro mice (n = 7) challenged with tumor cells in parallel developed 58 ± 24 foci (P < .001).
To determine whether the prometastatic phenotype of TMPro mice is also coupled to downstream procoagulant thrombin substrates (ie, components of the platelet-fibrinogen axis), we pretreated cohorts of TMPro and WT mice with an antiplatelet antibody (αIIb-specific monoclonal antibody 1B5)31 that eliminates > 95% of circulating platelets (data not shown), or nonimmune hamster IgG control antibodies, 24 hours before intravenous LLCGFP inoculation. Platelet depletion profoundly limited metastasis in both WT and TMPro mice (Figure 5C), indicating that the prometastatic effect of the TMGlu387Pro substitution is also critically dependent on at least one downstream thrombin target, platelets.
In addition to regulating hemostasis, TM suppresses inflammatory/immune responses independently of thrombin via its N-terminal lectin-like domain.3,7–11 Mice expressing a mutant form of TM lacking the lectin-like domain (TMLeD) exhibit a significant pro-inflammatory phenotype in response to several distinct experimental challenges.8,9,11 However, unchallenged TMLeD mice exhibit no evidence of hemostatic dysregulation and, even when challenged, are indistinguishable from WT mice with regards to multiple indicators of hemostatic system activation.8,11 To determine whether the TM lectin-like domain is important in metastasis, TMLeD and control mice were IV injected in parallel with 3.5 × 104 B16-F10 melanoma cells and pulmonary metastases evaluated 14 days later. In contrast to what was observed in TMPro mice, TMLeD expression had no significant impact on metastasis (Figure 5D). Taken together, these data are consistent with the conclusion that TM is coupled to metastasis primarily through a mechanism involving regulation of thrombin activity.
Discussion
These studies demonstrate for the first time that native thrombomodulin, a potent endothelial cell-associated regulator of thrombin activity and generation, is an important determinant of metastasis. Mice expressing a mutant form of TM that reduces thrombin binding affinity and thrombin-mediated protein C activation14 exhibited markedly enhanced support of hematogenous metastasis with several tumor cell lines. Notably, the growth of established tumors was not dependent on the integrity of TM. Rather, tumor cell fate studies revealed that TMGlu387Pro expression promoted metastatic potential by supporting the early survival of tumor cells newly localized to the lung. The prometastatic phenotype observed in TMPro mice was highly dependent on tumor cell-associated TF expression, circulating prothrombin, thrombin activity, and at least one distal thrombin target, platelets. Interestingly, the lectin-like domain of TM, a structure that has been shown to play a key role in suppressing inflammation and innate immune responses in multiple other contexts, had no effect on metastatic potential. While these findings do not formally exclude a thrombin-independent contribution of TM to malignancy, they imply that a dominant mechanism by which this multifunctional glycoprotein controls metastatic potential is by directly regulating thrombin procoagulant function and/or indirectly regulating thrombin generation through protein C activation. These studies add a fundamental new dimension to the general understanding of hemostatic factors and malignancy by directly establishing that modulators of thrombin activity/generation that are primarily endothelial cell-associated are major determinants of the malignant phenotype. Furthermore, these studies suggest that therapeutic strategies aimed at preserving or augmenting TM-mediated regulation of thrombin could limit tumor dissemination.
Several mechanisms could be envisioned for the profound influence of native TM on metastatic potential, including multiple mechanisms coupled to thrombin activity in distinct ways. One potential mechanism by which TMPro expression could support metastatic potential is by limiting TM-mediated aPC generation. A role for aPC in metastasis is suggested by previous studies showing that the administration of exogenous aPC and overexpression of the endothelial protein C receptor (EPCR) modestly limit metastasis in mice.33 Although we did not measure aPC levels in our studies, TMPro mice are known to have a reduced capacity to generate aPC,14 thereby reducing the presence of a protease with a spectrum of anticoagulant, antiproliferative, anti-inflammatory and cell-signaling properties, any of which could influence metastatic potential. In addition to loss of anticoagulant function, decreased aPC generation would be expected to limit any aPC-mediated signaling through PAR-1 and secondary alterations in endothelial barrier function and/or anti-inflammatory activities.34 Although PAR-1 signaling through aPC, thrombin or other proteases may be relevant to metastasis in selected settings, nontumor cell PAR-1 signaling does not appear to be uniformly important to tumor dissemination based on the finding that B16 melanoma cell metastasis is indistinguishable in WT and PAR-1–deficient mice.35 However, recent reports suggest aPC may also limit metastasis through sphingosine-1-phosphate receptor 1–mediated vascular endothelial barrier enhancement, thereby limiting tumor cell extravasation.26 The relative importance of aPC-mediated signaling versus anticoagulant function in malignancy remains to be fully explored. However, the findings here that diminution of circulating prothrombin, inhibition of thrombin function, and platelet depletion all supersede the prometastatic phenotype conferred by TMPro expression suggest that the control of prothrombotic processes by TM is at least one major determinant of the overall metastatic success of circulating tumor cells. This conclusion is consistent with studies suggesting that thrombin and multiple downstream procoagulant thrombin substrates (ie, platelet-associated PAR-4, fibrinogen, fXIII) can promote the survival of tumor cells newly localized to the lung.1,36
Beyond its role as a cofactor in aPC generation, the loss of other TM-mediated functions may contribute to the prometastatic phenotype observed in TMPro mice. For example, reduced TAFI carboxypeptidase activity in TMPro mice and potential secondary alterations in fibrinolysis, complement activity and other processes could augment metastasis,3–6 but any contribution of TAFI is likely to be limited based on previous reports showing that the genetic elimination of TAFI in mice had no significant impact on metastatic potential.37 A more attractive mechanism by which TM may influence metastasis independently of aPC generation is by serving as a local “sink for procoagulant thrombin.” Given that TM is a high affinity thrombin receptor that dramatically limits thrombin activity with multiple prothrombotic substrates, binding of thrombin to TM could limit metastasis by preventing thrombin-mediated activation of prothrombotic substrates known to be important in metastasis (eg, fibrinogen, platelet PARs, fXIII),1 The possibility that TM could essentially function as an anticoagulant independently of protein C is consistent with studies showing that soluble thrombomodulin is antithrombotic in vivo even in the presence of neutralizing protein C antibodies.38 In summary, there are many nonmutually exclusive mechanisms that may link TM to metastasis, and these could contribute in combination to overall malignant phenotype. The precise contribution of protein C-dependent and independent processes in tumor cell metastasis in TMPro mice will require more detailed study.
Consistent with previous reports showing that multiple thrombin targets are dispensable for the growth of established tumors, TMPro expression was shown to have no effect on tumor growth or tumor cell proliferation. Rather, TMPro expression was shown to promote metastatic success by supporting the survival of tumor cells newly localized to the lung. Loss of TM-mediated regulation of thrombin procoagulant function resulting from TMPro expression could promote the early survival of newly formed micrometastases by supporting the sustained adhesion of tumor cells to the vascular endothelium, promoting the extravasation of tumor cells, protecting tumor cells from innate immune surveillance mechanisms, or some combination of these. Interestingly, while TM was clearly shown to play an important role in hematogenous tumor metastases, this was not the case for metastasis via the lymphatic route. Like all endothelial cells, those of lymphatic origin also express detectable levels of TM.39 Lymph fluid also contains significant quantities of plasma proteins, including coagulation proteins, but not platelets.40 Given that hematogenous metastasis is highly dependent on platelets, the differential effect of TMPro expression on metastatic spread via the blood or lymphatic circulation may be explained by the distinct cellular makeup of these 2 circulations.
Another intriguing aspect of these studies was that the relative increase in hematogenous metastatic potential in the TMPro mice was greater when tumor cells were introduced intravenously than in spontaneous metastasis assays. Multiple challenges confront spontaneously metastasizing tumor cells, including transendothelial cell migration, transit within the circulation, stabilization within distant vascular beds, escape from innate immune surveillance, growth, and the establishment of a supportive vasculature. The alterations in thrombin function conferred by the TMGlu387Pro substitution might be advantageous at some steps of this process and a liability at others. For example, more robust tumor cell-associated platelet/fibrin deposition in TMPro mice might increase the stable adhesion and/or survival of circulating tumor cells once they have reached a distant vascular bed, whereas platelet/fibrin-rich matrices within a primary tumor might limit the success of individual tumor cells in gaining access to the circulation. Whatever the potential benefits and liabilities to specific steps in tumor dissemination conferred by TMPro expression, the data presented here establish that, in the balance, the functional prothrombotic shift in endothelial cells imposed by TMPro expression dramatically increases hematogenous metastatic potential.
In summary, these studies indicate that the interface between the hemostatic system and the malignant phenotype is not limited to tumor cell-associated and circulating hemostatic factors. Rather, this functional interface extends to properties of the endothelium controlling thrombin generation/activity. In addition to TM, endothelial cell factors controlling phosphatidylserine exposure, TF decryption, and platelet adhesion/activation are all potentially important to tumor cell metastasis. More detailed studies may reveal novel therapeutic strategies focused on the interface between endothelial cells and tumor cells that can effectively limit the development of metastatic disease without compromising vascular integrity.
Supplementary Material
Acknowledgments
This work was supported by National Institutes of Health grants R01-HL085545 (J.S.P.) and R01-HL085357 and R01-HL096126 (J.L.D.), as well as grants from the American Physicians Fellowship for Medicine in Israel (N.A.H.), the Dutch Cancer Foundation (C.A.S.), and the Canadian Institutes for Health Research (E.M.C.). E.M.C. holds a CSL Behring Research Chair and a Canada Research Chair in Endothelial Cell Biology and is an adjunct scientist with the Canadian Blood Services.
Footnotes
The online version of this article contains a data supplement.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Authorship
Contribution: N.A.H and J.S.P. designed and performed research, analyzed data, and wrote the manuscript; E.A.B, W.M.M., A.R.P, and K.E.T. performed research and were vital to the generation of gene-targeted mice; E.S.M. and M.J.F. helped design experiments and offered expert assistance; K.C.Q., K.S., and C.A.S. performed analyses in TMLeD mice; and B.P.M., E.M.C, H.W., and J.L.D. assisted in the design of the research and edited the manuscript.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Correspondence: Joseph S. Palumbo, MD, Division of Hematology, Cancer and Blood Diseases Institute, Cincinnati Children's Hospital Medical Center, 3333 Burnet Ave, Cincinnati, OH 45229; e-mail: joe.palumbo@cchmc.org.
References
- 1.Palumbo JS. Mechanisms linking tumor cell-associated procoagulant function to tumor dissemination. Semin Thromb Hemost. 2008;34(2):154–160. doi: 10.1055/s-2008-1079255. [DOI] [PubMed] [Google Scholar]
- 2.Palumbo JS, Talmage KE, Massari JV, et al. Tumor cell-associated tissue factor and circulating hemostatic factors cooperate to increase metastatic potential through natural killer cell-dependent and-independent mechanisms. Blood. 2007;110(1):133–141. doi: 10.1182/blood-2007-01-065995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Van de Wouwer M, Collen D, Conway EM. Thrombomodulin-protein C-EPCR system: integrated to regulate coagulation and inflammation. Arterioscler Thromb Vasc Biol. 2004;24(8):1374–1383. doi: 10.1161/01.ATV.0000134298.25489.92. [DOI] [PubMed] [Google Scholar]
- 4.Van de Wouwer M, Conway EM. Novel functions of thrombomodulin in inflammation. Crit Care Med. 2004;32(5 Suppl):S254–261. doi: 10.1097/01.ccm.0000128036.64448.9e. [DOI] [PubMed] [Google Scholar]
- 5.Weiler H. Mouse models of thrombosis: thrombomodulin. Thromb Haemost. 2004;92(3):467–477. doi: 10.1160/TH04-05-0307. [DOI] [PubMed] [Google Scholar]
- 6.Weiler H, Isermann BH. Thrombomodulin. J Thromb Haemost. 2003;1(7):1515–1524. doi: 10.1046/j.1538-7836.2003.00306.x. [DOI] [PubMed] [Google Scholar]
- 7.Abeyama K, Stern DM, Ito Y, et al. The N-terminal domain of thrombomodulin sequesters high-mobility group-B1 protein, a novel antiinflammatory mechanism. J Clin Invest. 2005;115(5):1267–1274. doi: 10.1172/JCI22782. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Conway EM, Van de Wouwer M, Pollefeyt S, et al. The lectin-like domain of thrombomodulin confers protection from neutrophil-mediated tissue damage by suppressing adhesion molecule expression via nuclear factor kappaB and mitogen-activated protein kinase pathways. J Exp Med. 2002;196(5):565–577. doi: 10.1084/jem.20020077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Geudens N, Van de Wouwer M, Vanaudenaerde BM, et al. The lectin-like domain of thrombomodulin protects against ischaemia-reperfusion lung injury. Eur Respir J. 2008;32(4):862–870. doi: 10.1183/09031936.00157107. [DOI] [PubMed] [Google Scholar]
- 10.Shi CS, Shi GY, Hsiao SM, et al. Lectin-like domain of thrombomodulin binds to its specific ligand Lewis Y antigen and neutralizes lipopolysaccharide-induced inflammatory response. Blood. 2008;112(9):3661–3670. doi: 10.1182/blood-2008-03-142760. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Van de Wouwer M, Plaisance S, De Vriese A, et al. The lectin-like domain of thrombomodulin interferes with complement activation and protects against arthritis. J Thromb Haemost. 2006;4(8):1813–1824. doi: 10.1111/j.1538-7836.2006.02033.x. [DOI] [PubMed] [Google Scholar]
- 12.Hanly AM, Winter DC. The role of thrombomodulin in malignancy. Semin Thromb Hemost. 2007;33(7):673–679. doi: 10.1055/s-2007-991969. [DOI] [PubMed] [Google Scholar]
- 13.Healy AM, Rayburn HB, Rosenberg RD, Weiler H. Absence of the blood-clotting regulator thrombomodulin causes embryonic lethality in mice before development of a functional cardiovascular system. Proc Natl Acad Sci U S A. 1995;92(3):850–854. doi: 10.1073/pnas.92.3.850. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Weiler-Guettler H, Christie PD, Beeler DL, et al. A targeted point mutation in thrombomodulin generates viable mice with a prethrombotic state. J Clin Invest. 1998;101(9):1983–1991. doi: 10.1172/JCI2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Hanly AM, Redmond M, Winter DC, et al. Thrombomodulin expression in colorectal carcinoma is protective and correlates with survival. Br J Cancer. 2006;94(9):1320–1325. doi: 10.1038/sj.bjc.6603098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Ogawa H, Yonezawa S, Maruyama I, et al. Expression of thrombomodulin in squamous cell carcinoma of the lung: its relationship to lymph node metastasis and prognosis of the patients. Cancer Lett. 2000;149(1–2):95–103. doi: 10.1016/s0304-3835(99)00348-1. [DOI] [PubMed] [Google Scholar]
- 17.Tamura A, Hebisawa A, Hayashi K, et al. Prognostic significance of thrombomodulin expression and vascular invasion in stage I squamous cell carcinoma of the lung. Lung Cancer. 2001;34(3):375–382. doi: 10.1016/s0169-5002(01)00261-6. [DOI] [PubMed] [Google Scholar]
- 18.Huang MT, Wei PL, Liu JJ, et al. Knockdown of thrombomodulin enhances HCC cell migration through increase of ZEB1 and decrease of E-cadherin gene expression. Ann Surg Oncol. 2010;17(12):3379–3385. doi: 10.1245/s10434-010-1163-4. [DOI] [PubMed] [Google Scholar]
- 19.Iino S, Abeyama K, Kawahara K, et al. The antimetastatic role of thrombomodulin expression in islet cell-derived tumors and its diagnostic value. Clin Cancer Res. 2004;10(18 Pt 1):6179–6188. doi: 10.1158/1078-0432.CCR-03-0750. [DOI] [PubMed] [Google Scholar]
- 20.Niimi S, Harashima M, Takayama K, et al. Thrombomodulin enhances the invasive activity of mouse mammary tumor cells. J Biochem. 2005;137(5):579–586. doi: 10.1093/jb/mvi070. [DOI] [PubMed] [Google Scholar]
- 21.Zhang Y, Weiler-Guettler H, Chen J, et al. Thrombomodulin modulates growth of tumor cells independent of its anticoagulant activity. J Clin Invest. 1998;101(7):1301–1309. doi: 10.1172/JCI925. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Hosaka Y, Higuchi T, Tsumagari M, Ishii H. Inhibition of invasion and experimental metastasis of murine melanoma cells by human soluble thrombomodulin. Cancer Lett. 2000;161(2):231–240. doi: 10.1016/s0304-3835(00)00617-0. [DOI] [PubMed] [Google Scholar]
- 23.Weijer S, Wieland CW, Florquin S, van der Poll T. A thrombomodulin mutation that impairs activated protein C generation results in uncontrolled lung inflammation during murine tuberculosis. Blood. 2005;106(8):2761–2768. doi: 10.1182/blood-2004-12-4623. [DOI] [PubMed] [Google Scholar]
- 24.Palumbo JS, Kombrinck KW, Drew AF, et al. Fibrinogen is an important determinant of the metastatic potential of circulating tumor cells. Blood. 2000;96(10):3302–3309. [PubMed] [Google Scholar]
- 25.Palumbo JS, Talmage KE, Massari JV, et al. Platelets and fibrin(ogen) increase metastatic potential by impeding natural killer cell-mediated elimination of tumor cells. Blood. 2005;105(1):178–185. doi: 10.1182/blood-2004-06-2272. [DOI] [PubMed] [Google Scholar]
- 26.Van Sluis GL, Niers TM, Esmon CT, et al. Endogenous activated protein C limits cancer cell extravasation through sphingosine-1-phosphate receptor 1-mediated vascular endothelial barrier enhancement. Blood. 2009;114(9):1968–1973. doi: 10.1182/blood-2009-04-217679. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Palumbo JS, Potter JM, Kaplan LS, Talmage K, Jackson DG, Degen JL. Spontaneous hematogenous and lymphatic metastasis, but not primary tumor growth or angiogenesis, is diminished in fibrinogen-deficient mice. Cancer Res. 2002;62(23):6966–6972. [PubMed] [Google Scholar]
- 28.Palumbo JS, Talmage KE, Liu H, La Jeunesse CM, Witte DP, Degen JL. Plasminogen supports tumor growth through a fibrinogen-dependent mechanism linked to vascular patency. Blood. 2003;102(8):2819–2827. doi: 10.1182/blood-2003-03-0881. [DOI] [PubMed] [Google Scholar]
- 29.Zhang H, Lowenberg EC, Crosby JR, et al. Inhibition of the intrinsic coagulation pathway factor XI by antisense oligonucleotides: a novel antithrombotic strategy with lowered bleeding risk. Blood. 2010;116(22):4684–4692. doi: 10.1182/blood-2010-04-277798. [DOI] [PubMed] [Google Scholar]
- 30.Mullins ES, Kombrinck KW, Talmage KE, et al. Genetic elimination of prothrombin in adult mice is not compatible with survival and results in spontaneous hemorrhagic events in both heart and brain. Blood. 2009;113(3):696–704. doi: 10.1182/blood-2008-07-169003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Lengweiler S, Smyth SS, Jirouskova M, et al. Preparation of monoclonal antibodies to murine platelet glycoprotein IIb/IIIa (alphaIIbbeta3) and other proteins from hamster-mouse interspecies hybridomas. Biochem Biophys Res Commun. 1999;262(1):167–173. doi: 10.1006/bbrc.1999.1172. [DOI] [PubMed] [Google Scholar]
- 32.Fidler IJ. Metastasis: guantitative analysis of distribution and fate of tumor embolilabeled with 125 I-5-iodo-2′-deoxyuridine. J Natl Cancer Inst. 1970;45(4):773–782. [PubMed] [Google Scholar]
- 33.Bezuhly M, Cullen R, Esmon CT, et al. Role of activated protein C and its receptor in inhibition of tumor metastasis. Blood. 2009;113(14):3371–3374. doi: 10.1182/blood-2008-05-159434. [DOI] [PubMed] [Google Scholar]
- 34.Mosnier LO, Zlokovic BV, Griffin JH. The cytoprotective protein C pathway. Blood. 2007;109(8):3161–3172. doi: 10.1182/blood-2006-09-003004. [DOI] [PubMed] [Google Scholar]
- 35.Camerer E, Qazi AA, Duong DN, Cornelissen I, Advincula R, Coughlin SR. Platelets, protease-activated receptors, and fibrinogen in hematogenous metastasis. Blood. 2004;104(2):397–401. doi: 10.1182/blood-2004-02-0434. [DOI] [PubMed] [Google Scholar]
- 36.Nierodzik ML, Karpatkin S. Thrombin induces tumor growth, metastasis, and angiogenesis: Evidence for a thrombin-regulated dormant tumor phenotype. Cancer Cell. 2006;10(5):355–362. doi: 10.1016/j.ccr.2006.10.002. [DOI] [PubMed] [Google Scholar]
- 37.Reijerkerk A, Meijers JC, Havik SR, Bouma BN, Voest EE, Gebbink MF. Tumor growth and metastasis are not affected in thrombin-activatable fibrinolysis inhibitor-deficient mice. J Thromb Haemost. 2004;2(5):769–779. doi: 10.1111/j.1538-7836.2004.00682.x. [DOI] [PubMed] [Google Scholar]
- 38.Tanaka KA, Fernandez JA, Marzec UM, et al. Soluble thrombomodulin is antithrombotic in the presence of neutralising antibodies to protein C and reduces circulating activated protein C levels in primates. Br J Haematol. 2006;132(2):197–203. doi: 10.1111/j.1365-2141.2005.05855.x. [DOI] [PubMed] [Google Scholar]
- 39.Maruyama I, Bell CE, Majerus PW. Thrombomodulin is found on endothelium of arteries, veins, capillaries, and lymphatics, and on syncytiotrophoblast of human placenta. J Cell Biol. 1985;101(2):363–371. doi: 10.1083/jcb.101.2.363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Miller GJ, Howarth DJ, Attfield JC, et al. Haemostatic factors in human peripheral afferent lymph. Thromb Haemost. 2000;83(3):427–432. [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.