Abstract
Autophagy is an intracellular process for the vacuolar degradation of cytoplasmic components and is important for nutrient recycling during starvation. Chloroplasts can be partially mobilized to the vacuole by autophagy via spherical bodies named Rubisco-containing bodies (RCBs). Although chloroplasts contain approximately 80% of total leaf nitrogen and represent a major carbon and nitrogen source for recycling, the relationship between leaf nutrient status and RCB production remains unclear. We analyzed the effects of nutrient factors on the appearance of RCBs in Arabidopsis leaves and postulated that a close relationship exists between the autophagic degradation of chloroplasts via RCBs and leaf carbon status but not nitrogen status in autophagy. The importance of carbohydrates in RCB production during leaf senescence can be further argued. During nitrogen-limited senescence, as leaf carbohydrates were accumulated, RCB production was strongly suppressed. During the life span of leaves, RCB production increased with the progression of leaf expansion and senescence, while the production declined in late senescent leaves with a remarkable accumulation of carbohydrates, glucose and fructose. These results suggest that RCB production may be controlled by leaf carbon status during both induced and natural senescence.
Key words: arabidopsis (Arabidopsis thaliana), autophagy, chloroplast, nutrient response, leaf senescence, carbohydrate
Autophagy is the major pathway by which proteins and organelles are delivered to the vacuole/lysosome for degradation in eukaryotic organisms. Plant autophagy is considered to play an important role in nutrient recycling under starvation, similar to its role previously noted in yeast and animals.1,2 For example, the creation of an autophagosome, a double-membrane autophagic vesicle, is induced by carbon or nitrogen starvation in plant heterotrophic tissues such as roots and hypocotyls.3–6 Autophagy-defective (atg) mutants of Arabidopsis (Arabidopsis thaliana) have been found to show accelerated leaf senescence under severe carbon or nitrogen starvation and cannot survive and respond to nutrient resupply.4–6 Transcription levels of autophagy genes (ATGs) are elevated during starvation-induced senescence.3,7,8 Recently, we demonstrated that chloroplasts are partially mobilized to the vacuole by autophagy via Rubisco-containing bodies (RCBs), a kind of autophagic vesicles containing chloroplast stroma.9,10 Although chloroplast proteins in leaves are the predominant source for autophagic recycling, it has not been clear how RCB production is affected by nutrient status in leaves.
We analyzed the effects of nutrient factors on the appearance of RCBs in leaves of transgenic Arabidopsis expressing stromatargeted fluorescent proteins.11 During incubation of excised leaves, RCB production was suppressed by the presence of metabolic sugars which were added externally or by light irradiation. The light-mediated suppression was relieved by inhibition of photosynthesis. Starch and sugar contents decreased in leaves incubated in darkness, but increased in light. To examine the effects of internal carbohydrate contents on RCB production, we analyzed RCB appearance during a diurnal cycle or in starch-related mutants. During a diurnal cycle, RCB production was higher in leaves excised at the end of the night with low starch content, and lower in leaves at the end of the day with high starch content. In starch-related mutants, starchless mutants, i.e., phosphoglucomutase (pgm-1) and ADP-Glc pyrophosphorylase1 (adg1-1), produced a large number of RCBs, while starch-excess mutants, i.e., starch-excess1 (sex1-1) and maltose-excess1 (mex1–3), produced fewer RCBs. These results suggest that carbon status is a major factor controlling RCB production in leaves.
Autophagy is induced by nitrogen starvation as well as by carbon starvation in plants.3–6 However, RCB production in nitrogen-limited plant leaves was significantly lower than that in nitrogen-sufficient plants from 1 day after treatment for nitrogen-limitation despite the accelerated loss of Rubisco and nitrogen contents.11 Quantitative analysis of carbohydrates showed that glucose, fructose and starch were remarkably accumulated in nitrogen-limited plant leaves, and that sucrose was also increased. We also analyzed RCB appearance and carbohydrate contents through the life span in fourth leaves of nitrogen-sufficient plants; from 5 days before bolting, the active stage of leaf expansion, to 10 days after bolting, the senescent stage (Fig. 1). RCB production increased in leaves from 5 days before bolting to 5 days after bolting, i.e., the early senescent stage, indicating leaf age is an important factor affecting RCB production. However, RCB production significantly decreased at 10 days after bolting, i.e., the later senescent stage (Fig. 1A). In leaf carbohydrate contents, glucose and fructose were remarkably accumulated, and sucrose gradually increased in late senescent leaves (Fig. 1B). Previous reports have also indicated a significant accumulation of soluble sugars in late senescent leaves.12,13 Sugars are not only energy metabolites but also important signaling molecules.14 Leaf senescence is accelerated by leaf carbon starvation,10,15 while some reports have indicated the possibility that sugar accumulation can promote leaf senescence or be a trigger for initiating senescence.12,13,16,17 Our studies suggest that RCB production may be regulated by leaf carbohydrate status, especially soluble sugars, during senescence. It is likely that leaf sugar starvation promotes RCB production such as individually darkened leaves,10 and that sugar accumulation suppresses RCB production during nitrogenlimited or natural senescence.
Figure 1.
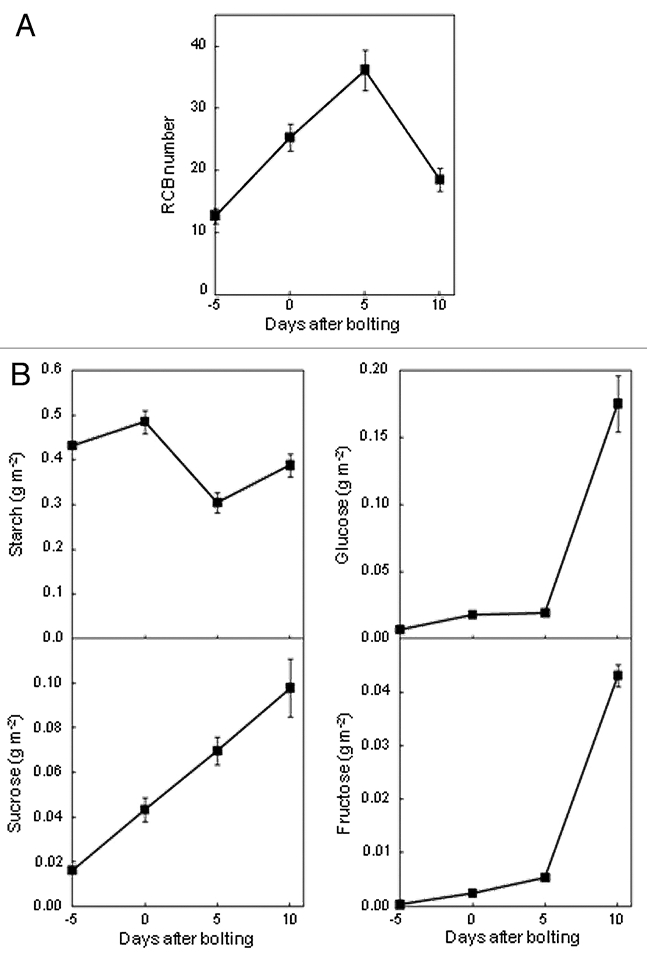
The changes of RCB appearance and leaf carbohydrate contents during the life span of leaves. (A) Changes of the appearance of RCBs. Transgenic Arabidopsis plants expressing chloroplast stroma-targeted GFP were grown in soil under 14 h-photoperiod condition. Fourth leaves from four independent plants were excised through the life span: 5 days before bolting, 0, 5 and 10 days after bolting, and incubated at 23°C for 20 h in 10 mM MES-NaOH (pH 5.5) with 1 µM concanamycin A and 100 µM E-64d in darkness in each period. After incubation, eight quadrangular regions (188 µm × 188 µm each) per leaf were monitored by laser-scanning confocal microscopy, and the number of accumulated RC Bs was counted. Data represent means ± SE (n = 32). (B) Changes of leaf carbohydrate contents. Changes in the starch, sucrose, glucose and fructose contents were measured in fourth leaves in each period. Data represent means ± SE (n = 3).
We also found that the response of the RCB production to nutrient conditions was not always the same as that of non-RCB-type autophagic vesicles, which contain cytoplasmic components other than chloroplasts, during incubation of excised leaves.11 Our results indicate the possibility that RCB production can be specifically controlled by novel mechanisms depending on leaf carbon status. Chloroplasts are plant-characteristic organelles derived from endosymbiosis of a free-living cyanobacterial progenitor. In higher plants, chloroplasts play central roles in plant metabolism via photosynthesis. In darkness or insufficient light conditions which cause energy limitation, stromal proteins such as Rubisco cannot function sufficiently; thus, excess proteins could be reasonable substrates for autophagy as an energy source for survival or adaptation of plants. It seems that RCBs can obtain stromal proteins during carbon-limited conditions without the loss of whole chloroplasts, which suggests a role of RCBs. However, the underpinning mechanisms of RCB production and regulation are still unknown. In yeast, some selective autophagy pathways and specific ATG genes required for respective pathways have been identified. Particularly, receptor proteins, which link targets and autophagic membranes, are important for specific cargo recognition, e.g., ATG19 for the cytoplasm-to-vacuole targeting (Cvt) pathway, ATG30 for pexophagy and ATG32 for mitophagy.18–21 Similar recognition mechanisms may be required for the autophagic degradation of chloroplasts. Identification of such genes is relevant for further understanding of the specific regulation of RCB production in autophagy.
Acknowledgements
This research was supported by KAKENHI (nos. 20200061, 20780044 and 22780054 for H.I. and 21-6184 for M.I.) and by Research Fellowships of JSPS for Young Scientists for M.I.
References
- 1.Thompson AR, Vierstra RD. Autophagic recycling: Lessons from yeast help define the process in plants. Curr Opin Plant Biol. 2005;8:165–173. doi: 10.1016/j.pbi.2005.01.013. [DOI] [PubMed] [Google Scholar]
- 2.Bassham DC, Laporte M, Marty F, Moriyasu Y, Ohsumi Y, Olsen LJ, et al. Autophagy in development and stress responses of plants. Autophagy. 2006;2:2–11. doi: 10.4161/auto.2092. [DOI] [PubMed] [Google Scholar]
- 3.Yoshimoto K, Hanaoka H, Sato S, Kato T, Tabata S, Noda T, et al. Processing of ATG8s, ubiquitin-like proteins and their deconjugation by ATG4s are essential for plant autophagy. Plant Cell. 2004;16:2967–2983. doi: 10.1105/tpc.104.025395. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Thompson AR, Doelling JH, Suttangkakul A, Vierstra RD. Autophagic nutrient recycling in Arabidopsis directed by the ATG8 and ATG12 conjugation pathways. Plant Physiol. 2005;138:2097–2110. doi: 10.1104/pp.105.060673. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Xiong Y, Contento AL, Bassham DC. AtATG18a is required for the formation of autophagosomes during nutrient stress and senescence in Arabidopsis thaliana. Plant J. 2005;42:535–546. doi: 10.1111/j.1365-313X.2005.02397.x. [DOI] [PubMed] [Google Scholar]
- 6.Phillips AR, Suttangkakul A, Vierstra RD. The ATG12-conjugating enzyme ATG10 is essential for autophagic vesicle formation in Arabidopsis thaliana. Genetics. 2008;178:1339–1353. doi: 10.1534/genetics.107.086199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.van der Graaff E, Schwacke R, Schneider A, Desimone M, Flügge UI, Kunze R. Transcription analysis of Arabidopsis membrane transporters and hormone pathways during developmental and induced leaf senescence. Plant Physiol. 2006;141:776–792. doi: 10.1104/pp.106.079293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Chung T, Suttangkakul A, Vierstra RD. The ATG autophagic conjugation system in maize: ATG transcripts and abundance of the ATG8-lipid adduct are regulated by development and nutrient availability. Plant Physiol. 2009;149:220–234. doi: 10.1104/pp.108.126714. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Ishida H, Yoshimoto K, Izumi M, Reisen D, Yano Y, Makino A, et al. Mobilization of Rubisco and stroma-localized fluorescent proteins of chloroplasts to the vacuole by an ATG gene-dependent autophagic process. Plant Physiol. 2008;148:142–155. doi: 10.1104/pp.108.122770. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Wada S, Ishida H, Izumi M, Yoshimoto K, Ohsumi Y, Mae T, et al. Autophagy plays a role in chloroplast degradation during senescence in individually darkened leaves. Plant Physiol. 2009;149:885–893. doi: 10.1104/pp.108.130013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Izumi M, Wada S, Makino A, Ishida H. The autophagic degradation of chloroplasts via Rubiscocontaining bodies is specifically linked to leaf carbon status but not nitrogen status in Arabidopsis. Plant Physiol. 2010;154:1196–1209. doi: 10.1104/pp.110.158519. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Pourtau N, Jennings R, Pelzer E, Pallas J, Wingler A. Effect of sugar-induced senescence on gene expression and implications for the regulation of senescence in Arabidopsis. Planta. 2006;224:556–568. doi: 10.1007/s00425-006-0243-y. [DOI] [PubMed] [Google Scholar]
- 13.Wingler A, Purdy S, MacLean JA, Pourtau N. The role of sugars in integrating environmental signals during the regulation of leaf senescence. J Exp Bot. 2006;57:391–399. doi: 10.1093/jxb/eri279. [DOI] [PubMed] [Google Scholar]
- 14.Rolland F, Baena-Gonzalez E, Sheen J. Sugar sensing and signaling in plants: Conserved and novel mechanisms. Annu Rev Plant Biol. 2006;57:675–709. doi: 10.1146/annurev.arplant.57.032905.105441. [DOI] [PubMed] [Google Scholar]
- 15.Weaver LM, Amasino RM. Senescence is induced in individually darkened Arabidopsis leaves but inhibited in whole darkened plants. Plant Physiol. 2001;127:876–886. [PMC free article] [PubMed] [Google Scholar]
- 16.Wingler A, Masclaux-Daubresse C, Fischer AM. Sugars, senescence and ageing in plants and heterotrophic organisms. J Exp Bot. 2009;60:1063–1066. doi: 10.1093/jxb/erp067. [DOI] [PubMed] [Google Scholar]
- 17.van Doorn WG. Is the onset of senescence in leaf cells of intact plants due to low or high sugar levels? J Exp Bot. 2008;59:1963–1972. doi: 10.1093/jxb/ern076. [DOI] [PubMed] [Google Scholar]
- 18.Kanki T, Wang K, Cao Y, Baba M, Klionsky DJ. Atg32 Is a mitochondrial protein that confers selectivity during mitophagy. Dev Cell. 2009;17:98–109. doi: 10.1016/j.devcel.2009.06.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Okamoto K, Kondo-Okamoto N, Ohsumi Y. Mitochondria-anchored receptor Atg32 mediates degradation of mitochondria via selective autophagy. Dev Cell. 2009;17:87–97. doi: 10.1016/j.devcel.2009.06.013. [DOI] [PubMed] [Google Scholar]
- 20.Lynch-Day MA, Klionsky DJ. The Cvt pathway as a model for selective autophagy. FEBS Lett. 2010;584:1359–1366. doi: 10.1016/j.febslet.2010.02.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Oku M, Sakai Y. Peroxisomes as dynamic organelles: Autophagic degradation. FEBS J. 2010;277:3289–3294. doi: 10.1111/j.1742-4658.2010.07741.x. [DOI] [PubMed] [Google Scholar]


