Abstract
To date, a number of studies have documented the toxic impacts of Al ions in plant cells. One of the key factors required for Al cytotoxicity is the generation of reactive oxygen species (ROS). Here we observed that Al treatments of suspension-cultured Arabidopsis thaliana cells resulted in biphasic superoxide generation monitored with chemiluminescence. Among six respiratory burst oxidase homologs (Atrbohs) coding for plant NADPH oxidase, AtrbohD was shown to be the only gene responsive to Al. As the expression of AtrbohD was rapid and long-lasting (1 min to 24 h). Al-induced superoxide generation, AtrbohD expression and cell death were all inhibited by NADPH oxidase inhibitor and superoxide dismutase. Interestingly, Al-induced AtrbohD expression and cell death were inhibited in the mutant and transgenic cell lines lacking salicylic acid biosyhthesis and accumulation (sid2 and NahG). Involvements of salicylic acid signaling in Al-induced AtrbohD expression and cell death development were also confirmed by the use of npr1 mutant cells and NPR1-overexpressing cells. Taken together, there would be a loop of SA signaling and SA-dependent expression of AtrbohD gene leading to prolonged ROS production and cell death development in the Al-exposed Arabidopsis cells.
Key words: Al toxicity, Arabidopsis, AtrbohD, ROS, salicylic acid
To date, a number of studies have documented the toxic impacts of Al ions on roots,1 hypocotyls2 and germinating pollens.3 It has been proposed that early effects of Al toxicity at the root apex, such as those on cell division, cell extension or nutrient transport, involve the direct intervention of Al on cell function.4 In addition to intact plants, the cell suspension cultures derived from model plant species such as tobacco (Nicotiana tabacum) have been frequently employed for elucdating the molecular component involved in the mechanism of metal toxicity5,6 and Al phytotoxicity.7,8
One key factor required for Al cytotoxicity is the generation of reactive oxygen species as observed in various materials.9 We found that Al3+ and other trivalent cations added to tobacco cells trigger the apoplastic generation of cytotoxic superoxide (O2•−).5,10 While earlier studies suggested the involvement of mitochondrial oxidative burst as the source of Al-induced ROS,11 our data were indicative of the involvement of NADPH oxidases (plant respiratory burst oxidase homologs, rbohs), since the Al-induced O2•− has been shown to be sensitive to an inhibitor of NADPH oxidase.10,12 Therefore, involvement of the rbohs in acute O2•− generation induced by Al3+ has been suggested10 by analogy to previously reported phenomena with various trivalent cations such as lanthanide ions (Ln3+).5 Despite pharmocological implications, no molecular biological data on Al-responsive rbohs has been reported to date.
In many occasions in plant responses to biotic and abiotic stresses including plant-microbe interactions, the involvement of rbohs in burst of ROS production has been documented.13 It has been suggested that plant cells naturally respond to a defense-related hormonal molecule, salicylic acid (SA), by inducing the rboh-dependent oxidative burst.14 Such rboh-dependent bursts in ROS production induced by SA is likely a late response that follows the earlier events involving apoplastic peroxidase-dependent acute O2•− generation.15,16
Here we show the molecular genetic evidence that Al treatment results in expression of an NADPH oxidase-coding gene known as AtrbohD, through SA signal transduction pathways, by employing the cell lines derived from Arabidopsis thaliana mutants lacking SA synthesis or SA-dependent signaling factors.
Suspension-cultured cells of Arabidopsis thaliana with ecotype Columbia backgroud namely wildtype (cell line, Col-0), mutants (cell lines, sid2 and npr1) and transgenic cell lines (cell lines, NahG, overexpressing bacterial SA hydroxylase; NPR-Ox, overexpressing NPR1 gene) were used for assessing the impact of Al treatement.
These cell lines were propagated in MS liquid medium (pH- 5.8) containing 30 mg/ml sucrose, 400 µg/ml myo-inositol, 4 µg/ml nicotinic acid, 4 µg/ml pyridoxine-HCl, 40 µg/ml thiamine-HCl and 0.2 µg/ml of 2,4-dichlorophenoxyacetic acid at 23°C with shaking on a gyratory shaker in darkness and sub-cultured once a week with a 5% (v/v) inoculum. The cells harvested three days after subculturing were used for experiments.
For Al treatment, AlCl3 was first dissolved in water and diluted with the same volume of 2x culture medium supplemented with 40 mM K-phosphate buffer (pH 5.8). To compare the effect of metal salts at the physiologically normal pH, the cells were suspended in the culture medium supplemented with K-phosphate buffer (pH 5.8) and incubated for at least half an hour prior to addition of AlCl3. Then the cell suspension (0.2 ml) was added with solutions (0.2 ml) of AlCl3.
Generation of O2•− in cell suspension culture was monitored by chemiluminescence of Cypridina luciferin analog as described previously in reference 15. The O2•−-specific chemiluminescence was measured with a luminometer (Luminescensor PSN AB-2200-R, Atto, Tokyo) and expressed as “rlu.”
Al-induced cell death in the cell suspension culture was determined by staining the dead cells with Evans blue (0.1%, w/v) by mixing and incubating the cells and the dye for 30 min. Unless indicated, 1 h of post-Al incubation was employed prior to addition of the dye to the cells. Then stained cells were observed under microscopes (SMZ800 and Labophoto, Nikon, Tokyo, Japan; VHX-100, Keyence, Tokyo, Japan). For statistic analysis, 3–4 different digital images of cells under the microscope (each covering 50–100 cells to be counted) were acquired and stained cells were counted.
RNA extraction was generally performed by using phenol/SDS and LiCl. The treated cell (0.1 g) was frozen in liquid N2 and ground using pestle and mortar. The ground cell was transfered to a 1.5 ml plastic tube and added with 0.3 ml of extraction buffer (100 mM Tris-HCl, pH 8, 100 mM EDTA, 100 mM LiCl, 1% SDS), 0.3 ml of phenol and 0.3 ml CIA (chloroform:isoamyl alcohol at 24:1). The sample was centrifuged at 14,000x g for 10 min at 4°C. Supernatant was transfered to a new 1.5 ml tube and added with 0.3 ml of phenol and 0.3 ml of CIA. Then the sample was centrifuged at 14,000x g for 10 min at 4°C. The upper phase was again transfered to a new 1.5 ml tube and added with 1/3 volume 10 M LiCl. Then the sample was incubated for 2 h at −30°C and centrifuged at 14,000x g for 30 min at 4°C. The pellet was dissolved in 0.8 ml of 2 M LiCl and centrifuged at 14,000x g for 10 min at 4°C. The resultant pellet was dissolved in 0.4 ml of TE baffer (10 mM Tris-HCl [pH 8.0], 1 mM EDTA [pH 8]) and added with phenol and CIA (each 0.2 ml). The sample was then centrifuged at 14,000x g for 10 min at 4°C. To the upper phase collected in a new tube, 0.2 ml of CIA was added and centrifuged at 14,000x g for 10 min at 4°C. To the upper phase collected in a new tube, 1/10 volume of 3 M sodium acetate (pH 5.2) and 2 volume 100% EtOH were added and mixed. The sample was then centrifuged at 14,000x g for 10 min at 4°C. The obtained pellet was washed with 50 µl of 70% EtOH and centrifuged at 14,000x g for 10 min at 4°C. The pellet was dried and dissolved by 10 µl of diethylpyrocarbonate (DEPC)-treated water.
For RT-PCR, the genomic DNA concomitantly present in the total RNA preparation was digested with Cloned DNase I (RNase-free; TaKaRa BIO). Briefly, digestion was performed for 10 µg of total RNA dissolved in DEPC-treated water (with 5 µl of 10x DNase buffer, 20 units of RNaes Inhibitor, 10 units of DNase I; total vol., 50 µl), by incubating for 30 min at 37°C. Onto the solution, further DEPC-treated water (50 µl) and phenol/chloroform (1:1; 100 µl) were added and mixed. Following centrifugation at 14,000x g for 10 min at 4°C, the upper phase was collected in a new 1.5 ml tube and added with 10 µl of 3 M sodium acetate and 250 µl of 100% EtOH. The sample was again centrifuged at 14,000x g for 10 min at 4°C to collect the pellet (which was washed with 50 µl of 70% EtOH and centrifuged at the same condition). Lastly, the obtained pellet was dried and dissolved in 10 µl of DEPC-treated water.
Prior to RT-PCR, the first-strand cDNA synthesis was performed using 2 µg of total RNA and SuperScript™ III Reverse Transcriptase (Invitrogen, Carlsbad, CA). The reaction mixture in each PCR tube contained 1 µl of 50 mM oligo(dT)20, 4 µl of 10 mM dNTP mix and 2 µg of total RNA in 13 µl of distilled water. The tube was heated at 65°C for 5 min and cooled down at 4°C for 1 min using Program Temp control System PC-320 (ASTEC, Fukuoka, Japan). The solution was added with 4 µl of 5x First-strand synthesis buffer, 1 µl of 0.1 M DTT, 1 µl of RNaseOUT Recombinant RNase Inhibitor and 1 µl of SuperScript II RT (200 units/µl) and heated to 50°C and kept for 60 min. The reaction was terminated by heating at 70°C for 15 min and 4°C for 15 min. The obtaiend cDNA solution was used for subsequent PCR.
PCR was performed by using 60 ng of first-strand cDNA and Takara Ex Taq™ (TAKARA BIO INC.). The primers used were listed in Table 1. For each sample, 30 cycles of PCR were performed with denaturing at 94°C for 1 min, annealing for 1 min and elongation at 72°C for 1 min.
Table 1.
List of primers used for RT-PCR
| Gene studied | Forward primers | Reverse primers |
| Actin2 | CTT ACA ATT TCC CGC TCT GC | GTT GGG ATG AAC CAG AAG GA |
| AtrbohA | CC A GG A GAT GAC TAC CTC | GAC ACG TGT TCC TGA CAC |
| AtrbohB | GG A CTA CG T CG A GAT CAC | GAT ACG ATG TCA ATG CCG |
| AtrbohC | ATT GG A CAC GAG CTC TCA AAG G | GCG ACT TCG TTC ATT ATG TTC |
| AtrbohD | ATG AAA ATG AGA CG A GGC AAT TC | GGA TA C TGA TCA TA G GCG TGG CTC CA |
| AtrbohE | GTT ACT GAG GTC GG A ATC | GTC TTG ATG GTA AGC AGC |
| AtrbohF | CTT CCG ATA TCC TCC AAC CAA CTC | GAG ATT GCC TTT ATA CTA TAA GTG |
Following addition of Al3+, an acute generation of O2•− was observed in wild-type cell line (Col-0). As previously reported for tobacco cells, an increase in chemiluminescence reflecting an acute Al-induced generation of O2•− was also confirmed in Arabidopsis cells. The yield of chemiluminescence was shown to be sensitive to the presence of two O2•− scavengers, Fe(III)-EDTA and Cu, Zn-superoxide dismutase (SOD) and an inhibitor of NADPH oxidase, diphenyleneiodonium chloride (DPI), suggesting the involvement of Al-activated O2•− generating reaction catalyzed by NADPH oxidase (Fig. 1A and B). Since the reaction observed here was immediate following Al treatment, no involvement of gene expression is likely required but pre-existing NADPH oxidase may take place. Interestingly, following above rapid response, the cells showed gradual increase in O2•− generating capacity spending a few hours (Fig. 1C and D). Effect of high Al dose (1 mM) could be observed only in the rapid phase since this concentration of Al was shown to be highly toxic and cell death was induced with time. Therefore we examined the induced expression of NADPH oxidase-coding genes in response to 0.1 mM Al treatment.
Figure 1.
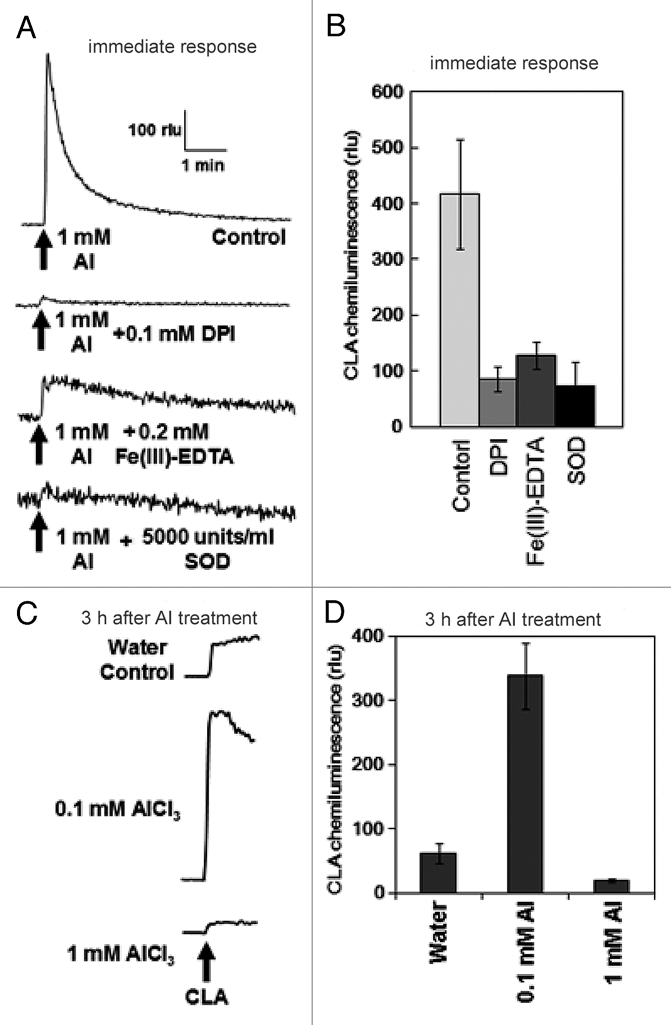
Induction of O2•− generation in Arabidopsis thaliana cell suspension culture by AlCl3. (A,B) Typical traces of AlCl3-induced immediate O2•− generation and effect of inhibitor and scavengers, namely 1 mM diphenyleneiodonium chloride (DPI), 0.2 mM Fe(III)-EDTA and 5,000 units/ml SOD. (C,D) Typical traces of AlCl3-induced increase in CLA-chemiluminescence reflecting the prolonged production of O2•− in Arabidopsis thaliana cells. Error bars reflect the mean and SD, (n = 3).
In Arabidopsis thaliana, six NADPH oxidase-coding genes (designated as AtrbohA-F) are known.17 Among Atrbohs, AtrbohA, B and C are known to be expressed only in the roots, especially in the elongating regions. AtrbohE is reportedly expressed in seeds and roots. AtrbohD and F are known to be expressed systemically in the plants.
Here, expression of Atrbohs was examined in the presence and absence of Al (Fig. 2). Among Atrboh isoforms, only AtrbohD was shown to be responsive to Al treatment. In addition, the expression of AtrbohD was shown to be both rapid (Fig. 2B, upper) and long-lasting (Fig. 2B, lower). It is noteworthy that the Al-induced expression of AtrbohD was shown to be sensitive to treatments with DPI and SOD (Fig. 2C), thus supporting our view that initial immediate oxidative burst catalyzed by Al-activated pre-existing NADPH oxidase triggers the subsequent induction of AtrbohD expression further contributing to the prolonged oxidative burst. Furthermore, timing of inhibitor addition is also important. Addition of DPI at 10 min after Al addition resulted in partial expression of AtrbohD (data not shown), suggesting the importance of the early phase oxidative burst catalyzed by preexisting NADPH oxidase.
Figure 2.
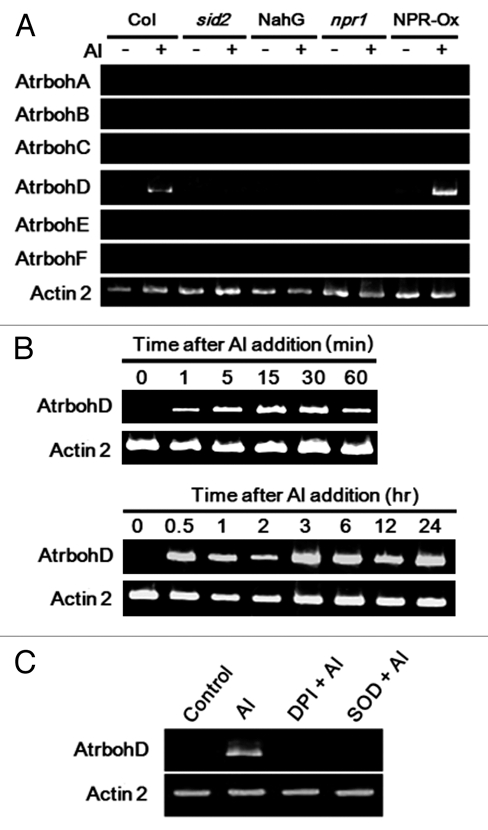
RT -PCR analysis of Al-responsive gene expressions in cell suspension. Typical RT -PCR profile of gene expressions in Al-treated cell suspension is shown. Total RNA was isolated from cell suspension and then RT-PCR was performed. Actin was used as an internal control. (A) Suspension-cultured cells of Arabidopsis thaliana with ecotype Columbia backgroud namely wildtype (cell line, Col-0), mutants (cell lines, sid2 and npr1) and transgenic cell lines (cell lines, NahG, overexpressing bacterial SA hydroxylase; NPR-Ox, overexpressing NPR1 gene) were treated with 0.1 mM AlCl3 for 3 h. (B) Cell suspension was treated with 0.1 mM AlCl3 for assessing the rapid and long-lasting gene expression. Upper, treated with 0.1 mM AlCl3 for 0, 1, 5, 15, 30, 60 min. Lower, treated with 0.1 mM AlCl3 for 0, 1, 2, 3, 6, 12, 24 h. (C) Cell suspension were treated with 1 mM DPI and 5,000 units/ml SOD for 5 min before treatment with 0.1 mM AlCl3 for 3 h.
In order to examine the involvement of SA signaling pathways in the cells of Arabidopsis thaliana, the cell lines lacking SA synthesis (transgenic NahG cell line and sid2 mutant cell line) and the cell lines with altered SA-dependent signaling factors (npr1 mutant cell line and transgenic NPR1-overexpressing cell line, NPR-Ox) were used for comparison. No expression of Atrboh genes could be observed in sid2, NahG and npr1 cells regardless of the presence of Al (Fig. 2A). In contrast, in the NPR-Ox cells, Al-induced expression of AtrbohD was shown to be enhanced while no expression of other Atrboh isoforms were observed. Above data clearly demonstrated that Al-induced AtrbohD expression requires the presence of SA biosynthesis and SA signaling. Taken together, there would be a loop of SA signaling and SA-dependent expression of AtrbohD gene leading to prolonged ROS production in the Al-exposed cells.
One of the likely consequences of Al-induced oxidative burst is induction of cell death. Following Al treatment, increase in cell death was observed (Fig. 3). Addition of NADPH oxidase inhibitor (DPI; Fig. 3A) and O2•−-scavenger (SOD, Fig. 3B) 5 min prior to Al treatment resulted in partial inhibition of Al-induced cell death. However, addition of these chemicals 10 min after Al treatment showed no inhibitory effect (Fig. 3A and B), suggesting that ROS production in the early phase of Al response plays a key role in the Al-dependent induction of cell death.
Figure 3.
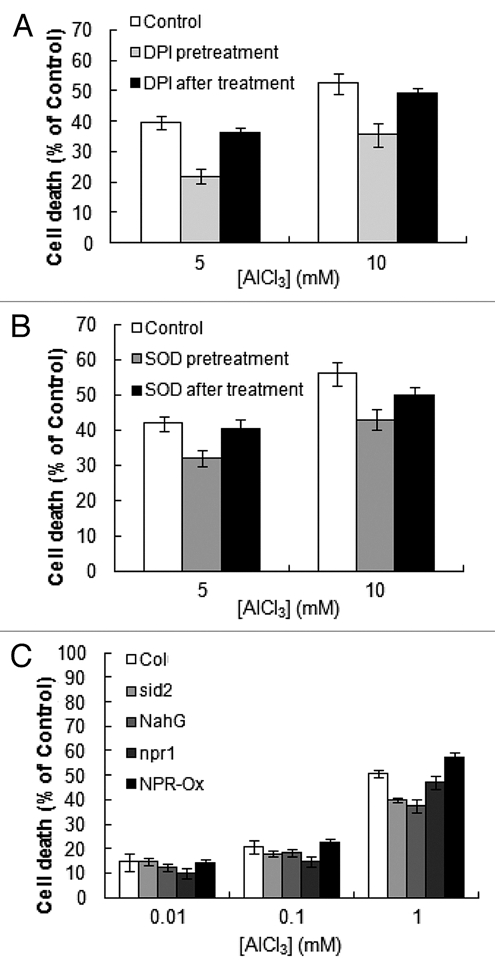
Al-induced cell death in suspension cultured cells. (A) Effect of DPI treatment on the Al-induced cell death. Cell suspension was treated with 1 mM DPI for 5 min before and 5, 10 min after addition of 0.1 mM AlCl3. (B) Effect of SOD treatment on the Al-induced cell death. Cell suspension was treated with 5,000 units/ml SOD for 5 min before and 10 min after addition of 5 and 10 mM AlCl3. (C) Dose-dependent progress of cells death after the Al treatment. Cells suspension were treated with 0.01, 0.1, 1 mM AlCl3 for 6 h. Cell death after 6 h of Al treatment was judged by Evans blue staining under microscopes. Each data point and error bar reflect the mean and SD, respectively (n = 3).
Furthermore, in the sid2 mutant cells and NahG transgenic cells both lacking the accumulation of SA, the level of Al-induced cell death was partially but significantly lower than that in wild-type cells (Fig. 3C), confirming that, at least partially, SA is involved in Al-induced cell death mechanism.
There are number of classical studies suggesting that NADPH oxidase from human neutrophils can be directly activated by metal cations. For instance, binding of monovalent and divalent cations reportedly results in spontaneous increase in the O2•− generating activity of the membrane-bound enzyme.18,19 There is a possibility that cation treatment of plant cells directly activates the O2•−-producing activity of pre-existing NADPH oxidase via similar manners that proposed for human neutrophil enzyme. In the case of Al response, the action of Al could be also indirect, possibly through the action of cytosolic Ca2+ since activation of AtrbohD by Ca2+ has been recently reported in reference 17. In fact, addition of Al to tobacco cells reportedly results in transient increase in cytosolic Ca2+ concentrations via activation of TPC1-type calcium channel.7,10,12 However, alternative mechanisms independent from calcium action are also likely to be involved since the Al-induced oxidative burst (CLA chemiluminescence) apparently precedes the changes in cytosolic Ca2+ concentrations (monitored with aequorin luminescence).10 Thus, the mechanism for immediate action of cations including Al3+,10 and Ln3+,5 on O2•− production can be both direct and indirect. These points must be critically discussed in the future study based on further data.
Lastly, we wish to propose a model for Al-dependent signaling path (Fig. 4). The series of signaling events is initiated by acute ROS production stimulated by Al, subsequently leading to the cell death and other downstream responses, to be mediated through SA biosynthesis and signaling by inducing the expression of rbohD. The SA-dependently activated or induced rbohD may further contribute to the oxidative burst (SA/rboh loop).
Figure 4.
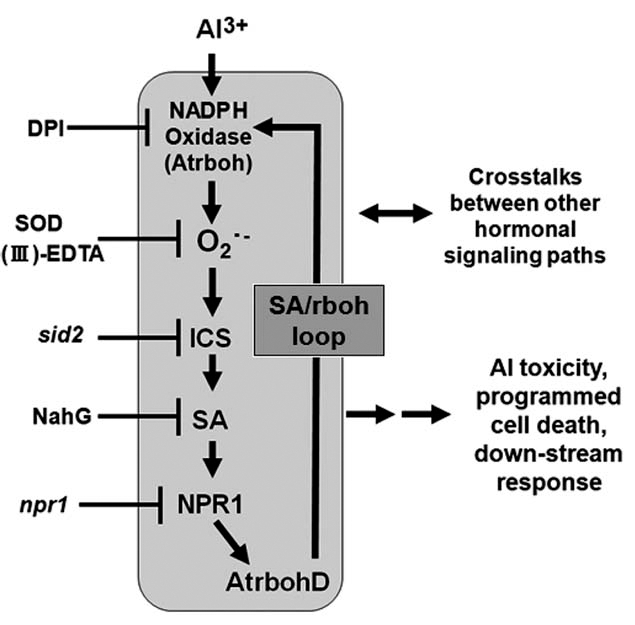
A model for mechanism of Al action in Arabidopsis thaliana cell suspension culture. SA signaling is activated in downstream of ROS. AtrbohD expression requires the NADPH oxidase-mediated oxidative burst and SA-dependent signaling.
Acknowledgements
Dr. T. Kadono is acknowledged for critical reading of our manuscript. This work was supported by a grant of Regional Innovation Cluster Program (Global 3 Type) implemented by Ministry of Education, Culture, Sports, Science and Technology (MEXT), Japan.
Abbreviations
- MS
murashige-skoog (culture medium)
- O2•−
superoxide anion
- rlu
relative luminescence units
- ROS
reactive oxygen species
References
- 1.Ma JF. Role of organic acids in detoxification of aluminum in higher plants. Plant Cell Physiol. 2000;41:383–390. doi: 10.1093/pcp/41.4.383. [DOI] [PubMed] [Google Scholar]
- 2.Ma JF, Yamamoto R, Nevin DJ, Matsumoto H, Brown PH. Al binding in the epidermis cell wall inhibits cell elongation of okra hypocotyl. Plant Cell Physiol. 1999;40:549–556. [Google Scholar]
- 3.Li GM, Qing SF, Zheng QY, Hua LZ, Fu SZ, Da YS. Does aluminum inhibit pollen germination via extracellular calmodulin. Plant Cell Physiol. 2000;41:372–376. doi: 10.1093/pcp/41.3.372. [DOI] [PubMed] [Google Scholar]
- 4.Lazof DB, Goldsmith JG, Rufty TW, Linton RW. Rapid uptake of aluminum into cells of intact soybean root tips. A microanalytical study using secondary ion mass spectrometry. Plant Physiol. 1994;106:1107–1114. doi: 10.1104/pp.106.3.1107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Kawano T, Kawano N, Muto S, Lapeyrie F. Cation-induced superoxide generation in tobacco cell suspension culture is dependent on ion valence. Plant Cell Environ. 2001;24:1235–1241. [Google Scholar]
- 6.Kawano T, Kawano N, Muto S, Lapeyrie F. Retardation and inhibition of the cation-induced superoxide generation in BY-2 tobacco cell suspension culture by Zn2+ and Mn2+ Physiol Plant. 2002;114:395–404. doi: 10.1034/j.1399-3054.2002.1140309.x. [DOI] [PubMed] [Google Scholar]
- 7.Lin C, Yu Y, Kadono T, Iwata M, Umemura K, Furuichi T, et al. Action of aluminum, novel TPC1-type channel inhibitor, against salicylate-induced and cold shock-induced calcium influx in tobacco BY-2 cells. Biochem Biophys Res Commun. 2005;332:823–830. doi: 10.1016/j.bbrc.2005.05.030. [DOI] [PubMed] [Google Scholar]
- 8.Ono K, Yamamoto Y, Hachiya A, Matsumoto H. Synergistic inhibition of growth by aluminium and iron of tobacco (Nicotiana tabacum L.) cells in suspension culture. Plant Cell Physiol. 1995;36:115–125. [Google Scholar]
- 9.Yamamoto Y, Kobayashi Y, Devi SR, Rikiishi S, Matsumoto H. Oxidative stress triggered by aluminum in plant roots. Plant Soil. 2003;255:239–243. [Google Scholar]
- 10.Kawano T, Kadono T, Furuichi T, Muto S, Lapeyrie F. Aluminum-induced distortion in calcium signaling involving oxidative bursts and channel regulations in tobacco BY-2 cells. Biochem Biophys Res Commun. 2003;308:35–42. doi: 10.1016/s0006-291x(03)01286-5. [DOI] [PubMed] [Google Scholar]
- 11.Yamamoto Y, Kobayashi Y, Devi SR, Rikiishi S, Matsumoto H. Aluminum toxicity is associated with mitochondrial dysfunction and the production of reactive oxygen species in plant cells. Plant Physiol. 2002;128:63–72. [PMC free article] [PubMed] [Google Scholar]
- 12.Kawano T, Kadono T, Fumoto K, Lapeyrie F, Kuse M, Isobe M, et al. Aluminum as a specific inhibitor of plant TPC1 Ca2+ channels. Biochem Biophys Res Commun. 2004;324:40–45. doi: 10.1016/j.bbrc.2004.09.015. [DOI] [PubMed] [Google Scholar]
- 13.Yoshioka H, Bouteau F, Kawano T. Discovery of oxidative burst in the field of plant immunity: Looking back at the early pioneering works and towards the future development. Plant Signaling and Behaviors. 2008;3:153–155. doi: 10.4161/psb.3.3.5537. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Yoshioka H, Sugie K, Park HJ, Maeda H, Tsuda N, Kawakita K, et al. Induction of plant gp91phox homolog by fungal cell wall, arachidonic acid and salicylic acid in potato. Mol Plant Microbe Interact. 2001;14:725–736. doi: 10.1094/MPMI.2001.14.6.725. [DOI] [PubMed] [Google Scholar]
- 15.Kawano T, Sahashi N, Takahashi K, Uozumi N, Muto S. Salicylic acid induces extracellular superoxide generation followed by an increase in cytosolic calcium ion in tobacco suspension culture. The earliest events in salicylic acid signal transduction. Plant Cell Physiol. 1998;39:721–730. [Google Scholar]
- 16.Kawano T, Furuichi T, Muso S. Controlled free salicylic acid levels and corresponding signaling mechanisms in plants. Plant Biotechnology. 2004;21:319–335. [Google Scholar]
- 17.Ogasawara Y, Kaya H, Hiraoka G, Yumoto F, Kimura S, Kadota Y, et al. Synergistic Activation of the Arabidopsis NADPH oxidase AtrbohD by Ca2+ and phosphorylation. J Biol Chem. 2008;283:8885–8892. doi: 10.1074/jbc.M708106200. [DOI] [PubMed] [Google Scholar]
- 18.Cross AR, Erichson R, Eliss BA, Curnutte JT. Spontaneous activation of NADPH oxidase in a cell-free system: unexpected multiple effects of magnesium ion concentrations. Biochemical Journal. 1999;338:229–233. [PMC free article] [PubMed] [Google Scholar]
- 19.Suzuki HP, Pabst MJ, Johnston R., Jr Enhancement by Ca2+ or Mg2+ of catalytic activity of the superoxide-producing NADPH oxidase in membrane fractions in human neutrophils and monocytes. J Biol Chem. 1985;260:3635–3639. [PubMed] [Google Scholar]


