Abstract
Drought, salinity and low temperature are major environmental factors that influence plant growth and development, and eventually limit crop yield and quality. To survive adverse stresses, plants have developed complex signaling networks to perceive external stimuli, and then manifest adaptive responses at molecular and physiological levels. Sucrose non-fermenting1-related protein kinase 2 (SnRK2) plays a critical role in plant sugar signaling via phosphorylation, while knowledge of specific functions of SnRK2s in wheat is still undiscovered. In this paper, we reviewed our recent studies on wheat SnRK2 members, TaSnRK2.4, TaSnRK2.7 and TaSnRK2.8, involved in abiotic stress responses. The results suggest that the three wheat kinases participate in sugar metabolic and stress signaling in wheat. Furthermore, we compare their distinct transcript levels in various tissues, expression patterns under diverse stress conditions and functions in transgenic Arabidopsis.
Key words: SnRK2, wheat, metabolism, stress signaling, stress response
The sucrose non-fermenting-1 (SNF1) protein kinase family comprises SNF1 itself in yeast, the AMP-activated protein kinases (AMPK) in mammals and the SNF1-related protein kinases (SnRKs) in plants. The plant SnRKs are divided into three subfamilies (SnRK1, SnRK2 and SnRK3) based on the amino acid sequence identity and expression patterns. SnRK2 is a type of serine/threonine protein kinase, including two typical domains, viz., an N-terminal catalytic domain and a C-terminal regulatory region, characterized by the presence of a short acidic patch. Based on the C-terminal acidic patch, the SnRK2 family is divided into two distinct subclasses, SnRK2a and SnRK2b. The acidic patch of SnRK2a is rich in aspartic acid residues, while that of SnRK2b enriches in glutamic acid residues.1,2 Increasing evidence shows that SnRK2s act within an intricate network that links metabolic and stress signaling in plants.1–4 Despite these important functions in plants, knowledge of specific functions of SnRK2s in wheat is fragmentary and the molecular mechanism of their activation is still enigmatic. In this review, we highlight the current view on the role of SnRK2 in plant carbohydrate metabolic and stress signaling pathways.
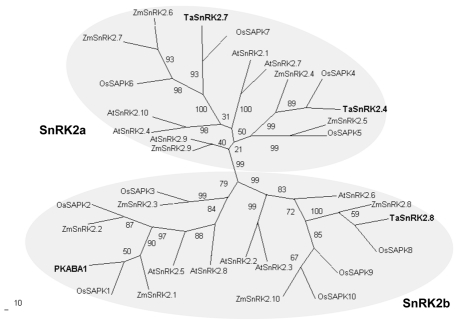
PKABA1, the first cloned SnRK2 member in wheat, was induced by ABA and dehydration stress, and it repressed the activities of gibberellic acid-inducible promoters when transiently overexpressed in barley aleurone layers.5,6 In our recent studies, three wheat SnRK2 members, TaSnRK2.4, TaSnRK2.7 and TaSnRK2.8 were cloned and characterized. Experimental evidence supported they were extensive regulatory factors in carbohydrate metabolism and stress signal transduction.7–9 Here, a phylogenetic tree was constructed with putative amino acid sequences of PKABA1, TaSnRK2.4, TaSnRK2.7, TaSnRK2.8 and SnRK2 family members in Arabidopsis, rice and maize (Fig. 1). TaSnRK2.4 and TaSnRK2.7 were clustered in the SnRK2a subclass and PKABA1 and TaSnRK2.8 fell into the SnRK2b subclass. Furthermore, the counterparts from Arabidopsis, rice and maize were clustered in the same clades, implying the emergence of SnRK2 occurred before the separation of monocots and dicots.
Figure 1.
Phylogenetic tree of four wheat SnRK2 members and SnRK2s from other plant species. Two distinct isoform groups are presented in grey. Ta, Triticum aestivum; Os, Oryza sativa; At, Arabidopsis thaliana; Zm, Zea mays. The phylogenetic tree was constructed with the PHYLIP 3.68 package; bootstrap values are in percentage.
Global Regulator of Carbohydrate Metabolism
Expression pattern is a direct indicator of a gene's involvement in developmental or differential events. In Arabidopsis, AtSRK2.8/AtSRK2C was identified as a root-specific protein kinase and AtSRK2.6/AtSRK2E/OST1 was confirmed to play a pivotal role in stomatal closure in leaves.10,11 Gene expression patterns in various wheat tissues showed that TaSnRK2.4, TaSnRK2.7 and TaSnRK2.8 were constitutively expressing genes; the highest expression of TaSnRK2.4 occurred in booting spindle, while that of TaSnRK2.7 and TaSnRK2.8 occurred in root. Moreover, all three proteins were present in the cell membrane, cytoplasm and nucleus. Thus, SnRK2 kinases existed extensively in plant cells and tissues.
It is well documented that yeast SNF1-kinase and mammalian AMPK have key roles in sugar metabolism.1,2 Similarly, our recently results showed that TaSnRK2.7 was mapped on chromosome 2AL with the flanking markers WMC179.4 and WMC401,12 which were co-located in the same or adjacent chromosome intervals with QTLs for phosphorus utilization efficiency13 and accumulation efficiency of stem water-soluble carbohydrates.14 To unravel the roles of the SnRK2 in the regulation of carbohydrate and energy metabolism, the three SnRK2s were transferred to Arabidopsis, respectively and the significant lower total soluble carbohydrate in transgenic Arabidopsis was identified. The results suggested that SnRK2 was involved in carbohydrate metabolism. As a result, it could function in plant growth and development, such as overexpression of TaSnRK2.4 in Arabidopsis resulted in the delayed seedling establishment and longer primary roots, and overexpressing TaSnRK2.7 and TaSnRK2.8 leaded to improved root growth and development, respectively.
Pivotal Factor in Stress Signal Transduction
Besides the prime carbon and energy source to plant growth and development, sugars can complement and interact with various hormones and growth factors signaling mechanisms to regulate metabolism and stress-resistance in complex systems. Recently, the pivotal roles of sugars in plant growth and development, and key players in sugar signaling network have been uncovered.15–17 As an integral component of the sugar signaling pathway, the plant SNF1 complex has been studied intensively. Currently, evidence suggested that the phosphatase PP2C acted as a constitutive negative regulator of SnRK2 kinases in the absence of the phytohormone abscisic acid (ABA) and the presence of ABA could enable the PYR/PYL/RCAR proteins to bind to and repress PP2C. Sequestration of PP2C permitted the auto-activation of SnRK2 kinases, which could phosphorylate downstream transcription factors (ABF/AREB) and facilitate transcription of ABA-responsive genes.18–20 These studies were focused on the plant hormone ABA, which was often recruited as the primary signal for increasing the transcription levels of the stress responsive genes, while some SnRK2 members might participate in ABA-independent signal transduction pathways.21,22 Until now, little is known in detail about its role in plant ABA-independent stress signaling.
In our research, although all the three members, TaSnRK2.4, TaSnRK2.7 and TaSnRK2.8, were involved in response to PEG, NaCl and cold stresses in wheat, they exhibited distinct expression patterns. Moreover, TaSnRK2.4 and TaSnRK2.8 could be induced by ABA treatment, whereas TaSnRK2.7 might be involved in ABA-independent pathway. Function analysis indicated that all plants overexpressing TaSnRK2.4, TaSnRK2.7 and TaSnRK2.8 exhibited the enhanced resistance to multi-abiotic stresses through increasing osmotic adjustment ability, promoting photosynthetic capability and strengthening seedling roots. These supported that wheat SnRK2 members might be involved in different stress signal pathways.
To elucidate the molecular mechanism of SnRK2s in stress response, the expression levels of the genes participated in ABA biosynthesis and signaling or those involved in stress protection were investigated in transgenic Arabidopsis. As expected, the transcript levels of ABA biosynthesis genes (ABA1, ABA2), ABA signaling genes (ABI3, ABI4, ABI5), stress-responsive genes including two ABA-dependent genes (RD29A, RD29B) and three ABA-independent genes (CBF1, CBF2, CBF3) were generally higher in transgenic TaSnRK2.8 plants than control plants under both normal and stress conditions. Intriguingly, the findings suggested that TaSnRK2.8 may act on the upstream regulators of these genes in stress tolerance, and thus directly or indirectly involved in ABA-dependent and ABA-independent signaling networks.
Conclusions and Perspectives
The results presented here demonstrate that (1) wheat SnRK2s were multifunctional regulatory factors, acted within an intricate network which linked metabolic and stress signaling in plant. Overexpression of TaSnRK2s in Arabidopsis remarkably enhances tolerance to drought, salt and cold stresses without growth retardation. Therefore, there is a potential to utilize them in the stress-tolerance improvement in crops. (2) Individual members of SnRK2 had evolved specifically for stress signaling and acquired distinct regulatory properties. (3) Each member might be involved in multiple signaling pathways. In future investigations, it will be interesting to determine the biochemical properties and precise roles of wheat SnRK2 in metabolic and stress signaling to further advance the understanding of its adaptive mechanisms under stress conditions.
Acknowledgements
We thank Dr. Frantisek Baluska for kindly inviting this review. This work was supported by the National Key Technologies R&D Program (2009ZX08002-012B).
References
- 1.Halford NG, Hardie DG. SNF1-related protein kinases: global regulators of carbon metabolism in plants? Plant Mol Biol. 1998;37:735–748. doi: 10.1023/a:1006024231305. [DOI] [PubMed] [Google Scholar]
- 2.Hardie DG, Carling D, Carlson M. The AMP-activated/SNF1 protein kinase subfamily: metabolic sensors of the eukaryotic cell? Annu Rev Biochem. 1998;67:821–855. doi: 10.1146/annurev.biochem.67.1.821. [DOI] [PubMed] [Google Scholar]
- 3.Halford NG, Hey SJ. Snf1-related protein kinases (SnRKs) act within an intricate network that links metabolic and stress signalling in plants. Biochem J. 2009;419:247–259. doi: 10.1042/BJ20082408. [DOI] [PubMed] [Google Scholar]
- 4.Zheng ZF, Xu XP, Crosley RA, Greenwalt SA, Sun YJ, et al. The protein kinase SnRK2.6 mediates the regulation of sucrose metabolism and plant growth in Arabidopsis. Plant Physiol. 2010;153:99–113. doi: 10.1104/pp.109.150789. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Anderberg RJ, Walker-Simmons MK. Isolation of a wheat cDNA clone for an abscisic acid-inducible transcript with homology to protein kinases. Proc Natl Acad Sci USA. 1992;89:10183. doi: 10.1073/pnas.89.21.10183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Gomez-Cadenas A, Verhey SD, Holappa LD, Shen Q, Ho TH, et al. An abscisic acid-induced protein kinase, PKABA1, mediates abscisic acid-suppressed gene expression in barley aleurone layers. Proc Natl Acad Sci USA. 1999;96:1767–1772. doi: 10.1073/pnas.96.4.1767. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Mao XG, Zhang HY, Tian SJ, Chang XP, Jing RL. TaSnRK2.4, an SNF1-type serine/threonine protein kinase of wheat (Triticum aestivum L.), confers enhanced multistress tolerance in Arabidopsis. J Exp Bot. 2010;61:683–696. doi: 10.1093/jxb/erp331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Zhang HY, Mao XG, Jing RL, Xie HM. Characterization of a common wheat (Triticum aestivum L.) TaSnRK2.7 gene involved in abiotic stress responses. J Exp Bot. 2011;62:975–988. doi: 10.1093/jxb/erq328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Zhang HY, Mao XG, Wang CS, Jing RL. Overexpression of a common wheat gene TaSnRK2.8 enhances tolerance to drought, salt and low temperature in Arabidopsis. PLOS One. 2010;5:16041. doi: 10.1371/journal.pone.0016041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Mustilli A, Merlot S, Vavasseur A, Fenzi F, Giraudat J. Arabidopsis OST1 protein kinase mediates the regulation of stomatal aperture by abscisic acid and acts upstream of reactive oxygen species production. Plant Cell. 2002;14:3089–3099. doi: 10.1105/tpc.007906. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Umezawa T, Yoshida R, Maruyama K, Yamaguchi-Shinozaki K, Shinozaki K. SRK2C, a SNF1-related protein kinase 2, improves drought tolerance by controlling stress-responsive gene expression in Arabidopsis thaliana. Proc Natl Acad Sci USA. 2004;101:17306–17311. doi: 10.1073/pnas.0407758101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Zhang HY, Mao MX, Wang CS, Wu XS, Jing RL. An abiotic stress response gene TaSnRK2.7-B in wheat accessions: genetic diversity analysis and gene mapping based on SNPs. (478).Gene. 2011;1(1–2):28–34. doi: 10.1016/j.gene.2011.01.011. [DOI] [PubMed] [Google Scholar]
- 13.Su JY, Zheng Q, Li HW, Li B, Jing RL, et al. Detection of QTLs for phosphorus use efficiency in relation to agronomic performance of wheat grown under phosphorus sufficient and limited conditions. Plant Science. 2009;176:824–836. [Google Scholar]
- 14.Yang DL, Jing RL, Chang XP, Li W. Identification of quantitative trait loci and environmental interactions for accumulation and remobilization of water-soluble carbohydrates in wheat (Triticum aestivum L.) stems. Genetics. 2007;176:571–584. doi: 10.1534/genetics.106.068361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Smith AM, Stitt M. Coordination of carbon supply and plant growth. Plant Cell Environ. 2007;30:1126–1149. doi: 10.1111/j.1365-3040.2007.01708.x. [DOI] [PubMed] [Google Scholar]
- 16.Leon P, Sheen J. Sugar and hormone connections. Trends Plant Sci. 2003;8:110–116. doi: 10.1016/S1360-1385(03)00011-6. [DOI] [PubMed] [Google Scholar]
- 17.Smeekens S. Sugar-induced signal transduction in plants. Annu Rev Plant Physiol Plant Mol Biol. 2000;51:49–81. doi: 10.1146/annurev.arplant.51.1.49. [DOI] [PubMed] [Google Scholar]
- 18.Furihata T, Maruyama K, Fujita Y, Umezawa T, Yoshida R, et al. Abscisic acid-dependent multisite phosphorylation regulates the activity of a transcription activator AREB1. Proc Natl Acad Sci USA. 2006;103:1988–1993. doi: 10.1073/pnas.0505667103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Fujii H, Chinnusamy V, Rodrigues A, Rubio S, Antoni R, et al. In vitro reconstitution of an abscisic acid signalling pathway. Nature. 2009;462:660–664. doi: 10.1038/nature08599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Park SY, Fung P, Nishimura N, Jensen DR, Fujii H, et al. Abscisic acid inhibits type 2C protein phosphatases via the PYR/PYL family of START proteins. Science. 2009;324:1068–1071. doi: 10.1126/science.1173041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Boudsocq M, Droillard MJ, Barbier-Brygoo H, Lauriere C. Different phosphorylation mechanisms are involved in the activation of sucrose non-fermenting 1 related protein kinases 2 by osmotic stresses and abscisic acid. Plant Molecular Biology. 2007;63:491–503. doi: 10.1007/s11103-006-9103-1. [DOI] [PubMed] [Google Scholar]
- 22.Kobayashi Y, Yamamoto S, Minami H, Kagaya Y, Hattori T. Differential activation of the rice sucrose nonfermenting1-related protein kinase2 family by hyperosmotic stress and abscisic acid. Plant Cell. 2004;16:1163–1177. doi: 10.1105/tpc.019943. [DOI] [PMC free article] [PubMed] [Google Scholar]