Abstract
Many lung and central nervous system disorders require robust and appropriate physiological responses to assure adequate breathing. Factors undermining the efficacy of ventilatory control will diminish the ability to compensate for pathology, threatening life itself. Although most of these same disorders are associated with systemic and/or neuroinflammation, and inflammation affects neural function, we are only beginning to understand interactions between inflammation and any aspect of ventilatory control (e.g. sensory receptors, rhythm generation, chemoreflexes, plasticity). Here we review available evidence, and present limited new data suggesting that systemic (or neural) inflammation impairs two key elements of ventilatory control: chemoreflexes and respiratory motor (vs. sensory) plasticity. Achieving an understanding of mechanisms whereby inflammation undermines ventilatory control is fundamental since inflammation may diminish the capacity for natural, compensatory responses during pathological states, and the ability to harness respiratory plasticity as a therapeutic strategy in the treatment of devastating breathing disorders, such as during cervical spinal injury or motor neuron disease.
Keywords: long-term facilitation, intermittent hypoxia, LPS, plasticity, respiratory motor neuron
1. INTRODUCTION
Accurate and robust ventilatory control is critical to maintain adequate breathing when confronted with many disorders of the lung or central nervous system (CNS). Factors that undermine the efficacy of ventilatory control will diminish the ability to compensate for pathology, threatening life itself (Mitchell, 2007). Most lung and CNS disorders are associated with systemic and/or neural inflammation, including chronic lung diseases (Stockley, 2009), traumatic, ischemic and degenerative neural disorders (Teeling and Perry, 2009) and obstructive sleep apnea. Inflammation in sleep apnea presumably results, at least in part, from severe intermittent hypoxia experienced in this disorder (Wills-Karp, 1999, Decramer et al., 2008, Gozal, 2009, McDonald et al., 2011). Although inflammation has profound effects on important neural functions, such as synaptic transmission and plasticity (Di Filippo et al., 2008), little is known concerning the impact of inflammation on the neural system controlling breathing.
Key elements in the ventilatory control system include rhythm generation, chemoreception (hypercapnic and hypoxic responses) and respiratory plasticity (reviewed in Feldman et al., 2003). Chemoreception and plasticity are critical elements of the ventilatory control system, enabling compensation for challenges to breathing capacity or stability presented by lung or neural disorders (Feldman et al., 2003, Mitchell and Johnson, 2003, Mitchell, 2007). Sporadic evidence has been accumulating in recent years, suggesting that systemic inflammation modulates several aspects of ventilatory control; such evidence is reviewed in the papers compiled in this special edition of Respiration Physiology and Neurobiology. In the present paper, our primary goal is to present evidence that inflammation impairs chemoreflexes and respiratory motor (vs. sensory) plasticity following acute intermittent hypoxia, which may leave an individual vulnerable to inadequate or unstable breathing during disease.
Systemic inflammation affects sensory receptors that modulate breathing, but can also trigger inflammatory responses in the central nervous system (CNS) through complex mechanisms. The primary CNS cells affected during systemic inflammation are microglia, the resident immune cells of the CNS, and astrocytes (Lehnardt, 2010). Since sensory processing and neuroplasticity are modulated by cell-cell interactions between neurons and microglia or neurons and astrocytes, factors that activate astrocyte and/or microglial inflammatory activities may alter respiratory chemoreflexes and/or plasticity.
In this review, we begin by discussing a common experimental model of systemic inflammation and its impact on the CNS. We then discuss major advances in our understanding of mechanisms whereby inflammation alters central neural processing of primary afferent neurons (particularly chronic pain), followed by consideration of how these advances relate to respiratory chemoreflexes (hypoxia/hypercapnia). Next, we discuss how inflammation affects hippocampal synaptic plasticity and spinal motor learning, followed by consideration of how these concepts relate to respiratory plasticity. We conclude by discussing the potential significance of interactions between inflammation and ventilatory control, and suggest areas where research is needed.
2. SYSTEMIC AND CNS INFLAMMATION
2.1 CNS inflammation: the role of microglia
Historically, the CNS was viewed as an immunologically privileged area that lacks traditional immune responses. Peripherally, the innate immune response activates signaling cascades that recruit immune cells (e.g. neutrophils and macrophages) to phagocytose foreign substances and release cytokines (Chen and Nunez, 2010). Cytokines trigger adaptive immune responses and activate lymphocytes. Collectively, these events eradicate foreign substances and promote tissue repair (Vivier et al., 2011). The CNS immune response differs in many respects since the blood-brain barrier, in most cases, prevents immune cell infiltration. Nevertheless, resident microglia trigger CNS inflammation (Carson et al., 2006, Graeber, 2010, Kaur et al., 2010).
Even when in their “resting state,” microglia are highly active, surveying their environment (Raivich, 2005, Parkhurst and Gan, 2010). When confronted with pathological conditions, such as neuronal injury/degeneration or bacterial/viral/fungal infection, they become “activated,” shifting from a stellate, ramified phenotype to an amoeboid shape (Kreutzberg, 1996). Activated microglia can be phagocytic, or they can release toxic and protective factors, including cytokines, prostaglandins, nitric oxide or neurotrophic factors (e.g. BDNF) (Kreutzberg, 1996, Graeber, 2010). Despite the importance of microglia in immune function, they are diffuse in the CNS (~70-90% of CNS cells are glia; microglia are ~5-10% of those cells). At this point, we still have little knowledge on the complex role played by microglia in systemic and/or neural disorders, let alone what role they play in respiratory-related regions of the CNS.
2.2 CNS inflammation: other cell types
Although there is general agreement that microglia are major contributors to CNS inflammatory responses, debate exists concerning the relative ability of neurons and/or astrocytes to release pro-inflammatory molecules in vivo. Recent reviews describe astrocytic and neuronal contributions to CNS inflammation, and toll-like receptor (TLR, see below) expression in many cell types (Rivest, 2001, Escartin and Bonvento, 2008, Griffiths et al., 2009, Miller et al., 2009, Okun et al., 2009, Whitney et al., 2009). The specific TLRs expressed differ among cell types. Neurons do not express TLR-4 in vivo (Chakravarty and Herkenham, 2005, Mishra et al., 2006), with the exception of gigantocellular neurons of the reticular formation (Mishra et al., 2006). Other neuronally expressed TLRs do not induce cytokine production (Okun et al., 2009). Thus, neurons probably play a minimal direct role in CNS inflammation. Astrocytes, on the other hand, contribute to the overall inflammatory response since they release cytokines, triggering nuclear factor-kappa B (NFκB) signaling elsewhere in the CNS. Further, they express many TLRs, including TLR-4, capable of eliciting an inflammatory response (Li and Stark, 2002, Farina et al., 2007, Johann et al., 2008). Given their relative abundance, astrocytes may play a key role in CNS inflammatory responses.
2.3 Induced versus endogenous inflammation
Many studies focus on (exogenously) induced systemic inflammation as an experimental model. However, it is not understood how these results relate to endogenous neuroinflammation (for example, during autoimmune diseases, spinal injury, neurodegenerative diseases or ischemic injury) since few studies directly compare induced versus endogenous inflammation. Available information suggests that induced and endogenous inflammation share many common features, and studies of induced inflammation have many experimental advantages (e.g. inflammation without attendant issues such as mechanical injury or degenerative disease). Thus, induced inflammation is a reasonable model to begin investigations concerning the impact of inflammatory activities on ventilatory control.
2.4 Lipopolysaccharide (LPS)
The most frequently studied model of induced systemic inflammation is administration of the bacterial endotoxin, LPS. Although LPS is a component of Gram-negative bacterial cell walls, its most relevant feature is that it initiates inflammation primarily via activation of CD14/TLR-4 receptors (Poltorak et al., 1998). This is important since naturally occurring proteins, such as certain heat shock proteins, are endogenous ligands for TLR-4s (Ohashi et al., 2000, Lehnardt et al., 2008). Thus, LPS is a reasonable model to study inflammation, and is relevant beyond Gram-negative bacterial infections. LPS also activates beta 2 integrins (e.g. CD11c and CD18) and scavenger receptors (Fenton and Golenbock, 1998, Triantafilou and Triantafilou, 2002).
While LPS does not cross the blood-brain barrier (Singh and Jiang, 2004, Qin et al., 2007), systemic LPS administration elicits CNS inflammation through complex mechanisms, including indirect effects mediated by cytokines or other inflammatory molecules that do cross into the CNS. Candidate molecules triggering CNS inflammatory activities following systemic LPS include interleukins (IL-1β), tumor necrosis factor alpha (TNFα) and prostaglandins produced by perivascular macrophages and/or endothelial cells that line the blood-brain barrier (Maier et al., 1998, Goehler et al., 1999, Laflamme et al., 1999, Blatteis and Li, 2000, Schnydrig et al., 2007, Rivest, 2009). Another means of transmission is via peripheral nerves (including the vagus nerves), which transmit inflammation into the CNS via unknown mechanisms (Ge et al., 2001, Roth and De Souza, 2001, Wieczorek et al., 2005, Blatteis, 2007).
2.5 Toll-Like Receptors (TLRs)
TLRs sense pathogens, quickly recognizing highly conserved pathogen-associated molecular patterns and triggering innate immune responses to eliminate the pathogen (e.g. bacteria, viruses, fungi, parasites) (Chen et al., 2007). TLRs (specifically TLR-2 and TLR-4) also recognize endogenously released damage-associated molecular patterns from necrotic or apoptotic cells (Chen et al., 2007). Thus, TLRs act as sensors for both exogenous (invading pathogens) and endogenous (cell death via apoptosis or necrosis) threats to tissue viability. While detailed signaling cascades triggered by endogenous versus exogenous inflammation are not fully understood, LPS is a viable model to begin studies of inflammation and ventilatory control since it is a TLR-4 ligand. Regardless, aspects of LPS-induced inflammation may not faithfully reflect inflammatory responses triggered by endogenous molecules.
TLR-4 receptors are cytokine family receptors that activate transcription factors, such as NFκB (Lu et al., 2008). NFκB regulates the expression of many inflammatory genes, including: IL-1β, -6 and -18, TNFα, cyclooxygenase-2 (COX-2) and inducible nitric oxide synthase (iNOS) (Ricciardolo et al., 2004, Nam, 2006). Endogenous molecules known to activate TLR-4 receptors include (but are not limited to) heat shock proteins (specifically HSP60, Ohashi et al., 2000, Lehnardt et al., 2008), fibrinogen, surfactant protein-A, fibronectin extra domain A, heparin sulfate, soluble hyaluronan, β-defensin 2 and HMGB1 (Chen et al., 2007).
2.6 Inflammatory gene expression in tissue homogenates
Cytokine release is a key factor initiating general CNS inflammation, and traditionally has been assessed in tissue homogenates. However, it is important to bear in mind that CNS homogenates are >50% astrocytes. Thus, inflammatory gene assessment in tissue homogenates may be dominated by astrocytes, with less influence from more diffuse cell types, including microglia. More specific methods are necessary to assess gene expression in less abundant cell types, such as analyses of microglia freshly isolated from CNS tissues.
Chronic intermittent hypoxia (CIH) stimulates CNS inflammation, increasing inflammatory molecules in tissue homogenates from the hippocampus (e.g. COX-2 and iNOS) (Li et al., 2003, Row et al., 2003, Li et al., 2004, Xu et al., 2004, Gozal and Kheirandish-Gozal, 2008). No information is currently available concerning the specific cell types involved in this inflammatory response. Further, little is known regarding LPS effects on microglia in vivo in any region of the CNS since most studies evaluate homogenates only. Thus, cell-specific isolation from distinct regions of the CNS is an important step to advance our understanding of the relative roles played by microglia versus other cell types in regions of interest to ventilatory control. At this time, significant gaps in our understanding include: 1) lack of knowledge concerning inflammatory gene expression and protein levels in identified cell types; 2) specific effects of inflammation in CNS regions relevant to ventilatory control (e.g. brainstem and cervical spinal cord), where microglia have different properties than cortical microglia; 3) a time course of LPS effects on inflammatory gene expression in different cell types and regions of interest; and 4) comparative data between LPS and other inflammatory stimuli (such as chronic intermittent hypoxia).
3. INFLAMMATION AND SENSORY SYSTEMS
3.1 Nociception
The role of inflammation (and specifically microglia) in chronic pain has been studied extensively (reviewed in Woolf and Salter, 2000, Trang et al., 2006, Mika, 2008, Abbadie et al., 2009, Baumbauer et al., 2009). A remarkable story has emerged, demonstrating the interplay between neurons, microglia, inflammation and plasticity in this spinal sensory system. In short, inflammation induces both peripheral and central sensitization, leading to allodynia (hypersensitivity to otherwise non-painful stimuli) and hyperalgesia (exaggerated or prolonged responses to a noxious stimulus) (Mika, 2008).
Plasticity at peripheral nerve terminals increases synaptic inputs to the CNS from primary afferent neurons associated with nociception. Inflammation increases Aβ-mediated synaptic input to the dorsal horn and activates spinal microglia through increased afferent input or cytokines crossing the blood brain barrier, and thus increases neuropeptide expression and pro-inflammatory gene expression in the spinal dorsal horn (Baumbauer et al., 2009, Latremoliere and Woolf, 2009). Additionally, inflammation also increases microglial P2X4 ATP receptor expression (Inoue, 2006), subsequently increasing expression of brain derived neurotrophic factor (BDNF; Coull et al., 2005). BDNF from these activated microglia down-regulates the chloride co-transporter KCC2 on second order nociceptive neurons (Coull et al., 2003), diminishing their chloride potential and, thus, the efficacy of inhibitory neurotransmission. Without the constraint of GABA/glycine inputs on nociceptive synaptic transmission, development of chronic pain results (Price et al., 2005).
Although scarcely explored, similar mechanisms may play major roles in ventilatory control, particularly in modulating sensory systems (e.g. chemoreflexes).
3.2 Chemoreflex control of breathing
An important aspect of ventilatory control susceptible to inflammatory modulation is the chemoreflex control of breathing. Chemoreflexes are critical for maintaining homeostasis of arterial blood gases via classical negative feedback (Mitchell et al., 2009), or acting as “teachers” that induce plasticity in the respiratory control system (Mitchell and Johnson, 2003). Major chemoreflexes include the hypoxic (Powell et al., 1998) and hypercapnic ventilatory responses (Nattie, 2001), arising predominantly from the peripheral arterial and central chemoreceptors (Lahiri and Forster, 2003).
3.2.1 Hypoxic ventilatory response
Systemic LPS decreases the hypoxic ventilatory response in cats, without change in ventilation during maximal chemoreceptor stimulation (Fernandez et al., 2008). Inflammation diminishes the hypoxic ventilatory response by a nitric oxide dependent mechanism in piglets (McDeigan et al., 2003). Further, TNFα diminishes carotid chemosensory discharge in vitro (Fernandez et al., 2008). Young rats treated with systemic LPS exhibit depressed hypoxic ventilatory responses (Ladino et al., 2007). Systemic LPS (3 mg/kg, i.p.) also reduces short-term hypoxic phrenic responses in anesthetized, vagotomized, paralyzed and ventilated adult rats (Vinit et al., 2011). Mechanisms whereby inflammation impairs the hypoxic response are not understood, and may involve multiple inflammatory molecules that influence peripheral and central chemoreceptors, or other respiratory neurons.
Another stimulus to plasticity in regions of interest to ventilatory control is chronic intermittent hypoxia (CIH). CIH increases carotid chemoreceptor responses to hypoxia, quite possibly due to peripheral chemoreceptor inflammation (Del Rio et al., 2010). In agreement, nocturnal CIH also augments the short-term hypoxic phrenic response in rats (Ling et al., 2001). However, the extent of CIH-induced inflammation in the brainstem and spinal cord, and the potential contributions of CNS inflammation to this form of plasticity remain unclear.
3.2.2. Hypercapnic ventilatory response
To date, no studies have reported the impact of systemic inflammation on hypercapnic responses. However, increased CO2 suppresses NFκB activation, possibly suppressing inflammatory gene expression (Taylor and Cummins, 2011). In fact, hypercapnia has been used to treat ischemia/reperfusion injury to decrease inflammation and reduce lung tissue damage (Laffey et al., 2000, O'Croinin et al., 2005, Curley et al., 2010, Li et al., 2010). In a rat model of Duchenne muscular dystrophy, where inflammation is a key component of disease progression, symptomatic mutant mice exhibit a diminished hypercapnic ventilatory response (Gosselin et al., 2003), consistent with the idea that inflammation may also impair hypercapnic ventilatory responses. Such impairment of ventilatory control may be due, at least in part, to impaired diaphragm mechanics resulting from increased TNFα expression. However, these studies do not rule out additional central neural effects of TNFα (Gosselin et al., 2003). Further work concerning the influence of systemic inflammation on hypercapnic ventilatory responses is warranted, particularly since impaired CO2 chemoreflexes would allow greater hypercapnia and minimize the ongoing inflammation; in this sense, impaired hypercapnic ventilatory responses during inflammation may (in part) be adaptive.
3.2.3. Inflammation and chemoreflexes in rats
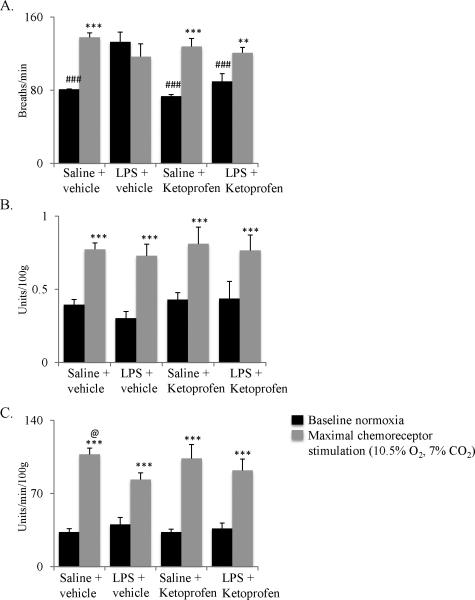
Here, we report new data concerning the impact of inflammation induced by systemic LPS on maximal chemoreflex stimulation of breathing in unanesthetized rats (Figure 1). Four treatment groups of adult, male Lewis rats were studied (each n=4): 1) vehicle controls (saline, 1ml/kg, i.p.), 2) LPS treated (5 mg/kg in saline, i.p.), 3) the non-steroidal anti-inflammatory, ketoprofen (12.5 mg/kg in 70% ethanol, s.c.), before and every 8 hours post-saline injection, and 4) ketoprofen before and every 8 hours post-LPS injection. 24 hours post-injection, ventilation was measured using whole-body plethysmography (Data Sciences International, St. Paul, MN, USA). Rectal temperature was measured before being placed in the chamber, and again at the end of protocols as rats were removed from the chamber. Due to a consistent equipment error in recording chamber temperature, we chose to express volumes in relative units (units/min/100g) rather than making a (small but) uncertain correction in absolute units (ml/min/100g). Regardless, chamber temperature measurement errors have no impact on recorded breathing frequencies, the main variable affected in these studies.
Figure 1.
Systemic LPS alters breathing frequency and minute ventilation in unanesthetized Lewis rats. A. Systemic LPS (5 mg/kg, i.p.) significantly increased baseline breathing frequency versus other baseline conditions; this effect was reversed by the non-steroidal anti-inflammatory drug, ketoprofen (12.5 mg/kg, s.c.). LPS also suppressed the frequency response to chemoreceptor stimulation (10.5% O2, 7% CO2, 15 min). B. Although chemoreceptor stimulation increased tidal volume in all groups, neither LPS nor ketoprofen had significant effects on this response. C. Although chemoreceptor stimulation increased minute ventilation in all treatment groups, LPS reduced this response versus saline + vehicle. Ketoprofen tended to restore maximal ventilation after LPS, but this change was not statistically significant. Ketoprofen controls had no significant effect on ventilation. ** p<0.01 *** p<0.001 indicates significant difference from baseline (black bars); ### p<0.001 indicates significant difference from LPS + vehicle baseline (black bar); @ p<0.01 indicates significant difference from LPS + vehicle (10.5% O2, 7% CO2, gray bar). Statistics: two-way, repeated measures ANOVA with Fisher's LSD post hoc test.
To determine ventilatory capacity in these rats, they were given maximal chemoreceptor stimulation by exposure to 10.5% inspired O2 with 7% inspired CO2 (balance N2) for 15 min. LPS significantly affected breathing frequency, but not tidal volume (Figure 1). Specifically, LPS increased baseline breathing frequency (breaths/min) versus other treatment groups (LPS + vehicle = 133±11; Saline + vehicle = 81±0.2; Saline + Ketoprofen = 74±1.4; LPS + Ketoprofen = 90±8.5) (Figure 1A). Increased frequency during chemoreceptor stimulation was not evident in rats treated with LPS (LPS + vehicle baseline = 133±11 breaths/min; LPS + vehicle maximal stimulation = 117±14 breaths/min). However, the frequency response to chemoreceptor stimulation was restored when LPS-treated rats were also treated with the nonsteroidal anti-inflammatory agent, ketoprofen (LPS + Ketoprofen baseline = 90±9 breaths/min; LPS + Ketoprofen maximal stimulation= 121±11 breaths/min). LPS-induced inflammation may influence respiratory rhythm by direct actions on brainstem centers controlling frequency (e.g. the pre-Bötzinger Complex), or via indirect effects mediated by sensory receptors that project to these rhythm generating neurons.
In contrast to LPS effects on breathing frequency, neither LPS nor ketoprofen affected tidal volume in baseline conditions or during maximal chemoreceptor stimulation (Figure 1B). Overall, LPS reduced pulmonary ventilation during chemoreceptor stimulation (Figure 1C), reflecting its effects on breathing frequency. Specifically, LPS decreased chemoreceptor stimulated ventilation versus vehicle controls (control: 107±6 units/min/100g; LPS: 83±6 units/min/100g, Figure 1C; p<0.05). Ketoprofen failed to reverse these LPS effects on chemoreceptor stimulated ventilation (92±11 units/min/100g; p>0.05). The lack of reversibility of LPS effects on breathing with ketoprofen may reflect an inadequate dose or the timing of ketoprofen administration since inflammatory molecule expression changes over time with different inflammatory mediators peaking in expression level at different times during an inflammatory response (Lund et al., 2006, Natoli et al., 2011).
From the limited data presented here, we cannot determine conclusively if blunted chemoreflex frequency responses result from effects on peripheral chemoreceptors (Iturriaga et al., 2009), vagal pulmonary receptors (Lai et al., 2002) or central neural mechanisms (e.g. rhythm generating neurons). However, it is unlikely they resulted from known effects of inflammation on respiratory muscles (Hussain, 1998) or lung mechanics (e.g. pulmonary edema) since these effects would be expressed as changes in tidal volume versus frequency. Further, since LPS (3 hrs post-injection) diminishes amplitude responses in the short-term hypoxic phrenic response of anesthetized, paralyzed, vagotomized and ventilated rats (Vinit et al., 2011), contributions from vagal afferent neurons, respiratory muscles or lung mechanics can be ruled out in this reduced experimental preparation; these diminished hypoxic responses must arise from LPS effects on peripheral chemoreceptors and/or the CNS.
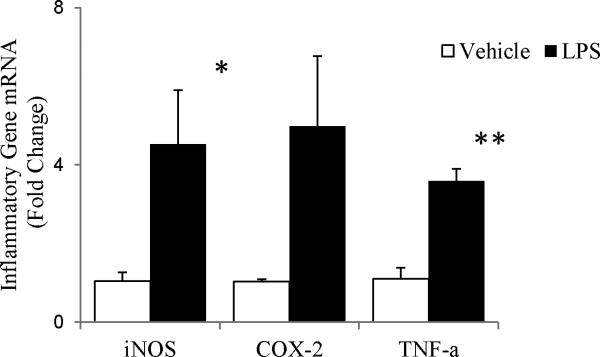
To confirm CNS inflammation 24 hours following systemic LPS, we examined changes in inflammatory gene expression in tissue homogenates from the brainstems of adult, male Sprague Dawley rats (iNOS, COX-2, and TNFα mRNA via quantitative RT-PCR). 24 hours post-LPS (10 mg/kg, i.p., n=3), iNOS (p=0.03) and TNFα (p=0.009) mRNA had increased; an apparent increase in COX-2 mRNA approached significance (p=0.054) (Fig 2). Thus, although LPS does not cross the blood-brain barrier, systemic LPS injection induces central neural inflammation in regions of interest to ventilatory control.
Figure 2.
Changes in inflammatory gene expression in brainstem homogenates from adult, male Sprague Dawley rats 24 hrs post-LPS (10 mg/kg, i.p.). iNOS and TNF-α increased significantly after LPS exposure (p=0.03 and p=0.009, respectively). An apparent increase in COX-2 was only marginally significant (p=0.054). Statistics: t-tests comparing vehicle vs. LPS for each gene.
4. INFLAMMATION AND NEUROPLASTICITY
4.1 Non-respiratory systems
Inflammatory molecules induce/maintain synaptic plasticity in some neural systems (Woolf and Salter, 2000, Beattie et al., 2002), but inhibit plasticity in others (Di Filippo et al., 2008). Hippocampal synaptic plasticity and hippocampus-dependent learning are inhibited by inflammation (Vereker et al., 2000, Shaw et al., 2001), including COX-2 regulated prostaglandin synthesis (Shaw et al., 2005). Inflammation also impairs: 1) spinal instrumental learning (Vichaya et al., 2009); 2) contextual fear conditioning, and spatial learning in the Morris water maze (Shaw et al., 2001); 3) memory processing in day old chicks (Sell et al., 2001); and 4) memory consolidation (Thomson and Sutherland, 2005). When inflammation inhibits recognition memory and LTP in the dentate gyrus, plasticity-associated changes in growth factor expression are blocked (Hennigan et al., 2007), giving some clue as to potential sites of impairment in the cellular cascades leading to memory. On the other hand, cytokines are also reported to be necessary for learning, memory and hippocampal synaptic plasticity (Bohme et al., 1993, Zhuo et al., 1993, Malen and Chapman, 1997, Pollmacher et al., 2002, Avital et al., 2003, Brennan et al., 2003, Goshen et al., 2007).
4.2 Respiratory plasticity
Hippocampal synaptic plasticity and spinal somatic motor learning share many common cellular mechanisms with phrenic long-term facilitation (pLTF) following acute intermittent hypoxia, the most frequently studied model of spinal respiratory motor plasticity (Mahamed and Mitchell, 2007, Mateika and Sandhu, 2011). Indeed, systemic LPS impairs pLTF (Vinit et al., 2011), an effect similar to hippocampal synaptic plasticity and spinal motor learning, but unlike plasticity the spinal dorsal horn where sensitization prevails (Woolf and Salter, 2000). Given our emerging awareness that inflammation has considerable impact on neuroplasticity in other regions of the CNS (Woolf and Salter, 2000, Di Filippo et al., 2008), an understanding of mechanisms whereby inflammation impairs respiratory motor plasticity is of considerable interest.
We have made considerable progress towards an understanding of cellular/synaptic mechanisms giving rise to pLTF induced by acute-intermittent hypoxia (AIH, 3 × 5 min 10.5% separated by 5 min normoxia) in vivo (Mahamed and Mitchell, 2007, Baker-Herman and Mitchell, 2008, Mateika and Sandhu, 2011). We have recently come to realize that multiple, distinct mechanisms give rise to long-lasting phrenic motor facilitation (pMF), where pMF is used as a general term that includes pLTF induced by AIH (Dale-Nagle et al., 2010). These pathways interact in complex and interesting ways, providing a range of potential responses in the face of changing physiological conditions or the onset of disease. A detailed understanding of cellular/synaptic mechanism(s) giving rise to pMF may guide the development of novel therapeutic strategies for severe breathing disorders, including obstructive sleep apnea (Mitchell, 2007). Thus, an understanding of mechanisms whereby inflammation undermines respiratory plasticity is of fundamental importance, since inflammation may diminish the capacity for natural, compensatory plasticity during pathological states and undermine the ability to harness respiratory plasticity as a therapeutic tool in the treatment of respiratory insufficiency (Mitchell, 2007). By understanding mechanisms whereby inflammation impairs respiratory plasticity, we may speed development of new strategies to restore breathing capacity in devastating ventilatory disorders such as cervical spinal injury or motor neuron disease. Unfortunately, we have only begun to appreciate the impact of inflammation on any form of respiratory plasticity (Di Filippo et al., 2008, Iturriaga et al., 2009, Vinit et al., 2011).
4.3 Do microglia contribute to impaired pLTF following AIH
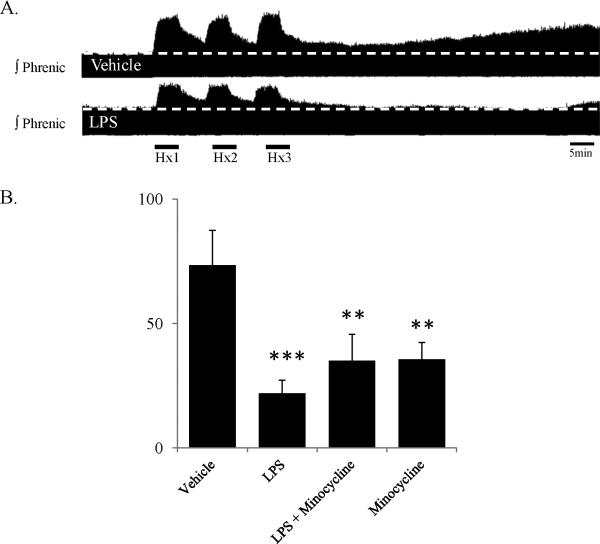
Here we report new data attempting to determine the role of microglia in LPS-induced impairment of AIH-induced pLTF using a “standard” approach to inhibiting microglial function. Specifically, we pretreated rats with minocycline, a semi-synthetic tetracycline known to inhibit microglial, with lesser effects on neuronal or astrocytic function (Kim and Suh, 2009). As we showed previously (Vinit et al., 2011), three hours post-LPS (3 mg/kg, i.p.), AIH-induced pLTF (3 × 5 min 10.5% O2, separated by 5 min normoxia) is impaired (control: 74±14%, n=8; LPS: 22±5%, n=12) (Figure 3). Unexpectedly, minocycline alone also impaired pLTF (minocycline: 36±7%, n=8, 30 mg/kg, i.v., Figure 3), and had no impact on LPS-induced pLTF impairment (LPS + minocycline: 35±11%, n=8). Based on these experiments, it is not possible to make conclusions regarding the role of microglia in the impairment of pLTF following LPS administration. Rather, we suggest that minocycline independently impairs pLTF, possibly by inhibition of relevant protein kinase C isoforms (Nikodemova et al., 2006).
Figure 3.
The effects of systemic LPS on pLTF, with and without a known microglial inhibitor (minocycline). Both LPS and minocycline diminish AIH-induced pLTF. A) Compressed phrenic neurograms from in vivo anesthetized, vagotomized, paralyzed, ventilated rats demonstrating typical AIH-induced pLTF (upper trace), and reduced pLTF 3 hrs post-LPS (3 mg/kg, i.p.; lower trace). B) Group data demonstrating significant reduction in pLTF with LPS, LPS and minocycline (30 mg/kg, i.v.) and minocycline alone. (** p<0.01, *** p<0.001 indicates significant difference from vehicle response). Statistics: one-way ANOVA with Fisher's LSD post hoc test.
5. INFLAMMATION AND RESPIRATORY RHYTHM GENERATION
To date, little is known concerning the impact of inflammation on respiratory rhythm generation. Since astrocytes play an important role in brainstem rhythm generation (Hulsmann et al., 2000, Grass et al., 2004, Haertel et al., 2009, Huxtable et al., 2010), and microglia are located in regions associated with rhythm generation, there is considerable potential for inflammation to alter cell-cell interactions and modulate this critical biological process. The increase in baseline breathing frequency in rats injected with LPS (5 mg/kg, i.p., Figure 2), and the failure to increase breathing frequency during chemoreceptor stimulation, suggest an influence of inflammation on brainstem centers controlling breathing frequency (see above). However, we do not yet know which inflammatory molecules may be responsible for these effects. It is essential to understand mechanisms whereby inflammation could disrupt respiratory rhythm generation, since infection and inflammation are implicated in apnea of prematurity and in devastating examples of respiratory arrest, such as sudden infant death syndrome (Blackwell et al., 2005, Blood-Siegfried, 2009, Marcus et al., 2009, Dale-Nagle et al., 2010).
6. SIGNIFICANCE, IMPLICATIONS AND FUTURE DIRECTIONS
A major implication of diminished chemoreflexes and respiratory plasticity with systemic inflammation is that, at a time when robust ventilatory control is needed the most (i.e. disease), the neural system controlling breathing may be compromised. A major goal should be to understand the extent and mechanisms compromising this critical homeostatic control system.
Although information is now becoming available concerning the impact of acute inflammation (<24 hrs) on ventilatory control, future investigations must explore longer time-domains. Longer time-domains are characteristic of chronic lung and neural diseases (Iturriaga et al., 2009, Del Rio et al., 2010). Further, inflammation is a dynamic process; specific combinations of inflammatory molecules expressed at any given time differ. Thus, it is not clear that acute and chronic inflammation will have the same impact on ventilatory control.
Here, we reviewed evidence that systemic inflammation activates brainstem and spinal inflammatory responses, impairing chemoreflexes and respiratory plasticity. However, most available evidence concerns exogenously induced models of inflammation, such as systemic LPS. Further research is necessary to confirm that this model reveals general principles applicable to endogenous inflammation characteristic of chronic lung disease (e.g. COPD), breathing disorders (e.g. sleep apnea) and neurological disorders, including traumatic, ischemic and neurodegenerative processes.
Sleep apnea and the attendant chronic intermittent hypoxia induce CNS inflammation and impair cognitive function (Gozal, 2009, McNicholas, 2009, Ryan et al., 2009, Inancli and Enoz, 2010, Kimoff et al., 2010). If chronic intermittent hypoxia-induced inflammation alters respiratory chemoreflexes and plasticity, then disease/ventilatory control interactions may contribute to the underlying pathophysiology. For example inflammation induced by sleep-disordered breathing may undermine spontaneous respiratory compensation, exacerbating the primary breathing disorder. Research concerning this possibility seems warranted.
In recent years, we have started to harness respiratory plasticity as a treatment for conditions associated with respiratory insufficiency, such as cervical spinal injury (Mitchell, 2007; Vinit et al., 2009). Inherent in these disorders is an element of (endogenous) inflammation, characterized by increased expression of pro- and anti-inflammatory molecules (Lehnardt, 2010). Patients with respiratory insufficiency are prone to greater rates of infection and generalized immune activation (Wills-Karp, 1999, Stockley, 2009, Oglesby et al., 2010). Because of the high incidence of inflammatory activity in respiratory disorders, a major obstacle in harnessing respiratory plasticity as a therapeutic tool may be overcoming the limits imposed by inflammation. Thus, therapeutic induction of respiratory (or other motor) plasticity may be optimized if the patients are first given anti-inflammatory agents. Before such combinatorial therapies can/should be applied, we need more information regarding mechanisms whereby inflammation impairs the neural control of breathing.
Overall, the theme of this special edition is quite novel in the context of respiratory neurobiology. We are only now beginning to appreciate the impact of inflammation on neural function in other regions of the nervous system (Di Filippo et al., 2008, Abbadie et al., 2009, Iturriaga et al., 2009). Although many human clinical conditions that require rigorous ventilatory control to assure adequate breathing are associated with inflammation, we are only at the beginning of our understanding concerning how inflammation impacts neural mechanisms that underlie any aspect of ventilatory control (e.g. rhythm generation, chemoreflexes, plasticity). We should move quickly to understand the impact of this common biological event (i.e. inflammation) on respiratory control.
Acknowledgements
Supported by NIH (HL80209 and HL69064).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
REFERENCES
- Abbadie C, Bhangoo S, De Koninck Y, Malcangio M, Melik-Parsadaniantz S, White FA. Chemokines and pain mechanisms. Brain Res Rev. 2009;60:125–134. doi: 10.1016/j.brainresrev.2008.12.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Avital A, Goshen I, Kamsler A, Segal M, Iverfeldt K, Richter-Levin G, Yirmiya R. Impaired interleukin-1 signaling is associated with deficits in hippocampal memory processes and neural plasticity. Hippocampus. 2003;13:826–834. doi: 10.1002/hipo.10135. [DOI] [PubMed] [Google Scholar]
- Baker-Herman TL, Mitchell GS. Determinants of frequency long-term facilitation following acute intermittent hypoxia in vagotomized rats. Respir Physiol Neurobiol. 2008;162:8–17. doi: 10.1016/j.resp.2008.03.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baumbauer KM, Young EE, Joynes RL. Pain and learning in a spinal system: contradictory outcomes from common origins. Brain Res Rev. 2009;61:124–143. doi: 10.1016/j.brainresrev.2009.05.004. [DOI] [PubMed] [Google Scholar]
- Beattie EC, Stellwagen D, Morishita W, Bresnahan JC, Ha BK, Von Zastrow M, Beattie MS, Malenka RC. Control of synaptic strength by glial TNFalpha. Science. 2002;295:2282–2285. doi: 10.1126/science.1067859. [DOI] [PubMed] [Google Scholar]
- Blackwell CC, Moscovis SM, Gordon AE, Al Madani OM, Hall ST, Gleeson M, Scott RJ, Roberts-Thomson J, Weir DM, Busuttil A. Cytokine responses and sudden infant death syndrome: genetic, developmental, and environmental risk factors. J Leukoc Biol. 2005;78:1242–1254. doi: 10.1189/jlb.0505253. [DOI] [PubMed] [Google Scholar]
- Blatteis CM. The onset of fever: new insights into its mechanism. Prog Brain Res. 2007;162:3–14. doi: 10.1016/S0079-6123(06)62001-3. [DOI] [PubMed] [Google Scholar]
- Blatteis CM, Li S. Pyrogenic signaling via vagal afferents: what stimulates their receptors? Auton Neurosci. 2000;85:66–71. doi: 10.1016/S1566-0702(00)00221-6. [DOI] [PubMed] [Google Scholar]
- Blood-Siegfried J. The role of infection and inflammation in sudden infant death syndrome. Immunopharmacol Immunotoxicol. 2009;31:516–523. doi: 10.3109/08923970902814137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bohme GA, Bon C, Lemaire M, Reibaud M, Piot O, Stutzmann JM, Doble A, Blanchard JC. Altered synaptic plasticity and memory formation in nitric oxide synthase inhibitor-treated rats. Proc Natl Acad Sci U S A. 1993;90:9191–9194. doi: 10.1073/pnas.90.19.9191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brennan FX, Beck KD, Servatius RJ. Low doses of interleukin-1beta improve the leverpress avoidance performance of Sprague-Dawley rats. Neurobiol Learn Mem. 2003;80:168–171. doi: 10.1016/s1074-7427(03)00060-1. [DOI] [PubMed] [Google Scholar]
- Carson MJ, Doose JM, Melchior B, Schmid CD, Ploix CC. CNS immune privilege: hiding in plain sight. Immunol Rev. 2006;213:48–65. doi: 10.1111/j.1600-065X.2006.00441.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chakravarty S, Herkenham M. Toll-Like Receptor 4 on Nonhematopoietic Cells Sustains CNS Inflammation during Endotoxemia, Independent of Systemic Cytokines. J Neurosci. 2005;25:1788–1796. doi: 10.1523/JNEUROSCI.4268-04.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen GY, Nunez G. Sterile inflammation: sensing and reacting to damage. Nat Rev Immunol. 2010;10:826–837. doi: 10.1038/nri2873. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen R, Alvero AB, Silasi DA, Mor G. Inflammation, cancer and chemoresistance: taking advantage of the toll-like receptor signaling pathway. Am J Reprod Immunol. 2007;57:93–107. doi: 10.1111/j.1600-0897.2006.00441.x. [DOI] [PubMed] [Google Scholar]
- Coull JA, Beggs S, Boudreau D, Boivin D, Tsuda M, Inoue K, Gravel C, Salter MW, De Koninck Y. BDNF from microglia causes the shift in neuronal anion gradient underlying neuropathic pain. Nature. 2005;438:1017–1021. doi: 10.1038/nature04223. [DOI] [PubMed] [Google Scholar]
- Coull JA, Boudreau D, Bachand K, Prescott SA, Nault F, Sik A, De Koninck P, De Koninck Y. Trans-synaptic shift in anion gradient in spinal lamina I neurons as a mechanism of neuropathic pain. Nature. 2003;424:938–942. doi: 10.1038/nature01868. [DOI] [PubMed] [Google Scholar]
- Curley G, Laffey JG, Kavanagh BP. Bench-to-bedside review: carbon dioxide. Crit Care. 2010;14:220. doi: 10.1186/cc8926. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dale-Nagle EA, Hoffman MS, MacFarlane PM, Satriotomo I, Lovett-Barr MR, Vinit S, Mitchell GS. Spinal plasticity following intermittent hypoxia: implications for spinal injury. Ann N Y Acad Sci. 2010;1198:252–259. doi: 10.1111/j.1749-6632.2010.05499.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Decramer M, Rennard S, Troosters T, Mapel DW, Giardino N, Mannino D, Wouters E, Sethi S, Cooper CB. COPD as a lung disease with systemic consequences--clinical impact, mechanisms, and potential for early intervention. COPD. 2008;5:235–256. doi: 10.1080/15412550802237531. [DOI] [PubMed] [Google Scholar]
- Del Rio R, Moya EA, Iturriaga R. Carotid body and cardiorespiratory alterations in intermittent hypoxia: the oxidative link. Eur Respir J. 2010;36:143–150. doi: 10.1183/09031936.00158109. [DOI] [PubMed] [Google Scholar]
- Di Filippo M, Sarchielli P, Picconi B, Calabresi P. Neuroinflammation and synaptic plasticity: theoretical basis for a novel, immune-centred, therapeutic approach to neurological disorders. Trends Pharmacol Sci. 2008;29:402–412. doi: 10.1016/j.tips.2008.06.005. [DOI] [PubMed] [Google Scholar]
- Escartin C, Bonvento G. Targeted activation of astrocytes: a potential neuroprotective strategy. Mol Neurobiol. 2008;38:231–241. doi: 10.1007/s12035-008-8043-y. [DOI] [PubMed] [Google Scholar]
- Farina C, Aloisi F, Meinl E. Astrocytes are active players in cerebral innate immunity. Trends Immunol. 2007;28:138–145. doi: 10.1016/j.it.2007.01.005. [DOI] [PubMed] [Google Scholar]
- Feldman JL, Mitchell GS, Nattie EE. Breathing: rhythmicity, plasticity, chemosensitivity. Annu Rev Neurosci. 2003;26:239–266. doi: 10.1146/annurev.neuro.26.041002.131103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fenton MJ, Golenbock DT. LPS-binding proteins and receptors. J Leukoc Biol. 1998;64:25–32. doi: 10.1002/jlb.64.1.25. [DOI] [PubMed] [Google Scholar]
- Fernandez R, Gonzalez S, Rey S, Cortes PP, Maisey KR, Reyes EP, Larrain C, Zapata P. Lipopolysaccharide-induced carotid body inflammation in cats: functional manifestations, histopathology and involvement of tumour necrosis factor-alpha. Exp Physiol. 2008;93:892–907. doi: 10.1113/expphysiol.2008.041152. [DOI] [PubMed] [Google Scholar]
- Ge X, Yang Z, Duan L, Rao Z. Evidence for involvement of the neural pathway containing the peripheral vagus nerve, medullary visceral zone and central amygdaloid nucleus in neuroimmunomodulation. Brain Res. 2001;914:149–158. doi: 10.1016/s0006-8993(01)02789-5. [DOI] [PubMed] [Google Scholar]
- Goehler LE, Gaykema RPA, Nguyen KT, Lee JE, Tilders FJH, Maier SF, Watkins LR. Interleukin-1beta in Immune Cells of the Abdominal Vagus Nerve: a Link between the Immune and Nervous Systems? J Neurosci. 1999;19:2799–2806. doi: 10.1523/JNEUROSCI.19-07-02799.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goshen I, Kreisel T, Ounallah-Saad H, Renbaum P, Zalzstein Y, Ben-Hur T, Levy-Lahad E, Yirmiya R. A dual role for interleukin-1 in hippocampal-dependent memory processes. Psychoneuroendocrinology. 2007;32:1106–1115. doi: 10.1016/j.psyneuen.2007.09.004. [DOI] [PubMed] [Google Scholar]
- Gosselin LE, Barkley JE, Spencer MJ, McCormick KM, Farkas GA. Ventilatory dysfunction in mdx mice: impact of tumor necrosis factor-alpha deletion. Muscle Nerve. 2003;28:336–343. doi: 10.1002/mus.10431. [DOI] [PubMed] [Google Scholar]
- Gozal D. Sleep, sleep disorders and inflammation in children. Sleep Med. 2009;10(Suppl 1):S12–16. doi: 10.1016/j.sleep.2009.07.003. [DOI] [PubMed] [Google Scholar]
- Gozal D, Kheirandish-Gozal L. Cardiovascular Morbidity in Obstructive Sleep Apnea: Oxidative Stress, Inflammation, and Much More. Am J Respir Crit Care Med. 2008;177:369–375. doi: 10.1164/rccm.200608-1190PP. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Graeber MB. Changing Face of Microglia. Science. 2010;330:783–788. doi: 10.1126/science.1190929. [DOI] [PubMed] [Google Scholar]
- Grass D, Pawlowski PG, Hirrlinger J, Papadopoulos N, Richter DW, Kirchhoff F, Hulsmann S. Diversity of functional astroglial properties in the respiratory network. J Neurosci. 2004;24:1358–1365. doi: 10.1523/JNEUROSCI.4022-03.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Griffiths MR, Gasque P, Neal JW. The multiple roles of the innate immune system in the regulation of apoptosis and inflammation in the brain. J Neuropathol Exp Neurol. 2009;68:217–226. doi: 10.1097/NEN.0b013e3181996688. [DOI] [PubMed] [Google Scholar]
- Haertel K, Schnell C, Hulsmann S. Astrocytic calcium signals induced by neuromodulators via functional metabotropic receptors in the ventral respiratory group of neonatal mice. Glia. 2009;57:815–827. doi: 10.1002/glia.20808. [DOI] [PubMed] [Google Scholar]
- Hennigan A, Trotter C, Kelly AM. Lipopolysaccharide impairs long-term potentiation and recognition memory and increases p75NTR expression in the rat dentate gyrus. Brain Res. 2007;1130:158–166. doi: 10.1016/j.brainres.2006.10.066. [DOI] [PubMed] [Google Scholar]
- Hulsmann S, Oku Y, Zhang W, Richter DW. Metabolic coupling between glia and neurons is necessary for maintaining respiratory activity in transverse medullary slices of neonatal mouse. Eur J Neurosci. 2000;12:856–862. doi: 10.1046/j.1460-9568.2000.00973.x. [DOI] [PubMed] [Google Scholar]
- Hussain SN. Respiratory muscle dysfunction in sepsis. Mol Cell Biochem. 1998;179:125–134. doi: 10.1023/a:1006864021783. [DOI] [PubMed] [Google Scholar]
- Huxtable AG, Zwicker JD, Alvares TS, Ruangkittisakul A, Fang X, Hahn LB, Posse de Chaves E, Baker GB, Ballanyi K, Funk GD. Glia contribute to the purinergic modulation of inspiratory rhythm-generating networks. J Neurosci. 2010;30:3947–3958. doi: 10.1523/JNEUROSCI.6027-09.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Inancli HM, Enoz M. Obstructive sleep apnea syndrome and upper airway inflammation. Recent Pat Inflamm Allergy Drug Discov. 2010;4:54–57. doi: 10.2174/187221310789895568. [DOI] [PubMed] [Google Scholar]
- Inoue K. ATP receptors of microglia involved in pain. Novartis Found Symp. 2006;276:263–272. discussion 273-281. [PubMed] [Google Scholar]
- Iturriaga R, Moya EA, Del Rio R. Carotid body potentiation induced by intermittent hypoxia: implications for cardiorespiratory changes induced by sleep apnoea. Clin Exp Pharmacol Physiol. 2009;36:1197–1204. doi: 10.1111/j.1440-1681.2009.05213.x. [DOI] [PubMed] [Google Scholar]
- Johann S, Kampmann E, Denecke B, Arnold S, Kipp M, Mey J, Beyer C. Expression of enzymes involved in the prostanoid metabolism by cortical astrocytes after LPS-induced inflammation. J Mol Neurosci. 2008;34:177–185. doi: 10.1007/s12031-007-9028-4. [DOI] [PubMed] [Google Scholar]
- Kaur G, Han SJ, Yang I, Crane C. Microglia and central nervous system immunity. Neurosurg Clin N Am. 2010;21:43–51. doi: 10.1016/j.nec.2009.08.009. [DOI] [PubMed] [Google Scholar]
- Kim HS, Suh YH. Minocycline and neurodegenerative diseases. Behav Brain Res. 2009;196:168–179. doi: 10.1016/j.bbr.2008.09.040. [DOI] [PubMed] [Google Scholar]
- Kimoff RJ, Hamid Q, Divangahi M, Hussain S, Bao W, Naor N, Payne RJ, Ariyarajah A, Mulrain K, Petrof BJ. Increased upper airway cytokines and oxidative stress in severe obstructive sleep apnoea. Eur Respir J. 2010 doi: 10.1183/09031936.00048610. [DOI] [PubMed] [Google Scholar]
- Kreutzberg GW. Microglia: a sensor for pathological events in the CNS. Trends Neurosci. 1996;19:312–318. doi: 10.1016/0166-2236(96)10049-7. [DOI] [PubMed] [Google Scholar]
- Ladino J, Bancalari E, Suguihara C. Ventilatory response to hypoxia during endotoxemia in young rats: role of nitric oxide. Pediatr Res. 2007;62:134–138. doi: 10.1203/PDR.0b013e318098721a. [DOI] [PubMed] [Google Scholar]
- Laffey JG, Tanaka M, Engelberts D, Luo X, Yuan S, Tanswell AK, Post M, Lindsay T, Kavanagh BP. Therapeutic hypercapnia reduces pulmonary and systemic injury following in vivo lung reperfusion. Am J Respir Crit Care Med. 2000;162:2287–2294. doi: 10.1164/ajrccm.162.6.2003066. [DOI] [PubMed] [Google Scholar]
- Laflamme N, Lacroix S, Rivest S. An Essential Role of Interleukin-1beta in Mediating NF-kappa B Activity and COX-2 Transcription in Cells of the Blood-Brain Barrier in Response to a Systemic and Localized Inflammation But Not During Endotoxemia. J Neurosci. 1999;19:10923–10930. doi: 10.1523/JNEUROSCI.19-24-10923.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lahiri S, Forster RE., 2nd CO2/H(+) sensing: peripheral and central chemoreception. Int J Biochem Cell Biol. 2003;35:1413–1435. doi: 10.1016/s1357-2725(03)00050-5. [DOI] [PubMed] [Google Scholar]
- Lai CJ, Ho CY, Kou YR. Activation of lung vagal sensory receptors by circulatory endotoxin in rats. Life Sci. 2002;70:2125–2138. doi: 10.1016/s0024-3205(01)01537-5. [DOI] [PubMed] [Google Scholar]
- Latremoliere A, Woolf CJ. Central sensitization: a generator of pain hypersensitivity by central neural plasticity. J Pain. 2009;10:895–926. doi: 10.1016/j.jpain.2009.06.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lehnardt S. Innate immunity and neuroinflammation in the CNS: the role of microglia in Toll-like receptor-mediated neuronal injury. Glia. 2010;58:253–263. doi: 10.1002/glia.20928. [DOI] [PubMed] [Google Scholar]
- Lehnardt S, Schott E, Trimbuch T, Laubisch D, Krueger C, Wulczyn G, Nitsch R, Weber JR. A vicious cycle involving release of heat shock protein 60 from injured cells and activation of toll-like receptor 4 mediates neurodegeneration in the CNS. J Neurosci. 2008;28:2320–2331. doi: 10.1523/JNEUROSCI.4760-07.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li AM, Quan Y, Guo YP, Li WZ, Cui XG. Effects of therapeutic hypercapnia on inflammation and apoptosis after hepatic ischemia-reperfusion injury in rats. Chin Med J (Engl) 2010;123:2254–2258. [PubMed] [Google Scholar]
- Li RC, Row BW, Gozal E, Kheirandish L, Fan Q, Brittian KR, Guo SZ, Sachleben LR, Jr., Gozal D. Cyclooxygenase 2 and Intermittent Hypoxia-induced Spatial Deficits in the Rat. Am J Respir Crit Care Med. 2003;168:469–475. doi: 10.1164/rccm.200211-1264OC. [DOI] [PubMed] [Google Scholar]
- Li RC, Row BW, Kheirandish L, Brittian KR, Gozal E, Guo SZ, Sachleben LR, Gozal D. Nitric oxide synthase and intermittent hypoxia-induced spatial learning deficits in the rat. Neurobiology of Disease. 2004;17:44–53. doi: 10.1016/j.nbd.2004.05.006. [DOI] [PubMed] [Google Scholar]
- Li X, Stark GR. NFkappaB-dependent signaling pathways. Exp Hematol. 2002;30:285–296. doi: 10.1016/s0301-472x(02)00777-4. [DOI] [PubMed] [Google Scholar]
- Ling L, Fuller DD, Bach KB, Kinkead R, Olson EB, Jr., Mitchell GS. Chronic intermittent hypoxia elicits serotonin-dependent plasticity in the central neural control of breathing. J Neurosci. 2001;21:5381–5388. doi: 10.1523/JNEUROSCI.21-14-05381.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lu Y-C, Yeh W-C, Ohashi PS. LPS/TLR4 signal transduction pathway. Cytokine. 2008;42:145–151. doi: 10.1016/j.cyto.2008.01.006. [DOI] [PubMed] [Google Scholar]
- Lund S, Christensen KV, Hedtjarn M, Mortensen AL, Hagberg H, Falsig J, Hasseldam H, Schrattenholz A, Porzgen P, Leist M. The dynamics of the LPS triggered inflammatory response of murine microglia under different culture and in vivo conditions. J Neuroimmunol. 2006;180:71–87. doi: 10.1016/j.jneuroim.2006.07.007. [DOI] [PubMed] [Google Scholar]
- MacFarlane PM, Mitchell GS. Respiratory long-term facilitation following intermittent hypoxia requires reactive oxygen species formation. Neuroscience. 2008;152:189–197. doi: 10.1016/j.neuroscience.2007.12.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MacFarlane PM, Wilkerson JE, Lovett-Barr MR, Mitchell GS. Reactive oxygen species and respiratory plasticity following intermittent hypoxia. Respir Physiol Neurobiol. 2008;164:263–271. doi: 10.1016/j.resp.2008.07.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mahamed S, Mitchell GS. Is there a link between intermittent hypoxia-induced respiratory plasticity and obstructive sleep apnoea? Exp Physiol. 2007;92:27–37. doi: 10.1113/expphysiol.2006.033720. [DOI] [PubMed] [Google Scholar]
- Maier SF, Goehler LE, Fleshner M, Watkins LR. The Role of the Vagus Nerve in Cytokine-to-Brain Communication. Annals of the New York Academy of Sciences. 1998;840:289–300. doi: 10.1111/j.1749-6632.1998.tb09569.x. [DOI] [PubMed] [Google Scholar]
- Malen PL, Chapman PF. Nitric oxide facilitates long-term potentiation, but not long-term depression. J Neurosci. 1997;17:2645–2651. doi: 10.1523/JNEUROSCI.17-07-02645.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marcus CL, Smith RJ, Mankarious LA, Arens R, Mitchell GS, Elluru RG, Forte V, Goudy S, Jabs EW, Kane AA, Katz E, Paydarfar D, Pereira K, Reeves RH, Richtsmeier JT, Ruiz RL, Thach BT, Tunkel DE, Whitsett JA, Wootton D, Blaisdell CJ. Developmental aspects of the upper airway: report from an NHLBI Workshop, March 5-6, 2009. Proc Am Thorac Soc. 2009;6:513–520. doi: 10.1513/pats.200905-024CB. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mateika JH, Sandhu KS. Experimental protocols and preparations to study respiratory long term facilitation. Respir Physiol Neurobiol. 2011;176:1–11. doi: 10.1016/j.resp.2011.01.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McDeigan GE, Ladino J, Hehre D, Devia C, Bancalari E, Suguihara C. The effect of Escherichia coli endotoxin infusion on the ventilatory response to hypoxia in unanesthetized newborn piglets. Pediatr Res. 2003;53:950–955. doi: 10.1203/01.PDR.0000064581.94126.1C. [DOI] [PubMed] [Google Scholar]
- McDonald VM, Simpson JL, Higgins I, Gibson PG. Multidimensional assessment of older people with asthma and COPD: clinical management and health status. Age Ageing. 2011;40:42–49. doi: 10.1093/ageing/afq134. [DOI] [PubMed] [Google Scholar]
- McNicholas WT. Obstructive sleep apnea and inflammation. Prog Cardiovasc Dis. 2009;51:392–399. doi: 10.1016/j.pcad.2008.10.005. [DOI] [PubMed] [Google Scholar]
- Mika J. Modulation of microglia can attenuate neuropathic pain symptoms and enhance morphine effectiveness. Pharmacol Rep. 2008;60:297–307. [PubMed] [Google Scholar]
- Miller RJ, Jung H, Bhangoo SK, White FA. Cytokine and chemokine regulation of sensory neuron function. Handb Exp Pharmacol. 2009:417–449. doi: 10.1007/978-3-540-79090-7_12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mishra BB, Mishra PK, Teale JM. Expression and distribution of Toll-like receptors in the brain during murine neurocysticercosis. J Neuroimmunol. 2006;181:46–56. doi: 10.1016/j.jneuroim.2006.07.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mitchell GS. Respiratory plasticity following intermittent hypoxia: a guide for novel therapeutic approaches to ventilatory control disorders. In: Gaultier C, editor. Genetic Basis for Respiratory Control Disorders. Springer Publishing Company; New York: 2007. pp. 291–311. [Google Scholar]
- Mitchell GS, Baker-Herman TL, McCrimmon DR, Feldman JL. Encyclopedia of Neuroscience. Vol. 8. Oxford Academic Press; 2009. pp. 121–130. [Google Scholar]
- Mitchell GS, Johnson SM. Neuroplasticity in respiratory motor control. J Appl Physiol. 2003;94:358–374. doi: 10.1152/japplphysiol.00523.2002. [DOI] [PubMed] [Google Scholar]
- Nam NH. Naturally occurring NF-kappaB inhibitors. Mini Rev Med Chem. 2006;6:945–951. doi: 10.2174/138955706777934937. [DOI] [PubMed] [Google Scholar]
- Natoli G, Ghisletti S, Barozzi I. The genomic landscapes of inflammation. Genes Dev. 2011;25:101–106. doi: 10.1101/gad.2018811. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nattie EE. Central chemosensitivity, sleep, and wakefulness. Respir Physiol. 2001;129:257–268. doi: 10.1016/s0034-5687(01)00295-x. [DOI] [PubMed] [Google Scholar]
- Nikodemova M, Duncan ID, Watters JJ. Minocycline exerts inhibitory effects on multiple mitogen-activated protein kinases and IkappaB alpha degradation in a stimulus-specific manner in microglia. Journal of Neurochemistry. 2006;96:314–323. doi: 10.1111/j.1471-4159.2005.03520.x. [DOI] [PubMed] [Google Scholar]
- O'Croinin D, Ni Chonghaile M, Higgins B, Laffey JG. Bench-to-bedside review: Permissive hypercapnia. Crit Care. 2005;9:51–59. doi: 10.1186/cc2918. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oglesby IK, McElvaney NG, Greene CM. MicroRNAs in inflammatory lung disease--master regulators or target practice? Respir Res. 2010;11:148. doi: 10.1186/1465-9921-11-148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ohashi K, Burkart V, Flohe S, Kolb H. Cutting edge: heat shock protein 60 is a putative endogenous ligand of the toll-like receptor-4 complex. J Immunol. 2000;164:558–561. doi: 10.4049/jimmunol.164.2.558. [DOI] [PubMed] [Google Scholar]
- Okun E, Griffioen KJ, Lathia JD, Tang SC, Mattson MP, Arumugam TV. Toll-like receptors in neurodegeneration. Brain Res Rev. 2009;59:278–292. doi: 10.1016/j.brainresrev.2008.09.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parkhurst CN, Gan WB. Microglia dynamics and function in the CNS. Curr Opin Neurobiol. 2010;20:595–600. doi: 10.1016/j.conb.2010.07.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pollmacher T, Haack M, Schuld A, Reichenberg A, Yirmiya R. Low levels of circulating inflammatory cytokines--do they affect human brain functions? Brain Behav Immun. 2002;16:525–532. doi: 10.1016/s0889-1591(02)00004-1. [DOI] [PubMed] [Google Scholar]
- Poltorak A, He X, Smirnova I, Liu M-Y, Huffel CV, Du X, Birdwell D, Alejos E, Silva M, Galanos C, Freudenberg M, Ricciardi-Castagnoli P, Layton B, Beutler B. Defective LPS Signaling in C3H/HeJ and C57BL/10ScCr Mice: Mutations in Tlr4 Gene. Science. 1998;282:2085–2088. doi: 10.1126/science.282.5396.2085. [DOI] [PubMed] [Google Scholar]
- Powell FL, Milsom WK, Mitchell GS. Time domains of the hypoxic ventilatory response. Respir Physiol. 1998;112:123–134. doi: 10.1016/s0034-5687(98)00026-7. [DOI] [PubMed] [Google Scholar]
- Price TJ, Cervero F, de Koninck Y. Role of cation-chloride-cotransporters (CCC) in pain and hyperalgesia. Curr Top Med Chem. 2005;5:547–555. doi: 10.2174/1568026054367629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Qin L, Wu X, Block ML, Liu Y, Breese GR, Hong J-S, Knapp DJ, Crews FT. Systemic LPS causes chronic neuroinflammation and progressive neurodegeneration. Glia. 2007;55:453–462. doi: 10.1002/glia.20467. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Raivich G. Like cops on the beat: the active role of resting microglia. Trends Neurosci. 2005;28:571–573. doi: 10.1016/j.tins.2005.09.001. [DOI] [PubMed] [Google Scholar]
- Ricciardolo FL, Sterk PJ, Gaston B, Folkerts G. Nitric oxide in health and disease of the respiratory system. Physiol Rev. 2004;84:731–765. doi: 10.1152/physrev.00034.2003. [DOI] [PubMed] [Google Scholar]
- Rivest S. How circulating cytokines trigger the neural circuits that control the hypothalamic-pituitary-adrenal axis. Psychoneuroendocrinology. 2001;26:761–788. doi: 10.1016/s0306-4530(01)00064-6. [DOI] [PubMed] [Google Scholar]
- Rivest S. Regulation of innate immune responses in the brain. Nat Rev Immunol. 2009;9:429–439. doi: 10.1038/nri2565. [DOI] [PubMed] [Google Scholar]
- Roth J, De Souza GE. Fever induction pathways: evidence from responses to systemic or local cytokine formation. Braz J Med Biol Res. 2001;34:301–314. doi: 10.1590/s0100-879x2001000300003. [DOI] [PubMed] [Google Scholar]
- Row BW, Liu R, Xu W, Kheirandish L, Gozal D. Intermittent Hypoxia Is Associated with Oxidative Stress and Spatial Learning Deficits in the Rat. Am J Respir Crit Care Med. 2003;167:1548–1553. doi: 10.1164/rccm.200209-1050OC. [DOI] [PubMed] [Google Scholar]
- Ryan S, Taylor CT, McNicholas WT. Systemic inflammation: a key factor in the pathogenesis of cardiovascular complications in obstructive sleep apnoea syndrome? Thorax. 2009;64:631–636. doi: 10.1136/thx.2008.105577. [DOI] [PubMed] [Google Scholar]
- Schnydrig S, Korner L, Landweer S, Ernst B, Walker G, Otten U, Kunz D. Peripheral lipopolysaccharide administration transiently affects expression of brain-derived neurotrophic factor, corticotropin and proopiomelanocortin in mouse brain. Neurosci Lett. 2007;429:69–73. doi: 10.1016/j.neulet.2007.09.067. [DOI] [PubMed] [Google Scholar]
- Sell KM, Crowe SF, Kent S. Lipopolysaccharide induces memory-processing deficits in day-old chicks. Pharmacol Biochem Behav. 2001;68:497–502. doi: 10.1016/s0091-3057(01)00463-4. [DOI] [PubMed] [Google Scholar]
- Shaw KN, Commins S, O'Mara SM. Lipopolysaccharide causes deficits in spatial learning in the watermaze but not in BDNF expression in the rat dentate gyrus. Behav Brain Res. 2001;124:47–54. doi: 10.1016/s0166-4328(01)00232-7. [DOI] [PubMed] [Google Scholar]
- Shaw KN, Commins S, O'Mara SM. Cyclooxygenase inhibition attenuates endotoxin-induced spatial learning deficits, but not an endotoxin-induced blockade of long-term potentiation. Brain Res. 2005;1038:231–237. doi: 10.1016/j.brainres.2005.01.035. [DOI] [PubMed] [Google Scholar]
- Singh AK, Jiang Y. How does peripheral lipopolysaccharide induce gene expression in the brain of rats? Toxicology. 2004;201:197–207. doi: 10.1016/j.tox.2004.04.015. [DOI] [PubMed] [Google Scholar]
- Stockley RA. Progression of chronic obstructive pulmonary disease: impact of inflammation, comorbidities and therapeutic intervention. Curr Med Res Opin. 2009;25:1235–1245. doi: 10.1185/03007990902868971. [DOI] [PubMed] [Google Scholar]
- Taylor CT, Cummins EP. Regulation of gene expression by carbon dioxide. J Physiol. 2011 doi: 10.1113/jphysiol.2010.201467. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Teeling JL, Perry VH. Systemic infection and inflammation in acute CNS injury and chronic neurodegeneration: underlying mechanisms. Neuroscience. 2009;158:1062–1073. doi: 10.1016/j.neuroscience.2008.07.031. [DOI] [PubMed] [Google Scholar]
- Thomson LM, Sutherland RJ. Systemic administration of lipopolysaccharide and interleukin-1beta have different effects on memory consolidation. Brain Res Bull. 2005;67:24–29. doi: 10.1016/j.brainresbull.2005.05.024. [DOI] [PubMed] [Google Scholar]
- Trang T, Beggs S, Salter MW. Purinoceptors in microglia and neuropathic pain. Pflugers Arch. 2006;452:645–652. doi: 10.1007/s00424-006-0074-5. [DOI] [PubMed] [Google Scholar]
- Triantafilou M, Triantafilou K. Lipopolysaccharide recognition: CD14, TLRs and the LPS-activation cluster. Trends in Immunology. 2002;23:301–304. doi: 10.1016/s1471-4906(02)02233-0. [DOI] [PubMed] [Google Scholar]
- Vereker E, Campbell V, Roche E, McEntee E, Lynch MA. Lipopolysaccharide inhibits long term potentiation in the rat dentate gyrus by activating caspase-1. J Biol Chem. 2000;275:26252–26258. doi: 10.1074/jbc.M002226200. [DOI] [PubMed] [Google Scholar]
- Vichaya EG, Baumbauer KM, Carcoba LM, Grau JW, Meagher MW. Spinal glia modulate both adaptive and pathological processes. Brain Behav Immun. 2009;23:969–976. doi: 10.1016/j.bbi.2009.05.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vinit S, Lovett-Barr MR, Mitchell GS. Intermittent hypoxia induces functional recovery following cervical spinal injury. Respir Physiol Neurobiol. 2009;169:210–217. doi: 10.1016/j.resp.2009.07.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vinit S, Windelborn JA, Mitchell GS. Lipopolysaccharide attenuates phrenic long-term facilitation following acute intermittent hypoxia. Respir Physiol Neurobiol. 2011 doi: 10.1016/j.resp.2011.02.008. PMID: 21334467. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vivier E, Raulet DH, Moretta A, Caligiuri MA, Zitvogel L, Lanier LL, Yokoyama WM, Ugolini S. Innate or adaptive immunity? The example of natural killer cells. Science. 2011;331:44–49. doi: 10.1126/science.1198687. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Whitney NP, Eidem TM, Peng H, Huang Y, Zheng JC. Inflammation mediates varying effects in neurogenesis: relevance to the pathogenesis of brain injury and neurodegenerative disorders. J Neurochem. 2009;108:1343–1359. doi: 10.1111/j.1471-4159.2009.05886.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wieczorek M, Swiergiel AH, Pournajafi-Nazarloo H, Dunn AJ. Physiological and behavioral responses to interleukin-1beta and LPS in vagotomized mice. Physiol Behav. 2005;85:500–511. doi: 10.1016/j.physbeh.2005.05.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilkerson JE, Satriotomo I, Baker-Herman TL, Watters JJ, Mitchell GS. Okadaic acid-sensitive protein phosphatases constrain phrenic long-term facilitation after sustained hypoxia. J Neurosci. 2008;28:2949–2958. doi: 10.1523/JNEUROSCI.5539-07.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wills-Karp M. Immunologic basis of antigen-induced airway hyperresponsiveness. Annu Rev Immunol. 1999;17:255–281. doi: 10.1146/annurev.immunol.17.1.255. [DOI] [PubMed] [Google Scholar]
- Woolf CJ, Salter MW. Neuronal plasticity: increasing the gain in pain. Science. 2000;288:1765–1769. doi: 10.1126/science.288.5472.1765. [DOI] [PubMed] [Google Scholar]
- Xu W, Chi L, Row BW, Xu R, Ke Y, Xu B, Luo C, Kheirandish L, Gozal D, Liu R. Increased oxidative stress is associated with chronic intermittent hypoxia-mediated brain cortical neuronal cell apoptosis in a mouse model of sleep apnea. Neuroscience. 2004;126:313–323. doi: 10.1016/j.neuroscience.2004.03.055. [DOI] [PubMed] [Google Scholar]
- Zhuo M, Small SA, Kandel ER, Hawkins RD. Nitric oxide and carbon monoxide produce activity-dependent long-term synaptic enhancement in hippocampus. Science. 1993;260:1946–1950. doi: 10.1126/science.8100368. [DOI] [PubMed] [Google Scholar]