Abstract
Respiratory failure is the leading cause of death after cervical spinal injury. We hypothesized that incomplete cervical spinal injuries would alter respiratory pattern and initiate plasticity in the neural control of breathing. Further, we hypothesized that the severity of cervical spinal contusion would correlate with changes in breathing pattern. Fourteen days after C4–C5 contusions, respiratory frequency and tidal volume were measured in unanesthetized Sprague Dawley rats in a whole body plethysmograph. Phrenic motor output was monitored in the same rats which were anesthetized, vagotomized, paralyzed and ventilated to eliminate and/or control sensory feedback that could alter breathing patterns. The extent of spinal injury was approximated histologically by measurements of the injury-induced cyst area in transverse sections; cysts ranged from 2 to 28% of spinal cross-sectional area, and had a unilateral bias. In unanesthetized rats, the severity of spinal injury correlated negatively with tidal volume (R2=0.85; p<0.001) and positively with breathing frequency (R2=0.65; p<0.05). Thus, the severity of C4–C5 spinal contusion dictates post-injury breathing pattern. In anesthetized rats, phrenic burst amplitude was decreased on the side of injury, and burst frequency correlated negatively with contusion size (R2=0.51; p<0.05). A strong correlation between unanesthetized breathing pattern and the pattern of phrenic bursts in anesthetized, vagotomized and ventilated rats suggests that changes in respiratory motor output after spinal injury reflect, at least in part, intrinsic neural mechanisms of CNS plasticity initiated by injury.
Keywords: Cervical contusion, respiratory recovery, phrenic nerve, plethysmography
INTRODUCTION
Spinal cord injuries (SCI) are most frequent in cervical spinal segments (NSCISC, 2010). Since cervical spinal segments contain descending bulbospinal premotor axons that convey inspiratory premotor drive to phrenic (C3–C5 in humans; C3–C6 in rats) and intercostal motoneurons (DeVries and Goshgarian, 1989, Goshgarian and Rafols, 1981, Keswani and Hollinshead, 1955, Kuzuhara and Chou, 1980, Vinit and Kastner, 2009, Fuller et al., 2008), most patients with SCI have at least some respiratory impairment; the extent of impairment increases with more severe and more rostral cervical injuries (Winslow and Rozovsky, 2003).
Despite the impact of cervical injuries on respiratory motor output, investigations concerning respiratory function after spinal injury in animal models are limited. Most reports using animal models to investigate spinal injury effects on breathing involve hemisection of the second cervical spinal segment (C2 hemisection; for review, see Goshgarian, 2003). The advantages of spinal hemisections are surgical precision, reproducibility and anatomical specificity. However, spontaneous hemisections are rarely encountered clinically. In many respects, experimental contusions more closely approximate clinical spinal injuries (Young, 2002).
Cervical contusions have been studied in rats (Choi, et al., 2005, El-Bohy, et al., 1998, Schrimsher and Reier, 1992, Soblosky, et al., 2001, Baussart et al., 2006; Lane et al., 2009) and cats (Bohlman, et al., 1981). However, only Choi et al. (2005) reported changes in breathing pattern in unanesthetized rats following contusion injury (Choi et al., 2005). Altered breathing patterns after spinal injury may reflect persistent changes in sensory input or processes of injury-induced central neural plasticity. For example, changes in pulmonary mechanics or gas exchange may underlie persistent changes in sensory feedback arising from lung or chest wall mechanoreceptors, as well as changes in chemoreceptor feedback. On the other hand, changes in sensory feedback attendant to spinal injury may initiate central neural plasticity, consolidating mechanisms that lead to persistent changes in respiratory motor output (Vinit et al., 2007). Our primary purpose was to test the hypothesis that respiratory motor plasticity contributes to altered breathing patterns after spinal injury. To accomplish this, we used an anesthetized rat preparation which enables more precise regulation of sensory inputs to the respiratory control system associated with lung stretch or changes in arterial blood gases. In this preparation, changes in respiratory output in rats with cervical contusion injury will reflect neural plasticity vs. altered afferent regulation of phrenic bursting and/or respiratory muscle plasticity. A strong positive correlation between unanesthetized breathing pattern and the pattern of phrenic bursts in anesthetized rats suggests that intrinsic neural mechanisms associated with CNS plasticity underlie at least part of the persistent change in respiratory pattern in rats with chronic cervical injuries.
METHODS
Animals
Fifty-three male Sprague Dawley rats (290 to 434 g, Harlan, Indianapolis, IN; and Charles River Laboratories, Wilmington, MA) were allocated to control (n=45) and spinally injured (n=8) groups. The initial experiment was designed to explore the relationship between the severity of cervical spinal contusion injuries on ventilation in unanesthetized rats at 2 and 14 days post-injury. The second series of experiments was performed on the same animals between 14 and 21 days post-injury, and was designed to investigate relationships between the severity of spinal injury and phrenic motor activity, as well as the relationship between breathing pattern in unanesthetized rats and phrenic motor output in anesthetized, vagotomized and pump ventilated animals. Animal husbandry and all procedures were in compliance with the Institutional Animal Care and Use Committee at the University of Wisconsin, Madison.
Spinal cord contusion
Rats were anesthetized with medetomidine (sedative and analgesic; 75 μg/kg, i.m.) and halothane in oxygen. After oro-tracheal intubation, anesthesia was maintained with halothane in oxygen and rats were mechanically ventilated. Dorsal laminectomies were made between the 3rd and 5th cervical vertebrae to expose the 4th and 5th cervical spinal segments (C4 and C5). Controlled midline or lateral 10g × 12.5 mm to 25 mm impactions at either C4 or C5 were created using a computer monitored weight drop model (New York University Impactor) (Young, 2002). Variation in injury size was created by altering impactor height and alignment with the dorsum of the spinal cord. After contusion, the surgical wound was closed using standard technique. Rats were extubated and mechanical ventilation was discontinued once the rats were able to breathe and swallow spontaneously. All animals received post-surgical pain control as previously described (Golder, et al., 2003).
Whole-body plethysmography
At 2 and 14 days post-injury, a flow through, barometric plethysmograph (Buxco Inc., Wilmington, NC) was used to quantify ventilation in unanesthetized spinally injured rats, and compared with control rats. The plethysmograph consisted of a Plexiglas chamber in which pressure, humidity and temperature were continuously monitored. Pressurized gas mixtures flowed through the chamber at 2 L/min, thereby allowing control of inspired gas composition and preventing CO2 buildup. Baseline recordings lasted 30–60 min and were made while the chamber was flushed with 21% oxygen. Rats were then exposed to 10 min of 7% inspired CO2 (21% O2, balance N2). A pressure calibration signal (obtained before placing a rat into the chamber), plethysmograph temperature, rat body temperature, ambient and chamber pressures were measured and then used in the Dorbaugh and Fenn equation (1955) to calculate tidal volume (ml/breath), frequency (breaths/min) and minute ventilation (ml/min). Rectal temperature was measured immediately prior to placing the animal in the chamber and immediately following removal from the chamber. If the post-experiment temperature differed from the pre-value by more than 0.2 °C, respiratory volumes during hypercapnia were corrected to the post-protocol temperature.
Plethysmography data were analyzed in 1-min bins. For baseline, normoxia conditions, data represent the average of 10 consecutive 1-min bins, measured just prior to the onset of hypercapnia. During hypercapnia, we report peak tidal volume, frequency and minute ventilation occurring over the course of each 10-min exposure, as well as the mean of each 1-min bin.
Neurophysiology
Fourteen to 21 days post-injury, phrenic motor output was recorded from the same spinally injured rats used in the plethysmography studies; however, separate control groups were used for these experiments. Anesthesia was induced using isoflurane in 50% oxygen (balance nitrogen) in a closed chamber and maintained via nose cone. The trachea was cannulated to permit mechanical ventilation throughout the experiment (2.0–2.5 ml; Rodent Respirator model 683; Harvard Apparatus, South Natick, MA). Bilateral cervical vagotomy was performed to prevent entrainment of respiratory nerve activity with the ventilator. Femoral vessels were cannulated to measure arterial blood pressure (Model P122, Grass Telefactor, West Warrick, RI), arterial blood pH and blood gases (ABL-500, Radiometer, Copenhagen, Denmark), and for fluid administration (5 ml.kg−1.hr−1 of a 50:50 mixture of lactated ringers and 6% hetastarch with 0.8% sodium bicarbonate, i.v.). End-tidal PCO2 was continuously measured using a mainstream CO2 monitor (Capnoguard, Novametrix Medical Systems, Wallingford, CT). After surgical instrumentation, rats were slowly converted to urethane anesthesia (1.6 g.kg−1 i.v.) while isoflurane was gradually discontinued. Pancuronium bromide (1 mg.kg−1 i.v.) was administered to create neuromuscular paralysis. The left and right phrenic nerves were isolated via a dorsal approach, desheathed, submerged in mineral oil, and placed on bipolar silver recording electrodes. Nerve activity was amplified (gain, 10,000; A-M Systems, Everett, WA), bandpass-filtered (100 Hz to 10 kHz), rectified, and processed with a moving averager (CWE 821 filter; Paynter, Ardmore, PA; time constant, 50 ms). The signal was then digitized, recorded, and analyzed using the WINDAQ data acquisition system (DATAQ Instruments, Akron, OH). At least 1 hour was allowed after conversion to urethane anesthesia (and isoflurane withdrawal) to allow stabilization of blood pressure and phrenic nerve activity.
At the beginning of the experimental protocol, rats were hyperventilated by increasing the ventilator pump rate until inspiratory bursting ceased in both nerves (CO2 apneic threshold). The ventilator pump rate was then gradually decreased until spontaneous inspiratory bursting reappeared in both phrenic nerves. The end-tidal PCO2 at which inspiratory activity resumed was designated as the “CO2 recruitment threshold;” end-tidal PCO2 was then raised 2 mmHg above this value to establish baseline conditions. Peak integrated burst amplitudes were recorded for 20 to 30-min at baseline conditions, during a 5-min episode of isocapnic hypoxia (FIO2=0.10–0.12), for an additional 5 minutes after baseline conditions were restored, and finally during a 5-min episode of hypercapnia (end-tidal PCO2 75–80 mmHg) to obtain near maximal hypercapnic nerve activity. Arterial blood samples (0.4 ml) were collected for pH and blood gas measurements during baseline conditions and hypoxia. Peak integrated (left and right) phrenic burst amplitude and frequency were measured for 30-s periods immediately prior to each blood sample.
Burst frequency and peak amplitude were averaged over 30-s sample periods immediately prior to blood sampling for blood gas measurements. Baseline burst amplitude and changes in amplitude during and after hypoxia are reported as absolute values and expressed as a percentage of hypercapnic maximal amplitude. Burst frequency is reported as absolute values and as a change from the baseline value.
Histological assessment of injury severity
Spinally injured rats were perfused through the ascending aorta with 300 ml heparinized saline (4 IU.ml−1) followed by 500 ml 4% paraformaldehyde (pH 7.35–7.40). The cervical spinal cord was removed and stored in 0.1 M phosphate buffer solution with 0.02% sodium azide at 4 °C. The cervical spinal cord was removed, and the C4–C5 spinal segment was sectioned (40 μm thick) and stained with cresyl violet. The extent of cervical spinal cord injury was assessed under light microscopy. For each animal, a camera lucida drawing was made of the section with the largest area occupied by injured tissue. The resultant image was scanned and Adobe Photoshop (version 7.0) was used to color code the uninjured (white) and injured components of the camera lucida drawings (black) for digital image analysis (Image-Pro Plus version 4.5.1.22, Silver Spring, MD). The number of pixels occupying the injured area was measured and expressed as a percentage of total section area (white + black).
Statistical analysis
The assumptions of normally distributed data with equal variance were confirmed before parametric analyses were performed. When these assumptions were not valid, nonparametric analysis was performed (Kruskal-Wallis ANOVAs followed by Mann-Whitney U tests). All other means were compared using repeated measures ANOVA, with individual comparisons made using the Student-Neuman-Keuls post hoc test. Differences were considered significant if p <0.05. All values are expressed as mean ± SEM.
RESULTS
Injury Severity
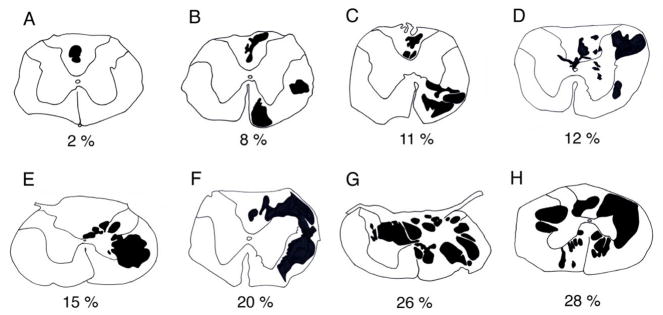
Histological examination of the injured spinal cord at 14-d post-contusion revealed pathology extending from the rostral C4 to caudal C5 spinal segments. Neural lesions involved the dorsal column, the lateral and ventral funiculus, and grey matter on the side of injury of varying magnitudes between rats. The lesion epicenters were characterized by partitioned cystic cavities. Cystic cavity size tapered rostral and caudal from the epicenters. The tissue section with the largest area occupied by cyst-like structures (range 2 to 28% of total section area) was identified in each animal and is represented in Figure 1A to H. Most animals predominantly had a unilateral injury, however, involvement of both sides was apparent in the two most severely injured rats (Fig. 1G & H).
Figure 1.
Camera lucida drawings from C4 to C5 spinal segments of spinally contused rats at 21 days post-injury. Sections depicted had the largest cross-sectional area occupied by cyst-like cavitations (black objects). Injury size ranged from 2 to 28% (A to H) of total section area and most injuries had a unilateral bias.
Breathing pattern
Minute volume was similar between control and spinally injured rats at 2-d and 14-d post-contusion during air breathing (Table 1). At 2-d post-injury, the breathing pattern in the spinally injured group was rapid and shallow, but returned to control patterns by 14-days post-injury (Table 1; p<0.05). During hypercapnia, minute volume increased in all groups, although injured rats at 2-d post-injury were not able to increase minute volume by the same magnitude as control and chronically injured rats (Table 1; p<0.05).
Table 1.
Grouped data for breathing pattern measurements during room air breathing and hypercapnic challenge in unanesthetized control and spinally injured rats at 2- and 14-d post-contusion.
| Control | Spinally Injured
|
||
|---|---|---|---|
| 2-days | 14-days | ||
| Tidal Volume (ml) | |||
| Room Air | 2.5 ± 0.1 | 1.6 ± 0.1 *† | 2.4 ± 0.1 |
| Hypercapnia | 4.3 ± 0.1 | 2.4 ± 0.3 *† | 3.8 ± 0.2 |
| Δ Volume | 1.8 ± 0.1 | 0.8 ± 0.3 *† | 1.4 ± 0.1 |
| Respiratory Rate (min−1) | |||
| Room Air | 77 ± 3 | 114 ± 6*† | 83 ± 3 |
| Hypercapnia | 147 ± 4 | 133 ± 7 | 147 ± 6 |
| Δ Respiratory Rate | 70 ± 4 | 19 ± 11 *† | 64 ± 6 |
| Minute Volume (ml.min−1) | |||
| Room Air | 188 ± 7 | 170 ± 113 | 189 ± 12 |
| Hypercapnia | 647 ± 28 | 316 ± 50*† | 554 ± 47 |
| Δ Minute Volume | 458 ± 28 | 146 ± 48 *† | 377 ± 29 |
Δ, the difference between room air and hypercapnic measurements;
, p<0.05 different to controls;
p<0.05, different to 14-d value.
Relationship between breathing pattern and injury severity
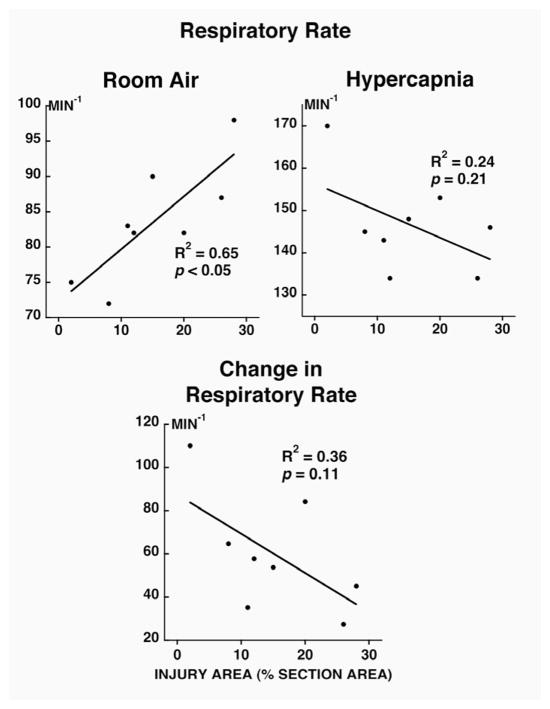
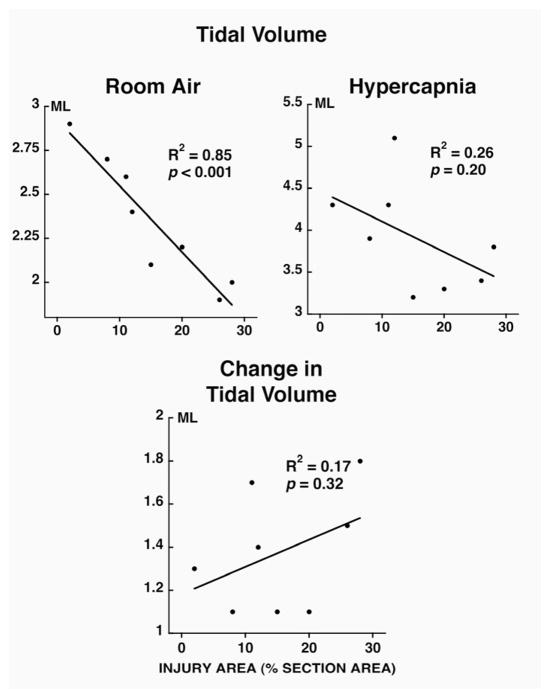
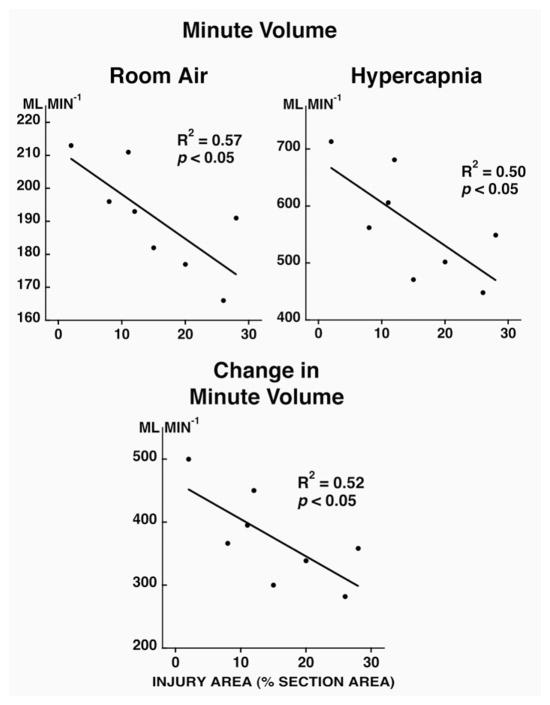
During room air breathing, respiratory rate (Fig. 2; p<0.05) was positively correlated and tidal volume (Fig. 3; p<0.05) and minute volume (Fig. 4; p<0.05) were negatively correlated with injury severity at 14 days post-contusion. During hypercapnia, only minute volume and the change in minute volume were negatively correlated to injury severity (Fig. 4; p<0.05).
Figure 2.
Relationship between injury area (% section area, x axis) and respiratory rate (during room air breathing and during hypercapnic challenge; top panels) and change in respiratory rate (bottom panel) in unanesthetized rats at 14 days post-contusion. Note that the respiratory rate is positively correlated at room air to the injury severity.
Figure 3.
Relationship between injury area (% section area; x axis) and tidal volume (room air breathing and hypercapnic challenge; top panels) and change in tidal volume (bottom panel) in unanesthetized rats at 14 days post-contusion. Note that the change in tidal volume is negatively correlated to the injury severity.
Figure 4.
Relationship between injury area (% section area; x axis) and minute volume (room air breathing and hypercapnic challenge; top panels) and change in minute volume (bottom panel) in unanesthetized rats at 14 days post-contusion. Note that the minute volume and the change in minute volume are negatively correlated to the injury severity.
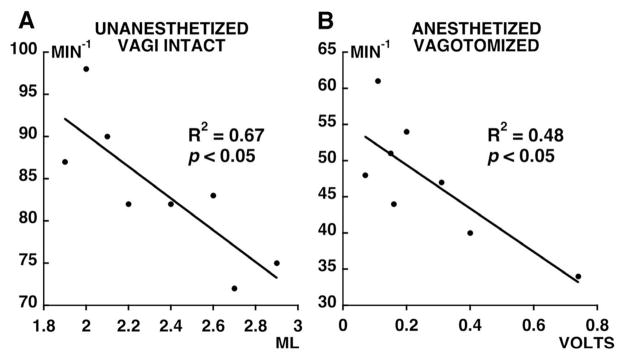
Respiratory rate was negatively correlated with tidal volume after spinal contusion (Fig. 5A; p<0.05). Thus, spinally injured rats with the lowest tidal volume (and greatest injury severity) had the highest respiratory rate. This relationship between rate and volume was not observed in control rats (p=0.49).
Figure 5.
Relationship between: A) respiratory rate and tidal volume during room air breathing in unanesthetized, vagally intact, and spinally injured rats at 14 days post-injury; and B) phrenic burst rate and integrated phrenic burst amplitude on the most injured side in the same rats when anesthetized, bilaterally vagotomized, and ventilated at 21 days post-injury. No relationship has been found in control rats for unanesthetized vagi intact and anesthetized vagotomized (p=0.49, not shown).
Phrenic Motor Output
Arterial pH, blood gas, and blood pressure measurements differed between control and spinally injured rats at 14 to 21-d post-injury (Table 2; p<0.05). Spinally injured rats had lower CO2 apneic thresholds versus control rats and, as a result, baseline PaCO2 was lower. In agreement, arterial pH was higher in animals with contusions. Arterial blood pressure was lower in injured rats. PaO2 during baseline conditions and changes in blood pressure, PaO2 and PaCO2 during hypoxia were not different between groups.
Table 2.
Arterial blood gas and blood pressure measurements in anesthetized control and spinally contused rats at 2 weeks post-injury.
| Control | Injured | |
|---|---|---|
| pH Baseline |
7.38 ± 0.01 | 7.41 ± 0.01* |
| Hypoxia | 7.34 ± 0.01 | 7.40 ± 0.01* |
|
PaCO2 mmHg Baseline |
41 ± 1 | 34 ± 2* |
| Hypoxia | 40 ± 1 | 34 ± 1* |
|
PaO2 mmHg Baseline |
257 ± 5 | 268 ± 11 |
| Hypoxia | 38 ± 1‡ | 37± 1‡ |
| MBP mmHg Baseline |
125 ± 4 | 100 ± 8* |
| Hypoxia | 101 ± 5‡ | 68 ± 9*‡ |
Pa, partial pressure in arterial blood; MBP, mean arterial blood pressure;
, p<0.05 different to controls;
p<0.05 different to baseline value.
Phrenic burst amplitude (volts) from spinally contused rats was lowest on the side with greatest injury compared to the contralateral side and compared to control rats during all experimental conditions (Table 3; p<0.05). When normalized to maximal amplitude during hypercapnia, baseline phrenic burst amplitudes were similar across groups (Table 3). During hypoxia and hypercapnia, phrenic burst amplitude increased bilaterally in control and spinally injured rats (Table 3; p<0.05). The change in amplitude from baseline to hypoxic conditions was decreased after spinal contusion, but only on the side of major injury (Table 3; p<0.05). In control rats, hypercapnic burst amplitude was larger than hypoxic burst amplitude reflecting the difference between these stimuli in changing respiratory drive (Table 3; p<0.05). After spinal contusion, hypoxic and hypercapnic burst amplitudes were not different (Table 3; p<0.05).
Table 3.
Integrated phrenic inspiratory burst amplitudes during baseline conditions, hypoxia, and hypercapnia from control and spinally injured rats at 2 weeks post-contusion.
| Control | Spinally Injured
|
||
|---|---|---|---|
| Minor injured Side | Major Injured Side | ||
| ∫ Phrenic Burst Amplitudes (volts) Baseline |
1.2 ± 0.1 | 0.8 ± 0.2 | 0.3 ± 0.1*† |
| Hypoxia | 2.1 ± 0.3 | 1.4 ± 0.4 | 0.6 ± 0.1*† |
| Hypercapnia | 3.1 ± 0.3 | 1.5 ± 0.4* | 0.7 ± 0.2*† |
| Δ ∫ Phrenic Burst Amplitudes (volts) Hypoxia |
1.0 ± 0.2 | 0.6 ± 0.2 | 0.3 ± 0.1* |
| Hypercapnia | 1.8 ± 0.2 | 0.8 ± 0.2* | 0.4 ± 0.1* |
| Amplitude (%Max) Baseline |
41 ± 2 | 47 ± 5 | 39 ± 6 |
| Hypoxia | 71 ± 4 | 90 ± 5* | 87 ± 6* |
| ? Amplitude (%Max) Hypoxia |
32 ± 2 | 45 ± 5* | 47 ± 6* |
∫, integrated; %Max, percentage of maximal amplitude during hypercapnia; ?, change in amplitude from baseline values;
, p<0.05 different to controls;
p<0.05 different to the minor injured side side.
In the spinally injured group, phrenic burst frequency (fictive respiratory rate) was negatively correlated to phrenic burst amplitude on the side with greatest injury (Fig. 5B; p<0.05). Thus, the relationship observed between respiratory rate and tidal volume in unanesthetized spinally injured rats was conserved after anesthesia, vagotomy and standardized arterial blood gases. No relationship existed between burst frequency and phrenic burst amplitude on the side with least injury.
Correlation of Phrenic and Ventilatory Measurements
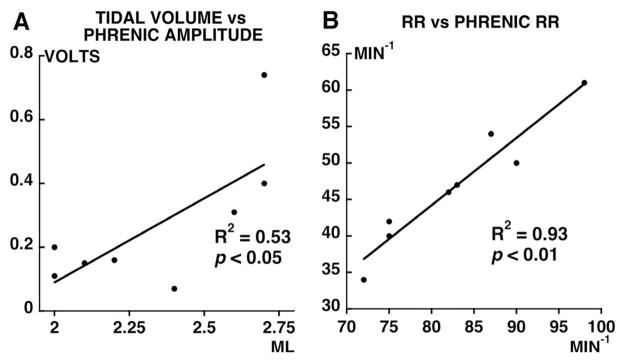
Relationships between tidal volume and respiratory rate in unanesthetized breathing were correlated with their phrenic motor output (integrated phrenic amplitude and burst frequency). Tidal volume was positively correlated with phrenic burst amplitude in the same rat on the side with greatest injury (Fig. 6A; p<0.05). Similarly, a positive correlation existed between respiratory rate and phrenic burst frequency in individual rats (Fig. 6B; p<0.05). Thus, ventilatory pattern changes associated with spinal injury were conserved despite anesthesia, vagotomy, paralysis and controlled levels of arterial blood gases.
Figure 6.
Relationship between: A) tidal volume in unanesthetized spinally injured rats and integrated phrenic burst amplitude on the most injured side from the same rats during baseline conditions and while anesthetized, bilaterally vagotomized, and ventilated; and B) respiratory rate in unanesthetized spinally injured rats and phrenic respiratory rate from the same rats during baseline conditions and while anesthetized, bilaterally vagotomized, and ventilated. Note that the ventilatory pattern changes associated with spinal injury were conserved despite anesthesia, vagotomy, paralysis and controlled levels of arterial blood gases (positive correlation in both A, p<0.05 and B, p<0.01).
DISCUSSION
Mid-cervical spinal contusion altered breathing pattern in proportion to injury severity. The relationship between neural damage and breathing was observed during room air breathing, but not during hypercapnia. Rapid breathing was evident in the same rats during anesthesia, even after vagotomy, paralysis and standardization of respiratory drive. Thus, breathing patterns after mid-cervical spinal contusion arise from intrinsic neural mechanisms that most likely represent neuroplasticity initiated post-injury.
Altered breathing pattern and phrenic motor output after mid-cervical contusion
2-d post-injury, spinally contused rats maintained similar minute ventilation as uninjured controls by using a rapid shallow breathing pattern; however, by 14-d, the breathing pattern was no longer different from controls. Respiratory rate and tidal volume were correlated with the injury severity in individual rats. Thus, full effects of spinal contusion were apparent only in individual animals compared to 2-d post-injury.
Spinal lesions involved the dorsal column, the lateral and ventral funiculus, and grey matter of varying magnitude between rats. Rat phrenic motoneurons are arranged as columns of cells parallel to the longitudinal axis of the spinal cord in the ventral horn of the C3 to C6 spinal segments (DeVries and Goshgarian, 1989; Goshgarian and Rafols, 1981; Kuzuhara and Chou, 1980; Boulenguez et al., 2007; Mantilla et al., 2009; Lee and Fuller, 2011). As such, cystic lesions of four spinally contused rats (Fig. 1; C, E, G, & H) may have involved space previously occupied by the phrenic nucleus. Motoneuron death has been reported following incomplete spinal contusion in rats (Choi, et al., 2005, Grossman, et al., 2001). Thus, injury-induced changes in tidal volume and phrenic burst amplitude in our study may, in part, reflect a decreased number of phrenic motoneurons. Phrenic motoneurons receive premotor drive from bulbospinal neurons whose cell bodies are located within various respiratory centers of the medulla (Feldman, et al., 2003). Respiratory premotor axons project to phrenic motoneurons via the lateral and ventral funiculi, and are diffusely distributed within the white matter (Lipski, et al., 1994; Fuller et al., 2009). These axons then arborize at multiple spinal segments to innervate more than one phrenic motoneuron (Lipski, et al., 1994). Thus, the white matter lesions which were noted in seven spinally contused rats (Fig. 1; all except A) likely contributed to decreases in tidal volume and phrenic burst amplitude. These changes could be due mainly to reduced input from descending bulbospinal pathways to phrenic motor neurons. The extent of spinal cord injury will also impact the degree of spontaneous neuroplasticity and the subsequent recovery. For example, following anatomically complete C2 hemisection injury a rapid, shallow pattern of breathing persists for months (Fuller et al., 2006; Fuller et al., 2008). In regard to our data, the discrepancy between spontaneous tidal volume versus phrenic nerve recovery could be explained by the contribution of other, respiratory-related muscles (eg. intercostals) in non-anaesthetized animals. We cannot exclude spontaneous recovery of different respiratory muscles which could contribute to the ventilatory response during respiratory challenges (Bolser et al., 2009).
In the short-term, respiratory rate is altered by multiple factors including respiratory muscle afferents ascending via the spinal dorsal columns, vagal afferent feedback and chemoreflexes. Since proprioceptor feedback from intercostal muscles decreases respiratory rate (Bolser, et al., 1988), dorsal column injuries in all spinally contused rats may increase post-injury respiratory rate by interrupting this proprioceptor feedback. Pulmonary receptors alter respiratory rate via vagal afferents (Coleridge and Coleridge, 1986). Spinal injuries may increase respiratory rate indirectly via altered vagal afferent feedback, for example due to diminished tidal volume (Haas, et al., 1986, Kokkola, et al., 1975, Ledsome and Sharp, 1981, Roth, et al., 1997), alveolar atelectasis, pneumonia, bronchitis, bronchospasm, chest wall spasticity (Jackson and Groomes, 1994), and reduced lung and chest wall compliance (De Troyer and Heilporn, 1980, Estenne, et al., 1983, Goldman, et al., 1988, Scanlon, et al., 1989). Phrenic afferents also control ipsilateral phrenic nerve activity following cervical hemisection (Vinit et al., 2007), but similar involvement cannot be assumed following cervical hemicontusion since intact vagal afferents were present in the only available study (Baussart et al., 2006).
Recovery of respiratory function after mid-cervical spinal contusions
In our study, breathing pattern improved with time post-injury, an effect also observed in spinally injured people (Loveridge, et al., 1992). Time-dependent changes in respiratory function have been divided into two stages (Haas, et al., 1985): 1) a rapid increase in respiratory function (Haas, et al., 1985, Ledsome and Sharp, 1981) attributed to resolution of spinal cord inflammation, edema, and spinal shock (Mansel and Norman, 1990); and 2) a slower progressive return of respiratory function attributed to changes in pulmonary mechanics (Haas, et al., 1985). These changes include increased accessory respiratory muscle strength (De Troyer, et al., 1986, McKinley, et al., 1969), increased stability of the chest wall due to spasticity (De Troyer and Heilporn, 1980, McMichan, et al., 1980), and increased abdominal compliance (Estenne and De Troyer, 1986).
The role of central respiratory plasticity in slow improvements of respiratory function in spinally injured people is unknown. However, time-dependent improvements in diaphragmatic function in spinally injured humans occur with concurrent improvements in shoulder and upper arm muscles, all of which receive innervation from motoneurons in C3 to C6 spinal segments (Axen, et al., 1985). Thus, delayed spontaneous improvements in diaphragmatic function may reflect improvements in diaphragm innervation. However, ascribing mechanisms to injury-induced changes in breathing pattern and ventilatory reflexes is difficult because the control of breathing involves complex peripheral (i.e., chemoreceptors, proprioreceptors, and effector muscles) and central factors (i.e. chemoreceptors, rhythm generator, sensory afferent processing centers and respiratory motor pathways), all of which may potentially be altered after spinal cord injury.
Relationship between respiratory rate and phrenic burst frequency after mid-cervical spinal contusions
Individual respiratory patterns used by spinally injured rats during unanesthetized spontaneous breathing at 14-d post-injury persisted after bilateral vagotomy, standardization of respiratory drive, neuromuscular paralysis and mechanical ventilation. Thus, breathing patterns at 14-d post-injury cannot be explained entirely by alterations in tidal volume or sensory feedback from the lung or respiratory muscles. Although not conclusive, we suggest that respiratory plasticity may contribute to the maintenance of “rapid shallow” breathing and diminished phrenic motor output following spinal injury. This suggestion does not preclude early post-injury changes due to altered vagal afferent feedback or dorsal column lesions after mid-cervical spinal contusion; in fact, we consider such an involvement to be likely.
Diminished phrenic responses to hypoxia and hypercapnia
The ability to increase phrenic burst amplitude in response to hypoxia and hypercapnia (phrenic motor reserve) was diminished on the side of major injury after cervical spinal contusion. An earlier study reached similar conclusions (El-Bohy, et al., 1998). El-Bohy et al. (1998) recorded phrenic motor output from anesthetized spinally contused rats. Baseline phrenic burst area was normalized to maximal phrenic burst area during asphyxic conditions. During baseline conditions, C2 lateral spinal contusion increased phrenic burst area as a percent of maximal phrenic burst area, but only on the side of injury. In contrast, C4–C5 midline contusion increased this variable bilaterally. Here, baseline phrenic burst amplitude was not elevated after injury when normalized to maximal amplitude during hypercapnia. This discrepancy between studies may reflect the means of data expression (area versus amplitude), the stimulus used to achieve maximal amplitude (asphyxia versus hypercapnia), the method of baseline standardization, or other uncontrolled factors such as rat genetics. In the study of El-Bohy et al. (1998), baseline conditions were not individualized to each animal; thus, injury-induced changes in apneic threshold were not identified. In our study, the CO2 apneic threshold decreased after contusion. If the same effect on apneic threshold was present in the study of El-Bohy et al. (1998), normalized baseline phrenic burst areas would be relatively elevated in the injured group. Similar results have been shown in a recent study of diaphragm activity during asphyxia (Baussart et al., 2006). After C2 contusion, ipsilateral diaphragm activity is enhanced during transient asphyxia; however they could not rule out activity due to spared ipsilateral pathways since, after an additional contralateral acute cervical injury, ipsilateral activity is diminished but remains present (Baussart et al., 2006).
After cervical SCI, the ventilatory response to CO2 is decreased in humans (Bergofsky, 1964, Kelling, et al., 1985, Lin, et al., 1998, Manning, et al., 1992, McCool, et al., 1988). Some argue that impaired ventilatory responses occur primarily through respiratory muscle limitations (Lin, et al., 1998), whereas others suggest such deficiencies occur through impaired central respiratory drive (Manning, et al., 1992). Multiple mechanisms probably interact to contribute to ventilatory deficiencies post-injury. In our study, hypercapnic ventilatory responses post-injury (as a group) were not different from controls. However, evidence was present for a central neural limitation to hypercapnic ventilation after injury, since: 1) the change in minute volume during hypercapnia was negatively correlated to injury severity, and 2) the change in phrenic motor output during hypercapnia was diminished bilaterally.
Research Highlights.
Severity of spinal injury correlated negatively with tidal volume
Severity of C4–C5 spinal contusion dictates post-injury breathing pattern
Correlation between unanesthetized breathing pattern and phrenic bursts
Changes in respiratory motor output reflect intrinsic plasticity initiated by injury
Acknowledgments
Supported by NIH HL69064 (FJG, GSM), the Christopher Reeve Paralysis Foundation (FJG), the Francis Families Foundation (DDF) and the Craig H. Neilsen Foundation (SV). We thank Dr. Adrianne Huxtable and Dr. Nicole Nichols for their helpful comments.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Axen K, Pineda H, Shunfenthal I, Haas F. Diaphragmatic function following cervical cord injury: neurally mediated improvement. Arch Phys Med Rehabil. 1985;66:219–222. doi: 10.1016/0003-9993(85)90146-7. [DOI] [PubMed] [Google Scholar]
- Baussart B, Stamegna JC, Polentes J, Tadié M, Gauthier P. A new model of upper cervical spinal contusion inducing a persistent unilateral diaphragmatic deficit in the adult rat. Neurobiol Dis. 2006;22(3):562–74. doi: 10.1016/j.nbd.2005.12.019. [DOI] [PubMed] [Google Scholar]
- Bergofsky EH. Quantiation of the Function of Respiratory Muscles in Normal Individuals and Quadriplegic Patients. Arch Phys Med Rehabil. 1964;45:575–580. [PubMed] [Google Scholar]
- Bohlman HH, Bahniuk E, Field G, Raskulinecz G. Spinal cord monitoring of experimental incomplete cervical spinal cord injury: a preliminary report. Spine. 1981;6:428–436. doi: 10.1097/00007632-198109000-00002. [DOI] [PubMed] [Google Scholar]
- Bolser DC, Jefferson SC, Rose MJ, Tester NJ, Reier PJ, Fuller DD, Davenport PW, Howland DR. Recovery of airway protective behaviors after spinal cord injury. Respir Physiol Neurobiol. 2009;169(2):150–6. doi: 10.1016/j.resp.2009.07.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bolser DC, Lindsey BG, Shannon R. Respiratory pattern changes produced by intercostal muscle/rib vibration. J Appl Physiol. 1988;64:2458–2462. doi: 10.1152/jappl.1988.64.6.2458. [DOI] [PubMed] [Google Scholar]
- Choi H, Liao WL, Newton KM, Onario RC, King AM, Desilets FC, Woodard EJ, Eichler ME, Frontera WR, Sabharwal S, Teng YD. Respiratory abnormalities resulting from midcervical spinal cord injury and their reversal by serotonin 1A agonists in conscious rats. J Neurosci. 2005;25:4550–4559. doi: 10.1523/JNEUROSCI.5135-04.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boulenguez P, Gestreau C, Vinit S, Stamegna JC, Kastner A, Gauthier P. Specific and artifactual labeling in the rat spinal cord and medulla after injection of monosynaptic retrograde tracers into the diaphragm. Neurosci Lett. 2007;417(2):206–11. doi: 10.1016/j.neulet.2007.02.047. [DOI] [PubMed] [Google Scholar]
- Coleridge HM, Coleridge JCG. Reflexes evoked from the tracheobronchial tree and lungs. In: Cherniack NS, Widdicome JG, editors. Handbook of Physiology: the respiratory system II. Vol. 2. American Physiological Society; Bethesda, MD: 1986. pp. 395–429. [Google Scholar]
- De Troyer A, Estenne M, Heilporn A. Mechanism of active expiration in tetraplegic subjects. N Engl J Med. 1986;314:740–744. doi: 10.1056/NEJM198603203141203. [DOI] [PubMed] [Google Scholar]
- De Troyer A, Heilporn A. Respiratory mechanics in quadriplegia. The respiratory function of the intercostal muscles. Am Rev Respir Dis. 1980;122:591–600. doi: 10.1164/arrd.1980.122.4.591. [DOI] [PubMed] [Google Scholar]
- DeVries KL, Goshgarian HG. Spinal cord localization and characterization of the neurons which give rise to the accessory phrenic nerve in the adult rat. Exp Neurol. 1989;104:88–90. doi: 10.1016/0014-4886(89)90013-7. [DOI] [PubMed] [Google Scholar]
- El-Bohy AA, Schrimsher GW, Reier PJ, Goshgarian HG. Quantitative assessment of respiratory function following contusion injury of the cervical spinal cord. Exp Neurol. 1998;150:143–152. doi: 10.1006/exnr.1997.6757. [DOI] [PubMed] [Google Scholar]
- Estenne M, De Troyer A. The effects of tetraplegia on chest wall statics. Am Rev Respir Dis. 1986;134:121–124. doi: 10.1164/arrd.1986.134.1.121. [DOI] [PubMed] [Google Scholar]
- Estenne M, Heilporn A, Delhez L, Yernault JC, De Troyer A. Chest wall stiffness in patients with chronic respiratory muscle weakness. Am Rev Respir Dis. 1983;128:1002–1007. doi: 10.1164/arrd.1983.128.6.1002. [DOI] [PubMed] [Google Scholar]
- Feldman JL, Mitchell GS, Nattie EE. Breathing: rhythmicity, plasticity, chemosensitivity. Annu Rev Neurosci. 2003;26:239–266. doi: 10.1146/annurev.neuro.26.041002.131103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fuller DD, Doperalski NJ, Dougherty BJ, Sandhu MS, Bolser DC, Reier PJ. Modest spontaneous recovery of ventilation following chronic high cervical hemisection in rats. Exp Neurol. 2008;211(1):97–106. doi: 10.1016/j.expneurol.2008.01.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fuller DD, Golder FJ, Olson EB, Jr, Mitchell GS. Recovery of phrenic activity and ventilation after cervical spinal hemisection in rats. J Appl Physiol. 2006;100(3):800–6. doi: 10.1152/japplphysiol.00960.2005. [DOI] [PubMed] [Google Scholar]
- Fuller DD, Sandhu MS, Doperalski NJ, Lane MA, White TE, Bishop MD, Reier PJ. Graded unilateral cervical spinal cord injury and respiratory motor recovery. Respir Physiol Neurobiol. 2009;165(2–3):245–53. doi: 10.1016/j.resp.2008.12.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Golder FJ, Fuller DD, Davenport PW, Johnson RD, Reier PJ, Bolser DC. Respiratory motor recovery after unilateral spinal cord injury: eliminating crossed phrenic activity decreases tidal volume and increases contralateral respiratory motor output. J Neurosci. 2003;23:2494–2501. doi: 10.1523/JNEUROSCI.23-06-02494.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goldman JM, Williams SJ, Denison DM. The rib cage and abdominal components of respiratory system compliance in tetraplegic patients. Eur Respir J. 1988;1:242–247. [PubMed] [Google Scholar]
- Goshgarian HG. The crossed phrenic phenomenon: a model for plasticity in the respiratory pathways following spinal cord injury. J Appl Physiol. 2003;94:795–810. doi: 10.1152/japplphysiol.00847.2002. [DOI] [PubMed] [Google Scholar]
- Goshgarian HG, Rafols JA. The phrenic nucleus of the albino rat: a correlative HRP and Golgi study. J Comp Neurol. 1981;201:441–456. doi: 10.1002/cne.902010309. [DOI] [PubMed] [Google Scholar]
- Grossman SD, Rosenberg LJ, Wrathall JR. Temporal-spatial pattern of acute neuronal and glial loss after spinal cord contusion. Exp Neurol. 2001;168:273–282. doi: 10.1006/exnr.2001.7628. [DOI] [PubMed] [Google Scholar]
- Haas F, Axen K, Pineda H. Aerobic capacity in spinal cord injured people. Cent Nerv Syst Trauma. 1986;3:77–91. doi: 10.1089/cns.1986.3.77. [DOI] [PubMed] [Google Scholar]
- Haas F, Axen K, Pineda H, Gandino D, Haas A. Temporal pulmonary function changes in cervical cord injury. Arch Phys Med Rehabil. 1985;66:139–144. [PubMed] [Google Scholar]
- Jackson AB, Groomes TE. Incidence of respiratory complications following spinal cord injury. Arch Phys Med Rehabil. 1994;75:270–275. doi: 10.1016/0003-9993(94)90027-2. [DOI] [PubMed] [Google Scholar]
- Kelling JS, DiMarco AF, Gottfried SB, Altose MD. Respiratory responses to ventilatory loading following low cervical spinal cord injury. J Appl Physiol. 1985;59:1752–1756. doi: 10.1152/jappl.1985.59.6.1752. [DOI] [PubMed] [Google Scholar]
- Keswani NH, Hollinshead WH. The phrenic nucleus. III. Organization of the phrenic nucleus in the spinal cord of the cat and man. Mayo Clin Proc. 1955;30:566–577. [PubMed] [Google Scholar]
- Kokkola K, Moller K, Lehtonen T. Pulmonary function in tetraplegic and paraplegic patients. Ann Clin Res. 1975;7:76–79. [PubMed] [Google Scholar]
- Kuzuhara S, Chou SM. Localization of the phrenic nucleus in the rat: a HRP study. Neurosci Lett. 1980;16:119–124. doi: 10.1016/0304-3940(80)90330-4. [DOI] [PubMed] [Google Scholar]
- Lane MA, Salazar K, Vavrousek JC, Mercier LM, O’Steen BE, Fuller DD, Reier PJ. Plasticity in the Phrenic Motor System following Contusion in Adult Rat Spinal Cord. Neurorehabilitation and Neural Repair; 13th International Symposium on Neural Regeneration (ISNR); 2009. p. 974. [Google Scholar]
- Ledsome JR, Sharp JM. Pulmonary function in acute cervical cord injury. Am Rev Respir Dis. 1981;124:41–44. doi: 10.1164/arrd.1981.124.1.41. [DOI] [PubMed] [Google Scholar]
- Lee KZ, Fuller DD. Neural control of phrenic motoneuron discharge. Respir Physiol Neurobiol. 2011 doi: 10.1016/j.resp.2011.02.014. in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lin KH, Wu HD, Chang CW, Wang TG, Wang YH. Ventilatory and mouth occlusion pressure responses to hypercapnia in chronic tetraplegia. Arch Phys Med Rehabil. 1998;79:795–799. doi: 10.1016/s0003-9993(98)90358-6. [DOI] [PubMed] [Google Scholar]
- Lipski J, Zhang X, Kruszewska B, Kanjhan R. Morphological study of long axonal projections of ventral medullary inspiratory neurons in the rat. Brain Res. 1994;640:171–184. doi: 10.1016/0006-8993(94)91871-6. [DOI] [PubMed] [Google Scholar]
- Loveridge B, Sanii R, Dubo HI. Breathing pattern adjustments during the first year following cervical spinal cord injury. Paraplegia. 1992;30:479–488. doi: 10.1038/sc.1992.102. [DOI] [PubMed] [Google Scholar]
- Manning HL, Brown R, Scharf SM, Leith DE, Weiss JW, Weinberger SE, Schwartzstein RM. Ventilatory and P0.1 response to hypercapnia in quadriplegia. Respir Physiol. 1992;89:97–112. doi: 10.1016/0034-5687(92)90074-7. [DOI] [PubMed] [Google Scholar]
- Mansel JK, Norman JR. Respiratory complications and management of spinal cord injuries. Chest. 1990;97:1446–1452. doi: 10.1378/chest.97.6.1446. [DOI] [PubMed] [Google Scholar]
- Mantilla CB, Zhan WZ, Sieck GC. Retrograde labelling of phrenic motor neurons by intrapleural injections. J Neurosci Methods. 2009;182(2):244–249. doi: 10.1016/j.jneumeth.2009.06.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McCool FD, Brown R, Mayewski RJ, Hyde RW. Effects of posture on stimulated ventilation in quadriplegia. Am Rev Respir Dis. 1988;138:101–105. doi: 10.1164/ajrccm/138.1.101. [DOI] [PubMed] [Google Scholar]
- McKinley AC, Auchincloss JH, Jr, Gilbert R, Nicholas JJ. Pulmonary function, ventilatory control, and respiratory complications in quadriplegic subjects. Am Rev Respir Dis. 1969;100:526–532. doi: 10.1164/arrd.1969.100.4.526. [DOI] [PubMed] [Google Scholar]
- McMichan JC, Michel L, Westbrook PR. Pulmonary dysfunction following traumatic quadriplegia. Recognition, prevention, and treatment. Jama. 1980;243:528–531. [PubMed] [Google Scholar]
- NSCISC. Spinal cord injury facts and figures at a glance. 2010. [PubMed] [Google Scholar]
- Roth EJ, Lu A, Primack S, Oken J, Nusshaum S, Berkowitz M, Powley S. Ventilatory function in cervical and high thoracic spinal cord injury. Relationship to level of injury and tone. Am J Phys Med Rehabil. 1997;76:262–267. doi: 10.1097/00002060-199707000-00002. [DOI] [PubMed] [Google Scholar]
- Scanlon PD, Loring SH, Pichurko BM, McCool FD, Slutsky AS, Sarkarati M, Brown R. Respiratory mechanics in acute quadriplegia. Lung and chest wall compliance and dimensional changes during respiratory maneuvers. Am Rev Respir Dis. 1989;139:615–620. doi: 10.1164/ajrccm/139.3.615. [DOI] [PubMed] [Google Scholar]
- Schrimsher GW, Reier PJ. Forelimb motor performance following cervical spinal cord contusion injury in the rat. Exp Neurol. 1992;117:287–298. doi: 10.1016/0014-4886(92)90138-g. [DOI] [PubMed] [Google Scholar]
- Soblosky JS, Song JH, Dinh DH. Graded unilateral cervical spinal cord injury in the rat: evaluation of forelimb recovery and histological effects. Behav Brain Res. 2001;119:1–13. doi: 10.1016/s0166-4328(00)00328-4. [DOI] [PubMed] [Google Scholar]
- Vinit S, Stamegna JC, Boulenguez P, Gauthier P, Kastner A. Restorative respiratory pathways after partial cervical spinal cord injury: role of ipsilateral phrenic afferents. Eur J Neurosci. 2007;25(12):3551–60. doi: 10.1111/j.1460-9568.2007.05619.x. [DOI] [PubMed] [Google Scholar]
- Vinit S, Kastner A. Descending bulbospinal pathways and recovery of respiratory motor function following spinal cord injury. Respir Physiol Neurobiol. 2009;169(2):115–22. doi: 10.1016/j.resp.2009.08.004. [DOI] [PubMed] [Google Scholar]
- Winslow C, Rozovsky J. Effect of spinal cord injury on the respiratory system. Am J Phys Med Rehabil. 2003;82:803–814. doi: 10.1097/01.PHM.0000078184.08835.01. [DOI] [PubMed] [Google Scholar]
- Young W. Spinal cord contusion models. Prog Brain Res. 2002;137:231–255. doi: 10.1016/s0079-6123(02)37019-5. [DOI] [PubMed] [Google Scholar]