Abstract
Receptor-like kinases (RLKs) are a family of transmembrane proteins with a variable ligand-binding extracellular domain and a cytoplasmic kinase domain. In Arabidopsis, there are ∼600 RLKs believed to have diverse functions during plant growth, development and interactions with the environment. Based on the variable extracellular domain, RLKs can be classified into different subfamilies. The CrRLK subfamily contains 17 members in Arabidopsis and characterization of some of its members suggests a role for these proteins in the regulation of growth and reproduction. This review focuses on the roles of CrRLKs in the regulation of polarized growth with emphasis on the newly identified signal transduction pathways activated downstream of CrRLKs. A picture is emerging where CrRLKs are part of a conserved signal transduction cascade important for growth maintenance in different cell types.
Key words: CrRLKs, FERONIA, RAC/ROP, ROS, polar growth
The ability of plants to perceive and process environmental and internal information into coordinated responses is crucial to their adaptability and survival in constantly changing environments. Most of signal perception occurs at the plasma membrane of cells where membrane-associated receptors receive signals to activate downstream signaling cascades that regulate growth and development. In plants and animals alike, receptor-like kinases (RLKs) mediate many of the signaling events at the cell surface and in the model plant Arabidopsis they comprise a monophyletic family with more than 600 members.1 RLKs are transmembrane proteins with a variable N-terminal extracellular domain and a Ser/Thr intracellular kinase domain. The diversity of their extracellular domains suggests involvement in the transduction of a wide range of signals and allows them to be classified into different sub-families.2 The CrRLK1L subfamily (from here on referred to as CrRLK) is named after the first member characterized in Catharanthus roseus cell cultures3 and contains 17 members in Arabidopsis.4 Several members of this family have now been implicated in growth regulatory processes.
THESEUS1 (THE1) was identified through a suppressor screen of a cellulose-deficient mutant (prc1-1) which has a short hypocotyl phenotype.5 Loss of THE1 function resulted in reduced growth inhibition in the prc1-1 the1 double mutant. Interestingly, the the1 mutation itself has no effect in wild type background, thus leading to the suggestion that THE1 functions as a sensor of cell wall integrity in situations where the cell wall is weakened and organ elongation would be detrimental for the plant.4,5
A second CrRLK, FERONIA (FER), was first implicated in the regulation of female control of fertility. In the female gametophyte FER is involved in sensing pollen tube arrival and promoting its rupture which is necessary for double fertilization to occur.6,7 FER is in fact involved in several processes depending on the tissue where it is expressed. In hypocotyls, FER is involved in the integration of ethylene and brassinosteroid (BR) signals to regulate hypocotyl elongation in the dark.8 Moreover, FER, THE1 and the closely related HERCULES1 (HERK1), were found to regulate cell elongation by interacting with BR signaling.9 More recently, roles for FER in the regulation of root hair development and fungal invasion have been established.10,11 The pollen-specific ANXUR1 (ANX1) and ANXUR2 (ANX2) are closely related to FER and act redundantly to maintain pollen tube growth integrity during its journey through the style and ensure against precocious pollen tube rupture before reaching the ovule.12,13
Apparently with different biological roles, all the CrRLK members analyzed thus far have an effect on the growth of plant cells. The present review focuses on their role during cell growth with emphasis on polar cell growth and the downstream pathways activated by CrRLKs.
Regulation of Polar Cell Growth
Polarized cell growth is an important process in many aspects of plant development. The classic cases are the growth of root hairs on the root surface and of pollen tubes en route to deliver sperm cells to the female gametophyte. In these cells, growth occurs only at the tip of the cell and relies on signaling cascades that allow the polarized delivery of material to the tip cell surface. Many of the downstream events in these signaling pathways are known but the molecules responsible for perception at the cell surface are poorly characterized.14,15 Recent studies have provided strong evidence for a role of CrRLKs as important sensors during polar growth regulation in plants: they may act in the cells where they are expressed to maintain tip growth or interact with and control the growth of other invading tip growing cells.6,7,10–13,16,17
Cross cell regulation: FER controls the growth of invading tip-growing cells.
FER is almost constitutively expressed in the plant, pollen being a notable exception.16 Roles for FER in different tissues have been described and it is likely that FER activity will depend on the tissue where it is expressed.
In the female gametophyte FER is necessary to sense pollen tube arrival. In feronia (fer) plants, pollen tube growth and guidance throughout the mutant female tissues remains unaffected and they are able to enter the ovules but, instead of rupturing in order to release the male gametes, pollen tubes continue to grow and fertilization does not occur. Moreover, more than one pollen tube is able to enter each fer ovule.6,7 Interestingly, in synergid cells, FER is polarly localized in the filiform apparatus, a thickened cell wall region with an abundance of synergid cell membrane close to the pollen tube entrance site.6,16 FER is therefore ideally located to sense pollen tube arrival, to regulate pollen tube reception and rupture and to deter the arrival of other pollen tubes.
There are striking similarities between fertilization and fungal invasion.18 Both invading cells (pollen tubes and fungal hyphae) elongate by exclusively growing at the tip and along a substrate secreted by the tissues they invade. Upon reaching their target (ovule for a pollen tube or host cell for a pathogenic fungus) they are specifically recognized by the host tissues and in the case of a compatible reaction allowed to invade the tissue. This triggers a series of events that cause tip growth arrest, tube rupture and the consequent release of sperm cells or spores.18 Recently Kessler et al. (2010) reported an exciting finding that further revealed conserved mechanisms behind these processes.11 They found that fer mutants are more resistant to fungal infection in part due to reduced entry rates of fungal hyphae and reduced spore production.11 Interestingly, host cell entry is not as severely affected as the establishment of spore producing structures suggesting that FER is acting at the later stages of infection including hypha rupture to release spores.11 These observations suggest that FER may act as a cell membrane sensor to perceive the polar growth of the incoming pollen tube or fungal hyphae and induce the release of the signals necessary to regulate their growth.
Same cell regulation: ANX1/ANX2 and FER are required for the establishment and maintenance of tip growth.
Interestingly, CrRLKs are not only involved in arresting the growth of invading tip growing cells but are also important in maintaining tip growth. In elongating pollen tubes, the CrRLKs ANX1 and ANX2 were found to be necessary for maintaining pollen tube integrity and the anx1/anx2 double mutant is male sterile.12,13 Pollen tubes of anx1/anx2 plants burst when germinated in vitro and precociously in the pistil, failing to reach the female gametophyte. In addition, the tips of the arrested anx1/anx2 pollen tubes are bulged suggesting a disruption in the polar growth process.13 ANX1 and ANX2 proteins were found to be localized in the plasma membrane at the tip of the pollen tube.12,13 Because both FER and ANX1/ANX2 are in contact with the same environment at the ovule entrance, one attractive possibility is that both compete for the same ligands. Conceivably, FER could sequester the ligands preventing ANX1/ANX2-mediated signaling in the pollen tube ultimately causing its rupture.17 Alternatively, upon arrival, the pollen tube may release a signal that is recognized by FER, triggering a signaling cascade within the synergid cell that ultimately leads to the release of another signal that is recognized by ANX1/ANX2 and causes pollen tube growth arrest and bursting (Fig. 1B).
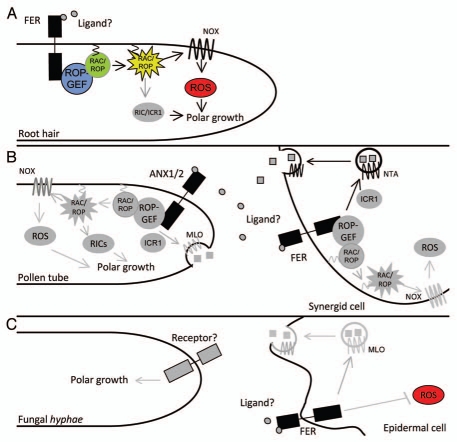
Figure 1.
Proposed models for CrRLK-dependent signaling pathways in plant cells. Identified components of the pathway are shown in black/color and putative/proposed ones in gray. (A) In root hairs, FER is activated by a yet unidentified ligand. This activation recruits ROPGEF1 that in turn activates the RAC/ROP protein. RAC/ROP activation leads to a series of downstream events including the activation of the NADPH oxidase (NOX) that stimulates reactive oxygen species (ROS) production. ROS, possibly acting together with other cellular pathways mediated by other RAC/ROP effectors such as RICs (ROP interactive CRIB motif-containing proteins),38 and ICR1 (Interactor of constitutive active ROPs),39 regulate root hair polar growth. (B) Model for the pollen tube-synergid cell interaction. In the elongating pollen tube ANX1/ANX2 signaling is necessary to support polar growth. We propose that ANX1/ANX2 activate RAC/ROP signaling pathways as is the case in root hairs and this will in turn activate many downstream effectors and ROS production essential for the maintenance of polar growth. On the synergid cell side, FER is inactive and synergid cell integrity is maintained. Upon pollen tube arrival, FER is activated leading to synergid cell degeneration and this may be mediated by RAC/ROP-dependent ROS production. In addition, FER triggers the relocalization of NTA from the endomembrane system to the cell membrane. This might promote the release of signals or extracellular matrix material and could be mediated by ICR1 through the activation of RAC/ROP signaling. One possible scenario is that ANX1/ANX2 and FER compete for the same ligands; upon pollen tube arrival, FER sequesters the ligand, preventing ANX1/ANX2 activity causing pollen tube rupture. Another possibility is that activated FER triggers a signaling cascade leading to the release of a signal that causes pollen tube rupture. (C) Model for the role of FER during fungal hyphae infection. FER regulates hyphae penetration possibly through the activation of MLO proteins and the inhibition of ROS production through a yet unknown mechanism.
Root hairs also elongate by tip growth and a role for FER during this process has recently been described.10 The root hairs and trichomes of fer mutants are severely defective with many root hairs collapsing after emergence, demonstrating that FER is also important for polar growth of root hairs.10
Another example of polar growing cells are the protonemal filaments in the alga Physcomytrella patens.19 It will be interesting to determine if any of the six Physcomitrella patens CrRLKs20 are also involved in the polar growth of these structures. So far no homologues for CrRLKs have been identified in fungi.20
From these data a picture is emerging where plasma-membrane localized CrRLKs are important components of polar growth establishment and maintenance in the tissues where they are expressed. Due to the broad expression pattern of some CrRLK family members such as FER, it is likely that more growth-related roles will be described.
In addition, these observations also underscore the fact that some CrRLKs may assume different functions. The fact that even a single CrRLK like FER may assume two seemingly opposing roles, one to promote growth the other to arrest growth, also suggests that growth promotion or inhibition might depend on in which cells they are expressed and/or on the availability of ligands and effectors.
Signaling Events Downstream of CrRLK Activation
Until very recently, the downstream signaling events triggered by CrRLK activation were largely unknown. Two recent studies revealed new, and somewhat intriguing, components of the FER, and thus potentially of other CrRLKs, signaling pathways.
RAC/ROP activation of NADPH oxidase (NOX)-dependent reactive oxygen species (ROS) production.
ROS are important secondary messengers in a variety of signaling pathways during growth and development. ROS secreted to the apoplast are important regulators of cell wall properties while a tip-focused ROS gradient is necessary for polar growth maintenance.21 Upon pathogen infection, ROS are important second messengers in the activation of plant responses.22 Studies using the the1 mutant revealed that many genes involved in the protection against ROS are upregulated in the the1 mutant and that THE1 controls ectopic lignin production in the presence of ROS. These observations suggest that ROS production is triggered downstream of THE1 signaling.4,5 Recent studies of FER disclosed direct evidence for the activation of ROS signaling pathways.10 The root hairs of fer-deficient plants showed decreased NOX-dependent ROS accumulation and the opposite was observed in cells overexpressing FER.10 On the other hand and by a yet unknown mechanism, the leaves of the fer mutant show a phenotype of spontaneous cell death and high H2O2 accumulation even in the absence of fungal infection.11 The multiple pathways and complexity of chemical reactions underlying production of ROS (a collection of different oxygenated species), the complexity of CrRLK signaling pathways and the overall growth defects in fer mutants probably underline the seemingly opposite effects of FER on ROS accumulation observed in different tissues.
Plant RAC/ROP GTPases are membrane associated proteins involved in many signaling processes, perhaps their role during polar growth being the best characterized.15,16 In a yeast two-hybrid screen, FER was found to interact with ROPGEF1, an upstream activator of RAC/ROPs.10 fer seedlings maintain reduced levels of active RAC/ROPs and upregulating RAC/ROP signaling in fer restores auxin-regulated ROS accumulation and root hair growth. The inactive form of RAC/ROP was preferentially pulled-down by FER suggesting that FER activates ROPGEF1, which in turn recruits the inactive RAC/ROP, stimulating GDP/GTP exchange leading to RAC/ROP activation. In root hairs, active RAC/ROP stimulates NOX activity leading to ROS accumulation at the tip and consequently maintenance of polar growth10 (Fig. 1A). The role of RAC/ROPs signaling downstream of CrRLKs is likely not to be restricted to root hair growth. In fact, RAC/ROP regulates NOX-dependent ROS production during defense responses in rice23 and given the role of FER in powdery mildew resistance,11 it may also act through the activation of a RAC/ROP signaling cascade. In addition, RAC/ROP activity is crucial for the polar growth of pollen tubes and the role for ROS during this process has been suggested.24 It is thus plausible that ANX1/ANX2 regulate pollen tube growth by the activation of RAC/ROP-dependent ROS signaling pathways (Fig. 1B). Moreover, ROS are important during secondary cell wall formation. At least in cotton fibers, this mechanism seems to be dependent on RAC/ROP GTPases,25 suggesting that the THE1-dependent ectopic lignification may also result from RAC/ROP-mediated ROS production.
RAC/ROP GTPases activate many diverse signaling pathways through a wide range of effectors.14 It will be interesting to determine if the various CrRLKs activate different RAC/ROPs leading to distinct signaling pathways in the plant. In addition, given FER's function in regulating female fertility6,7,16 and that a role for RAC/ROPs in female reproductive cells has not been implicated before, discovery of FER as an upstream regulator for RAC/ROP reveals yet another key role for these small GTPases in plant development.
MLO proteins.
Aiming to identify new components of the FER signaling pathway activated when the pollen tube reaches the synergid cells, Kessler and colleagues unexpectedly identified NORTIA (NTA), a mildew resistance locus o protein (MLO).11 MLO proteins were first identified as membrane-associated susceptibility factors during powdery mildew infection in barley.26 Just like what is observed in fer mutants, pollen tubes fail to rupture when reaching the synergids of a nta ovule and continue to grow inside the mutant female gametophyte and fertilization does not occur. NTA is expressed only in synergid cells where it localizes to the endomembrane system. In the wild type ovule, upon pollen tube arrival, NTA becomes polarized to the plasma membrane at the site of pollen tube entrance and this re-localization is dependent on FER.11 These findings underscore the similarities between pollen tube perception and fungal invasion and further support a role for CrRLKs in the maintenance of polar growth. The role of MLOs during defense is not established but they seem to be involved in the delivery of cargo vesicles to the plasma membrane.27 In this context, upon pollen tube arrival, FER would initiate a signaling cascade leading to the re-localization of NTA-containing vesicles to the plasma membrane at the point of pollen tube contact (Fig. 1B). Potentially these vesicles would contain the factors necessary to inhibit pollen tube growth and promote their rupture. Although NTA is only expressed in synergid cells, the other members of the MLO family (15 in Arabidopsis) are expressed in different tissues and in response to different stimuli28 and this would provide functional specificity for FER in different tissues (Fig. 1C). Interestingly, together with MLO proteins, RAC/ROPs are also involved in actin-dependent cell polarity establishment during powdery mildew fungal infection in barley.29–31
Potential Ligands for CrRLKs
Discovery of ligand(s) responsible for CrRLK activation will be the next exciting frontier to further our understanding of these RLKs. There are some attractive possibilities.
Mutations in LORELEI (LRE), a glycosylphosphatidylinositol (GPI)-anchored cysteine-rich protein, induce similar reproductive defects as fer in that lre female gametophyte also fails to mediate rupture of the penetrating pollen tube.32,33 LRE is expressed in a narrow developmental window in the ovary, around peak reception time and accumulates in the synergid cells just prior to pollination. As GPI-anchored proteins, they would be secreted and possibly remain associated with the outer leaflet of the cell membrane sharing the extracellular environment with CrRLKs, suggesting potential interactions as ligand-receptor or co-receptors for other ligands.
Domain homology analysis using the extracellular domain of FER revealed that it has two domains with similarity with Malectin proteins that in mammalian systems bind small oligosaccharides (Cheung AY, unpublished data; reviewed in ref. 34). This is very interesting as cell wall-derived oligosaccharides have been implicated in pathogen defense responses.35 In addition, cell-wall derived oligosaccharides are ligands for a group of cell-wall associated kinases (WAKs) that are important during growth and development.36 It is also well established that glycoproteins are important for pollen tube growth and guidance along the female tissues.37 It is therefore plausible that extracellular matrix (ECM)-derived oligosaccharides are ligands for CrRLKs. This hypothesis is attractive because the ECM composition is different in different tissues and this, together with the presence of different glycoenzymes would allow for diverse signaling environments. The same CrRLK might be exposed to different ligands in different tissues to elicit distinct responses and/or different CrRLKs might bind different ligands providing them with functional specificity.
Future Perspectives
Despite the recent increase in activity and interest in studying CrRLK-mediated signaling pathways, many questions remain unanswered. As mentioned above, the identification of CrRLK ligands is crucial for the understanding of their function in different developmental and environmental contexts. RAC/ROPs are important mediators of hormone signaling pathways that impact on many of the developmental and defense processes, functions also mediated by CrRLKs. It will be interesting to determine how these signaling pathways are integrated. Another important area of research is the identification of additional downstream pathways activated by RAC/ROP-mediated CrRLK signaling and the events triggered, e.g. by ROS production, in different cell and tissue types. Finally the role of NTA-dependent vesicle trafficking during FER signaling remains to be established. Do these vesicles carry the ligand for the CrRLKs in the pollen tube/hyphae side? Answering these questions will be critical for furthering our knowledge on how CrRLKs function as sensors during plant growth, development and interaction with the environment.
Acknowledgements
Research carried out in A.Y.C.'s laboratory is supported by grants from the United States National Science Foundation (IOB0544222).
References
- 1.Morris ER, Walker JC. Receptor-like protein kinases: the keys to response. Curr Opin Plant Biol. 2003;6:339–342. doi: 10.1016/s1369-5266(03)00055-4. [DOI] [PubMed] [Google Scholar]
- 2.Shiu SH, Bleecker AB. Expansion of the receptor-like kinase/Pelle gene family and receptor-like proteins in Arabidopsis. Plant Physiol. 2003;132:530–543. doi: 10.1104/pp.103.021964. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.SchulzeMuth P, Irmler S, Schroder G, Schroder J. Novel type of receptor-like protein kinase from a higher plant (Catharanthus roseus)—cDNA, gene intramolecular autophosphorylation and identification of a threonine important for auto- and substrate phosphorylation. J Biol Chem. 1996;271:26684–26689. doi: 10.1074/jbc.271.43.26684. [DOI] [PubMed] [Google Scholar]
- 4.Hematy K, Hofte H. Novel receptor kinases involved in growth regulation. Curr Opin Plant Biol. 2008;11:321–328. doi: 10.1016/j.pbi.2008.02.008. [DOI] [PubMed] [Google Scholar]
- 5.Hematy K, Sado PE, Van Tuinen A, Rochange S, Desnos T, Balzergue S, et al. A receptor-like kinase mediates the response of Arabidopsis cells to the inhibition of cellulose synthesis. Curr Biol. 2007;17:922–931. doi: 10.1016/j.cub.2007.05.018. [DOI] [PubMed] [Google Scholar]
- 6.Huck N, Moore JM, Federer M, Grossniklaus U. The Arabidopsis mutant feronia disrupts the female gametophytic control of pollen tube reception. Development. 2003;130:2149–2159. doi: 10.1242/dev.00458. [DOI] [PubMed] [Google Scholar]
- 7.Rotman N, Rozier F, Boavida L, Dumas C, Berger F, Faure JE. Female control of male gamete delivery during fertilization in Arabidopsis thaliana. Curr Biol. 2003;13:432–436. doi: 10.1016/s0960-9822(03)00093-9. [DOI] [PubMed] [Google Scholar]
- 8.Deslauriers SD, Larsen PB. FERONIA is a key modulator of brassinosteroid and ethylene responsiveness in Arabidopsis hypocotyls. Molec Plant. 2010;3:626–640. doi: 10.1093/mp/ssq015. [DOI] [PubMed] [Google Scholar]
- 9.Guo HQ, Li L, Ye HX, Yu XF, Algreen A, Yin YH. Three related receptor-like kinases are required for optimal cell elongation in Arabidopsis thaliana. Proc Natl Acad Sci USA. 2009;106:7648–7653. doi: 10.1073/pnas.0812346106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Duan QH, Kita D, Li C, Cheung AY, Wu HM. FERONIA receptor-like kinase regulates RHO GTPase signaling of root hair development. Proc Natl Acad Sci USA. 2010;107:17821–17826. doi: 10.1073/pnas.1005366107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Kessler SA, Shimosato-Asano H, Keinath NF, Wuest SE, Ingram G, Panstruga R, et al. Conserved molecular components for pollen tube reception and fungal invasion. Science. 2010;330:968–971. doi: 10.1126/science.1195211. [DOI] [PubMed] [Google Scholar]
- 12.Boisson-Dernier A, Roy S, Kritsas K, Grobei MA, Jaciubek M, Schroeder JI, et al. Disruption of the pollen-expressed FERONIA homologs ANXUR1 and ANXUR2 triggers pollen tube discharge. Development. 2009;136:3279–3288. doi: 10.1242/dev.040071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Miyazaki S, Murata T, Sakurai-Ozato N, Kubo M, Demura T, Fukuda H, et al. ANXUR1 and 2, sister gGenes to FERONIA/SIRENE, are male factors for coordinated fertilization. Curr Biol. 2009;19:1327–1331. doi: 10.1016/j.cub.2009.06.064. [DOI] [PubMed] [Google Scholar]
- 14.Lee YJ, Yang Z. Tip growth: signaling in the apical dome. Curr Opin Plant Biol. 2008;11:662–671. doi: 10.1016/j.pbi.2008.10.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Nibau C, Wu HM, Cheung AY. RAC/ROP GTPases: “hubs” for signal integration and diversification in plants. Trends Plant Sci. 2006;11:309–315. doi: 10.1016/j.tplants.2006.04.003. [DOI] [PubMed] [Google Scholar]
- 16.Escobar-Restrepo JM, Huck N, Kessler S, Gagliardini V, Gheyselinck J, Yang WC, et al. The FERONIA receptor-like kinase mediates male-female interactions during pollen tube reception. Science. 2007;317:656–660. doi: 10.1126/science.1143562. [DOI] [PubMed] [Google Scholar]
- 17.Kanaoka MM, Torii KU. FERONIA as an upstream receptor kinase for polar cell growth in plants. Proc Natl Acad Sci USA. 2010;107:17461–17462. doi: 10.1073/pnas.1013090107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Dresselhaus T, Márton ML. Micropylar pollen tube guidance and burst: adapted from defense mechanisms? Curr Opin Plant Biol. 2009;12:773–780. doi: 10.1016/j.pbi.2009.09.015. [DOI] [PubMed] [Google Scholar]
- 19.Eklund DM, Svensson EM, Kost B. Physcomitrella patens: a model to investigate the role of RAC/ROP GTPase signalling in tip growth. J Exp Bot. 2010;61:1917–1937. doi: 10.1093/jxb/erq080. [DOI] [PubMed] [Google Scholar]
- 20.Lehti-Shiu MD, Zou C, Hanada K, Shiu SH. Evolutionary history and stress regulation of plant receptor-like kinase/Pelle genes. Plant Physiol. 2009;150:12–26. doi: 10.1104/pp.108.134353. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Swanson S, Gilroy S. ROS in plant development. Physiol Plant. 2010;138:384–392. doi: 10.1111/j.1399-3054.2009.01313.x. [DOI] [PubMed] [Google Scholar]
- 22.Torres MA. ROS in biotic interactions. Physiol Plant. 2010;138:414–429. doi: 10.1111/j.1399-3054.2009.01326.x. [DOI] [PubMed] [Google Scholar]
- 23.Wong HL, Pinontoan R, Hayashi K, Tabata R, Yaeno T, Hasegawa K, et al. Regulation of rice NADPH oxidase by binding of Rac GTPase to its N-terminal extension. Plant Cell. 2007;19:4022–4034. doi: 10.1105/tpc.107.055624. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Potocký M, Jones MA, Bezvoda R, Smirnoff N, Žárský V. Reactive oxygen species produced by NADPH oxidase are involved in pollen tube growth. New Phytol. 2007;174:742–751. doi: 10.1111/j.1469-8137.2007.02042.x. [DOI] [PubMed] [Google Scholar]
- 25.Potikha TS, Collins CC, Johnson DI, Delmer DP, Levine A. The involvement of hydrogen peroxide in the differentiation of secondary walls in cotton fibers. Plant Physiol. 1999;119:849–858. doi: 10.1104/pp.119.3.849. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Büschges R, Hollricher K, Panstruga R, Simons G, Wolter M, Frijters A, et al. The barley MLO gene: a novel control element of plant pathogen resistance. Cell. 1997;88:695–705. doi: 10.1016/s0092-8674(00)81912-1. [DOI] [PubMed] [Google Scholar]
- 27.Panstruga R. Serpentine plant MLO proteins as entry portals for powdery mildew fungi. Biochem Soc Trans. 2005;33:389–392. doi: 10.1042/BST0330389. [DOI] [PubMed] [Google Scholar]
- 28.Chen Z, Hartmann H, Wu MJ, Friedman E, Chen JG, Pulley M, et al. Expression analysis of the AtMLO; gene family encoding plant-specific seven-transmembrane domain proteins. Plant Molec Biol. 2006;60:583–597. doi: 10.1007/s11103-005-5082-x. [DOI] [PubMed] [Google Scholar]
- 29.Opalski KS, Schultheiss H, Kogel KH, Huckelhoven R. The receptor-like MLO protein and the RAC/ROP family G-protein RACB modulate actin reorganization in barley attacked by the biotrophic powdery mildew fungus Blumeria graminis f.sp hordei. Plant J. 2005;41:291–303. doi: 10.1111/j.1365-313X.2004.02292.x. [DOI] [PubMed] [Google Scholar]
- 30.Schultheiss H, Hensel G, Imani J, Broeders S, Sonnewald U, Kogel KH, et al. Ectopic expression of constitutively activated RACB in barley enhances susceptibility to powdery mildew and abiotic stress. Plant Physiol. 2005;139:353–362. doi: 10.1104/pp.105.066613. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Schultheiss H, Preuss J, Pircher T, Eichmann R, Hückelhoven R. Barley RIC171 interacts with RACB in planta and supports entry of the powdery mildew fungus. Cell Microbiol. 2008;10:1815–1826. doi: 10.1111/j.1462-5822.2008.01167.x. [DOI] [PubMed] [Google Scholar]
- 32.Tsukamoto T, Qin Y, Huang Y, Dunatunga D, Palanivelu R. A role for LORELEI, a putative glycosylphosphatidylinositol-anchored protein, in Arabidopsis thaliana double fertilization and early seed development. Plant J. 2010;62:571–588. doi: 10.1111/j.1365-313X.2010.04177.x. [DOI] [PubMed] [Google Scholar]
- 33.Capron A, Gourgues M, Neiva LS, Faure JE, Berger F, Pagnussat G, et al. Maternal control of male-gamete delivery in Arabidopsis involves a putative GPI-anchored protein encoded by the LORELEI gene. Plant Cell. 2008;20:3038–3049. doi: 10.1105/tpc.108.061713. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Schallus T, Feher K, Sternberg U, Rybin V, Muhle-Goll C. Analysis of the specific interactions between the lectin domain of malectin and diglucosides. Glycobiology. 2010;20:1010–1020. doi: 10.1093/glycob/cwq059. [DOI] [PubMed] [Google Scholar]
- 35.Vorwerk S, Somerville S, Somerville C. The role of plant cell wall polysaccharide composition in disease resistance. Trends Plant Sci. 2004;9:203–209. doi: 10.1016/j.tplants.2004.02.005. [DOI] [PubMed] [Google Scholar]
- 36.Brutus A, Sicilia F, Macone A, Cervone F, De Lorenzo G. A domain swap approach reveals a role of the plant wall-associated kinase 1 (WAK1) as a receptor of oligogalacturonides. Proc Natl Acad Sci USA. 2010;107:9452–9457. doi: 10.1073/pnas.1000675107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Lord EM. Adhesion and guidance in compatible pollination. J Exp Bot. 2003;54:47–54. doi: 10.1093/jxb/erg015. [DOI] [PubMed] [Google Scholar]
- 38.Wu G, Gu Y, Li S, Yang Z. A genome-wide analysis of Arabidopsis Rop-interactive CRIB motif-containing proteins that act as Rop GTPase targets. Plant Cell. 2001;13:2841–2856. doi: 10.1105/tpc.010218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Lavy M, Bloch D, Hazak O, Gutman I, Poraty L, Sorek N, et al. A Novel ROP/RAC effector links cell polarity, root-meristem maintenance and vesicle trafficking. Curr Biol. 2007;17:947–952. doi: 10.1016/j.cub.2007.04.038. [DOI] [PubMed] [Google Scholar]