Abstract
Context
In 2005, HEDIS introduced a quality measure to assess the receipt of disease modifying anti-rheumatic drugs (DMARDs) among patients with rheumatoid arthritis (RA).
Objective
To identify sociodemographic, community, and health-plan factors associated with DMARD receipt among Medicare managed care enrollees.
Design, Setting, and Patients
We analyzed individual-level HEDIS data for 93,143 patients ≥ 65 years old with at least 2 diagnoses of RA within a measurement year (during 2005–2008). Logistic regression models with generalized estimating equations were used to determine factors associated with DMARD receipt. We used logistic regression to adjust health plan performance for case-mix.
Main Outcome Measure
DMARD receipt (yes/no).
Results
The mean age of patients was 74 years; 75% were female, and 82% were white. Overall performance on the HEDIS RA measure was 59% in 2005, rising to 67% in 2008 (p for trend <.01). The largest difference in performance was based on age: patients ≥ 85 had a 30% (29%, 32%), p<.001) point lower rate of DMARD receipt compared to patients 65–69, even after adjusting for other factors. Males (−3%, 95% CI (−5%, −2%), p<.001), blacks (−4%, 95% CI (−6%, −2%), p<.001), patients with low personal income(−6%, 95% CI (−8%,−5%), p<.001), and those with the lowest ZIP-code-based socioeconomic status (SES) (−4%, 95% CI (−6%,−2%), p<.001) were also found to have lower percentage point rates, as were patients in the Middle (−7%, 95% CI (−13%,−2%), p<.001) and South Atlantic regions (−11%, 95% CI (−20%,−3%), p<.001, compared to the Pacific) and patients enrolled in for-profit health plans (−4%, 95% CI (−7%,−0%), p<.001). Performance varied widely by health plan, ranging from 16% to 87%.
Conclusions
Among Medicare managed care enrollees carrying a diagnosis of RA between 2005 and 2008, 63% received a DMARD; DMARD receipt varied based on demographic factors, SES, geographic location, and health plan.
Despite evidence-based guidelines recommending early and aggressive treatment of active rheumatoid arthritis (RA),1,2 recent population-based studies of disease modifying anti-rheumatic drug (DMARD) utilization in RA patients report consistently low rates of DMARD receipt (30–52%).3,4,5,6,7 One limitation of the existing literature is that U.S. studies have only examined groups with low socioeconomic status (SES) covered by state-funded insurance programs or within a single health plan and geographic area, so these data may not be broadly generalizable. 4,5,6
In 2005, the National Committee for Quality Assurance revised HEDIS (Healthcare Effectiveness Data and Information Set), a set of performance indicators used by health plans to report on their quality of care, to include a measure assessing whether patients with RA received a prescription for a DMARD. Medicare managed care (MMC) plans (alternatives to the traditional Medicare fee-for-service programs that provide hospital, outpatient, and pharmacy coverage to over 8 million Medicare beneficiaries) are required to report their performance on all HEDIS measures. Therefore, HEDIS data from MMC plans provide a nationally-representative sample of the managed care population over age 65 and avoid the selection bias associated with studies of patients in specialty care or in health plans with voluntary reporting.
In this study, we examined variations in DMARD receipt in a large cohort of managed care patients diagnosed with RA. To our knowledge, this is the first comprehensive study to assess sociodemographic, community, and health-plan factors associated with DMARD receipt.
Methods
Study population and data sources
We obtained individual-level HEDIS data for all MMC plans from Centers for Medicare and Medicaid Services (CMS) for 4 reporting years (2006 to 2009), covering clinical care delivered between 2005 and 2008. Each observation included an individual’s Health Identification Code, health plan, and variables indicating eligibility for and receipt of care consistent with the HEDIS RA measure.
Information about data collection and CMS-sponsored audits has been published previously.8,9 Using the Health Identification Code, we matched beneficiaries eligible for the HEDISRA measure with the Medicare denominator file for the corresponding year. This file contains demographic information on race, age, sex, ZIP code, and county of residence. The file also contains information on whether beneficiaries had received “state buy-in” assistance for their Medicare Part B premiums or copayments, a marker of low personal income.10 We achieved a match rate of 98%. Zip-code-based SES was calculated based on Census 2000 variables using the Agency for Healthcare Research and Quality’s SES index score.11 Health professional shortage area (HPSA) counties (counties with a complete or partial shortage of physicians) were defined using the Area Resource File.12 We obtained health plan characteristics (model type, plan age, enrollment size, and tax status) from the Interstudy Competitive Edge data set or by contacting the health plans directly.13
Inclusion criteria for the study were eligibility for the HEDIS RA measure per the specification (see below), age 65 or greater, residence in the 50 U.S. states, and survival through the measurement year. There were 180,153 observations that met these criteria. We excluded 94 health plans (with 12,862 observations) that appeared to have incomplete reporting, as evidenced by missing data for >10% of eligible enrollees or <10% performance on the HEDIS RA measure. Because the dataset covers multiple years, individuals could have up to four observations; we selected one observation at random from each individual in order to avoid multiple levels of clustering in the statistical analysis (see below). The final study sample included 93,143 observations from 299 health plans.
Measure specification
The National Committee for Quality Assurance’s HEDIS RA measure aimed to assess “whether patients diagnosed with RA have had at least one ambulatory prescription dispensed for a DMARD [during the measurement year].” Patients in the denominator for the measure (1) were continuously enrolled in a MMC plan during the measurement year (no more than one 45-day gap in enrollment allowed), (2) had both medical and pharmacy benefits, and (3) had at least 2 face-to-face physician encounters with different dates of service in an ambulatory or non-acute patient setting during the measurement year with any diagnosis of RA (ICD-9 codes 714.0, 714.1, 714.2, or 714.81). Patients were excluded from the measure if they were pregnant or carried a diagnosis of HIV during the measurement year. Accepted drugs included both traditional and biologic DMARDs: abatacept, adalimumab, anakinra, azathioprine, cyclophosphamide, cyclosporine, etanercept, gold, hydroxychloroquine, infliximab, leflunomide, methotrexate, minocycline, penicillamine, rituximab, staphylococcal protein A, and sulfasalazine. The numerator for the measure was a dichotomous measure of DMARD receipt (yes/no); the names of the specific DMARDs received were not recorded.
Study variables
The dependent variable was DMARD receipt among eligible enrollees. Independent variables included age, race (white, black, other), sex, low personal income (as proxied by the state buy-in variable), ZIP-code-based SES index score (divided into quintiles), residence in a physician shortage county, geographic region, and health-plan variables (model type, plan age, enrollment size, and tax status). Information on race in the Medicare denominator file was derived from Social Security Administration data obtained at the time of an individual’s application for a new or replacement Social Security card and has been shown to be reliable for persons designated as white or black.14,15
Statistical analysis
We assessed demographic, socioeconomic, and health-plan characteristics of patients in the final study sample and used chi-square or t-tests to compare patients receiving DMARDs to those who were not. We determined the overall and yearly performance on the HEDIS RA measure. For each subgroup of patients (defined by demographic, socioeconomic, and health plan characteristics), we calculated performance on the HEDIS RA measure and the absolute difference in performance compared to the relevant referent group.
To determine adjusted performance differences among subgroups, we fitted linear models with generalized estimating equations to predict DMARD receipt adjusted for covariates and accounting for the clustering of individuals within health plans.16 Variables in the multivariate models were determined a priori based on prior studies of DMARD utilization and HEDIS measure fulfillment.8 All covariates were tested to ensure non-collinearity. We fitted three versions of these models: Model 1 adjusted for age, race, sex, income, and year; Model 2 included all variables in Model 1 in addition to ZIP-code-based SES, geographic region, and residence in a physician shortage county; Model 3 included all variables in Model 2 in addition to health plan variables (model type, plan age, enrollment size, and tax status). Results of the models were reported as percentage point differences. We performed additional sensitivity analyses: (1) using the first year of data contributed by a given individual, (2) using the last year of data contributed by an individual, and (3) using the “best-case” year of data contributed by an individual (i.e., if an individual received a DMARD in some years but not others, categorizing her as having received a DMARD).
Last, we assessed the variability in the performance of different health plans on the HEDIS RA measure. Health plans with fewer than 20 beneficiaries in the final study sample were excluded, leaving 245 plans. Health plan performance on the measure was calculated by aggregating the individual-level data. We assessed performance on the HEDIS RA measure with logistic regression models to adjust health plan performance for case-mix (including variables for age, race, sex, income as proxied by “state buy-in”, ZIP-code-based SES, residence in a physician shortage county, and geographic region). This process has been described at length elsewhere.17 Briefly, we applied regression coefficients from multivariate logistic regressions to calculate the predicted probability of DMARD receipt in each health plan for every individual in the combined sample. By averaging the predicted probabilities by health plan, we calculated a directly standardized adjusted performance rate defined as the predicted performance for each plan if every plan had the same distribution of member characteristics.
Statistical tests were 2-sided with p <.05 considered statistically significant. We were able to detect a minimum difference of 2.2 percentage points in the individual subgroup analyses with 80% power. All analyses were performed using SAS (version 9.2, Cary, NC.). Our study protocol was approved by the Institutional Review Boards of Stanford University and the University of California—San Francisco and by the CMS Privacy Board; an exemption to informed consent was granted because the dataset used was de-identified.
Results
The characteristics of the patients and health plans in the study sample are listed in Table 1. Overall performance on the HEDIS RA measure in the study sample was 63%. In 2005, 59% of the sample received a DMARD; in 2006, 58%; in 2007, 62%; in 2008, 67% (p for trend <.01).
Table 1.
Sociodemographic characteristics of patients eligible for the HEDIS rheumatoid arthritis measure and characteristics of health plans.
| Characteristics (No. (%) except where otherwise noted) | Health Plans† (n = 245) | All Enrollees (n = 93,143) | Receiving DMARD (n = 58,506) | Not receiving DMARD (n = 34,637) |
|---|---|---|---|---|
| Female | 70,186 (75.4) | 44,565 (76.2) | 25621 (74.0) | |
|
| ||||
| Age (mean (SD)) | 74.4 (6.7) | 73.4 (6.2) | 76.0 (7.1) | |
| 65–69 | 26,440 (28.4) | 18,991 (32.5) | 7.449 (21.5) | |
| 70–74 | 24,356 (26.2) | 16,106 (27.5) | 8,250 (23.9) | |
| 75–79 | 20,930 (22.5) | 12,722 (21.8) | 8,208 (23.7) | |
| 80–84 | 13,609 (14.6) | 7,458 (12.7) | 6,151 (17.8) | |
| ≥ 85 | 7,808 (8.4) | 3,249 (5.5) | 4,559 (13.2) | |
|
| ||||
| Race | ||||
| White | 76,275 (81.9) | 48,858 (83.5) | 27,417 (79.2) | |
| Black | 10,229 (11.0) | 5,806 (9.9) | 4,423 (12.8) | |
| Other | 6,639 (7.1) | 3,862 (6.6) | 2.777 (8.0) | |
|
| ||||
| Low personal income | 12,297 (13.2) | 6,762 (11.6) | 5,535 (16.0) | |
|
| ||||
| SES indicator (ZIP-code) (mean (SD)) | 50.9 (3.8) | 51.0 (3.8) | 50.6 (3.9) | |
|
| ||||
| Health Professional Shortage Area (part or whole) | 79,201 (85.1) | 49,129 (84.0) | 30,072 (86.9) | |
|
| ||||
| Geographic division | ||||
| New England | 4,087 (4.4) | 2,734 (4.7) | 1,353 (3.9) | |
| Middle Atlantic | 17,746 (19.0) | 10,461 (17.8) | 7,285 (21.1) | |
| East North Central Midwest | 7,838 (8.4) | 5,423 (9.3) | 2,415 (7.0) | |
| West North Central Midwest | 5.719 (6.1) | 4,067 (7.0) | 1,652 (4.8) | |
| South Atlantic | 16,035 (17.2) | 8,280 (14.2) | 7,755 (22.4) | |
| East South Central | 3,626 (3.9) | 2,212 (3.8) | 1,414 (4.1) | |
| West South Central | 6,712 (7.2) | 4,134 (7.1) | 2,578 (7.4) | |
| Mountain | 8,015 (8.6) | 5,584 (9.5) | 2,431 (7.0) | |
| Pacific | 23,365 (25.1) | 15,631 (26.7) | 7,734 (22.3) | |
|
| ||||
| Model type | ||||
| Group/staff | 131 (53.5) | 45,373 (48.7) | 28,146 (48.1) | 17,227 (49.7) |
| IPA | 79 (32.2) | 41,810 (44.9) | 26,428 (45.1) | 15,382 (44.5) |
| Mixed/Network/Other | 35 (14.3) | 5,960 (6.4) | 3,952 (6.8) | 2,008 (5.8) |
|
| ||||
| HMO started operation | ||||
| After 2000 | 71 (29.0) | 13,968 (15.0) | 8,892 (15.2) | 5,076 (14.7) |
| 1980 – 1999 | 136 (55.5) | 48,894 (52.5) | 29,211 (49.9) | 19.683 (56.9) |
| Before 1980 | 38 (15.5) | 30,281 (32.5) | 20,423 (34.9) | 9.858 (28.4) |
|
| ||||
| Plan size | ||||
| 0–24,999 | 155 (63.3) | 16,955 (18.2) | 10,835 (18.5) | 6,120 (17.7) |
| 25,000–99,999 | 77 (31.4) | 41,107 (44.1) | 25,972 (44.4) | 15,135 (43.7) |
| ≥ 100,000 | 13 (5.3) | 35, 081 (37.7) | 21,719 (37.1) | 13,362 (38.6) |
|
| ||||
| Profit status | ||||
| Not for profit | 70 (28.6) | 31, 508 (33.8) | 21,185 (36.2) | 10,323 (29.8) |
| For profit | 175 (71.4) | 61,635 (66.2) | 37,341 (63.8) | 24,294 (70.2) |
refers to health plans used in health plan analysis (Figure 1)
A chi-square or t-test was performed to compare the characteristics of individuals who received to those who did not receive DMARDs; all tests resulted in p values < .001
The largest difference in performance on the HEDIS RA measure was based on age: patients ≥ 85 years had a 30% (95% CI 29%, 32%) point lower rate of DMARD receipt compared to patients 65–69 years old, even after adjusting for other factors (Table 2). Males, non-whites, individuals with low personal income, and those in lower SES ZIP-codes were also less likely to receive a DMARD, as were individuals in the Middle and South Atlantic regions. Patients living in a health professional shortage area had slightly lower performance (3% point lower, 95% CI 1%, 5%). In addition, patients enrolled in a for-profit health plan had a 4% (95% CI 0%, 7%) point lower rate of DMARD receipt compared with patients enrolled in a not-for-profit health plan.
Table 2.
Observed and adjusted performance rates on the HEDIS RA measure.
| Characteristics | N | Observed rate (%) | Unadjusted difference (percentage points) | Adjusted difference (percentage points) | ||
|---|---|---|---|---|---|---|
| Model 1* | Model 2* | Model 3* | ||||
| Gender | ||||||
| Female | 70186 | 63.5 | referent | referent | referent | referent |
| Male | 22957 | 60.9 | −2.6 | −4.0 (−5.6, −2.4) | −3.5 (−4.9, −2.0) | −3.3 (−4.7, −1.9) |
|
| ||||||
| Age | ||||||
| 65–69 | 26449 | 71.8 | referent | referent | referent | referent |
| 70–74 | 24359 | 66.0 | −5.8 | −5.2 (−6.2, −4.2) | −5.2 (−6.1, −4.2) | −5.0 (−5.9, −4.2) |
| 75–79 | 20923 | 60.8 | −11.0 | −10.3 (−11.7, −9.0) | −10.5 (−11.7, −9.4) | −10.4 (−11.4, −9.3) |
| 80–84 | 13607 | 54.8 | −17.0 | −16.3 (−18.2, −14.4) | −16.7 (−18.2, −15.1) | −16.6 (−18.0, −15.0) |
| ≥ 85 | 7805 | 41.5 | −30.3 | −29.6 (−31.3, −27.9) | −30.3 (−31.8, −28.9) | −30.3 (−31.7, −28.7) |
|
| ||||||
| Race | ||||||
| White | 76275 | 64.0 | referent | referent | referent | referent |
| Black | 10229 | 56.6 | −7.4 | −7.5 (−9.2, −5.8) | −4.1 (−6.5, −2.7) | −4.3 (−6.1, −2.5) |
| Other | 6639 | 58.2 | −5.8 | −4.2 (−6.8, −1.6) | −4.6 (−6.5, −2.7) | −5.0 (−7.2, −2.7) |
|
| ||||||
| Personal income | ||||||
| Low | 80851 | 64.0 | referent | referent | referent | referent |
| Not low | 12292 | 55.0 | −9.0 | −7.8 (−9.3, −6.4) | −6.6 (−8.3, −4.9) | −6.3 (−7.9, −4.8) |
|
| ||||||
| Zip-code-based SES | ||||||
| Quintile 1 (low) | 19696 | 59.8 | −7.2 | −4.5 (−6.4, −2.6) | −4.0 (−5.8, −2.3) | |
| Quintile 2 | 19205 | 60.5 | −6.5 | −4.2 (−5.7, −2.6) | −3.7 (−5.2, −2.2) | |
| Quintile 3 | 18236 | 62.9 | −4.1 | −2.4 (−3.8, −0.9) | −2.0 (−3.5, −0.6) | |
| Quintile 4 | 17862 | 64.3 | −2.7 | −1.8 (−3.1, −0.1) | −1.6 (−2.7, 0.0) | |
| Quintile 5 (high) | 18144 | 67.0 | referent | referent | referent | |
|
| ||||||
| Geographic division | ||||||
| New England | 4086 | 67.0 | 0.1 | 0.0 (−3.8, 4.7) | 0.0 (−4.8, 5.8) | |
| Middle Atlantic | 17744 | 58.8 | −8.1 | −9.4 (−14.0, −4.7) | −7.6 (−12.9, −2.2) | |
| East North Central Midwest | 7839 | 69.2 | 2.3 | 0.2 (−4.1, 4.5) | 2.6 (−1.6, 6.9) | |
| West North Central Midwest | 5719 | 71.1 | 4.2 | 2.2 (−3.3, 7.6) | 2.5 (−4.1, 9.0) | |
| South Atlantic | 16037 | 51.6 | −15.3 | −15.1 (−26.0, −4.2) | −11.3 (−19.7, −2.8) | |
| East South Central | 3626 | 61.0 | −5.9 | −8.3 (−17.0, 0.4) | −5.3 (−14.3, 3.8) | |
| West South Central | 6713 | 61.6 | −5.3 | −6.3 (−10.7, −1.9) | −2.6 (−7.6, 2.3) | |
| Mountain | 8015 | 69.7 | 2.8 | 0.1 (−3.2, 5.8) | 2.9 (−1.3, 7.1) | |
| Pacific | 23364 | 66.9 | referent | referent | referent | |
|
| ||||||
| Health professional shortage area | ||||||
| No shortage | 13873 | 67.4 | referent | referent | referent | |
| Shortage | 79203 | 62.0 | −5.4 | −3.4 (−5.2, −1.6) | −2.8 (−4.6, −1.2) | |
|
| ||||||
| Model type | ||||||
| Group/staff | 45373 | 62.0 | referent | referent | ||
| IPA | 41814 | 63.2 | 1.2 | 2.7 (−1.6, 7.1) | ||
| Mixed/Network/Other | 5956 | 66.3 | 4.3 | 1.4 (−2.2, 5.0) | ||
|
| ||||||
| HMO started operation | ||||||
| After 2000 | 13969 | 63.4 | −4.1 | −1.2 (−6.6, 4.3) | ||
| 1980 – 1999 | 48893 | 59.7 | −7.8 | −4.2 (−8.4, 0.0) | ||
| Before 1980 | 30281 | 67.5 | referent | referent | ||
|
| ||||||
| Plan size | ||||||
| 0–24,999 | 16958 | 63.9 | 2.0 | 1.0 (−4.2, 6.2) | ||
| 25,000–99,999 | 41104 | 63.1 | 1.2 | 1.4 (−3.3, 6.1) | ||
| ≥ 100,000 | 35081 | 61.9 | referent | referent | ||
|
| ||||||
| Profit status | ||||||
| Not for profit | 31506 | 67.2 | referent | referent | ||
| For profit | 61637 | 60.6 | −6.6 | −3.7 (−7.2, −0.2) | ||
adjusted for all listed variables in addition to calendar year
A model similar to Model 3 in which the separate measures of personal and neighborhood SES were replaced by one categorical variable representing the four possible combinations for personal income (low/not low) and lowest quintile of ZIP-code based SES (yes/no) revealed a stepped relationship: individuals with both personal and neighborhood poverty had the lowest performance on the HEDIS RA measure (−7.1%, 95% CI −9.8%, −4.4%) compared to those without low personal income and living in any of the top 4 quintiles of SES ZIP-codes; those with only one type of poverty were slightly better off (low personal income only, −6.9%(95% CI −8.9%, −5.0%); lowest SES ZIP-codes only, −2.4%(95% CI −4.1%, −0.8%).
Sensitivity analyses where we used (1) the first or (2) the most recent year of data contributed by an individual did not change the results of the multivariate models. Similarly, using (3) the “best-case” year of data showed that overall, 66% of patients received a DMARD; the results of the multivariate models were unchanged.
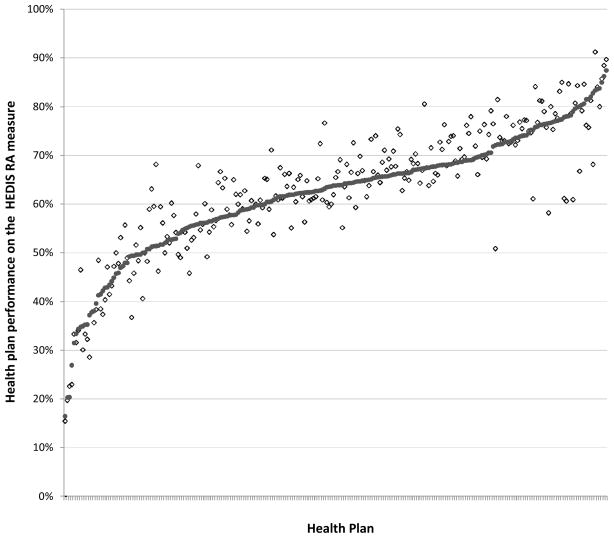
Figure 1 shows the performance of each health plan. Performance varied widely by health plan, with rates ranging from 16% to 87%, even after adjusting for case-mix. The range of adjustment due to case-mix was −16% to +21% with mean −1% and interquartile range of −4% to +1%. The case mix adjustments were smaller than the standard error of the rates for the majority of the plans; in other words, the variation introduced into the performance rates by case mix was smaller than the sampling variability, but not negligible. A sensitivity analysis using the “best-case” year of data only changed adjusted health plan performance slightly, with a range of 15% to 89%.
Figure 1.
Observed and case-mix-adjusted rate of performance by health plans on the HEDIS rheumatoid arthritis measure.
Each health plan has a solid circle representing the case-mix adjusted rate for the plan, and a empty diamond representing the observed rate for the plan (n=245). Plans with fewer than 20 observations were excluded.
Discussion
In this nationally representative sample of patients in Medicare managed care with a diagnosis of RA, we found wide variations in DMARD receipt based on sociodemographics, geographic location, and health plan. Prior research has found that disparities in outcomes for patients with RA exist on the basis of race and socioeconomic status.18–21 While RA was once an inevitably deforming and disabling condition, the development of new DMARDs and support for their early use has dramatically improved clinical outcomes for many patients.1,22,23,24 Our study suggests that one mechanism for the sociodemographic disparities in RA outcomes in the United States may relate to differences in DMARD receipt.
While we found that over one third of patients in this cohort were not receiving a DMARD, the optimal rate of DMARD receipt for this cohort is difficult to estimate. Certainly, some patients in the HEDIS denominator for this measure may have declined treatment, had quiescent disease, or had contraindications to all 17 eligible drugs. In a recent study using the German Biologic Register, Ziegler et al. report that up to 19% of patients did not receive a DMARD within a calendar year and that up to 11% had quiescent disease or relative contraindications to available drugs.25 In our study, the proportion of patients in these categories may be even higher because of an older mean age and a possibly higher rate of RA misdiagnosis (because RA diagnoses were drawn from administrative sources).
We found that DMARD receipt varied significantly with sex and age; the oldest patients had the lowest rate of DMARD receipt and males had slightly lower DMARD receipt compared to females. Although there is conflicting data around disparities based on sex, recent studies have shown that older patients are less likely to receive DMARDs. 3,4,7,26,27,28 Age differences may be due to age-bias,26 increased prevalence of comorbidities that may represent contraindications to DMARD use,4 patient preferences against DMARD receipt, or a milder or different clinical course among older patients. Future studies using a large clinical dataset could further elucidate these issues.
In addition, we found that individuals with low personal income and those living in low SES neighborhoods had reduced DMARD receipt. Lacaille et al. report similar findings in a population-based study from Canada.27 Low neighborhood SES exerted an independent negative effect on DMARD receipt beyond that of individual low income; this finding has been described before in studies of physical and mental health outcomes and access to care in patients with other chronic conditions.29,30
We found significant variation in performance on the HEDIS RA measure based on geography, with patients in the Middle and South Atlantic using DMARDs at rates 10% points lower than patients in other areas of the country, even after adjusting for characteristics of individual patients. Such geographic disparities have been noted in other analyses of Medicare quality and spending.31–33 We also found a small but significant increase in DMARD receipt in enrollees of not-for-profit health plans compared to enrollees of for-profit health plans, which has also been reported for other HEDIS measures.34
The dramatic variations in performance on the HEDIS RA measure among different health plans are concerning. We found a 70% point spread between the best and worst performing plans. Possible explanations for these differences include variations in the availability or accessibility of specialty care within a health plan or differences in the ability of the health plan to appropriately identify patients in the numerator and denominator for the measure. At least one other study has found that health plans can influence quality of care independent of the selection of physician organizations with which they contract.35
The primary strength of our study is that it provides the first examination of DMARD receipt in a nationally representative sample of patients in managed care plans. Because we enhanced data obtained from HEDIS with multiple other data sources, we were able to investigate the influence of individual, community, and health-care system factors on DMARD receipt. However, the study has several limitations. RA diagnoses were drawn from administrative sources. Gabriel’s population-based study measuring the accuracy of administrative diagnoses for RA showed only moderate (57%) positive predictive value of a single diagnostic code for RA compared to a gold-standard that included medical record review.36 Identification of RA patients in our study were based on 2 RA diagnosis codes from 2 face-to-face physician encounters with different dates of service; still, some patients in our study may have been misclassified as having RA when in fact they carried an alternate diagnosis or had quiescent disease that might not require DMARDs.
In addition, we lacked detailed clinical information on factors such as disease activity, co-morbidities, contraindications to DMARD use, or patient preferences. However, the number of patients with contraindications or intolerance to all 17 available DMARDs (including hydroxychloroquine and sulfasalazine) was likely to be small, as demonstrated by very high rates of DMARD receipt reported for patients seen in specialty clinics.25 We did not have information on the type of physician who treated each individual patient, or the number of rheumatology specialists serving a particular health plan. Because physician type has been identified as an important predictor of quality care in other studies,4,5,27 this represents a possible explanation for the disparities we observed in this population. However, we did include a variable (health professional shortage areas) that assessed the density of physicians, including specialists, in a patient’s county and found that patients residing in a physician shortage county were significantly less likely to receive a DMARD.
Finally, we were not able to assess the generosity of the prescription drug benefits for each health plan, which may be an important factor associated with DMARD receipt. Although annual out-of-pocket costs among patients in MMC plans for biologic DMARDs can exceed $4,000,37 drugs such as hydroxychloroquine or methotrexate cost less than $1,000 per year. Still, patients with state buy-in or living in lower SES neighborhoods might be less likely to receive a DMARD because they are unable to afford co-payments or other forms of cost-sharing for a DMARD.38 If patients with lowest SES were clustered within a few health plans, cost burden may also be a mechanism for the variation found in health plan performance.
In summary, we found significant differences in DMARD receipt based on individual, community, and health plan characteristics. Given the enormous individual and societal costs associated with RA, and growing, substantial evidence that DMARDs can reduce these costs, variations in DMARD receipt based on demographics, SES, and geography, are unacceptable. Because optimizing DMARD utilization is the primary mechanism to decrease the significant public health impact of RA in the U.S., targeting educational and quality improvement interventions to patients that are under-using DMARDs and their providers will be important to eliminate these disparities. Additional studies of population-wide cohorts that include clinical data and disease activity measures are needed to validate our findings.
Acknowledgments
Funding: ACR/REF Physician-Scientist Development Award, National Center for Research Resources, Grant Number: 5-M01-RR-00079, Rosalind Russell Medical Research Center for Arthritis, NIH (Grant Number: R01-AR-44804), State of California Lupus Fund, Arthritis Foundation, Agency for Healthcare Research and Quality, National Institute of Arthritis and Musculoskeletal and Skin Diseases (Grant Number: 1-R01-HS-013893, P60-AR-053308, K24-AR-AR055989, R01-AR-056215)
Role of the Sponsors: The funding organizations had no role in the design and conduct of the study, in the collection, analysis, and interpretation of the data, or in the preparation, review, or approval of the manuscript.
We would like to thank Alan Zaslavsky PhD (Department of Health Care Policy, Harvard Medical School) for sharing his case-mix adjustment macro. Dr. Zaslavsky was not compensated for this contribution.
Footnotes
Author Contributions: Dr. Schmajuk had full access to all of the data in the study and takes responsibility for the integrity of the data and the accuracy of the data analysis.
Design and conduct of study: Schmajuk, Trivedi, Yazdany
Acquisition of data: Schmajuk, Yazdany
Management of data: Schmajuk
Analysis and interpretation of data: Schmajuk, Trivedi, Solomon, Yelin, Trupin, Chakravarty, Yazdany
Preparation of manuscript: Schmajuk, Yazdany
Review and approval of the manuscript: Schmajuk, Trivedi, Solomon, Yelin, Trupin, Chakravarty, Yazdany
References
- 1.Guidelines for the management of RA: 2002 update. American College of Rheumatology Ad Hoc Committee on Clinical Guidelines. Arthritis Rheum. 2002;46:328. [Google Scholar]
- 2.Saag KG, Teng GG, Patkar NM, Anuntiyo J, Finney C, Curtis JR, Paulus HE, Mudano A, Pisu M, Elkins-Melton M, Outman R, Allison JJ, Suarez Almazor M, Bridges SL, Jr, Chatham WW, Hochberg M, MacLean C, Mikuls T, Moreland LW, O’Dell J, Turkiewicz AM, Furst DE American College of Rheumatology. American College of Rheumatology 2008 recommendations for the use of nonbiologic and biologic disease-modifying antirheumatic drugs in rheumatoid arthritis. Arthritis Rheum. 2008;59:762–784. doi: 10.1002/art.23721. [DOI] [PubMed] [Google Scholar]
- 3.Edwards CJ, Arden NK, Fisher D, Saperia JC, Reading I, Van Staa TP, Cooper C. The changing use of disease-modifying anti-rheumatic drugs in individuals with rheumatoid arthritis from the United Kingdom General Practice Research Database. Rheumatology (Oxford) 2005 Nov;44(11):1394–8. doi: 10.1093/rheumatology/kei024. [DOI] [PubMed] [Google Scholar]
- 4.Schmajuk G, Schneeweiss S, Katz JN, Weinblatt ME, Setoguchi S, Avorn J, Levin R, Solomon DH. Treatment of older adult patients diagnosed with rheumatoid arthritis: improved but not optimal. Arthritis Rheum. 2007 Aug 15;57(6):928–34. doi: 10.1002/art.22890. [DOI] [PubMed] [Google Scholar]
- 5.Khanna R, Smith MJ. Utilization and costs of medical services and prescription medications for rheumatoid arthritis among recipients covered by a state Medicaid program: a retrospective, cross-sectional, descriptive, database analysis. Clin Ther. 2007 Nov;29(11):2456–67. doi: 10.1016/j.clinthera.2007.11.009. [DOI] [PubMed] [Google Scholar]
- 6.Grijalva CG, Chung CP, Stein CM, Mitchel EF, Jr, Griffin MR. Changing patterns of medication use in patients with rheumatoid arthritis in a Medicaid population. Rheumatology (Oxford) 2008 Jul;47(7):1061–4. doi: 10.1093/rheumatology/ken193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Widdifield J, Bernatsky S, Paterson JM, Thorne JC, Cividino A, Pope J, Gunraj N, Bombardier C. Quality care in seniors with new-onset rheumatoid arthritis: A Canadian perspective. Arthritis Care Res (Hoboken) 2010 Aug 30; doi: 10.1002/acr.20304. [Epub ahead of print] [DOI] [PubMed] [Google Scholar]
- 8.Trivedi AN, Zaslavsky AM, Schneider EC, Ayanian JZ. Trends in the quality of care and racial disparities in Medicare Managed Care. N Engl J Med. 2005;353:692–700. doi: 10.1056/NEJMsa051207. [DOI] [PubMed] [Google Scholar]
- 9.Health Care Financing Administration, US Department of Health and Human Services. [accessed August 2, 2010.];Medicare HEDIS 3.0/1998 Data Audit Report. http://permanent.access.gpo.gov/websites/www.hcfa.gov/quality/3i2.htm.
- 10.Koroukian SM, Dahman B, Copeland G, Bradley CJ. The utility of the state buy-in variable in the Medicare denominator file to identify dually eligible Medicare-Medicaid beneficiaries: a validation study. Health Serv Res. 2010 Feb;45(1):265–82. doi: 10.1111/j.1475-6773.2009.01051.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Final Report AHRQ Publication No 08-0029-EF. Agency for Healthcare Research and Quality; Rockville, MD: Jan, 2008. [accessed February 11, 2010]. Creation of New Race-Ethnicity Codes and Socioeconomic Status (SES) Indicators for Medicare Beneficiaries. http://www.ahrq.gov/qual/medicareindicators/ [Google Scholar]
- 12.Best AE. Secondary data bases and their use in outcomes research: a review of the area resource file and the Healthcare Cost and Utilization Project. J Med Syst. 1999 Jun;23(3):175–81. doi: 10.1023/a:1020515419714. [DOI] [PubMed] [Google Scholar]
- 13.The InterStudy Competitive Edge 12.1 [database] St Paul, Minn: InterStudy Publications; 2001. [Google Scholar]
- 14.Sequist TD, Schneider EC. Addressing racial and ethnic disparities in health care. Health Serv Res. 2006;41:1451–1468. doi: 10.1111/j.1475-6773.2006.00549.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Arday SL, Arday DR, Monroe S, Zhang J. HCFA’s racial and ethnic data: current accuracy and recent improvements. Health Care Financ Rev. 2000;21:107–116. [PMC free article] [PubMed] [Google Scholar]
- 16.Liang KY, Zeger SL. Longitudinal data analysis using generalized linear models. Biometrika. 1986;73(1):13–22. [Google Scholar]
- 17.Zaslavsky AM, Epstein AM. How patients’ sociodemographic characteristics affect comparisons of competing health plans in California on HEDIS quality measures. Int J Qual Health Care. 2005 Feb;17(1):67–74. doi: 10.1093/intqhc/mzi005. [DOI] [PubMed] [Google Scholar]
- 18.Groessl EJ, Ganiats TG, Sarkin AJ. Sociodemographic differences in quality of life in RA. Pharmacoeconomics. 2006;24(2):109–21. doi: 10.2165/00019053-200624020-00002. [DOI] [PubMed] [Google Scholar]
- 19.Bruce B, Fries JF, Murtagh KN. Health status disparities in ethnic minority patients with RA: a cross-sectional study. J Rheumatol. 2007;34(7):1475–9. [PubMed] [Google Scholar]
- 20.Iren UT, Walker MS, Hochman E, Brasington R. A Pilot Study to Determine Whether Disability and Disease Activity Are Different in African-American and Caucasian Patients with RA in St. Louis, Missouri, USA. J Rheumatol. 2005;32(4):602–8. [PubMed] [Google Scholar]
- 21.Brunner HI, Taylor J, Britto MT, et al. Differences in disease outcomes between Medicaid and privately insured children: possible health disparities in juvenile rheumatoid arthritis. Arthritis Rheum. 2006;55(3):378–84. doi: 10.1002/art.21991. [DOI] [PubMed] [Google Scholar]
- 22.Pincus T, O’Dell JR, Kremer JM. Combination therapy with multiple disease-modifying antirheumatic drugs in RA: a preventive strategy. Ann Intern Med. 1999;131(10):768–774. doi: 10.7326/0003-4819-131-10-199911160-00009. [DOI] [PubMed] [Google Scholar]
- 23.Pisetsky DS, St Clair EW. Progress in the Treatment of RA. JAMA. 2001;286:2787–90. doi: 10.1001/jama.286.22.2787. [DOI] [PubMed] [Google Scholar]
- 24.Strand V, Singh JA. Improved health-related quality of life with effective disease-modifying antirheumatic drugs: evidence from randomized controlled trials. Am J Manag Care. 2008;14(4):234–54. [PubMed] [Google Scholar]
- 25.Ziegler S, Huscher D, Karberg K, Krause A, Wassenberg S, Zink A. Trends in treatment and outcomes of rheumatoid arthritis in Germany 1997–2007: results from the National Database of the German Collaborative Arthritis Centres. Ann Rheum Dis. 2010;69:1803–8. doi: 10.1136/ard.2009.122101. [DOI] [PubMed] [Google Scholar]
- 26.Tutuncu A, Reed G, Kremer J, Kavanaugh A. Do patients with older onset RA receive less aggressive treatment than younger patients. Ann Rheum Dis. 2006;65(9):1226–9. doi: 10.1136/ard.2005.051144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Lacaille D, Anis AH, Guh DP, Esdaile JM. Gaps in care for RA: a population study. Arthritis Rheum. 2005;53(2):241–8. doi: 10.1002/art.21077. [DOI] [PubMed] [Google Scholar]
- 28.Sokka T, Toloza S, Cutolo M, Kautiainen H, Makinen H, Gogus F, et al. Women, men, and rheumatoid arthritis: analyses of disease activity, disease characteristics, and treatments in the QUEST-RA study. Arthritis Res Ther. 2009;11(1):R7. doi: 10.1186/ar2591. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Brown AF, Ang A, Pebley AR. The relationship between neighborhood characteristics and self-rated health for adults with chronic conditions. Am J Public Health. 2007;97:926–32. doi: 10.2105/AJPH.2005.069443. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Trupin L, Tonner MC, Yazdany J, et al. The role of neighborhood and individual socioeconomic status in outcomes of systemic lupus erythematosus. J Rheumatol. 2008 Sep;35(9):1782–8. [PMC free article] [PubMed] [Google Scholar]
- 31.Zuckerman S, Waidmann T, Berenson R, Hadley J. Clarifying sources of geographic differences in Medicare spending. N Engl J Med. 2010 Jul 1;363(1):54–62. doi: 10.1056/NEJMsa0909253. [DOI] [PubMed] [Google Scholar]
- 32.Song Y, Skinner J, Bynum J, Sutherland J, Wennberg JE, Fisher ES. Regional variations in diagnostic practices. N Engl J Med. 2010 Jul 1;363(1):45–53. doi: 10.1056/NEJMsa0910881. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Keenan PS, Cleary PD, O’Malley AJ, Landon BE, Zaborski L, Zaslavsky AM. Geographic area variations in the Medicare health plan era. Med Care. 2010 Mar;48(3):260–6. doi: 10.1097/MLR.0b013e3181ca410a. [DOI] [PubMed] [Google Scholar]
- 34.Schneider EC, Zaslavsky AM, Epstein AM. Quality of care in for-profit and not-for-profit health plans enrolling Medicare beneficiaries. Am J Med. 2005 Dec;118(12):1392–400. doi: 10.1016/j.amjmed.2005.05.032. [DOI] [PubMed] [Google Scholar]
- 35.Baker LC, Hopkins DS. The contribution of health plans and provider organizations to variations in measured plan quality. Int J Qual Health Care. 2010 Jun;22(3):210–8. doi: 10.1093/intqhc/mzq011. [DOI] [PubMed] [Google Scholar]
- 36.Gabriel SE. The sensitivity and specificity of computerized databases for the diagnosis of rheumatoid arthritis. Arthritis Rheum. 1994 Jun;37(6):821–3. doi: 10.1002/art.1780370607. [DOI] [PubMed] [Google Scholar]
- 37.Polinski JM, Mohr PE, Johnson L. Impact of Medicare Part D on access to and cost sharing for specialty biologic medications for beneficiaries with rheumatoid arthritis. Arthritis Rheum. 2009 Jun 15;61(6):745–54. doi: 10.1002/art.24560. [DOI] [PubMed] [Google Scholar]
- 38.Gleason PP, Starner CI, Gunderson BW, Schafer JA, Sarran HS. Association of prescription abandonment with cost share for high-cost specialty pharmacy medications. J Manag Care Pharm. 2009 Oct;15(8):648–58. doi: 10.18553/jmcp.2009.15.8.648. [DOI] [PMC free article] [PubMed] [Google Scholar]