Abstract
Low temperature could significantly induce anthocyanin accumulation in the presence of light. Recently, two bZIP transcription factors LONG HYPOCOTYL 5 (HY5) and HOMOLOG OF HY5 (HYH) were identified to play an important role in the process of low temperature-induced anthocyanin accumulation. However, the mechanism by which HY5/HYH regulates anthocyanin accumulation under low temperature still remains unclear. Here, we found that the gibberellins (GAs) could decrease but PAC (endogenous GAs biosynthesis inhibitor) increases the low temperature-induced anthocyanin accumulation, implying that GAs signaling may involve in this process. Furthermore, the transcript level of GA2ox1, encoding a major member of bioactive GAs-deactivating enzymes, was significantly upregulated by low temperature in a HY5/HYH-dependent manner. Moreover, hy5 hyh mutant was insensitive to PAC in enhancing anthocyanin accumulation under low temperature. From these data we propose that, together with HY5/HYH, GA signaling may play an important role during low temperature-induced anthocyanin accumulation.
Key words: anthocyanin, GA, HY5, light, low temperature
Light, as the most influential environmental factor, not only provides the source of energy for plant life, but also acts as a signal affecting plant growth and development throughout the entire life cycle from germination to flowering.1,2 Light perceived by photoreceptors will induce activation of genes in photomorphogenesis as strikingly characterized by short hypocotyls, cotyledon expansion and greening.3 Lots of downstream components of photoreceptors have been identified, such as Long Hypocotyl 5 (HY5) and HY5 Homolog (HYH).4,5 HY5, a bZIP protein, is the first known and most extensively studied transcription factor involved in promoting photomorphogenesis.4 Mutation in HY5 will cause a defect in inhibition of the hypocotyls elongation under all light conditions, suggesting that HY5 acts downstream of all photoreceptors and HY5 is crucial in light signaling.2,4
Phytohormone gibberellins (GAs) control a wide range of processes during plant development, including seed germination, leaf expansion, stem and root elongation, flowering time, and flower and fruit development.6 GA signaling is generally believed to hinge to DELLA proteins, a family of nuclear growth-repressing proteins.7 The production of active GAs is regulated through modulation of biosynthesis and inactivation. GA 2-oxidases (GA2ox) can inactivate most active GAs.6,7 Reduced bioactive GA levels will cause an increase of DELLA proteins.7,8 It has been clearly proven that DELLAs are major components of the adaptively significant mechanism via which light regulates plant growth during photomorphogenesis.9–12 Interestingly, recent reports indicated an important function of DELLAs under environmental stresses. It was found that salt stress partially induces DELLA protein stabilization and accumulation and restricts plant growth.13–15 Moreover, DELLA proteins will accumulate to a relative high level after cold treatment and also will confer higher freezing tolerance in Arabidopsis plants.16
Anthocyanins are often induced by many environmental factors, such as light, low temperature and so on.17 It has been shown that HY5/HYH play an important role during low temperature- induced anthocyanin accumulation.18 However, how HY5/HYH function in this process still remains elusive. Given that GAs are involved in both light singling and low temperature response,9,10,16 whether GA signaling is involved in low temperature-induced anthocyanin accumulation becomes a question to be addressed. Very interestingly, it was found that exogenous GA3 (an active form of GAs) significantly reduced the level of low temperature-induced anthocyanin, whereas PAC conversely increased anthocyanin level (Fig. 1B). As a control, GA3 and PAC only slightly affect the anthocyanin levels at normal temperature (23°C) (Fig. 1A). This result at least suggests that GAs could negatively regulate low temperature-induced anthocyanin accumulation, although it's still unknown whether GAs regulates this process directly or indirectly through deregulation of DELLAs. However, Achard et al. recently indicated that RGA, a main DELLA member in Arabidopsis, accumulated after low temperature treatment,16 implying that the negative regulation of low temperature-induced anthocyanin accumulation by GAs is likely achieved by DELLAs degradation. Low temperature was able to stimulate GA2ox1 expression, as a result, endogenous GAs decreased and DELLAs accumulated.16 We also found that low temperature markedly upregulated the GA2ox1 expression (Fig. 2A). Moreover, it was intriguing that this upregulation was strongly impaired in hy5 and hy5 hyh mutants (Fig. 2B), indicating that the effect of low temperature treatment is HY5/HYH-dependent.
Figure 1.
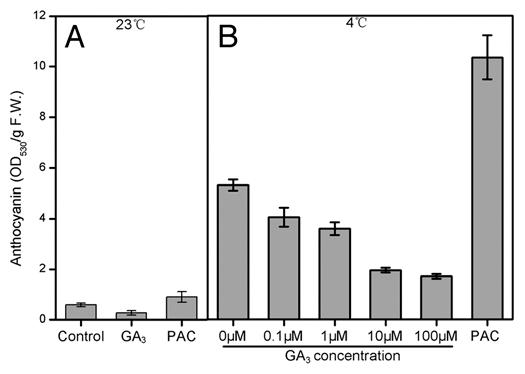
Effects of exogenous GA3 and PAC on anthocyanin accumulation. Col-0 seedlings grown at control condition for four days were treated by exogenously supplied 10 µM GA3 and 1 µM PAC at 23°C (A), or different concentration of GA3 and 10 µM PAC at 4°C (B) for 3 days. Data are mean values ± SE of at least four independent experiments.
Figure 2.
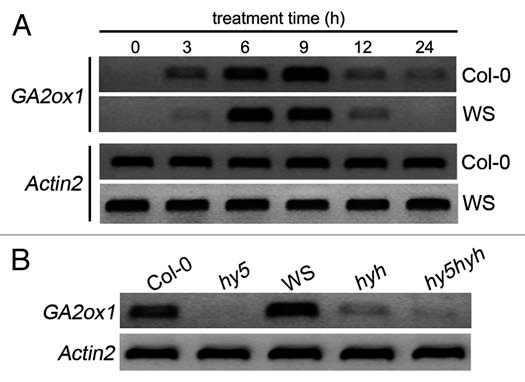
Effect of low temperature (4°C) on the expression of GA2ox1. (A) Col-0 and WS plants grown for four days in the control condition were treated under low temperature in the presence of constant white light for indicated time. (B) Low temperature induced upregulation of GA2ox1 is impaired in hy5, hyh and hy5 hyh mutants. Four-day-old seedlings of Col-0, WS, hy5, hyh and hy5 hyh were treated under low temperature (4°C) in the presence of constant white light for 6 h. The gel images shown here are representative of up to three independent experiments.
So, it was proposed that the DELLAs accumulation under low temperature might also be HY5/HYH-dependent. Accordingly, PAC enhanced anthocyanin level under low temperature was impaired in hy5 and hy5 hyh mutants (Fig. 3), implying that the effect of DELLAs on anthocyanin accumulation is HY5/HYH-dependent. In other words, the reduction of GAs enhanced anthocyanin accumulation under low temperature may depend on HY5/HYH. Interestingly, it was recently found that GAs reduction in Arabidopsis seedlings could enhance HY5/HYH activity.10 We assume that the accumulation of DELLAs under low temperature could enhance the anthocyanin accumulation through enhancing the HY5/HYH transcription activity. In a word, HY5/HYH plays a central role during this process. Figure 4 represents a proposed model of how HY5/HYH and GA signaling regulate low temperature-induced anthocyanin accumulation: in the presence of light, low temperature elevates GA2ox1 expressions in the presence of HY5/HYH; increased GA2ox1 expression results in decrease of endogenous active GAs and induction of DELLA protein accumulation, which in turn enhance the transcription activity of HY5 and HYH on the expression of DFR. However, more investigation is still needed to clearly identify the role of both HY5/HYH and GA signaling in low temperature inducing anthocyanin accumulation.
Figure 3.
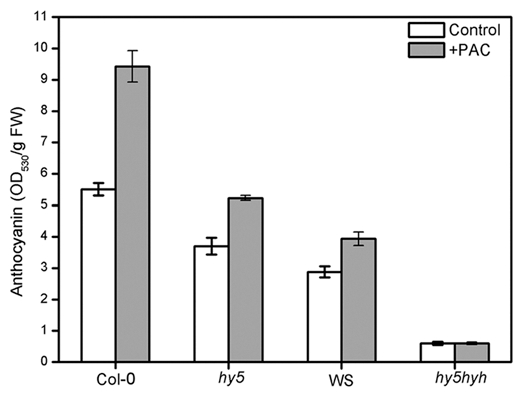
Effect of PAC (1 µM) on anthocyanin contents in Col-0, hy5, WS and hy5 hyh at 4°C. Four-day-old seedlings were treated at 4°C in the presence of constant white light for three days, and anthocyanin contents of the seedlings were determined. Data are mean values ± SE of at least four independent experiments.
Figure 4.
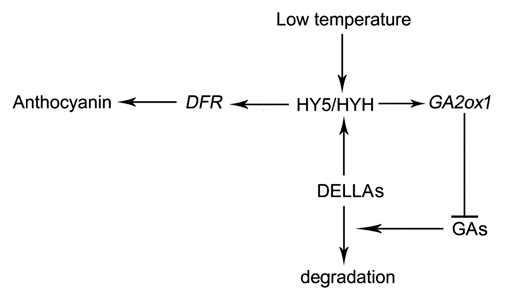
A flow scheme based on the results obtained from our studies and others showing components involved in signal transduction under low temperature inducing anthocyanin accumulation. Arrowheads and tees indicate positive and negative regulation, respectively.
Acknowledgements
The authors thank Prof. Xing-Wang Deng of Yale University for providing the seeds of Ararbidopsis mutants hy5, hyh and hy5 hyh. This work was supported by the National High Technology Research and Development Program (863 Program) (2007AA021401), the Major Project of Cultivating New Varieties of Transgenic Organisms (2009ZX08009-029B), and the Natural Science Foundation of China (No. 90917019).
References
- 1.Jiao Y, Lau OS, Deng XW. Light-regulated transcriptional networks in higher plants. Nat Rev Genet. 2007;8:217–230. doi: 10.1038/nrg2049. [DOI] [PubMed] [Google Scholar]
- 2.Lee J, He K, Stolc V, Lee H, Figueroa P, Gao Y, et al. Analysis of transcription factor HY5 genomic binding sites revealed its hierarchical role in light regulation of development. Plant Cell. 2007;19:731–749. doi: 10.1105/tpc.106.047688. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Lorrain S, Genoud T, Fankhauser C. Let there be light in the nucleus! Curr Opin Plant Biol. 2006;9:509–514. doi: 10.1016/j.pbi.2006.07.013. [DOI] [PubMed] [Google Scholar]
- 4.Oyama T, Shimura Y, Okada K. The Arabidopsis HY5 gene encodes a bZIP protein that regulates stimulus-induced development of root and hypocotyl. Genes Dev. 1997;11:2983–2995. doi: 10.1101/gad.11.22.2983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Holm M, Ma LG, Qu LJ, Deng XW. Two interacting bZIP proteins are direct targets of COP1-mediated control of light-dependent gene expression in Arabidopsis. Genes Dev. 2002;16:1247–1259. doi: 10.1101/gad.969702. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Yamaguchi S. Gibberellin metabolism and its regulation. Annu Rev Plant Biol. 2008;59:225–251. doi: 10.1146/annurev.arplant.59.032607.092804. [DOI] [PubMed] [Google Scholar]
- 7.Hartweck LM. Gibberellin signaling. Planta. 2008;229:1–13. doi: 10.1007/s00425-008-0830-1. [DOI] [PubMed] [Google Scholar]
- 8.Achard P, Genschik P. Releasing the brakes of plant growth: How GAs shutdown DELLA proteins. J Exp Bot. 2009;60:1085–1092. doi: 10.1093/jxb/ern301. [DOI] [PubMed] [Google Scholar]
- 9.Achard P, Liao L, Jiang C, Desnos T, Bartlett J, Fu X, et al. DELLAs contribute to plant photomorphogenesis. Plant Physiol. 2007;143:1163–1172. doi: 10.1104/pp.106.092254. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Alabadí D, Gallego-Bartolomé J, Orlando L, Gárcia-Carcel L, Rubio V, Martínez C, et al. Gibberellins modulate light signaling pathways to prevent Arabidopsis seedling de-etiolation in darkness. Plant J. 2008;53:324–335. doi: 10.1111/j.1365-313X.2007.03346.x. [DOI] [PubMed] [Google Scholar]
- 11.de Lucas M, Davière JM, Rodríguez-Falcón M, Pontin M, Iglesias-Pedraz JM, Lorrain S, et al. A molecular framework for light and gibberellin control of cell elongation. Nature. 2008;451:480–484. doi: 10.1038/nature06520. [DOI] [PubMed] [Google Scholar]
- 12.Feng S, Martinez C, Gusmaroli G, Wang Y, Zhou J, Wang F, et al. Coordinated regulation of Arabidopsis thaliana development by light and gibberellins. Nature. 2008;451:475–479. doi: 10.1038/nature06448. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Achard P, Cheng H, De Grauwe L, Decat J, Schoutteten H, Moritz T, et al. Integration of plant responses to environmentally activated phytohormonal signals. Science. 2006;311:91–94. doi: 10.1126/science.1118642. [DOI] [PubMed] [Google Scholar]
- 14.Achard P, Renou JP, Berthomé R, Harberd NP, Genschik P. Plant DELLAs restrain growth and promote survival of adversity by reducing the levels of reactive oxygen species. Curr Biol. 2008;18:656–660. doi: 10.1016/j.cub.2008.04.034. [DOI] [PubMed] [Google Scholar]
- 15.Magome H, Yamaguchi S, Hanada A, Kamiya Y, Oda K. The DDF1 transcriptional activator upregulates expression of a gibberellin-deactivating gene, GA2ox7, under high-salinity stress in Arabidopsis. Plant J. 2008;56:613–626. doi: 10.1111/j.1365-313X.2008.03627.x. [DOI] [PubMed] [Google Scholar]
- 16.Achard P, Gong F, Cheminant S, Alioua M, Hedden P, Genschik P. The cold-inducible CBF1 factor-dependent signaling pathway modulates the accumulation of the growth-repressing DELLA proteins via its effect on gibberellin metabolism. Plant Cell. 2008;20:2117–2129. doi: 10.1105/tpc.108.058941. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Hatier JH, Gould KS. Foliar anthocyanins as modulators of stress signals. J Theor Biol. 2008;253:625–627. doi: 10.1016/j.jtbi.2008.04.018. [DOI] [PubMed] [Google Scholar]
- 18.Zhang Y, Zheng S, Liu Z, Wang L, Bi Y. Both HY5 and HYH are necessary regulators for low temperature-induced anthocyanin accumulation in Arabidopsis seedlings. J Plant Physiol. 2011;168:367–374. doi: 10.1016/j.jplph.2010.07.025. [DOI] [PubMed] [Google Scholar]


