Abstract
Aquatic Utricularia species usually grow in standing, nutrient-poor humic waters. They take up all necessary nutrients either directly from the water by rootless shoots or from animal prey by traps. The traps are hollow bladders, 1–6 mm long with elastic walls and have a mobile trap door. The inner part of the trap is densely lined with quadrifid and bifid glands and these are involved in the secretion of digestive enzymes, resorption of nutrients and pumping out the water. The traps capture small aquatic animals but they also host a community of microorganisms considered as commensals. How do these perfect traps function, kill and digest their prey? How do they provide ATP energy for their demanding physiological functions? What are the nature of the interactions between the traps and the mutualistic microorganisms living inside as commensals? In this mini review, all of these questions are considered from an ecophysiologist's point of view, based on the most recent literature data and unpublished results. A new concept on the role of the commensal community for the plants is presented.
Key words: aquatic carnivorous plants, bladderwort, bladders, firing, resetting, enzyme secretion, water pumping, microbial commensals
Introduction
Aquatic carnivorous plants comprise the species Aldrovanda vesiculosa L. (Droseraceae) and about 50 species of the genus Utricularia L. (Lentibulariaceae2,3). Aquatic Utricularia species usually grow in shallow, standing humic waters which are usually poor in N and P, but occasionally also in K.3,4 They take up all necessary nutrients either directly from the water by their shoots or from animal prey by traps. Their entirely rootless shoots are mostly linear and, under favorable conditions, they exhibit very rapid apical shoot growth of 3–4 leaf nodes per day while their shoot bases decay at this same high rate.5–7
Although the Utricularia traps are the smallest among those of carnivorous plants, they are arguably the most sophisticated and intricate ones. They have always fascinated scientists.1 One composed leaf of Utricularia usually bears dozens to hundreds; of oval-shaped, fluid-filled traps of foliar origin (Fig. 1). These bladders are typically 1–6 mm long with elastic walls two cell layers thick and have a mobile trap door (Fig. 2).1 The inner part of the trap is densely lined by large glands of two types: quadrifid glands cover almost the whole inner surface and take part in the secretion of digestive enzymes and in the resorption of released nutrients, while the smaller bifid glands, which are located near the door, take part in pumping out the water. The traps capture small aquatic animals, usually 0.5–2 mm long, and these are mostly zooplankton.1,14
Figure 1.

Functional trap of Utricularia reflexa. Trap length is about 4 mm. The bubble inside the trap was aspirated in instead of water during the trap manipulation.
Figure 2.
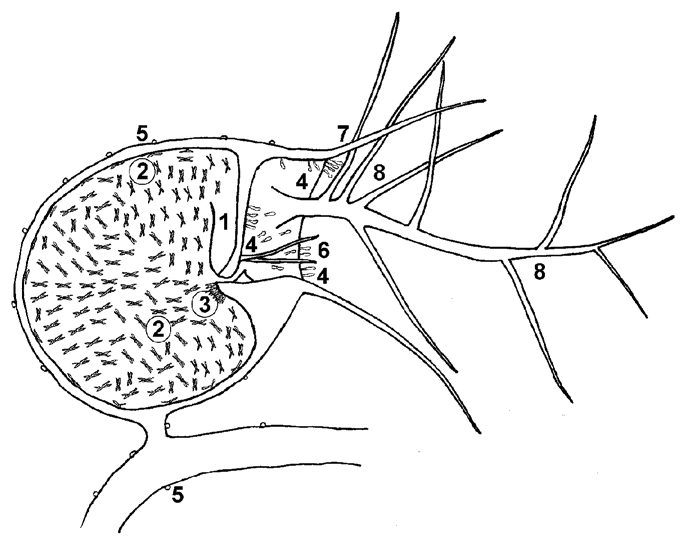
Schematic longitudinal section through a trap of Utricularia with glands and other structures 1, trap door; 2, quadrifid glands; 3, bifid glands; 4, stalked mucilage glands; 5, spherical sessile glands; 6, trigger hairs; 7, rostrum; 8, antennae. Prepared by J. Vrba.
How do these perfect traps function, then kill and digest their prey? How do they provide ATP energy for these demanding physiological functions? What are the nature of the interactions between traps and the mutualistic microorganisms living inside them as commensals? In this mini-review, all of these questions are addressed from an ecophysiologist's point of view, based on the most recent literature data. Challenges and inspirations for further research on this topic are also suggested.
Trap Functioning in “Classic” Studies
Almost all of the knowledge of the biophysical nature of Utricularia trap functioning was published only in a series of four studies conducted between 1973–1985.8–11 These studies are based on tricky measurements of negative pressures inside the trap and water outflow by piercing the isolated trap with fine glass capillaries (outer tip diam. 20–50 µm). The trap thickness was simultaneously optically or mechanically monitored. The trap thickness, as an easily and accurately measured parameter, correlated closely with the magnitude of the negative pressure during trap resetting and became a very reliable measure of trap water flow and partly also of the negative pressure. It is very curious that nobody has successfully repeated this simple capillary method as water leakage along capillary tips has always since occurred.
The trap is hermetically sealed and when fully reset, a negative pressure of about −16 kPa relative to the ambient water is maintained inside.8–11 When a prey species touches sensory hairs situated on the trap door it opens, the small animal is aspirated into the trap and the door closes again. This process of firing is complete within 10–15 ms and is the most rapid plant movement known.8 Immediately after firing, the negative pressure inside the trap drops to zero, but is soon restored by the rapid removal of ca. 40% of the water from the fired trap until the original concave shape is reached. This aspect of trap resetting lasts about 25–30 min and the trap is ready to fire again; the full resetting process lasts much longer. Using very inventive methods, the authors8–11 have also found that water is pumped out of the traps by an active process associated with a consumption of the metabolic energy of ATP. When inhibitors of aerobic respiration were added to the trap fluid, the water pumping and trap narrowing processes were very markedly blocked.
How is water pumped out of the traps? The authors8–11 have found that bifid glands attached close to the trap door (Fig. 2) take part in water pumping and that water is exuded from the pavement epithilium close to the door. Electrophysiological measurements revealed an electrical potential difference between the interior of the intact trap and the ambient solution of about +130 mV (trap interior positive). Additionally, an unusually high potential difference of about −300 mV between the cells of the inner trap wall layer and the internal trap fluid (i.e., local electrical cell membrane potential; usual values in plant cells are only within −60 to −200 mV11) was also found. These findings have led to the hypothesis9,10 that Cl− ions are actively taken up from the trap fluid by the bifid glands and, due to their movement, osmotically drag water molecules. Monovalent cations (Na+, K+) accompany the Cl- fluxes, while divalent cations (Ca2+, Mg2+) rather inhibit them. The second part of the water pathway is, however, veiled in mystery as it appears that the water is expelled from the cells of the pavement epithelium by the turgor pressure through a very leaky plasmalemma (for solutes <600 Daltons).9 Traps can also pump out water in moist air or when immersed in a liquid paraffin oil.10
Food Web Inside the Traps
It must be mentioned that traps of aquatic Utricularia possess one significant ecological trait which is their principal difference from snapping Aldrovanda traps. This offers unexpected ecological possibilities for the plants. In spite of its tiny volume, the trap fluid in Utricularia plants is inhabited permanently by various commensal microorganisms—bacteria, cyanobacteria, microfungi, algae, euglens, dinophytes, protozoa (ciliates) and rotifers—which live in a mutualistic interaction with the plant.13–19 These organisms enter the originally sterile traps from the immediate ambient environment, propagate inside the traps and take part in prey decomposition. Quite recently, evidence for the formation of a miniature food web has been provided in two Belizean Utricularia species.17 Similar food webs, in addition to a participation of specialised dipteran insect larvae, have also been described in digestive fluids of large pitcher-shaped traps of several species and genera of terrestrial carnivorous plants (Darlingtonia californica, Sarracenia purpurea, Nepenthes spp).1,20–22 In Utricularia traps, due to methodical limitation, it may not be clear which microorganisms found inside the traps are living as commensals and which are prey.18,19
Oxygen Regime Inside the Traps and Trap Respiration
Oxygen concentration in the Utricularia trap fluid may be considered the crucial characteristic, not only for trap respiration and water pumping but also for life and propagation of the commensal communities and the death of the prey. Using a miniature oxygen sensor, almost zero [O2] were measured in the trap fluid of mature empty traps of six aquatic Utricularia species.23 The median steady-state [O2] values were within 0.0–4.7 µM, but usually only within 0.0–1.4 µM (i.e., 0.0–0.5% of O2 saturation), both in isolated traps and those on intact shoots bathed in stirred, aerated media. These results are independent of irradiance and O2 oversaturation in shoot tissues. The redox potential ranged from −24 to −105 mV, further confirming the almost zero [O2]. Thus, even in spite of some leakage of oxygenated water into the measured traps and O2 diffusion from the trap wall intercellulars, the internal trap structures are able to consume the O2 rapidly to cause anoxia. After the minisensor tip had been inserted into the trap lumen, the trap fluid [O2] declined linearly almost to zero within 10–100 min. The linear rate of the decline is a measure of the respiration rates of internal trap structures and commensal organisms offset by O2 influx from the trap walls. It may be inferred that under natural growth conditions, long periods (hours to 20 h24–26) of anoxia inside the traps are interrupted by short periods (20–100 min) of higher [O2] after accidental firings. Therefore, captured organisms either die of O2 deprivation and are prey, or are able to tolerate anoxia and are commensals. Utricularia traps likely kill their prey by suffocation. Prey digestion inside the traps did not increase the dark respiration rate (RD) of traps12 but RD of halved traps was 27% greater than that of intact ones in U. reflexa23 but were unchanged in U. vulgaris.11 Though the photosynthetic regime in Utricularia shoots (i.e., O2 oversaturation in the shoot tissues) leads to a greater O2 influx to the trap lumen than from a stirred, aerated ambient medium in darkness, the steady-state [O2] in the trap fluid is zero in both cases.23 In conclusion, the anoxic trap fluid of intact traps is well isolated from the oxygenated ambient water from the trap walls such that O2 from the ambient water is not transferred inside the traps.
The discovery of internal trap anoxia raises some important ecophysiological questions. Due to their very demanding functions, Utricularia traps are metabolically very active and their RD per unit fresh or dry weight is 2–3 times greater than that of leaves or shoots bearing these traps.12 Moreover, as found in the classic studies, pumping the water out of the traps must require the participation of aerobic respiration inside the traps.8–11 It can be assumed that it is the high RD of internal bifid and quadrifid glands and pavement epithelium that is responsible for the high trap RD11,12,23 due to the presence of abundant mitochondria and transfer cells in all these structures.10,27 In halved U. vulgaris traps, the aerobic RD value per unit fresh weight of the parts containing bifid glands near the trap door (6.8 mmol.kg−.h−1) was 50% greater than that of the opposite side.11 From a biochemical point of view, however, it is unclear how the internal trap glands provide ATP energy to maintain their demanding physiological functions (water pumping) under anoxic conditions for many hours or even permanently.25,26,28 Based on molecular findings, a link between faster reaction kinetics of Utricularia traps and a mutation occurring in the mitochondrial respiratory chain enzyme cytochrome c oxidase has been suggested.29 Within Lentibulariaceae, this mutation has only been detected in Utricularia, but not in the sister genera Pinguicula and Genlisea which have immobile traps. The authors30 further hypothesise the decoupling of mitochondrial proton pumping from electron transfer, which could be a rich source of ATP energy after trap firing during the aerobic period. Such decoupling would allow the traps to optimise power output during times of need, although with a 20% decrease in total energy efficiency of the respiratory chain. For some important reasons, it seems improbable that the traps provide most of their ATP energy need from anaerobic fermentation (e.g., the magnitude of redox potential23). On the contrary, it is more probable that the inner trap structures possess an extremely high O2 affinity (well below 0.5–1 µM, which is beyond the resolution of the Clark-type O2 sensor), using the permanent O2 influx from the trap wall intercellulars. The above mentioned mutation in the cytochrome c oxidase could account for such a high O2 affinity.
In wide-spread U. australis, traps usually represent only about one-third of dry biomass of mature shoot segments as a structural investment in carnivory (i.e., structural cost),6,7,31 but the total trap RD amounted to 67% of the total shoot RD. If the net photosynthetic rate of traps of aquatic Utricularia (per unit fresh weight) reached only 7–10% of the values for leaves or shoots even under optimum conditions, then this combination means that traps represent a very high energetic (metabolic) cost for the plants. Moreover, as found recently,31,32 the traps also represent a high mineral cost, especially for N, P and K. Therefore, the proportion of trap biomass to the total plant biomass is under a purposeful ecological regulation.5–7,31,33
Production of Digestive Enzymes
Little is still known about prey digestion in Utricularia traps though standard biochemical techniques revealed the presence of proteases in the trap fluid as early as the 1920s.1 Later, pro-tease, (acid) phosphatase and esterase were localised cytochemically in the digestive quadrifid glands34–36 with phosphatase also on their surfaces.16,37 Activities of five hydrolases (phosphatase, aminopeptidase, β-hexosaminidase, α- and β-glukosidase) were measured microfluorimetrically directly in the filtered trap fluid collected from both empty traps of four aquatic Utricularia species and their culture water.16 Phosphatase invariably exhibited the highest activity, while the activities of the other enzymes were usually lower by one or two orders of magnitude. As the activities of the other enzymes in the trap fluid were usually lower than in the ambient culture water, the enzymes could have entered the trap from the ambient water. Phosphatase activity could partly decrease due to dilution after trap firings. However, the trap fluid phosphatase exhibited its highest activity at a pH between 4.7–5.5, while that in the ambient water occurred at a higher pH of 9.0. It has been confirmed recently for some Utricularia species that traps produce the phosphatase continuously and independently of prey capture,16 loading with N or P salts or enrichment of the culture water in mineral N and P.38 Trap age was shown to be the key factor in the patterns of phosphatase production.16,17,38 As ultrastructural changes in the quadrifid glands occur during different stages of trap development36 it is possible that old, still functional traps shift their function from enzyme production towards nutrient uptake.17 Except for U. foliosa, trap fluid pH in several species was usually within a narrow range from 4.8 to 5.1 and seemed to be regulated by the traps.16,17,38
One surprising aspect of these enzyme studies should be pointed out. In all species studied (excepting U. foliosa38) and under all experimental conditions, very low or even zero activity of aminopeptidase (i.e., protease) was found in the fluid in traps with or without prey16,17,38 although it is expected (reviewed in ref. 1 and 3) that proteinaceous N is the main N source from prey. Moreover, the aminopeptidase found inside the traps had its optimum pH between 7.0 to 9.0, but zero activity at pH 4.7 and much resembled that found in the ambient water; the activities in both environments were also very similar.16,38 Thus, a great deal of the very low aminopeptidase activity enters the traps from the ambient water. The absence of aminopeptidases in the fluid could be compensated for by the autolysis of dead prey tissues.39 Yet the discrepancy between the findings of very low or zero protease activity in the trap fluid with or without prey, and in the presence of large secretory vesicles (Golgi apparatus) rich in proteases in quadrifid glands34–36 still cannot be explained. Similarly, the same discrepancy exists between the invariably high phosphatase activity found in the trap fluid and a very low activity of enzyme labelled fluorescence (ELF) of phosphatase usually detected on the surface of quadrifid glands.16 At least a part of this discrepancy might be explained by methodical limitation of the ELF method (cf. 16 and 37). Moreover, it is not clear which proportion of any enzyme activity in the filtered trap fluid is produced by trap glands alone or various trap commensals, in addition to an unknown part of the activity gained from the ambient water. Evidence has recently been provided using the ELF method that some commensals in the trap fluid (algae, bacteria) exhibit a considerable phosphatase activity on their surface.17,37 Consistently high trap fluid activities of phosphatases in all species imply that P uptake from prey or detritus might be more important than that of N for the plant.
The Importance of Commensals in Utricularia Traps
As mentioned above, an abundant community of commensal microorganisms occurs inside aquatic Utricularia traps and the older the traps are, the denser is the community.17 Due to green euglens or other algae, they are greenish. Though the first data on species composition of the community are over 130 years old (reviewed in ref. 1 and 16–19) their importance for Utricularia ecophysiology is still quite unclear. One must emphasise that, in spite of their undeniable perfect functional features, aquatic Utricularia traps capture relatively little animal prey in barren, nutrient-poor waters though a high abundance of commensal organisms also occur in empty traps.6,14,17,18,31 Starting with the novel study by Richards,14 it has therefore been assumed that a mutualistic interaction between the plant and trap commensal community prevails over catching animal prey for mineral nutrient uptake in these barren waters.17–19,38
Trap commensal microorganisms are not strictly specialized to living inside the traps. They can live either as periphyton on the external plant surface or freely as plankton in the ambient water and may be considered generalists.17–19 Some recent studies have suggested a considerable potential importance of phytoplankon for mineral nutrition of European aquatic Utricularia species.18,19 A high proportion of traps contained the same planktonic algae as in the ambient water but about 90% of them were dead and thus served as prey.18 They entered the traps due to an incidental firing. That is why the authors have named their paper appropriately: “Utricularia—a vegetarian carnivorous plant?” In the second study from NE Germany, 60% of all animals found inside traps were ciliates.19 In species with dimorphic shoots (U. intermedia, U. ochroleuca), the carnivorous shoots of which grow down to a loose peaty substrate, an incidental aspiration of brown detritus (rich in humic acids) could also be of a similar nutritional importance.
Another study has proven a great accumulation of nutrients inside the traps without prey. In U. foliosa and U. purpurea growing at an oligotrophic site in Belize, surprisingly high concentrations of both organic and mineral dissolved substances were found in the filtered trap fluid in all trap age categories (in mg l−1): C, 64–307; N, 7–25; P, 0.2–0.6.17 Nevertheless, the total content of both C, N and P in the trap fluid, including mainly the particulate form (i.e., the commensal organisms and detritus), was several times greater (in mg l−1): C, 632–1570; N, 21–81; P, 0.9–4.2. The total nutrient content usually correlated with the increasing trap age. On the basis of phospholipid fatty acid analysis of the trap commensal biomass, the occurrence of a complex microbial food web in the trap fluid was revealed, with bacteria forming >75% of the viable microbial biomass. The authors thus assumed that trap commensals could play a role in N and P uptake by the traps in barren waters without prey.
In a two-day experiment on U. australis and U. vulgaris using 13C, a great proportion of newly fixed CO2 was allocated from shoot bases not only to shoot apices, but also to mature shoot segments.40 Total carbon allocation in plant tissues rapidly decreased with increasing age of the shoot segments but the ratio of C exuded into the trap fluid to that in plant tissues increased markedly with age—twice as much newly fixed C was allocated into the trap fluid than the plant tissue in the oldest analyzed segments. Overall, 20–25% of the newly fixed C was allocated into the trap fluid. In this way, Utricularia plants supply easily available organic C to the traps to support the development of the prevailingly heterotrophic microbial commensal community. Preliminary analyses of the trap fluid in several aquatic Utricularia species revealed relatively high concentrations of simple sugars (1–120 mg l−1; mainly sucrose, glucose and fructose) and organic acids (mainly lactic acid).28,40 This extensive C supply to the trap fluid is undoubtedly an important additional maintenance (energetic) cost of traps. It is thus possible to conclude that the dominant part of C in the commensal organisms is provided by the plant.17,40 Yet, on the basis of record high photosynthetic rate of photosynthetic Utricularia shoots,12 it seems that the plants can afford such “gardening” despite their very rapid growth. Beneficially, the plant could gain growth-limiting N and P from phytoplankton and detritus decomposed in the trap fluid. Nevertheless, this N and P input from the ambient medium to the traps has never been quantified (but see below).
Spontaneous Firings and Permanent Water Flow
New important challenges for Utricularia trap ecophysiology have very recently arisen from the discovery of spontaneous firing in Utricularia traps.24–26 Using both a high-speed camera for intact shoots24,25 and a linear position sensor for excised traps,26 both groups of authors confirmed a more or less regular trap firing without any mechanical stimulation in three aquatic Utricularia species and in two trap age categories. Spontaneous firings occurred 0.3–2.4 times during the 1-day resting period and the mean time between two spontaneous firings varied between 5–16 h. Quantitatively (trap thickness increase due to firing, resetting rate), spontaneous firings resembled mechanically stimulated ones.26 It was also found that the traps pumped water out after firing for at least 5–10 h until a steady-state was attained (cf. 8–10 and 26). It is probable that a spontaneous firing occurs when the negative pressure in the reset traps is minimal (i.e., the trap thickness is minimal) and the trap door is no longer able to withstand the ambient water pressure, thus functioning as a “safety valve” protecting the integrity of the trap door.26 The unique use of the high-speed camera further confirmed a mechanical deformation (buckling) of the trap door during single phases of the firing process.24,25 As opposed to eight who postulated a concept of an internal negative pressure sensor regulating water pumping in the trap, the most recent data review in reference 28 suggest a permanent pumping out of water from the traps. These data are based mainly on the fact that no lag-period in water pumping from the trap was detected within the first 2 s after trap firing, which occurred after a 3-h resetting period. This can mean that either the water permanently recirculates through some leaks under the trap door or the mechanism of water pumping becomes thermodynamically inefficient at high negative pressure though it runs permanently.
Ecological Consequences of Water Flow
Obviously, the most recent findings on trap operation—spontaneous firing and water recirculation—can better explain how growth-limiting N and P enter the traps from the ambient medium and become a substrate for the microbial food web. It is therefore evident that spontaneous firing of each trap, which occurs on average several times a day, could lead to a substantial gain of N and P for the traps. During its three-week life-span, each trap can aspirate around 15 times its own volume in surrounding water (40% trap volume × 2-daily spontaneous firings ×20-day life-span). If the trap commensals help to decompose this “non-animal” prey, it could theoretically imply a significant N and P gain for the plant, especially for those plants in barren waters which trap few animals. The literature assumed that this N and P gain by empty traps could be ecologically important but no quantification has been given so far.7,14,17–19,26,40 When, however, one considers the published data on the mean total N and P content (both dissolved and particulate, see above)17 within a middle-aged trap and the mean total N and P content at natural oligo- and mesotrophic sites of U. australis in the Czech Republic,31 a simple budget model of N and P gain can be made. When water recirculation through the traps is accounted for then, surprisingly, only a very slow accumulation of total N and P within empty traps can occur. [Model data: trap volume 5 µl, water recirculation rate 1 µl h−1/after 10, 11, 28/for 20 h a day, only NH4-N and PO4-P influx from the ambient water, mean natural concentrations of NH4-N and PO4-P at the sites31 ]. In other words, it would take between 40–70 days for an empty trap to accumulate the measured N or between 15–23 days for them to accumulate the P if there were only spontaneous firing and water recirculation mechanisms occurring. By the application of the model data for the relationship between trap volume and biomass and for shoot N and P content in U. australis,6,17,28,31 one can further estimate that the total N and P amount inside the traps17 represents only about 3.5% of total plant N and 1.2% of P.
What conclusions can be drawn from these model data? (A) The calculated N and P input rates from the ambient water due to both spontaneous firings and hypothetical water recirculation are so low that each trap without prey can only reach these nutrient levels after a long time, which is comparable with or even longer than the trap life-span. Thus, such a low N and P gain cannot be ecologically important for the plant mineral nutrition at all but the rapid turnover of N and P inside the traps can be. (D) Since even young Utricularia traps (ca. 1 week old) contained a relatively high total N and P content comparable with that in the older traps,17 the traps could take up neither N nor P from the trap fluid for the plant itself as the total N and P content in the trap fluid would have been much lower. (C) To account for all of these findings, it is evident that the traps exude not only organic C into the fluid,40 but also N and P to enhance the microbial community. This idea can be supported by the finding of a growth reduction of terrestrial U. uliginosa after addition of euglens to peaty soil.41 It is possible that inoculation of young traps by microorganisms stimulates the traps to exude both organic C and also N and P. Therefore, it seems that Utricularia traps without animal prey have no nutritional benefit from the trap commensal community and that the trap microorganisms behave rather as parasites than commensals. The extent of this N and P exudation to the trap fluid can only be a few % of the total plant N and P amount. (D) Utricularia species commonly grow in very oligotrophic, barren waters with very low prey availability.4–6,14,17,18,31 It can be inferred that the N and P uptake affinity of Utricularia shoots is very high (the limits for uptake should be below 1 µM for NH4+ and 0.1 µM for phosphate), while that for N- and P-containing substances in the traps without prey can be very low or zero.17,28 (E) If the trap microorganisms are beneficial for the plant, the ecological benefit can occur only in traps with captured prey to facilitate prey digestion. The trap microorganisms in traps represent additional ecological costs for trap maintenance. It may be hypothesised that plants keep up their trap commensal community (“gardening”) by exudation of organic and mineral nutrients into all traps, as an offset for better prey digestion only in the traps with captured animal prey and, thus, for greater uptake of mineral nutrients from prey. This should lead to a final ecological nutritional benefit for the plant. The real cost:benefit ratio depends on the proportion of traps which capture animal prey during their life. (F) Aquatic Utricularia species invest more in trap production at low tissue N and/or P content in shoots.31,33,39 As the greater proportion of trap biomass usually leads to increasing the total prey capture, ecological benefit associated with the greater proportion of trap biomass should obviously prevail over the cost associated with the structure and maintenance of the traps, including the support of the commensal community. (G) If capturing animal prey is crucially important for the nutritional benefit of carnivory in Utricularia, the strategy of prey capturing could “drive” the evolution of traps in aquatic Utricularia species, while the interaction with the trap commensal organisms could be more marginal.
Concluding Remarks:Inspiration for Further Research
The aim of this mini review was to summarize the most recent data and concepts (some unpublished) on trap functioning of aquatic Utricularia species including also the importance of trap commensal microorganisms. It is undeniable that the newest data on the water flow in the traps (spontaneous firings and water recirculation),24–26,28 have markedly changed the classic view of trap function.8–11 It follows from this review, in light of unpublished data and concepts, that it is necessary to reject the commonly accepted view that there is a great nutritional importance on the trap commensal community for the plant in barren waters with low prey availability.14,17–19,39,40 The microbial trap community in traps without animal prey may now be considered as parasites rather then commensals. However, this revised view on the role of trap microorganisms in Utricularia traps does not contradict the commonly accepted role of these microorganisms in facilitating prey digestion as commensals but only clearly excludes a nutritional benefit for the plant from their living in traps without prey.
To obtain further insight into the ecophysiology of trap functioning in (aquatic) Utricularia species, the following directions of research could be considered and the questions raised could be answered.
Electrophysiological studies should resolve the crucial question as to whether Utricularia traps are stimulated to fire via the conventional electrophysiological signaling pathway (including a rise in action potential in the sensory hairs) or purely mechanically (reviewed in ref. 8).
As the dominant part of ecophysiological research on Utricularia traps has been conducted on aquatic species (they have much larger traps) do the same processes run also in the terrestrial species (i.e., water flows, secretion of enzymes, respiration characteristics, interaction with the commensals, etc.,)?
The discrepancy between very low protease activity found in the trap fluid16,38 and high activity found inside secretory vesicles in the quadrifid glands34–36 should be elucidated. Moreover, what is the role of commensal microorganisms in prey digestion for different enzyme classes? What is the uptake efficiency of the main mineral nutrients (N, P, K) from prey in the traps?
As opposed to the almost zero [O2] in the trap fluid, the internal trap glands can provide sufficient ATP energy for their demanding functions. Which adaptation allows these glands to provide sufficient ATP energy?
The biochemical mechanism of water pumping out of the traps has been insufficiently studied.8–11 Can this mechanism be determined using modern experimental approaches (e.g., patch-clamp, vibration probe, ion-sensitive microelectrodes)?
Biophysical aspects of trap firing and resetting should be studied in association with the measurement or setting of negative pressure inside the traps as a possible regulatory factor for water pumping. The study should verify the recent concept of continuous water pumping from the traps and water recirculation.
The importance of phytoplankton and detritus as a potential nutrient (N, P, K) source for Utricularia in barren waters could be elucidated on the basis of estimation of the matter in the trap fluid and modeling.
What is the role of the commensal community in the nutrient interactions within the plant having traps with or without prey? Obviously, the use of sterile plants and their inoculation with commensal microorganisms could be one of the promising approaches.
Acknowledgements
This paper is dedicated to Prof. Katsuhiko Kondo (Tokyo University of Agriculture, Atsugi City, Japan) for his great merits on studying carnivorous plants inclusive Utricularia. This study was partly funded by the Czech Scientific Foundation (Research Project No. P504/11/0783) and the Research Programme of the Academy of Sciences of the Czech Republic (No. AV0Z60050516). Sincere thanks are due to Dr. Brian G. McMillan (Glasgow, Scotland) for correction of the language.
References
- 1.Juniper BE, Robins RJ, Joel DM. The carnivorous plants. London: Academic Press Ltd.; 1989. pp. 1–353. [Google Scholar]
- 2.Taylor P. The genus Utricularia: A taxonomic monograph. Vol. 4. Kew: Kew Bulletin, Additional Series; 1989. pp. 1–724. [Google Scholar]
- 3.Adamec L. Mineral nutrition of carnivorous plants: A review. Bot Rev. 1997;63:273–299. [Google Scholar]
- 4.Guisande C, Granado-Lorencio C, Andrade-Sossa C, Duque SR. Bladderworts. Funct Plant Sci Biotechnol. 2007;1:58–68. [Google Scholar]
- 5.Friday LE. Rapid turnover of traps in Utricularia vulgaris L. Oecologia. 1989;80:272–277. doi: 10.1007/BF00380163. [DOI] [PubMed] [Google Scholar]
- 6.Adamec L. Photosynthetic CO2 affinity of the aquatic carnivorous plant Utricularia australis (Lentibulariaceae) and its investment in carnivory. Ecol Res. 2009;24:327–333. [Google Scholar]
- 7.Adamec L, Sirová D, Vrba J. Contrasting growth effects of prey capture in two carnivorous plant species. Fundam Appl Limnol. 2010;176:153–160. [Google Scholar]
- 8.Sydenham PH, Findlay GP. The rapid movement of the bladder of Utricularia sp. Aust J Biol Sci. 1973;26:1115–1126. [Google Scholar]
- 9.Sydenham PH, Findlay GP. Transport of solutes and water by resetting bladders of Utricularia. Aust J Plant Physiol. 1975;2:335–351. [Google Scholar]
- 10.Sasago A, Sibaoka T. Water extrusion in the trap bladders of Utricularia vulgaris I. A possible pathway of water across the bladder wall. Bot Mag. 1985;98:55–66. [Google Scholar]
- 11.Sasago A, Sibaoka T. Water extrusion in the trap bladders of Utricularia vulgaris II. A possible mechanism of water outflow. Bot Mag. 1985;98:113–124. [Google Scholar]
- 12.Adamec L. Respiration and photosynthesis of bladders and leaves of aquatic Utricularia species. Plant Biol. 2006;8:765–769. doi: 10.1055/s-2006-924540. [DOI] [PubMed] [Google Scholar]
- 13.Mette N, Wilbert N, Barthlott W. Food composition of aquatic bladderworts (Utricularia, Lentibulariaceae) in various habitats. Beitr Biol Pfl. 2000;72:1–13. [Google Scholar]
- 14.Richards JH. Bladder function in Utricularia purpurea (Lentibulariaceae): is carnivory important? Am J Bot. 2001;88:170–176. [PubMed] [Google Scholar]
- 15.Jobson RW, Morris EC. Feeding ecology of a carnivorous bladderwort (Utricularia uliginosa, Lentibulariaceae) Aust Ecol. 2001;26:680–691. [Google Scholar]
- 16.Sirová D, Adamec L, Vrba J. Enzymatic activities in traps of four aquatic species of the carnivorous genus Utricularia. New Phytol. 2003;159:669–675. doi: 10.1046/j.1469-8137.2003.00834.x. [DOI] [PubMed] [Google Scholar]
- 17.Sirová D, Borovec J, Cerná B, Rejmánková E, Adamec L, Vrba J. Microbial community development in the traps of aquatic Utricularia species. Aquat Bot. 2009;90:129–136. [Google Scholar]
- 18.Peroutka M, Adlassnig W, Volgger M, Lendl T, Url WG, Lichtscheidl IK. Utricularia: a vegetarian carnivorous plant? Algae as prey of bladderwort in oligotrophic bogs. Plant Ecol. 2008;199:153–162. [Google Scholar]
- 19.Alkhalaf IA, Hübener T, Porembski S. Prey spectra of aquatic Utricularia species (Lentibulariaceae) in northeastern Germany: The role of planktonic algae. Flora. 2009;204:700–708. [Google Scholar]
- 20.Cochran-Stafira DL, von Ende CN. Integrating bacteria into food webs: Studies with Sarracenia purpurea inquilines. Ecology. 1998;79:880–898. [Google Scholar]
- 21.Kneitel JM, Miller TE. Resource and top-predator regulation in the pitcher plant (Sarracenia purpurea) inquiline community. Ecology. 2002;83:680–688. [Google Scholar]
- 22.Gray SM, Miller TE, Mouquet N, Daufresne T. Nutrient limitation in detritus-based microcosms in Sarracenia purpurea. Hydrobiologia. 2006;573:173–181. [Google Scholar]
- 23.Adamec L. Oxygen concentrations inside the traps of the carnivorous plants Utricularia and Genlisea (Lentibulariaceae) Ann Bot. 2007;100:849–856. doi: 10.1093/aob/mcm182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Marmottant P, Vincent O, Quilliet C. Cayenne, French Guiana: Book of Abstracts, Plant Biomechanics Conference. 2009. Study of the ultrafast trap of an aquatic carnivorous plant. [Google Scholar]
- 25.Vincent O, Weisskopf C, Poppinga S, Masselter T, Speck T, Joyeux M, et al. Ultra-fast underwater suction traps. Proc R Soc B. 2011 doi: 10.1098/rspb.2010.2292. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Adamec L. The comparison of mechanically stimulated and spontaneous firings in traps of aquatic carnivorous Utricularia species. Aquat Bot. 2011;94:44–49. [Google Scholar]
- 27.Płachno JB, Jankun A. Transfer cell wall architecture in secretory hairs of Utricularia intermedia traps. Acta Biol Cracov Ser Bot. 2004;46:193–200. [Google Scholar]
- 28.Adamec L. 2010. Unpublished results.
- 29.Jobson RW, Nielsen R, Laakkonen L, Wikström M, Albert VA. Adaptive evolution of cytochrome c oxidase: Infrastructure for a carnivorous plant radiation. Proc Natl Acad Sci USA. 2004;101:18064–18068. doi: 10.1073/pnas.0408092101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Laakkonen L, Jobson RW, Albert VA. A new model for the evolution of carnivory in the bladderwort plant (Utricularia): adaptive changes in cytochrome c oxidase (COX) provide respiratory power. Plant Biol. 2006;8:758–764. doi: 10.1055/s-2006-924459. [DOI] [PubMed] [Google Scholar]
- 31.Adamec L. Mineral nutrient relations in the aquatic carnivorous plant Utricularia australis and its investment in carnivory. Fund Appl Limnol. 2008;171:175–183. [Google Scholar]
- 32.Adamec L. Mineral cost of carnivory in aquatic carnivorous plants. Flora. 2010;205:618–621. [Google Scholar]
- 33.Kibriya S, Jones JI. Nutrient availability and the carnivorous habit in Utricularia vulgaris. Freshwater Biol. 2007;52:500–509. [Google Scholar]
- 34.Heslop-Harrison Y. Enzyme release in carnivorous plants. In: Dingle JT, Dean RT, editors. Lysozymes in biology and pathology. Amsterdam: North Holland Publishing; 1975. pp. 525–578. [PubMed] [Google Scholar]
- 35.Parkes DM. Adaptive mechanisms of surfaces and glands in some carnivorous plants. Monash University: Clayton, Victoria, Australia; 1980. MSc thesis. [Google Scholar]
- 36.Vintéjoux C, Shoar-Ghafari A. Glandes digestives de l'Utriculaire: ultrastructures et fonctions. Acta Bot Gall. 2005;152:131–145. (Fre). [Google Scholar]
- 37.Płachno BJ, Adamec L, Lichtscheidl IK, Peroutka M, Adlassnig W, Vrba J. Fluorescence labelling of phosphatase activity in digestive glands of carnivorous plants. Plant Biol. 2006;8:813–820. doi: 10.1055/s-2006-924177. [DOI] [PubMed] [Google Scholar]
- 38.Adamec L, Sirová D, Vrba J, Rejmánková E. Enzyme production in the traps of aquatic Utricularia species. Biologia. 2010;65:273–278. [Google Scholar]
- 39.Adamec L. Ecophysiological look at plant carnivory: Why are plants carnivorous? In: Seckbach J, Dubinski Z, editors. All flesh is grass. Plant-animal interrelationships. Cellular origin, life in extreme habitats and astrobiology. Dordrecht, Heidelberg, London, New York: Springer Science + Business Media BV; 2011. pp. 455–489. [Google Scholar]
- 40.Sirová D, Borovec J, Šantrucková H, Šantrucek J, Vrba J, Adamec L. Utricularia carnivory revisited: Plants supply photosynthetic carbon to traps. J Exp Bot. 2010;61:99–103. doi: 10.1093/jxb/erp286. [DOI] [PubMed] [Google Scholar]
- 41.Jobson RW, Morris EW, Burgin S. Carnivory and nitrogen supply affect the growth of the bladderwort Utricularia uliginosa. Aust J Bot. 2000;48:549–560. [Google Scholar]


