Abstract
Substantial in vitro and in vivo evidence of neurotrophic and neuroprotective effects of lithium suggests that it may also have considerable potential for the treatment of neurodegenerative conditions. Lithium's main mechanisms of action appear to stem from its ability to inhibit glycogen synthase kinase-3 activity and also to induce signaling mediated by brain-derived neurotrophic factor. This in turn alters a wide variety of downstream efectors, with the ultimate effect of enhancing pathways to cell survival. In addition, lithium contributes to calcium homeostasis. By inhibiting N-methyl-D-aspartate receptor-mediated calcium influx, for instance, it suppresses the calcium-dependent activation of pro-apoptotic signaling pathways. By inhibiting the activity of phosphoinositol phosphatases, it decreases levels of inositol 1,4,5-trisphosphate, a process recently identified as a novel mechanism for inducing autophagy. These mechanisms alow therapeutic doses of lithium to protect neuronal cells from diverse insults that would otherwise lead to massive cell death. Lithium, moreover, has been shown to improve behavioral and cognitive deficits in animal models of neurodegenerative diseases, including stroke, amyotrophic lateral sclerosis, fragile X syndrome, and Huntington's, Alzheimer's, and Parkinson's diseases. Since lithium is already FDA-approved for the treatment of bipolar disorder, our conclusions support the notion that its clinical relevance can be expanded to include the treatment of several neurological and neurodegenerative-related diseases.
Keywords: brain-derived neurotrophic factor, CNS disorders, glutamate excitotoxicity, glycogen synthase kinase-3, lithium, neurodegenerative diseases, neuroprotection
1 INTRODUCTION
For more than 60 years, lithium has been the standard pharmacological treatment for bipolar disorder (BD), a chronic mental iilness characterized by cycling between moods of mania and depression[1]. In fact, current treatment guidelines frequently recommend lithium as the first-line treatment against acute mania and prophylactically for recurrent manic and depressive episodes. Clinically, lithium can be used adjunctively with other mood stabilizers, antidepressants, and antipsychotic medications to facilitate, enhance, or prolong both treatment response and remission[2]. While lithium's mood-stabilizing effects have been associated with a number of actions[3], the underlying biochemical mechanisms involved have yet to be defined.
Neuronal atrophy and reduced cellular density, as well as reduced grey matter volume were found in various brain regions of patients with BD[4], and MRI studies of the prefrontal cortex in patients with BD show abnormally low levels of the neuronal integrity marker N-acetyl-asparate (NAA)[5]. It is interesting to note that BD patients who receive chronic lithium treatments show consistently higher NAA levels and reduced loss of grey matter volume[6-8]. In fact, significant attention has focused on lithium's neurotrophic and neuroprotective effects during the last decade, and considerable research has been conducted on its efficacy as a novel therapeutic in various disease models.
The neuroprotective effects of lithium against glutamate-induced excitotoxicity have been extensively studied in various cellular and animal models. Glutamate excitotoxicity has been implicated in a variety of neurodegenerative diseases such as stroke, Huntington's disease (HD), amyotrophic lateral sclerosis (ALS), brain trauma, cerebellar degeneration, spinal cord injury, and possibly Alzheimer's disease (AD) and Parkinson's disease (PD)[9-10]. Lithium has also been shown to protect against insults to neurons in the central nervous system (CNS) and neurally related cell lines; these insults include endoplasmic reticulum (ER) stress[11-12], apoptosis induced by withdrawal of growth factor[13], β-amyloid (Aβ)[14], or colchicines[15], high potassium deprivation[16], exposure to heat shock[17], and supratherapeutic concentrations of anticonvulsants (phenytoin and carbamazepine)[18] . This article reviews recent findings regarding potential target involved in lithium's neuroprotective effects and their implications fo the treatment of human disorders of the CNS.
2 MECHANISMS UNDERLYING LITHIUM'S NEUROPROTECTIVE EFFECT
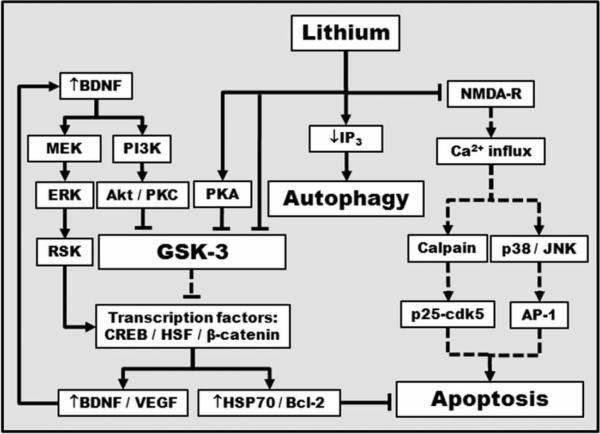
The fact that lithium's beneficial effects normally become evident only after long-term treatment and that these effects are no immediately reversed after discontinuation of the drug suggests that the drug works by altering signaling pathways and gene expression in the CNS. Fig. 1 shows the many signaling pathways and mechanisms of action implicated to date in lithium's neuroprotective effects.
Fig. 1. A schematic iilustration of proposed mechanisms underlying lithium's neuroprotective effects.
Lithium can directly and indirectly inhibit constitutively activated glycogen synthase kinase-3 (GSK-3) by multiple mechanisms, leading to disinhibition of several transcription factors, including cyclic AMP-response element binding protein (CREB), heat-shock factor-1 (HSF-1), and β-catenin, and subsequent induction of major cytoprotective proteins such as brain-derived neurotrophic factor (BDNF), vascular endothelial growth factor (VEGF), heat shock protein (HSP)70, and B-cell lymphoma/leukemia-2 protein (Bcl-2). Lithium-induced neurotrophic factors such as BDNF, in turn, activate its cell surface receptor and the downstream phosphoinositide 3-kinase (PI3K)/Akt and MAP kinase kinase (MEK)/extracellular-signal regulated kinase (ERK) pathways. BDNF induction is an early and essential step for neuroprotection against glutamate excitotoxicity and may contribute to lithium-induced neurogenesis. Lithium also indirectly inhibits GSK-3 activity via PI3K-dependent activation of protein kinase C (PKC) and cAMP-dependent activation of protein kinase A (PKA). The ability of lithium to decrease inositol 1,4,5-trisphosphate (IP3) levels is a novel route for inducing autophagy. Furthermore, lithium inhibits N-methyl-D-aspartate (NMDA) receptor-mediated calcium influx, which in turn decreases subsequent activation of c-Jun N-terminal kinase (JNK), p38 kinase, and transcription factor activator protein-1 (AP-1). Inhibition of intracellular calcium increase not only suppreses cellular stress, but also reduces the activity of calpain and calpain-mediated activation of pro-apoptotic cyclin-dependent kinase 5 (Cdk5)/p25 kinase. Lines with solid arrows represent stimulatory connections; lines with flattened ends represent inhibitory connections. Dashed lines represent pathways with reduced activity as a result of lithium treatment.
2.1 Protection against glutamate-induced excitotoxicity
In cultured rat CNS neurons that included cerebellar granule cells (CGCs) and cerebral cortical and hippocampal neurons[19], chronic lithium treatment was found to robustly reduce glutamate-induced excitotoxicity mediated by NMDA receptors. This effect was at least partly due to lithium's ability to inhibit the influx of calcium, which mediates activity in NMDA receptor. Studies further indicate that the mechanism of action results from the attenuation of constitutive phosphorylation at Tyr1 472 of the NR2B subunit of the NMDA receptor, which is catalyzed by Fyn, a member of the Src tyrosine kinase family[20-21]. Brain ischemia is known to increase Src-mediated tyrosine phosphorylation of NR2A[22-23] and to increase the interaction of NR2A with Src and Fyn, which is mediated by postsynaptic density protein 95 (PSD-95)[24]. Lithium blocks increases in both ischemia-induced NR2A phosphorylation and PSD-95 interaction[25].
Cdk5 also regulates signaling mediated by NMDA receptors, either directly through phosphorylation of the NR2B subunit or indirectly through phosphorylation of PSD-95[26-27]. Cdk5 activity is primarily regulated by its co-activator p35, but when it binds to p25 (the product of calpain-mediated cleavage of p35), Cdk5 becomes pro-apoptotic and its activity becomes dysregulated[28-29]. Sustained activation of Cdk5 in neurons is believed to be involved in many neurodegenerative diseases[30]. In cultured rat CGCs, lithium pretreatment prevents colchicine-induced apoptosis, associated increases in Cdk5 expression, and fragmentation of p35 to p25[31]. In cultured primary brain neurons and rat brains moreover, pretreatment with lithium also reduces intracellular calcium increase calpain activity Cdk5 activation and cellular death induced by 3-nitropropionic acid (3-NPA)[32]—a succinate dehydrogenase inhibitor used to induce striatal pathology similar to that observed in HD[33].
Using cultured rat CGCs as a model to investigate the mechanisms underlying human neuropathology, researchers have associated excitotoxicity with down-regulation of the cytoprotective Bcl-2 protein and also with up-regulation of pro-apoptotic proteins such as Bax and p53[34]. Apoptotic death in cultured rat CGCs, furthermore, was found to require activation of both JNK and p38 mitogen-activated protein kinase (MAP kinase), which led to a robust increase in AP-1 binding before apoptotic death[35]. Long-term treatment with therapeutic concentrations of lithium, however, was found to prevent both the signaling events and the sharp increase in apoptosis.
2.2 Inhibition of GSK-3 and stabilization of β-catenin
Under non-stimulated basal conditions, GSK-3, an enzyme with α and β isoforms that is pro-apoptotic and appears to be a major regulator of inflammation, is considered to be constitutively active. Dysfunction of this enzyme, moreover has been implicated in the pathophysiology of mood disorders, AD, diabetes, cancer, and inflammatory and autoimmune diseases[36-37]. It has recently been suggested that lithium's mood-stabilizing, neurogenetic, neurotrophic, neuroprotective and anti-inflammatory effects stem, at least in part, from its ability to inhibit the kinase activity of GSK-3[36, 38-39]. This ability arises from the fact that lithium is a competitive inhibitor of magnesium. Since GSK-3 catalysis is dependent on ATP-magnesium, lithium can inhibit its kinase activity directly[40-41].
Lithium also inhibits GSK-3 activity indirectly. At therapeutic concentrations, it has been shown to enhance phosphorylation of GSK-3α at Ser21 and GSK-3β at Ser9. Researchers have identified a number of mechanisms that contribute to this effect including cAMP-dependent activation of PKA[3, 42], PI3K-dependent activation of PKC[43] and Akt[44] and auto-regulation involving inhibitor-2 complex activity, which enhances the inhibition of protein phosphatase-l[45]. Others have shown that in vitro and in vivo, lithium treatment can decrease GSK-3β transcription[46]. It also inhibits GSK-3 by negatively regulating the calcium-dependent protease calpain, whose N-terminal cleavage upregulates the activity of GSK-3β kinase[47]. The fact that GSK-3 activation has been linked to apoptotic cell death induced by a variety of neural insults including glutamate excitotoxicity[48] makes it highly likely that neuroprotective effect of lithium stems mainly from its ability to inhibit GSK-3. In fact, when RNA interference depletes either of the 2 GSK-3 isoforms in neurons cultured from the rat cerebral cortex, glutamate-induced excitotoxicity is blocked[49]. By the same token, transfection with isoform-specific dominant-negative mutants of GSK-3 or treatment with other non-selective pharmacological GSK-3 inhibitors also results in lithium-like neuroprotection against glutamate excitotoxicity. Although GSK-3α and GSK-3β could have distinct roles in transcriptional regulation and cell survival[49-50], these results strongly suggest that both are involved in the execution of glutamate-induced neuronal death, and that both isoforms are initial targets of lithium-induced neuroprotection.
The transcription factor β-catenin is a substrate of GSK-3 and is part of the Wnt pathway. Its cytoplasmic levels are negatively regulated by constitutively active GSK-3. After being phosphorylated by GSK-3, β-catenin undergoes proteasomal degradation[51]. Increases in cytoplasmic accumulations of β-catenin facilitate its translocation into the nucleus. There it conjoins with T-cell-specific transcription factor (Tcf)/lymphoid enhancer binding factor (Lef), enhances the transcription of growth factors[52-53], and enhances genes involved in apoptotic inhibition[54]. Rsults such as these have led some to propose elevating β-catenin as a novel therapeutic strategy for treating mood disorders. In support of this theory, treatment with lithium increases β-catenin levels both in vitro[11] and in vivo[55-56], and promotes β-catenin-dependent transcriptional events[51, 56]. These results indicate that lithium-induced accumulation of β-catenin could be relevant to its neuroprotective and therapeutic effects.
2.3 Induction of survival molecules in the brain
In addition to the prophylactic qualities described above, in rat brains and cultured CGCs chronic lithium treatment has been found to induce Bcl-2 expression in the frontal cortex[11, 34]. Bcl-2 is an anti-apoptotic protein that inhibits the release of cytochrome c from mitochondria by regulating the permeability of the mitochondrial outer membrane[57, 58]. The ability to maintain calcium homeostasis in the ER is another cytoprotective action of Bcl-2[59-60]. We have associated chronic lithium's ability to induce upregulation of Bcl-2 in PC12 cells with its cytoprotective effects against Aβ peptide and thapsigargin-induced ER stress[12, 61]. In the rat brain, chronic treatment with valproate—a mood-stabilizing drug anticonvulsant, and histone deacetylase inhibitor[62-64] often used in BD patients with poor response to lithium—also upregulates Bcl-2[11]. A recent study shows that, in SH-SY5Y cells, Bcl-2 translation is directly inhibited by expresion of the specific microRNA miR-34a[65]. In the rat hippocampus and in primary cultures of hippocampal neurons, chronic treatment with lithium or valproate decreases levels of several microRNAs, including miR-34a[66], suggesting a common regulator shared by these structuraly dissimilar mood stabilizers and indicating that a novel target accounts for their therapeutic efficacy.
BDNF, a major neurotrophin essential for cortical development, synaptic plasticity, and neuronal survival, is likely one of the mediators of the clinical efficacy of antidepressants and anxiolytics[67-68]. Long-term treatment of cultured cortical neurons with lithium induces BDNF, which in turn increases phosphorylation at the Tyr490 residue and activates its tyrosine receptor kinase B (TrkB) receptor[69]. Chronic treatment of rats with lithium also increases protein levels of BDNF in various brain regions, but without altering the expression of TrkB[70-71]. Recent study in cultured cortical neurons further reveals that treatment with lithium or valproate at therapeutic concentrations for 48 hours selectively increases the levels of exon IV (formerly rat exon III)-containing BDNF mRNA, and the activity of BDNF promoter IV[72]. Notably, this effect can be mimicked by the pharmacological inhibition of GSK-3 or by the siRNA-mediated gene silencing of either the GSK-3α or GSK-3β isoform. By the same token, adding the Trk-tyrosine kinase inhibitor K252a, or a BDNF-neutralizing antibody, counteracts lithium's ability to protect neurons from excitotoxicity[69]. In cultured cortical neurons, heterozygous or homozygous knock-out of the BDNF gene also blocks lithium's neuroprotective effect completely.
Researches in vitro and in vivo have further shown that lithium treatment increases the expression of VEGF[52, 73-74], in all probability by inhibiting GSK-3β and stabilizing β-catenin signaling. VEGF promotes cell proliferation[75], proneuronal differentiation of newly born cells[76], migration of immature neuroblasts[76-77], and neurovascular remodeling after stroke[74, 76, 78]. By upregulating VEGF, lithium treatment optimizes skeletal myoblast functions for cellular cardiomyoplasty in vitro[79] and prevent stress-induced reductions in VEGF level[52], and promotes angiogenic and anti-apoptotic signaling in rat ischemic preconditioned myocardium[74].
HSPs are a group of molecular chaperones that promote the folding of proteins and refolding of misfolded proteins. They also inhibit protein aggregate formation and, through the ubiquitin-proteasome system, facilitate the degradation of abnormally folded proteins[80-81]. Among HSPs, HSP70 exerts a wide variety of neuroprotective effects against apoptosis[82]. In various animal models, overexpression of HSP70 has been recognized as a potential therapeutic target against ischemic neuronal injury[83-85]. The expression of HSP70 is regulated by HSF-1[86], a transcription factor negatively regulated by GSK-3β-dependent phosphorylation[87]. Not surprisingly, therefore, GSK-3β activity correlates negatively with both DNA binding activity of HSF-1 and HSF-1-dependent transcription[86, 88]. In light of the fact that lithium's inhibition of GSK-3 is associated with the activation of HSF-1, upregulation of the heat-shock response may account for some part of the neuroprotective effect of lithium.
2.4 Induction of autophagy
Autophagy—a physiological process for degrading cytoplasmic proteins or organeles in bulk—has recently been recognized as a principal response to cellular stress and an important regulator of neuronal function and survival. As a ‘quality control’ process, autophagy is believed to be particularly beneficial in neurodegenerative disorders (AD, PD, ALS, spinocerebellar ataxia type 3, and HD) characterized by the accumulation of misfolded disease-causing proteins[89-92]. Authophagy appear to be negatively regulated by the mammalian target of rapamycin (mTOR). By inhibiting mTOR, rapamycin upregulates autophagy and this has been show to be beneficial in various models of neurodegenerative diseases[89-91]. Other mechanisms for inducing autophagy include inhibiting inositol monophosphatase and inositol transporters[93]. Lithium's ability to deplete free inositol and subsequently decrease IP3 levels was recently identified as a novel route (independent of mTOR) for inducing autophagy[94-95] and its attendant benefits.
2.5 Induction of neurogenesis
Lithium was found to stimulate progenitor proliferation in cultured brain neurons and to prevent the loss of proliferation induced by glutamate or glucocorticoids[96]. In addition, chronic lithium treatment not only enhances neurogenes is in the hippocampus of normal mice[97], but also restores neurogenesis in the brain in an animal model of Down syndrome[98].
In primary rat hippocampal progenitor cultures, long-term lithium treatment promotes the conversion of these progenitor cells into neurons through the GSK-3β inhibition/β-catenin activation pathway[99-100]. In a rat model of stroke, chronic lithium treatment upregulates the generation and survival of newborn cells in the hippocampus by the ERK pathway, and improves the behavioral performance of rats after transient global cerebral ischemia[101]. One possible common downstream event related to neurogenesis is lithium-induced upregulation of BDNF, which is necesary for hippocampal neurogenesis[102].
3 CLINICAL IMPLICATIONS AND APPLICATIONS
3.1 BD
Because lithium has been the mainstay of treatment for bipolar disorder, understanding the mechanisms underlying its neuroprotective effects could well provide insights into potential causes of the disease. With few exceptions, for instance, drugs prescribed to treat BD work by conferring some measure of neuroprotection[103-104]. As observed in rodent models, the antidepressant and antimanic effects of lithium are most likely due to the inhibition of the kinase GSK-3[56, 105-106], whose overexpression in mice produces behavioral corelates of hyperactivity and mania[107]. Drugs or genetic approaches that inactivate GSK-3β also alleviate depressive-like behaviors in mice expressing a mutant form of the brains serotonin-synthesizing enzyme[108], while administering lentiviral-mediated GSK-3β shRNA into the dentate gyrus of mice subjected to chronic stress appears to have an antidepressant-like effect[109]. By the same token, genetic inactivation of GSK-3α in mice appears to have a similar antidepressant-like effect as measured by decreased immobility time and fewer aggressive-like behaviors in behavioral tests[110]. A recent study further reveals that mice deficient in the inhibitory serine-phosphorylation of GSK-3 increases susceptibility to mood disturbances, and serine-phosphorylation of GSK-3 is reduced during both stress-related behavioral responses in wild-type mouse brain and in blood cells from patients with BD[111]. It is also interesting to not that lithium, valproate, and lamotrigine all enhance the serine phosphorylation of GSK-3[39, 112]. These findings not only support the hypothesis that lithium's therapeutic effects stem primarily from its inhibition of GSK-3, they also support the targeting of GSK-3-linked pathways in our search for new ways to treat BD.
3.2 Stroke
Most strokes are caused by cerebral ischemia, which is the interruption of blood supply to the brain. Long-term pretreatment with lithium has been reported to decrease infarct volume and reduce neurological deficits, not only in a model induced by permanent middle-cerebral artery occlusion (MCAO)[113], but also in transient MCAO models followed by reperfusion[114], which more closely approximate the pathophysiology of acute stroke. The complex mechanisms underlying lithium's neuroprotective effects may include inactivation of NMDA receptors[25], downregulation of pro-apoptotic p53 and upregulation of anti-apoptotic Bcl-2 and HSP70[115], resulting in reduced apoptotic cell death[114], activation of the PI3K/Akt cell survival pathway[44] and inhibition of hypoxia-induced activation of GSK-3[116]. When administered up to three hours after the onset of ischemia post-insult treatment with therapeutic doses of lithium also markedly decreases infarct volume. In a rat model of transient MCAO, lithium has been shown to suppress neurological deficits as measured by sensory, motor, and reflex tests[117]. These beneficial effects are associated with the activation of HSF-1 and induction of the cytoprotective protein HSP70 in ischemic brain hemispheres. A functional MRI study further showed that even delayed chronic lithium treatment (administered up to 12 hours after the onset of ischemia and followed by daily injections for 2 weeks) significantly improved functional MRI response magnitude, which is dependent on blood oxygenation levels, and vascular formation[118]. The ability of lithium to affect neurovascular remodeling may be related to its ability to increase protein levels of matrix metalloproteinase 9 (MMP-9) and VEGF[73]. VEGF has, in fact, been linked to angiogenesis, neurogenesis, and neuroprotection[119]. These preliminary demonstrations of lithium's pre- and post-insult beneficial effects suggest that it may ultimately become a valuable clinical tool for both the prevention and treatment of acute stroke.
3.3 HD
HD is an inherited, autosomal-dominant, neurodegenerative disease characterized by irreversible physical and mental deterioration[120]. It is caused by abnormal expansion of a trinucleotide CAG-repeat in the gene that encodes a polyglutamine stretch in the N-terminus of huntingtin, the disease-causing protein[121]. This abnormal expansion result in a selective loss of neurons in the striatum and cortex[9, 122]. Transcriptional dysregulation also plays a central role in the pathogenesis and pathophysiology of this disease[123]. HD is lethal, and curently there is no treatment proven to arrest or reverse its course.
Because the supersensitivity (or hyperactivation) of NMDA receptor appears to contribute to the pathophysiology of HD[124], lithium's protective properties against glutamate toxicity would seem to make it ideally suited to treat this disease. In the rat excitotoxic model induced by quinolinic acid (QA), lithium treatment markedly reduces the size of QA-induced striatal lesions[61] and the loss of striatal medium-sized neurons[125] . This lithium protection is correlated with upregulation of cytoprotective Bcl-2 and downregulation of caspase-3 activation. In a cell model of HD, the protective effects of lithium in reducing mutant huntingtin aggregates and cell death are mimicked, either by treatment with a GSK-3β inhibitor or by overexpression of a dominant-negative GSK-3β mutant[126]. In Drosophila, a GSK-3β inhibitor mimics lithium-induced protection against the toxicity of aggregate-prone proteins[127]. Lithium pretreatment also stimulates the proliferation of striatal cells near the site of QA-induced injuries, and some of these replicating cells have the phenotype of neurons or astroglia[125]. In a rat 3-NP model of HD, lithium treatment reduces striatal neurodegeneration by preventing the activation of calpain and Cdk5[33]. In Drosophila and R6/2 mouse models of HD, systemic administration of rapamycin induces autophagy and reduces toxicity of polyglutamine expansions[90]. Moreover, in cellular and Drosophil models of HD, lithium combined with rapamycin induces autophagy and shows greater protection against neurodegeneration than either pathway alone[128]. In R6/2 mice, although lithium treatment administered post- (but not pre-) symptomatically significantly improves rotarod performance, it appears to have no effect on survival overall[129]. However, in the N171-82Q and YAC128 mouse models of HD, pre-symptomatic co-treatment with lithium and valproate produces more robust improvements in motor deficits and stronger anxiolytic and antidepressant-like effects than either drug alone[130]. Evidence of these neuroprotective properties in models of HD suggests that lithium, especialy in combination with other medications, may prove useful as a treatment for HD.
3.4 AD
AD is characterized by progressive memory loss and personality changes, ultimately leading to dementia. The neuropathological hallmarks of AD are an abnormal accumulation of Aβ and neurofibrillary tangles (tauopathies) resulting from hyper-phosphorylation of tau, a microtubule-binding protein[131]. The association of pathogenesis and neuronal death in AD with abnormal increases in GSK-3 levels and activity[132] suggests a possible role for lithium in treating this disorder[133]. In vivo and in vitro, lithium reduces tau phosphorylation by inhibiting GSK-3[134-135]. Tau phosphorylation levels are also regulated by protein phosphatase 2 A (PPPA) [136], and reduced PP2A activity in the brain has been reported in individuals with AD[137]. In rats, lithium treatment has been shown to increase PP2A activity[138], decrease tau phosphorylation, and facilitate its destruction[139]. In cultured cortical neurons, lithium was also recently shown to downregulate tau transcription[140]. Chronic lithium treatment also blocks Aβ production through GSK-3 inhibition[141]. Aβ peptide is derived from amyloid precursor protein (APP) by sequential secretase-dependent proteolytic procesing. In the brains of mice overproducing APP, chronic lithium treatment blocks Aβ accumulation, presumably by interfering with the reaction of γ-secretase[142]. In cultured neurons and neurally related cells, chronic lithium treatment largely suppreses exogenous Aβ-induced hyper-phosphorylation of tau, downregulation of Bcl-2, and neuronal death[14, 134, 143]. It is further interesting to note that the protein level of Bcl-2 in the brains of a mouse model of AD is inversely correlated with the expression of miR-34a[65], a microRNA that has recently emerged as a common lithium and valproate target[66]. These findings suggest a novel mechanism for lithium's protective effects against AD in which the downregulation of miR-34a indirectly upregulates Bcl-2.
Experiments with various animal models of AD have shown a number of other benefits from lithium. In mouse models of tauopathies, chronic lithium treatment not only inhibits tau phosphorylation and neuronal degeneration mediated by GSK-3[144], it also decreases tau lesions by promoting ubiquitination[145]. In addition, in mutant tau transgenic mice with advanced neurofibrillary pathology, chronic lithium treatment decreases aggregation of mutant tau proteins[146] and arrests the development of neurofibrillary tangles[147]. Chronic lithium treatment in rats has also been shown to activate the Wnt/β-catenin pathway, and thereby to protect against Aβ-induced hippocampal neurodegeneration[148]. With regard to lithium's behavioral effects, Drosophila models of tauopathies show that its inhibition of GSK-3β reverses locomotor deficits[149]. In rats injected with preformed Aβ fibrils, chronic lithium treatment improves spatial learning deficits[148]. In transgenic mice overexpressing human APP, 3 months of treatment with lithium have been shown to recuce the burden of Aβ, tau hyper-phosphorylation, and neurodegeneration in the cortex and hippocampus. In addition, the inhibition of GSK-3β signaling normalizes deficits in water-maze performance[150]. Clinically, a preliminary study in individuals with BD found that a history of lithium treatment resulted in significantly better cognition and memory scores compared with individuals receiving other treatments[151]. In elderly BD patients, moreover, chronic lithium treatment reduced the prevalence of AD[152]. Taken together, these results suggest a promising therapeutic role for lithium in the treatment of AD.
3.5 PD
PD is a prevalent neurodegenerative disease characterized by resting tremor, muscular rigidity, bradykinesia, and postural instability asociated with a relatively selective loss of dopaminergic neurons in the substantia nigra. PD is another neurodegenerative condition characterized by aggregates of mutant protein (Lewy bodies), mainly α-synuclein[153-154]. In animal models, neurotoxins such as rotenone, 6-hydroxydopamine (6-OHDA), l-methyl-4-phenylpyridinium (MPPM+), and the MPP+ precursor N-methyl-4-phenyl-1, 2, 3, 6 tetrahydropyridine (MPTP) can trigger PD-asociated neurochemical changes. In these models, therapeutic concentrations of lithium have been shown to facilitate clearance of the mutant form of α-synuclein, an autophagy substrate[94]. In cultured human neuroblastoma cells, GSK-3β activation facilitates the activation of caspase-3 induced by rotenone, a mitochondrial complexI-inhibitor, or by MPP+. By the same token, lithium treatment inhibits the activation of caspase-3 in a PI3K-dependent manner[155]. In cultured neurons, it prevents 6-OHDA[156] and MPP+-induced neuronal death. In addition, chronic lithium treatment in mice prevents MPTP-induced neurotoxicity, normalizes the downregulation of Bcl-2, and normalizes the upregulation of Bax elicited by MPTP in the striatum of the mouse brain[157]. Experimental evidence of these protective effects suggests that lithium may have substantial therapeutic potential in the treatment of PD.
3.6 Fragile X syndrome (FXS)
FXS is caused by abnormal expansion of the trinucleotide (CGG) repeat-mediated transcriptional silencing of the fragile X mental retardation-1 (FMR1) gene[158] that encodes the fragile X mental retardation protein (FMRP)[159]. A recent study found that in FVB/NJ FMR1 knockout mice, the inhibitory serine-phosphorylation of GSK-3 is impaired[160], suggesting a possible therapeutic role for lithium. In a Drosophila model of FXS, adulthood lithium treatment increases naive courtship and restores short-term memory[161]. Moreover, in the Drosophila model, treatment with metabotropic glutamate receptor (mGluR) antagonists or lithium prevents age-related cognitive impairments and continuous treatment during aging effectively rescues these deficits[162]. Mouse models of FXS display certain FXS- and autism-relevant behavioral phenotypes[163-165], several of which are ameliorated with lithium treatment[160, 166]. Chronic lithium treatment of FXS mice largely blocks aberrant dendritic spine morphology and reduces anxiety levels, deficient social interactions and impaired learning ability[167]. Lithium's beneficial effects on FXS mouse brains are associated with normalization of hypo-phosphorylation of GSK-3β at Ser9. A pilot clinical study has confirmed similar benefits from lithium treatment in FXS patients aged 6-23 years, who showed improvements in behavior, adaptive skiils, and cognition[168].
3.7 ALS
ALS is an adult-onset neurodegenerative disease characterized by progressive loss of motor neurons (MNs) in the brain, brain stem, and spinal cord, resulting in generalized weakness, muscle atrophy, paralysis, and eventual mortality within 5 years of disease onset[169]. Mice expressing mutant Cu/Zn superoxide dismutase 1 (SOD1) exhibit ALS-like phenotypes, including the formation of intracellular aggregates of SOD1 in the brain and spinal cord, behavioral abnormalities, and premature death. In organotypic slice cultures of spinal cord, chronic treatment with lithium dose-dependently prevents excitotoxic cell death of MNs by inhibiting the GSK-3β signaling pathway[170]. Treatment with either lithium alone or in conjunction with an antioxidant has been shown to improve motor function and slow disease progression in a mouse model of ALS[171-173]. Combined treatment of ALS mice with lithium and valproate produces a greater and more consistent effect than monotreatment with either drug in delaying the onset of disease symptoms, decreasing neurological deficit scores, and prolonging life span[174]. Moreover, a 15-month pilot clinical trial in randomized ALS patients found that patients treated with lithium and riluzole together showed markedly reduced mortality than patients treated with riluzole alone[172]. Since inconsistent results have also been reported[175-177], however, further studies are needed to clarify these discrepancies.
3.8 Multiple sclerosis (MS)
MS is the most common inflammatory demyelinating disease of the CNS, which causes demyelination and neurodegeneration with lesions predominantly in the white matter[178]. The most frequently used animal model of MS is experimental autoimmune encephalomyelitis (EAE)[179] induced in mammals by systemic injection of myelin oligodendrocyte glycoprotein (MOG), myelin basic protein, or proteolipid protein[180]. A recent study demonstrates that in knock-in mice expressing constitutively active GSK-3, EAE develops more rapidly and is more severe[181], suggesting that GSK-3 kinase may be a potential therapeutic target for the treatment of MS. Administration of GSK-3 inhibitors in mice has been shown to control several inflammatory and immune conditions in both the periphery and the CNS[36]. Notably, lithium pretreatment at therapeutically relevant doses not only abolishes the onset of EAE but also greatly reduces demyelination, microglia activation, and leukocyte infiltration in the spinal cord[181]. In addition, lithium treatment suppresses MOG peptide-induced immune responses in vitro and decreases the production of several proinflammatory cytokines by splenocytes stimulated with MOG peptide after isolation from EAE mice. These results suggest that lithium may be useful for therapeutic intervention in autoimmune and inflammatory diseases such as MS, which afflict the CNS.
4 CONCLUSION
Studies from various laboratories confirm that, in a vast number of cellular and animal models of brain disorders, lithium has robust therapeutic effects. It is also becoming increasingly clear that lithium's inhibition of GSK-3, whose hyperactivity is involved in cell death and the pathophysiology of many neurodegenerative conditions, accounts for much of its ability to protect and even increase neurons. GSK-3 inhibition plays a prominent role in activating signaling pathways and inducing anti-apoptotic and neurotrophic proteins. The lithium-induced inhibition of the metabolism of phosphoinositide and production of IP3 also appears to be involved in upregulating autophagy—a process critical for the clearance of protein aggregates associated with neurodegenerative diseases. Emerging evidence suggests that the mood-stabilizers lithium and valproate target specific microRNAs that regulate the expression of anti-apoptotic proteins and are perhaps involved in the pathophysiology of brain disorders. Further micro-RNA research is therefore needed to investigate the etiology of these diseases and elucidate lithium's mechanisms of action.
As can be seen from the review provided above, research with animal models has confirmed the beneficial effects of lithium treatment in an increasing number of CNS disorders. Many preclinical studies report evidence of significantly decreased neurodegeneration, enhanced neurogenesis, improved behavioral performance, improved cognitive function, and prolonged survival. Based on promising preclinical results and its long history of safe clinical use in humans, lithium is currently being tested as a treatment for a variety of human brain disorders. Results to date are mixed. While some clinical studies report promising improvement, others indicate no treatment response. Resolving discrepancies such as these requires large-scale clinical trials of long duration—an expensive undertaking difficult to envision in this era of restricted budgets. Yet, in light of the results from recently completed preclinical studies, combined treatment with lithium and other neuroprotective drug(s) is recommended for adequate clinical testing to ameliorate the devastating effects of neurodegenerative diseases and psychiatric disorders that currently exact so great a human toll.
Acknowledgements
The authors thank Dr. Elizabeth Sherman and Mr. Peter Leeds of the NIMH, NIH, for critical review and editorial assistance with this manuscript.
Foundation items This work was supported by the Intramural Research Program of the National Institute of Mental Health (NIMH) and the National Institute of Health (NIH).
Biography
Biography Chi-Tso CHIU, Ph. D., mainly engaged in the research of molecular neurobiology.
Footnotes
De-Maw Chuang, Ph. D., professor of psychiatry and molecular neurobiology, has been mainly engaged in research involving neurodegenerative disorders for the past decades. His research has involved programmed cell death (apoptosis) of neurons, neurotransmitter receptor regulation, mechanisms and actions of mood stabiiizers and psychotropic drugs. He has been involved in characterization of potential roles of lithium and HDACIs in neuropsychiatric disorders including mood and anxiety disorders and stroke. He was awarded as Academician of Academia Sinica of Taiwan in 2006, and wan the Distinguished Investigator Award of National Aliance for Research on Schizophrenia and Depression (NARSAD) in 2002, the National Institute of Mental Health (NIMH) Director's Mentor of the Year Award in 2010. He is currently the Chief of Molecular Neurobiology Section, National Institute of Mental Health (NIMH), National Institute of Health (NIH).
REFERENCES
- 1.Manji HK, Lenox RH. Lithium: a molecular transducer of mood-stabilization in the treatment of bipolar disorder[J]. Neuropsychopharmacology. 1998;19(3):161–166. doi: 10.1016/S0893-133X(98)00021-9. [DOI] [PubMed] [Google Scholar]
- 2.Goodwin FK. Rationale for using lithium in combination with other mood stabilizers in the management of bipolar disorder [J]. J Clin Psychiatry. 2003;64(Suppl 5):18–24. [PubMed] [Google Scholar]
- 3.Jope RS. Anti-bipolar therapy: mechanism of action of lithium [J]. Mol Psychiatry. 1999;4(2):117–128. doi: 10.1038/sj.mp.4000494. [DOI] [PubMed] [Google Scholar]
- 4.Sassi RB, Brambilla P, Hatch JP, et al. Reduced left anterior cingulate volumes in untreated bipolar patients [J]. Biol Psychiatry. 2004;56(7):467–475. doi: 10.1016/j.biopsych.2004.07.005. [DOI] [PubMed] [Google Scholar]
- 5.Brambilla P, Stanley JA, Nicoletti MA, et al. 1H magnetic resonance spectroscopy investigation of the dorsolateral prefrontal cortex in bipolar disorder patients [J]. J Affect Disord. 2005;86(1):61–67. doi: 10.1016/j.jad.2004.12.008. [DOI] [PubMed] [Google Scholar]
- 6.Bearden CE, Thompson PM, Dalwani M, et al. Greater cortical gray matter density in lithium-treated patients with bipolar disorder [J]. Biol Psychiatry. 2007;62(1):7–16. doi: 10.1016/j.biopsych.2006.10.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Moore GJ, Bebchuk JM, Wilds IB, et al. Lithium-induced increase in human brain grey matter [J]. Lancet. 2000;356(9237):1241–1242. doi: 10.1016/s0140-6736(00)02793-8. [DOI] [PubMed] [Google Scholar]
- 8.Moore GJ, Bebchuk JM, Hasanat K, et al. Lithium increases N-acetyl-aspartate in the human brain: in vivo evidence in support of bcl-2's neurotrophic effects? [J]. Biol Psychiatry. 2000;48(1):1–8. doi: 10.1016/s0006-3223(00)00252-3. [DOI] [PubMed] [Google Scholar]
- 9.Friedlander RM. Apoptosis and caspases in neurodegenerative diseases [J]. N Engl J Med. 2003;348(14):1365–1375. doi: 10.1056/NEJMra022366. [DOI] [PubMed] [Google Scholar]
- 10.Mattson MP, Kroemer G. Mitochondria in cell death: novel targets for neuroprotection and cardioprotection [J]. Trends Mol Med. 2003;9(5):196–205. doi: 10.1016/s1471-4914(03)00046-7. [DOI] [PubMed] [Google Scholar]
- 11.Chen G, Zeng WZ, Yuan PX, et al. The mood-stabilizing agents lithium and valproate robustly increase the levels of the neuroprotective protein bcl-2 in the CNS [J]. J Neurochem. 1999;72(2):879–882. doi: 10.1046/j.1471-4159.1999.720879.x. [DOI] [PubMed] [Google Scholar]
- 12.Hiroi T, Wei H, Hough C, et al. Protracted lithium treatment protects against the ER stress elicited by thapsigargin in rat PC12 cells: roles of intracellular calcium, GRP78 and Bcl-2 [J]. Pharmacogenomics J. 2005;5(2):102–111. doi: 10.1038/sj.tpj.6500296. [DOI] [PubMed] [Google Scholar]
- 13.Bhat RV, Shanley J, Correll MP, et al. Regulation and localization of tyrosine216 phosphorylation of glycogen synthase kinase-3beta in celular and animal models of neuronal degeneration [J]. Proc Natl Acad Sci USA. 2000;97(20):11074–11079. doi: 10.1073/pnas.190297597. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Alvarez G, Munoz-montano JR, Satrustegui J, et al. Regulation of tau phosphorylation and protection against beta-amyloid-induced neurodegeneration by lithium. Possible implications for Alzheimer's disease [J]. Bipolar Disord. 2002;4(3):153–165. doi: 10.1034/j.1399-5618.2002.01150.x. [DOI] [PubMed] [Google Scholar]
- 15.Jorda EG, Verdaguer E, Morano A, et al. Lithium prevents colchicine-induced apoptosis in rat cerebellar granule neurons [J]. Bipolar Disord. 2004;6(2):144–149. doi: 10.1046/j.1399-5618.2003.00090.x. [DOI] [PubMed] [Google Scholar]
- 16.D'mello SR, Anelli R, Calissano P. Lithium induces apoptosis in immature cerebellar granule cells but promotes survival of mature neurons [J]. Exp Cell Res. 1994;211(2):332–338. doi: 10.1006/excr.1994.1095. [DOI] [PubMed] [Google Scholar]
- 17.Bijur GN, De SP, Jope RS. Glycogen synthase kinase-3 beta facilitates staurosporine- and heat shock-induced apoptosis. Protection by lithium [J]. J Biol Chem. 2000;275(11):7583–7590. doi: 10.1074/jbc.275.11.7583. [DOI] [PubMed] [Google Scholar]
- 18.Nonaka S, Katsube N, Chuang DM. Lithium protects rat cerebellar granule cells against apoptosis induced by anticonvulsants, phenytoin and carbamazepine [J]. J Pharmacol Exp Ther. 1998;286(1):539–547. [PubMed] [Google Scholar]
- 19.Nonaka S, Hough CJ, Chuang DM. Chronic lithium treatment robustly protects neurons in the central nervous system against excitotoxicity by inhibiting N-methyl-D-aspartate receptor-mediated calcium influx [J]. Proc Natl Acad Sci USA. 1998;95(5):2642–2647. doi: 10.1073/pnas.95.5.2642. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Hashimoto R, Hough C, Nakazawa T, et al. Lithium protection against glutamate excitotoxicity in rat cerebral cortical neurons: involvement of NMDA receptor inhibition posibly by decreasing NR2B tyrosine phosphorylation [J]. J Neurochem. 2002;80(4):589–597. doi: 10.1046/j.0022-3042.2001.00728.x. [DOI] [PubMed] [Google Scholar]
- 21.Hashimoto R, Fujimaki K, Jeong MR, et al. Lithium-induced inhibition of Src tyrosine kinase in rat cerebral cortical neurons: a role in neuroprotection against N-methyl-D-aspartate receptor-mediated excitotoxicity [J]. FEBS Lett. 2003;538(1/3):145–14. doi: 10.1016/s0014-5793(03)00167-4. [DOI] [PubMed] [Google Scholar]
- 22.Liu Y, Zhang G, Gao C, et al. NMDA receptor activation results in tyrosine phosphorylation of NMDA receptor subunit 2A (NR2A) and interaction of Pyk2 and Src with NR2A after transient cerebral ischemia and reperfusion [J]. Brain Res. 2001;909(1/2):51–58. doi: 10.1016/s0006-8993(01)02619-1. [DOI] [PubMed] [Google Scholar]
- 23.Takagi N, Shinno K, Teves L, et al. Transient ischemia differentially increases tyrosine phosphorylation of NMDA receptor subunits 2A and 2B [J]. J Neurochem. 1997;69(3):1060–1065. doi: 10.1046/j.1471-4159.1997.69031060.x. [DOI] [PubMed] [Google Scholar]
- 24.Hou XY, Zhang GY, Yan JZ, et al. Activation of NMDA receptors and L-type voltage-gated calcium channels mediates enhanced formation of Fyn-PSD95-NR2A complex after transient brain ischemia[J]. Brain Res. 2002;955(1/2):123–132. doi: 10.1016/s0006-8993(02)03376-0. [DOI] [PubMed] [Google Scholar]
- 25.Ma J, Zhang GY. Lithium reduced N-methyl-D-aspartate receptor subunit 2A tyrosine phosphorylation and its interactions with Src and Fyn mediated by PSD-95 in rat hippocampus following cerebral ischemia[J]. Neurosci Lett. 2003;348(3):185–189. doi: 10.1016/s0304-3940(03)00784-5. [DOI] [PubMed] [Google Scholar]
- 26.Morabito MA, Sheng M, Tsai LH. Cyclin-dependent kinase 5 phosphorylates the N-terminal domain of the postsynaptic density protein PSD-95 in neurons[J]. J Neurosci. 2004;24(4):865–876. doi: 10.1523/JNEUROSCI.4582-03.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Zhang S, Edelmann L, Liu J, et al. Cdk5 regulates the phosphorylation of tyrosine 1472 NR2B and the surface expression of NMDA receptors[J]. J Neurosci. 2008;28(2):415–424. doi: 10.1523/JNEUROSCI.1900-07.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Lee MS, Kwon YT, Li M, et al. Neurotoxicity induces cleavage of p35 to p25 by calpain[J]. Nature. 2000;405(6784):360–364. doi: 10.1038/35012636. [DOI] [PubMed] [Google Scholar]
- 29.Patrick GN, Zukerberg L, Nikolic M, et al. Conversion of p35 to p25 deregulates Cdk5 activity and promotes neurodegeneration[J]. Nature. 1999;402(6762):615–622. doi: 10.1038/45159. [DOI] [PubMed] [Google Scholar]
- 30.Cruz JC, Tsai LH. A Jekyll and Hyde kinase: roles for Cdk5 in brain development and disease [J]. Curr Opin 2004, Neurobiol. 2004;14(3):390–394. doi: 10.1016/j.conb.2004.05.002. [DOI] [PubMed] [Google Scholar]
- 31.Jorda EG, Verdaguer E, Canudas AM, et al. Implication of cyclin-dependent kinase 5 in the neuroprotective properties of lithium[J]. Neuroscience. 2005;134(3):1001–1011. doi: 10.1016/j.neuroscience.2005.04.061. [DOI] [PubMed] [Google Scholar]
- 32.Crespo-Biel N, Camins A, Pallas M, et al. Evidence of calpain/cdk5 pathway inhibition by lithium in 3-nitropropionic acid toxicity in vivo and in vitro [J]. Neuropharmacology. 2009;56(2):422–428. doi: 10.1016/j.neuropharm.2008.09.012. [DOI] [PubMed] [Google Scholar]
- 33.Brouillet E, Conde F, Beal MF, et al. Replicating Huntington's disease phenotype in experimental animals[J]. Prog Neurobiol. 1999;59(5):427–468. doi: 10.1016/s0301-0082(99)00005-2. [DOI] [PubMed] [Google Scholar]
- 34.Chen RW, Chuang DM. Long term lithium treatment suppresses p53 and Bax expression but increases Bcl-2 expresion. A prominent role in neuroprotection against excitotoxicity[J]. J Biol Chem. 1999;274(10):6039–6042. doi: 10.1074/jbc.274.10.6039. [DOI] [PubMed] [Google Scholar]
- 35.Chen RW, Qin ZH, Ren M, et al. Regulation of c-Jun N-terminal kinase, p38 kinase and AP-1 DNA binding in cultured brain neurons : roles in glutamate excitotoxicity and lithium neuroprotection[J]. J Neurochem. 2003;84(3):566–575. doi: 10.1046/j.1471-4159.2003.01548.x. [DOI] [PubMed] [Google Scholar]
- 36.Beurel E, Michalek SM, Jope RS. Innate and adaptive immune responses regulated by glycogen synthase kinase-3 (GSK3)[J]. Trends Immunol. 2010;31(1):24–31. doi: 10.1016/j.it.2009.09.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Jope RS, Yuskaitis CJ, Beurel E. Glycogen synthase kinase-3 (GSK3): inflammation, diseases, and therapeutics[J]. Neurochem Res. 2007;32(4/5):577–595. doi: 10.1007/s11064-006-9128-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Rowe MK, Chuang DM. Lithium neuroprotection: molecular mechanisms and clinical implications [J]. Expert Rev Mol Med. 2004;6(21):1–18. doi: 10.1017/S1462399404008385. [DOI] [PubMed] [Google Scholar]
- 39.Rowe MK, Wiest C, Chuang DM. GSK-3 is a viable potential target for therapeutic intervention in bipolar disorder[J]. Neurosci Biobehav Rev. 2007;31(6):920–931. doi: 10.1016/j.neubiorev.2007.03.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Klein PS, Melton DA. A molecular mechanism for the effect of lithium on development[J]. Proc Natl Acad Sci USA. 1996;93(16):8455–8459. doi: 10.1073/pnas.93.16.8455. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Stambolic V, Ruell, Woodgett JR. Lithium inhibits glycogen synthase kinase-3 activity and mimics wingless signalling in intact cells[J]. Curr Biol. 1996;6(12):1664–1668. doi: 10.1016/s0960-9822(02)70790-2. [DOI] [PubMed] [Google Scholar]
- 42.Jope RS. A bimodal model of the mechanism of action of lithium[J]. Mol Psychiatry. 1999;4(1):21–25. doi: 10.1038/sj.mp.4000444. [DOI] [PubMed] [Google Scholar]
- 43.Kirshenboim N, Plotkin B, Shlomo SB, et al. Lithium-mediated phosphorylation of glycogen synthase kinase-3beta involves PI3 kinase-dependent activation of protein kinase C-alpha[J]. J Mol Neurosci. 2004;24(2):237–245. doi: 10.1385/JMN:24:2:237. [DOI] [PubMed] [Google Scholar]
- 44.Chalecka-Franaszek E, Chuang DM. Lithium activates the serine/threonine kinase Akt-1 and suppresses glutamate-induced inhibition of Akt-1 activity in neurons[J]. Proc Natl Acad Sci USA. 1999;96(15):8745–8750. doi: 10.1073/pnas.96.15.8745. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Zhang F, Phiel CJ, Spece L, et al. Inhibitory phosphorylation of glycogen synthase kinase-3 (GSK-3) in response to lithium. Evidence for autoregulation of GSK-3[J]. J Biol Chem. 2003;278(35):33067–33077. doi: 10.1074/jbc.M212635200. [DOI] [PubMed] [Google Scholar]
- 46.Mendes CT, Mury FB, De Sa ME, et al. Lithium reduces Gsk3b mRNA levels: implications for Alzheimer Disease [J]. Eur Arch Psychiatry Clin Neurosci. 2009;259(1):16–22. doi: 10.1007/s00406-008-0828-5. [DOI] [PubMed] [Google Scholar]
- 47.Goni-Oliver P, Lucas JJ, Avila J, et al. N-terminal cleavage of GSK-3 by calpain: a new form of GSK-3 regulation[J]. J Biol Chem. 2007;282(31):22406–22413. doi: 10.1074/jbc.M702793200. [DOI] [PubMed] [Google Scholar]
- 48.Grimes CA, Jope RS. The multifaceted roles of glycogen synthase kinase 3beta in cellular signaling[J]. Prog Neurobiol. 2001;65(4):391–26. doi: 10.1016/s0301-0082(01)00011-9. [DOI] [PubMed] [Google Scholar]
- 49.Liang MH, Chuang DM. Regulation and function of glycogen synthase kinase-3 isoforms in neuronal survival[J]. J Biol Chem. 2007;282(6):3904–3917. doi: 10.1074/jbc.M605178200. [DOI] [PubMed] [Google Scholar]
- 50.Liang MH, Chuang DM. Differential roles of glycogen synthase kinase-3 isoforms in the regulation of transcriptional activation[J]. J Biol Chem. 2006;281(41):30479–30484. doi: 10.1074/jbc.M607468200. [DOI] [PubMed] [Google Scholar]
- 51.Jope RS, Johnson GV. The glamour and gloom of glycogen synthase kinase-3[J]. Trends Biochem Sci. 2004;29(2):95–102. doi: 10.1016/j.tibs.2003.12.004. [DOI] [PubMed] [Google Scholar]
- 52.Silva R, Martins L, Longato-Filho A, et al. Lithium prevents stress-induced reduction of vascular endothelium growth factor levels[J]. Neurosci Lett. 2007;429(1):33–38. doi: 10.1016/j.neulet.2007.09.062. [DOI] [PubMed] [Google Scholar]
- 53.Sinha D, Wang Z, Rrchalski KL, et al. Lithium activates the Wnt and phosphatidylinositol 3-kinase Akt signaling pathways to promote cell survival in the absence of soluble survival factors[J]. Am J Physiol Renal Physiol. 2005;288(4):F703–F713. doi: 10.1152/ajprenal.00189.2004. [DOI] [PubMed] [Google Scholar]
- 54.Huelsken J, Behrens J. The Wnt signalling pathway[J]. J Cell Sci. 2002;115(Pt 21):3977–3978. doi: 10.1242/jcs.00089. [DOI] [PubMed] [Google Scholar]
- 55.Gould TD, Chen G, Manji HK. In vivo evidence in the brain for lithium inhibition of glycogen synthase kinase-3[J]. Neuropsychopharmacology. 2004;29(1):32–38. doi: 10.1038/sj.npp.1300283. [DOI] [PubMed] [Google Scholar]
- 56.O'brien WT, Harper AD, Jove F, et al. Glycogen synthase kinase-3beta haploinsufficiency mimics the behavioral and molecular effects of lithium[J]. J Neurosci. 2004;24(30):6791–6798. doi: 10.1523/JNEUROSCI.4753-03.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Maiuri MC, Zalckvar E, Kimchi A, et al. Self-eating and self-killing: crosstalk between autophagy and apoptosis[J]. Nat Rev Mol Cell Biol. 2007;8(9):741–752. doi: 10.1038/nrm2239. [DOI] [PubMed] [Google Scholar]
- 58.Youle RJ, Strasser A. The BCL-2 protein family: opposing activities that mediate cell death[J]. Nat Rev Mol Cell Biol. 2008;9(1):47–59. doi: 10.1038/nrm2308. [DOI] [PubMed] [Google Scholar]
- 59.He H, Lam M, Mccormick TS, et al. Maintenance of calcium homeostasis in the endoplasmic reticulum by Bcl-2[J]. J Cell Biol. 1997;138(6):1219–1228. doi: 10.1083/jcb.138.6.1219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Lam M, Dubyak G, Chen L, et al. Evidence that BCL-2 represses apoptosis by regulating endoplasmic reticulum-associated Ca2+ fluxes[J]. Proc Natl Acad Sci USA. 1994;91(14):6569–6573. doi: 10.1073/pnas.91.14.6569. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Wei H, Qin ZH, Senatorov VV, et al. Lithium suppresses excitotoxicity-induced striatal lesions in a rat model of Huntington's disease[J]. Neuroscience. 2001;106(3):603–612. doi: 10.1016/s0306-4522(01)00311-6. [DOI] [PubMed] [Google Scholar]
- 62.Chuang DM, Leng Y, Marinova Z, et al. Multiple roles of HDAC inhibition in neurodegenerative conditions[J]. Trends Neurosci. 2009;32(11):591–601. doi: 10.1016/j.tins.2009.06.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Gottlicher M, Minuccl S, Zhu P, et al. Valproic acid defines a novel class of HDAC inhibitors inducing differentiation of transformed cells[J]. EMBO J. 2001;20(24):6969–6978. doi: 10.1093/emboj/20.24.6969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Phiel CJ, Zhang F, Huang EY, et al. Histone deacetylase is a direct target of valproic acid, a potent anticonvulsant, mood stabilizer, and teratogen[J]. J Biol Chem. 2001;276(39):36734–36741. doi: 10.1074/jbc.M101287200. [DOI] [PubMed] [Google Scholar]
- 65.Wang X, Liu P, Zhu H, et al. miR-34a, a microRNA up-regulated in a double transgenic mouse model of Alzheimer's disease, inhibits bcl2 translation[J]. Brain Res Bull. 2009;80(4/5):268–273. doi: 10.1016/j.brainresbull.2009.08.006. [DOI] [PubMed] [Google Scholar]
- 66.Zhou R, Yuan P, Wang Y, et al. Evidence for selective microRNAs and their effectors as common long-term targets for the actions of mood stabilizers [J]. Neuropsychopharmacology. 2009;34(6):1395–1405. doi: 10.1038/npp.2008.131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Manji HK, Quiroz JA, Sporn J, et al. Enhancing neuronal plasticity and celular resilience to develop novel, improved therapeutics for difficult-to-treat depression[J]. Biol Psychiatry. 2003;53(8):707–742. doi: 10.1016/s0006-3223(03)00117-3. [DOI] [PubMed] [Google Scholar]
- 68.Woo NH, Lu B. Regulation of cortical interneurons by neurotrophins: from development to cognitive disorders[J]. Neuroscientist. 2006;12(1):43–56. doi: 10.1177/1073858405284360. [DOI] [PubMed] [Google Scholar]
- 69.Hashimoto R, Takei N, Shimazu K, et al. Lithium induces brain-derived neurotrophic factor and activates TrkB in rodent cortical neurons: an essential step for neuroprotection against glutamate excitotoxicity[J]. Neuropharmacology. 2002;43(7):1173–1179. doi: 10.1016/s0028-3908(02)00217-4. [DOI] [PubMed] [Google Scholar]
- 70.Fukumoto T, Morinobu S, Okamoto Y, et al. Chronic lithium treatment increases the expression of brain-derived neurotrophic factor in the rat brain[J]. Psychopharmacology (Berl) 2001;158(1):100–106. doi: 10.1007/s002130100871. [DOI] [PubMed] [Google Scholar]
- 71.Jacobsen JP, Mork A. The effect of escitalopram, desipramine, electroconvulsive seizures and lithium on brain-derived neurotrophic factor mRNA and protein expression in the rat brain and the correlation to 5-HT and 5-HIAA levels[J]. Brain Res. 2004;1024(1/2):183–192. doi: 10.1016/j.brainres.2004.07.065. [DOI] [PubMed] [Google Scholar]
- 72.Yasuda S, Liang MH, Marinova Z, et al. The mood stabilizers lithium and valproate selectively activate the promoter IV of brain-derived neurotrophic factor in neurons[J]. Mol Psychiatry. 2009;14(1):51–59. doi: 10.1038/sj.mp.4002099. [DOI] [PubMed] [Google Scholar]
- 73.Guo S, Arai K, Stins MF, et al. Lithium upregulates vascular endothelial growth factor in brain endothelial cells and astrocytes[J]. Stroke. 2009;40(2):652–655. doi: 10.1161/STROKEAHA.108.524504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Kaga S, Zhan L, Altaf E, et al. Glycogen synthase kinase-3beta/beta-catenin promotes angiogenic and anti-apoptotic signaling through the induction of VEGF, Bcl-2 and survivin expression in rat ischemic preconditioned myocardium[J]. J Mol Cell Cardiol. 2006;40(1):138–147. doi: 10.1016/j.yjmcc.2005.09.009. [DOI] [PubMed] [Google Scholar]
- 75.Jin K, Zhu Y, Sun Y, et al. Vascular endothelial growth factor (VEGF) stimulates neurogenesis in vitro and in vivo[J]. Proc Natl Acad Sci USA. 2002;99(18):11946–11950. doi: 10.1073/pnas.182296499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Meng H, Zhang Z, Zhang R, et al. Biphasic effects of exogenous VEGF on VEGF expression of adult neural progenitors[J]. Neurosci Lett. 2006;393(2/3):97–101. doi: 10.1016/j.neulet.2005.09.044. [DOI] [PubMed] [Google Scholar]
- 77.Zhang H, Vutskits L, Pepper MS, et al. VEGF is a chemoattractant for FGF-2-stimulated neural progenitors[J]. J Cell Biol. 2003;163(6):1375–1384. doi: 10.1083/jcb.200308040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Chen J, Zhang C, Jiang H, et al. Atorvastatin induction of VEGF and BDNF promotes brain plasticity after stroke in mice[J]. J Cereb Blood Flow Metab. 2005;25(2):281–290. doi: 10.1038/sj.jcbfm.9600034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Du WJ, Li JK, Wang QY, et al. Lithium chloride preconditioning optimizes skeletal myoblast functions for cellular cardiomyoplasty in vitro via glycogen synthase kinase-3beta/beta-catenin signaling[J]. Cells Tissues Organs. 2009;190(1):11–19. doi: 10.1159/000167699. [DOI] [PubMed] [Google Scholar]
- 80.Fink AL. Chaperone-mediated protein folding[J]. Physiol Rev. 1999;79(2):425–449. doi: 10.1152/physrev.1999.79.2.425. [DOI] [PubMed] [Google Scholar]
- 81.Hendrick JP, Hartl FU. Molecular chaperone functions of heat-shock proteins[J]. Annu Rev Biochem. 1993;62:349–384. doi: 10.1146/annurev.bi.62.070193.002025. [DOI] [PubMed] [Google Scholar]
- 82.Takayama S, Reed JC, Homma S. Heat-shock proteins as regulators of apoptosis[J]. Oncogene. 2003;22(56):9041–9047. doi: 10.1038/sj.onc.1207114. [DOI] [PubMed] [Google Scholar]
- 83.Hoehn B, Ringer TM, Xu L, et al. Overexpression of HSP72 after induction of experimental stroke protects neurons from ischemic damage[J]. J Cereb Blood Flow Metab. 2001;21(11):1303–1309. doi: 10.1097/00004647-200111000-00006. [DOI] [PubMed] [Google Scholar]
- 84.Majda BT, Meloni BP, Rixon N, et al. Suppression subtraction hybridization and northern analysis reveal upregulation of heat shock, trkB, and sodium calcium exchanger genes following global cerebral ischemia in the rat[J]. Brain Res Mol Brain Res. 2001;93(2):173–179. doi: 10.1016/s0169-328x(01)00203-0. [DOI] [PubMed] [Google Scholar]
- 85.Rajdev S, Hara K, Kokubo Y, et al. Mice overexpressing rat heat shock protein 70 are protected against cerebral infarction[J]. Ann Neurol. 2000;47(6):782–791. [PubMed] [Google Scholar]
- 86.Bijur GN, Jope RS. Opposing actions of phosphatidylinositol 3-kinase and glycogen synthase kinase-3beta in the regulation of HSF-1 activity[J]. J Neurochem. 2000;7(6):2401–2408. doi: 10.1046/j.1471-4159.2000.0752401.x. [DOI] [PubMed] [Google Scholar]
- 87.Chu B, Soncin F, Price BD, et al. Sequential phosphorylation by mitogen-activated protein kinase and glycogen synthase kinase 3 represses transcriptional activation by heat shock factor-1[J]. J Biol Chem. 1996;271(48):30847–30857. doi: 10.1074/jbc.271.48.30847. [DOI] [PubMed] [Google Scholar]
- 88.Xavier IJ, Mercier PA, Mcloughlin CM, et al. Glycogen synthase kinase 3beta negatively regulates both DNA-binding and transcriptional activities of heat shock factor 1[J]. J Biol Chem. 2000;275(37):29147–29152. doi: 10.1074/jbc.M002169200. [DOI] [PubMed] [Google Scholar]
- 89.Berger Z, Ravikumar B, Menzies FM, et al. Rapamycin alleviates toxicity of different aggregate-prone proteins[J]. Hum Mol Genet. 2006;15(3):433–442. doi: 10.1093/hmg/ddi458. [DOI] [PubMed] [Google Scholar]
- 90.Ravikumar B, Vacher C, Berger Z, et al. Inhibition of mTOR induces autophagy and reduces toxicity of polyglutamine expansions in fly and mouse models of Huntington disease[J]. Nat Genet. 2004;36(6):585–595. doi: 10.1038/ng1362. [DOI] [PubMed] [Google Scholar]
- 91.Rubinsztein DC, Gestwicki JE, Murphy LO, et al. Potential therapeutic applications of autophagy[J]. Nat Rev Drug Discov. 2007;6(4):304–312. doi: 10.1038/nrd2272. [DOI] [PubMed] [Google Scholar]
- 92.Webb JL, Ravikumar B, Atkins J, et al. Alpha-Synuclein is degraded by both autophagy and the proteasome[J]. J Biol Chem. 2003;278(27):25009–25013. doi: 10.1074/jbc.M300227200. [DOI] [PubMed] [Google Scholar]
- 93.Phiel CJ, Klein PS. Molecular targets of lithium action[J]. Annu Rev Pharmacol Toxicol. 2001;41:789–813. doi: 10.1146/annurev.pharmtox.41.1.789. [DOI] [PubMed] [Google Scholar]
- 94.Sarkar S, Flotora RA, Berger Z, et al. Lithium induces autophagy by inhibiting inositol monophosphatase[J]. J Cell Biol. 2005;170(7):1101–1111. doi: 10.1083/jcb.200504035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Sarkar S, Rubinsztein DC. Inositol and IP3 levels regulate autophagy: biology and therapeutic speculations[J]. Autophagy. 2006;2(2):132–134. doi: 10.4161/auto.2387. [DOI] [PubMed] [Google Scholar]
- 96.Hashimoto R, Senatorov V, Kanai H, et al. Lithium stimulates progenitor proliferation in cultured brain neurons[J]. Neuroscience. 2003;117(1):55–61. doi: 10.1016/s0306-4522(02)00577-8. [DOI] [PubMed] [Google Scholar]
- 97.Chen G, Rajkowska G, Du F, et al. Enhancement of hippocampal neurogenesis by lithium[J]. J Neurochem. 2000;75(4):1729–1734. doi: 10.1046/j.1471-4159.2000.0751729.x. [DOI] [PubMed] [Google Scholar]
- 98.Bianchi P, Ciani E, Contestabile A, et al. Lithium restores neurogenesis in the subventricular zone of the Ts65Dn mouse, a model for Down syndrome[J]. Brain Pathol. 2010;20(1):106–118. doi: 10.1111/j.1750-3639.2008.00246.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Boku S, Nakagawa S, Masuda T, et al. Glucocorticoids and lithium reciprocally regulate the proliferation of adult dentate gyrus-derived neural precursor cells through GSK-3beta and beta-catenin/TCF pathway[J]. Neuropsychopharmacology. 2009;34(3):805–815. doi: 10.1038/npp.2008.198. [DOI] [PubMed] [Google Scholar]
- 100.Wexler EM, Geschwind DH, Palmer TD. Lithium regulates adult hippocampal progenitor development through canonical Wnt pathway activation[J]. Mol Psychiatry. 2008;13(3):285–292. doi: 10.1038/sj.mp.4002093. [DOI] [PubMed] [Google Scholar]
- 101.Yan XB, Hou HL, Wu LM, et al. Lithium regulates hippocampal neurogenesis by ERK pathway and facilitates recovery of spatial learning and memory in rats after transient global cerebral ischemia[J]. Neuropharmacology. 2007;53(4):487–495. doi: 10.1016/j.neuropharm.2007.06.020. [DOI] [PubMed] [Google Scholar]
- 102.Rossi C, Angelucci A, Costantin L, et al. Brain-derived neurotrophic factor (BDNF) is required for the enhancement of hippocampal neurogenesis following environmental enrichment[J]. Eur J Neurosci. 200624(7):1850–1856. doi: 10.1111/j.1460-9568.2006.05059.x. [DOI] [PubMed] [Google Scholar]
- 103.Li X, Keter TA, Frye MA. Synaptic, intracelular, and neuroprotective mechanisms of anticonvulsants: are they relevant for the treatment and course of bipolar disorders?[J]. J Affect Disord. 2002;69(1/3):1–14. doi: 10.1016/s0165-0327(00)00361-x. [DOI] [PubMed] [Google Scholar]
- 104.Manji HK, Duman RS. Impairments of neuroplasticity and cellular resilience in severe mood disorders: implications for the development of novel therapeutics[J]. Psychopharmacol Bull. 2001;35(2):5–49. [PubMed] [Google Scholar]
- 105.Beaulieu JM, Sotnikova TD, Yao WD, et al. Lithium antagonizes dopamine-dependent behaviors mediated by an AKT/glycogen synthase kinase 3 signaling cascade[J]. Proc Natl Acad Sci USA. 2004;101(14):5099–5104. doi: 10.1073/pnas.0307921101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Gould TD, Einat H, Bhat R, et al. AR-A014418, a selective GSK-3 inhibitor, produces antidepressant-like effects in the forced swim test[J]. Int J Neuropsychopharmacol. 2004;7(4):387–390. doi: 10.1017/S1461145704004535. [DOI] [PubMed] [Google Scholar]
- 107.Prickaerts J, Moechars D, Cryns K, et al. Transgenic mice overexpressing glycogen synthase kinase 3beta: a putative model of hyperactivity and mania[J]. J Neurosci. 2006;26(35):9022–9029. doi: 10.1523/JNEUROSCI.5216-05.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Beaulieu JM, Zhang X, Rodriguiz RM, et al. Role of GSK3 beta in behavioral abnormalities induced by serotonin deficiency[J]. Proc Natl Acad Sci USA. 2008;105(4):13331–338. doi: 10.1073/pnas.0711496105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Omata N, Chiu CT, Moya PR, et al. Lentivirally mediated GSK-3beta silencing in the hippocampal dentate gyrus induces antidepressant-like effects in stressed mice[J]. Int J Neuropsychopharmacol. 2011;14(5):711–717. doi: 10.1017/S1461145710000726. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Kaidanovich-Beilin O, Lipina TV, Takao K, et al. Abnormalities in brain structure and behavior in GSK-3alpha mutant mice[J]. Mol Brain. 2009;2(1):35. doi: 10.1186/1756-6606-2-35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Polter A, Beurel E, Yang S, et al. Deficiency in the inhibitory serine-phosphorylation of glycogen synthase kinase-3 increases sensitivity to mood disturbances[J]. Neuropsychopharmacology. 2010;35(8):1761–1774. doi: 10.1038/npp.2010.43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Jope RS. Lithium and GSK-3: one inhibitor, two inhibitory actions, multiple outcomes[J]. Trends Pharmacol Sci. 2003;24(9):441–443. doi: 10.1016/S0165-6147(03)00206-2. [DOI] [PubMed] [Google Scholar]
- 113.Nonaka S, Chuang DM. Neuroprotective effects of chronic lithium on focal cerebral ischemia in rats[J]. Neuroreport. 1998;9(9):2081–2084. doi: 10.1097/00001756-199806220-00031. [DOI] [PubMed] [Google Scholar]
- 114.Xu J, Culman J, Blume A, et al. Chronic treatment with a low dose of lithium protects the brain against ischemic injury by reducing apoptotic death[J]. Stroke. 2003;34(5):1287–1292. doi: 10.1161/01.STR.0000066308.25088.64. [DOI] [PubMed] [Google Scholar]
- 115.Bian Q, Shi T, Chuang DM, et al. Lithium reduces ischemia-induced hippocampal CA1 damage and behavioral deficits in gerbils[J]. Brain Res. 2007;1184:270–276. doi: 10.1016/j.brainres.2007.09.054. [DOI] [PubMed] [Google Scholar]
- 116.Roh MS, Eom TY, Zmijewska AA, et al. Hypoxia activates glycogen synthase kinase-3 in mouse brain in vivo: protection by mood stabilizers and imipramine[J]. Biol Psychiatry. 2005;57(3):278–286. doi: 10.1016/j.biopsych.2004.10.039. [DOI] [PubMed] [Google Scholar]
- 117.Re M, Senatorov VV, Che RW, et al. Postinsult treatment with lithium reduces brain damage and facilitates neurological recovery in a rat ischemia/reperfusion model[J]. Proc Natl Acad Sci USA. 2003;100(10):6210–6215. doi: 10.1073/pnas.0937423100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Kim YR, Van Meer MP, Tejima E, et al. Functional MRI of delayed chronic lithium treatment in rat focal cerebral ischemia[J]. Stroke. 2008;39(2):439–47. doi: 10.1161/STROKEAHA.107.492215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Fan Y, Yang GY. Therapeutic angiogenesis for brain ischemia: a brief review[J]. J Neuroimmune Pharmacol. 2007;2(3):284–289. doi: 10.1007/s11481-007-9073-3. [DOI] [PubMed] [Google Scholar]
- 120.Martin JB, Gusella JF. Huntington's disease. Pathogenesis and management[J]. N Engl J Med. 1986;315(20):1267–1276. doi: 10.1056/NEJM198611133152006. [DOI] [PubMed] [Google Scholar]
- 121.Macdonald ME, Ambrose CM, Duyao MP, et al. A novel gene containing a trinucleotide repeat that is expanded and unstable on Huntington's disease chromosomes[J]. Cell. 1993;72(6):971–983. doi: 10.1016/0092-8674(93)90585-e. [DOI] [PubMed] [Google Scholar]
- 122.Hickey MA, Chesselet MF. Apoptosis in Huntington's disease[J]. Prog Neuropsychopharmacol Biol Psychiatry. 2003;27(2):255–265. doi: 10.1016/S0278-5846(03)00021-6. [DOI] [PubMed] [Google Scholar]
- 123.Sugars KL, Rubinsztein DC. Transcriptional abnormalities in Huntington disease[J]. Trends Genet. 2003;19(5):233–238. doi: 10.1016/S0168-9525(03)00074-X. [DOI] [PubMed] [Google Scholar]
- 124.Taylor-Robinson SD, Weeks RA, Bryant DJ, et al. Proton magnetic resonance spectroscopy in Huntington's disease: evidence in favour of the glutamate excitotoxic theory[J]. Mov Disord. 1996;11(2):167–173. doi: 10.1002/mds.870110209. [DOI] [PubMed] [Google Scholar]
- 125.Senatorov VV, Ren M, Kanai H, et al. Short-term lithium treatment promotes neuronal survival and proliferation in rat striatum infused with quinolinic acid, an excitotoxic model of Huntington's disease[J]. Mol Psychiatry. 2004;9(4):371–385. doi: 10.1038/sj.mp.4001463. [DOI] [PubMed] [Google Scholar]
- 126.Carmichael J, Sugars KL, Bao YP, et al. Glycogen synthase kinase-3beta inhibitors prevent cellular polyglutamine toxicity caused by the Huntington's disease mutation[J]. J Biol Chem. 2002;277(37):33791–33798. doi: 10.1074/jbc.M204861200. [DOI] [PubMed] [Google Scholar]
- 127.Berger Z, Ttofi EK, Michel CH, et al. Lithium rescues toxicity of aggregate-prone proteins in Drosophila by perturbing Wnt pathway[J]. Hum Mol Genet. 2005;14(20):3003–3011. doi: 10.1093/hmg/ddi331. [DOI] [PubMed] [Google Scholar]
- 128.Sarkar S, Krishna G, Imarisio S, et al. A rational mechanism for combination treatment of Huntington's disease using lithium and rapamycin[J]. Hum Mol Genet. 2008;17(2):170–178. doi: 10.1093/hmg/ddm294. [DOI] [PubMed] [Google Scholar]
- 129.Wood NI, Morton AJ. Chronic lithium chloride treatment has variable effects on motor behaviour and survival of mice transgenic for the Huntington's disease mutation[J]. Brain Res Bull. 2003;61(4):375–383. doi: 10.1016/s0361-9230(03)00141-2. [DOI] [PubMed] [Google Scholar]
- 130.Chiu CT, Liu G, Leeds P, et al. Combined treatment with the mood stabilizers lithium and valproate produces multiple beneficial effects in transgenic mouse models of Huntington's disease[J]. Neuropsychopharmacology. 2011 doi: 10.1038/npp.2011.128. in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Selkoe DJ. Alzheimer's disease: genes, proteins, and therapy[J]. Physiol Rev. 2001;81(2):741–766. doi: 10.1152/physrev.2001.81.2.741. [DOI] [PubMed] [Google Scholar]
- 132.Munoz-Montano JR, Lim F, Moreno FJ, et al. Glycogen Synthase Kinase-3 Modulates Neurite Outgrowth in Cultured Neurons: Possible Implications for Neurite Pathology in Alzheimer's Disease[J]. J Alzheimers Dis. 1999;1(6):361–378. doi: 10.3233/jad-1999-1602. [DOI] [PubMed] [Google Scholar]
- 133.Huang HC, Klein PS. Multiple roles for glycogen synthase kinase-3 as a drug target in Alzheimer's disease[J]. Curr Drug Targets. 2006;7(11):1389–1397. doi: 10.2174/1389450110607011389. [DOI] [PubMed] [Google Scholar]
- 134.Hong M, Chen DC, Klein PS, et al. Lithium reduces tau phosphorylation by inhibition of glycogen synthase kinase-3[J]. J Biol Chem. 1997;272(40):25326–25332. doi: 10.1074/jbc.272.40.25326. [DOI] [PubMed] [Google Scholar]
- 135.Munoz-Montano JR, Moreno FJ, Avila J, et al. Lithium inhibits Alzheimer's disease-like tau protein phosphorylation in neurons[J]. FEBS Lett. 1997;411(2/3):183–188. doi: 10.1016/s0014-5793(97)00688-1. [DOI] [PubMed] [Google Scholar]
- 136.Tanaka T, Zhong J, Iqbal K, et al. The regulation of phosphorylation of tau in SY5Y neuroblastoma cells: the role of protein phosphatases[J]. FEBS Lett. 1998;426(2):248–254. doi: 10.1016/s0014-5793(98)00346-9. [DOI] [PubMed] [Google Scholar]
- 137.Trojanowski JQ, Lee VM. Phosphorylation of paired helical filament tau in Alzheimer's disease neurofibrillary lesions: focusing on phosphatases[J]. FASEB J. 1995;9(15):1570–1576. doi: 10.1096/fasebj.9.15.8529836. [DOI] [PubMed] [Google Scholar]
- 138.Tsuji S, Morinobu S, Tanaka K, et al. Lithium, but not valproate, induces the serine/threonine phosphatase activity of protein phosphatas 2A in the rat brain without affecting its expression[J]. J Neural Transm. 2003;110(4):413–425. doi: 10.1007/s00702-002-0798-0. [DOI] [PubMed] [Google Scholar]
- 139.Rametti A, Esclair F, Yardin C, et al. Linking alterations in tau phosphorylation and cleavage during neuronal apoptosis[J]. J Biol Chem. 2004;279(52):54518–54528. doi: 10.1074/jbc.M408186200. [DOI] [PubMed] [Google Scholar]
- 140.Rametti A, Esclair F, Yardin C, et al. Lithium down-regulates tau in cultured cortical neurons: a possible mechanism of neuroprotection[J]. Neurosci Lett. 2008;434(1):93–98. doi: 10.1016/j.neulet.2008.01.034. [DOI] [PubMed] [Google Scholar]
- 141.Sun X, Sato S, Murayama O, et al. Lithium inhibits amyloid secretion in COS7 cells transfected with amyloid precursor protein C100[J]. Neurosci Lett. 2002;321(1-2):61–64. doi: 10.1016/s0304-3940(01)02583-6. [DOI] [PubMed] [Google Scholar]
- 142.Phiel CJ, Wilson CA, Lee VM, et al. GSK-3alpha regulates production of Alzheimer's disease amyloid-beta peptides[J]. Nature. 2003;423(6938):435–439. doi: 10.1038/nature01640. [DOI] [PubMed] [Google Scholar]
- 143.Wei H, Leeds PR, Qian Y, et al. beta-amyloid peptide-induced death of PC 12 cells and cerebellar granule cell neurons is inhibited by long-term lithium treatment[J]. Eur J Pharmacol. 2000;392(3):117–123. doi: 10.1016/s0014-2999(00)00127-8. [DOI] [PubMed] [Google Scholar]
- 144.Noble W, Planel E, Zehr C, et al. Inhibition of glycogen synthase kinase-3 by lithium correlates with reduced tauopathy and degeneration in vivo[J]. Proc Natl Acad Sci USA. 2005;102(19):6990–6995. doi: 10.1073/pnas.0500466102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145.Nakashima H, Ishihara T, Suguimoto P, et al. Chronic lithium treatment decreases tau lesions by promoting ubiquitination in a mouse model of tauopathies [J]. Acta Neuropathol. 2005;110(6):547–556. doi: 10.1007/s00401-005-1087-4. [DOI] [PubMed] [Google Scholar]
- 146.Perez M, Hernandez F, Lim F, et al. Chronic lithium treatment decreases mutant tau protein aggregation in a transgenic mouse model[J]. J Alzheimers Dis. 2003;5(4):301–308. doi: 10.3233/jad-2003-5405. [DOI] [PubMed] [Google Scholar]
- 147.Leroy K, Ando K, Heraud C, et al. Lithium treatment arrests the development of neurofibrillary tangles in mutant tau transgenic mice with advanced neurofibrillary pathology [J]. J Alzheimers Dis. 2010;19(2):705–719. doi: 10.3233/JAD-2010-1276. [DOI] [PubMed] [Google Scholar]
- 148.De Ferrari GV, Chacon MA, Barria MI, et al. Activation of Wnt signaling rescues neurodegeneration and behavioral impairments induced by beta-amyloid fibrils[J]. Mol Psychiatry. 2003;8(2):195–208. doi: 10.1038/sj.mp.4001208. [DOI] [PubMed] [Google Scholar]
- 149.Mudher A, Shepherd D, Newman TA, et al. GSK-3beta inhibition reverses axonal transport defects and behavioural phenotypes in Drosophila[J]. Mol Psychiatry. 2004;9(5):522–530. doi: 10.1038/sj.mp.4001483. [DOI] [PubMed] [Google Scholar]
- 150.Rockenstein E, Torance M, Adame A, et al. Neuroprotective effects of regulators of the glycogen synthase kinase-3beta signaling pathway in a transgenic model of Alzheimer's disease are associated with reduced amyloid precursor protein phosphorylation[J]. J Neurosci. 2007;27(8):1981–1991. doi: 10.1523/JNEUROSCI.4321-06.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 151.Terao T, Nakano H, Inou Y, et al. Lithium and dementia: a preliminary study[J]. Prog Neuropsychopharmacol Biol Psychiatry. 2006;30(6):1125–1128. doi: 10.1016/j.pnpbp.2006.04.020. [DOI] [PubMed] [Google Scholar]
- 152.Nunes PV, Forlenza OV, Gattaz WF. Lithium and risk for Alzheimer's disease in elderly patients with bipolar disorder[J]. Br J Psychiatry. 2007;190:359–360. doi: 10.1192/bjp.bp.106.029868. [DOI] [PubMed] [Google Scholar]
- 153.Kruger R, Kuhn W, Muller T, et al. Ala30Pro mutation in the gene encoding alpha-synuclein in Parkinson's disease[J]. Nat Genet. 1998;18(2):106–108. doi: 10.1038/ng0298-106. [DOI] [PubMed] [Google Scholar]
- 154.Polymeropoulos MH, lavedan C, Leroy E, et al. Mutation in the alpha-synuclein gene identified in families with Parkinson's disease[J]. Science. 1997;276(5321):2045–2047. doi: 10.1126/science.276.5321.2045. [DOI] [PubMed] [Google Scholar]
- 155.King TD, Bijur GN, Jope RS. Caspase-3 activation induced by inhibition of mitochondrial complex I is facilitated by glycogen synthase kinase-3beta and attenuated by lithium[J]. Brain Res. 2001;919(1):106–114. doi: 10.1016/s0006-8993(01)03005-0. [DOI] [PubMed] [Google Scholar]
- 156.Chen G, Bower KA, Ma C, et al. (GSK3beta) mediates 6-hydroxydopamine-induced neuronal death[J]. Glycogen synthase kinase 3beta. FASEB J. 2004;18(10):1162–1164. doi: 10.1096/fj.04-1551fje. [DOI] [PubMed] [Google Scholar]
- 157.Youdim MB, Arraf Z. Prevention of MPTP (N-methyl-4-phenyl-1, 2, 3, 6-tetrahydropyridine) dopaminergic neurotoxicity in mice by chronic lithium: involvements of Bcl-2 and Bax[J]. Neuropharmacology. 2004;46(8):1130–1140. doi: 10.1016/j.neuropharm.2004.02.005. [DOI] [PubMed] [Google Scholar]
- 158.Crawford DC, Meadows KL, Newman JL, et al. Prevalence of the fragile X syndrome in African-Americans[J]. Am J Med Genet. 2002;110(3):226–233. doi: 10.1002/ajmg.10427. [DOI] [PubMed] [Google Scholar]
- 159.Garber K, Smith KT, Reines D, et al. Transcription, translation and fragile X syndrome[J]. Curr Opin Genet Dev. 2006;16(3):270–275. doi: 10.1016/j.gde.2006.04.010. [DOI] [PubMed] [Google Scholar]
- 160.Min WW, Yuskaitis CJ, Yan Q, et al. Elevated glycogen synthase kinase-3 activity in Fragile X mice: key metabolic regulator with evidence for treatment potential[J]. Neuropharmacology. 2009;56(2):463–472. doi: 10.1016/j.neuropharm.2008.09.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 161.Mcbride SM, Choi CH, Wang Y, et al. Pharmacological rescue of synaptic plasticity, courtship behavior, and mushroom body defects in a Drosophila model of fragile X syndrome[J]. Neuron. 2005;45(5):753–764. doi: 10.1016/j.neuron.2005.01.038. [DOI] [PubMed] [Google Scholar]
- 162.Choi CH, Mcbride SM, Schoenfeld BP, et al. Age-dependent cognitive impairment in a Drosophila fragile X model and its pharmacological rescue [J]. Biogerontology. 2010;11(3):347–362. doi: 10.1007/s10522-009-9259-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 163.Liu ZH, Smith CB. Dissociation of social and nonsocial anxiety in a mouse model of fragile X syndrome [J]. Neurosci Lett. 2009;454(1):62–66. doi: 10.1016/j.neulet.2009.02.066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 164.Qin M, Kang J, Smith CB. Increased rates of cerebral glucose metabolism in a mouse model of fragile X mental retardation[J]. Proc Natl Acad Sci USA. 2002;99(24):15758–15763. doi: 10.1073/pnas.242377399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 165.The Dutch-Belgian Fragile X Consortium Fmr1 knockout mice: A model to study fragile X mental retardation[J]. Cell. 1994;78(1):23–33. [PubMed] [Google Scholar]
- 166.Yuskaitis CJ, Mines MA, King MK, et al. Lithium ameliorates altered glycogen synthase kinase-3 and behavior in a mouse model of fragile X syndrome[J]. Biochem Pharmacol. 2010;79(4):632–646. doi: 10.1016/j.bcp.2009.09.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 167.Liu ZH, Chuang DM, Smith CB. Lithium ameliorates phenotypic deficits in a mouse model of fragile X syndrome[J]. Int J Neuropsychopharmacol. 2011;14(5):618–630. doi: 10.1017/S1461145710000520. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 168.Bery-Kravis E, Sumis A, Hervey C, et al. Open-label treatment trial of lithium to target the underlying defect in fragile X syndrome[J]. J Dev Behav Pediatr. 2008;29(4):293–302. doi: 10.1097/DBP.0b013e31817dc447. [DOI] [PubMed] [Google Scholar]
- 169.Rowland LP. Amyotrophic lateral sclerosis [J]. Curr Opin Neurol. 1994;7(4):310–315. doi: 10.1097/00019052-199408000-00006. [DOI] [PubMed] [Google Scholar]
- 170.Caldero J, Brunet N, Tarabal O, et al. Lithium prevents excitotoxic cell death of motoneurons in organotypic slice cultures of spinal cord [J]. Neuroscience. 2010;165(4):1353–1369. doi: 10.1016/j.neuroscience.2009.11.034. [DOI] [PubMed] [Google Scholar]
- 171.Ferrussi M, Spalloni A, Bartalucci A, et al. A systematic study of brainstem motor nuclei in a mouse model of ALS, the effects of lithium[J]. Neurobiol Dis. 2010;37(2):370–383. doi: 10.1016/j.nbd.2009.10.017. [DOI] [PubMed] [Google Scholar]
- 172.Fornai F, Longone P, Cafaro L, et al. Lithium delays progression of amyotrophic lateral sclerosis [J]. Proc Natl Acad Sci USA. 2008;105(6):2052–2057. doi: 10.1073/pnas.0708022105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 173.Shin JH, Cho SI, Lim HR, et al. Concurrent administration of Neu2000 and lithium produces marked improvement of motor neuron survival, motor function, and mortality in a mouse model of amyotrophic lateral sclerosis [J]. Mol Pharmacol. 2007;71(4):965–975. doi: 10.1124/mol.106.030676. [DOI] [PubMed] [Google Scholar]
- 174.Feng HL, Leng Y, Ma CH, et al. Combined lithium and valproate treatment delays disease onset, reduces neurological deficits and prolongs survival in an amyotrophic lateral sclerosis mouse model[J]. Neuroscience. 2008;155(3):567–572. doi: 10.1016/j.neuroscience.2008.06.040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 175.Aggarwal SP, Zinman L, Simpaon E, et al. Safety and efficacy of lithium in combination with riluzole for treatment of amyotrophic lateral sclerosis: a randomised, double-blind, placebo-controled trial[J]. Lancet Neurol. 2010;9(5):481–488. doi: 10.1016/S1474-4422(10)70068-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 176.Gill A, Kidd J, Vieira F, et al. No benefit from chronic lithium dosing in a sibling-matched, gender balanced, investigator-blinded trial using a standard mouse model of familial ALS [J]. PLoS One. 2009;4(8):e6489. doi: 10.1371/journal.pone.0006489. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 177.Pizzasegola C, Caron I, Daleno C, et al. Treatment with lithium carbonate does not improve disease progression in two different strains of SOD1 mutant mice[J]. Amyotroph Lateral Scler. 2009;10(4):221–228. doi: 10.1080/17482960902803440. [DOI] [PubMed] [Google Scholar]
- 178.Hafler DA. Multiple sclerosis[J]. J Clin Invest. 2004;113(6):788–794. doi: 10.1172/JCI21357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 179.Noseworthy JH, Lucchinetti C, Rodriguez M, et al. Multiple sclerosis[J]. N Engl J Med. 2000;343(13):938–952. doi: 10.1056/NEJM200009283431307. [DOI] [PubMed] [Google Scholar]
- 180.Baxter AG. The origin and application of experimental auto-immune encephalomyelitis[J]. Nat Rev Immunol. 2007;7(11):904–912. doi: 10.1038/nri2190. [DOI] [PubMed] [Google Scholar]
- 181.De Sarno P, Axtell RC, Raman C, et al. Lithium prevents and ameliorates experimental autoimmune encephalomyelitis[J]. J Immunol. 2008;181(1):338–345. doi: 10.4049/jimmunol.181.1.338. [DOI] [PMC free article] [PubMed] [Google Scholar]