Abstract
There is a growing body of evidence that flavonoids do not primarily function as UV-B screening pigments in photoprotection. Recent findings support the idea that excess light stress, irrespective of the relative proportions of the solar wavebands reaching the leaf surface, upregulates the biosynthesis of dihydroxy B-ring-substituted flavonoid glycosides, as a consequence of and aimed at countering the generation of ROS. Intriguingly, the very conditions that lead to the inactivation of antioxidant enzymes can also upregulate the biosynthesis of antioxidant flavonoids, which suggests flavonoids constituting a secondary ROS-scavenging system in plants exposed to severe/prolonged stress conditions. H2O2 may diffuse out of the chloroplast at considerable rates and be transported to the vacuole, the storing site for flavonoids, by tonoplast intrinsic proteins, under severe excess light conditions. We suggest that the unanticipated key role of the vacuole in the ROS homeostasis might be mediated by flavonoids.
Key words: antioxidant enzymes, excess light stress, flavonol metabolism, hydrogen peroxide (H2O2), mesophyll flavonoids, quercetin glycosides, oxidative stress
The ancient and widespread flavonol metabolism has been widely reported to be mostly involved in the response mechanisms of plants to a wide range of stressful conditions.1 The loss of mycosporin-like aminoacid (MAA) in favor of flavonol metabolism is a strong evidence that flavonoids did not likely serve a primary UV-B screening function during the evolution of early land plants.2,3 In fact (1) MAA are excellent UV-B absorbers and flavonols are less effective UV-B attenuators with respect to most flavonoid structures;4 (2) antioxidant flavonols accumulate to a great extent as a consequence of sunlight irradiance in the absence of UV-radiation.5,6 These findings lead to the hypothesis that excess light stress, irrespective of the relative proportions of the solar wavebands reaching the leaf surface, upregulates the biosynthesis of flavonoids, as a consequence of and aimed at countering the generation of reactive oxygen species (ROS).3 In other words, flavonoids behave mostly as antioxidants in photoprotection.3,7
We have recently reported that mild root-zone salinity stress and UV-irradiance increased to a very similar degree the biosynthesis of dihydroxy B-ring-substituted flavonoid glycosides (i.e., the antioxidant flavonoid structures usually encountered in leaf tissues)8 in Ligustrum vulgare leaves.9 Our findings are consistent with the expression of genes of the biosynthesis of antioxidant flavonols (e.g., quercetin 3-O-glycosides), i.e., FLS (flavonol synthase) and F3′H (flavonoid 3′-hydroxylase) being strongly induced by a plethora of abiotic stresses,10–12 including UV-B radiation.13 Since different stresses have been reported to generate ROS, it has been speculated that stress-induced changes in ROS/REDOX homeostasis activate the biosynthesis of antioxidant flavonols,3,14,15 this idea conforming to R2R3MYB transcriptor factors, which regulate the biosynthesis of flavonols, being themselves REDOX-controlled.16
There is a large consensus for flavonoids to function as ROS scavengers, as they may inhibit the generation and reducing ROS once formed,17 but the actual significance of their antioxidant function in a planta situation has been strongly criticized. These criticisms originate from the observations that (1) flavonoids occur almost exclusively in the vacuoles of epidermal cells, and hence, physically separated from the main sources of ROS;18 (2) plants posses a highly efficient antioxidant machinery to keep the level of ROS under a tight control.19
However, we have recently given compelling evidence that antioxidant flavonoids accumulate to a large extent in the vacuole of mesophyll cells in leaves experiencing severe excess light stress,6,9 and mesophyll anthocyanins have been reported to reduce hydrogen peroxide (H2O2) generated upon mechanical wounding.20 Furthermore, the view that the “constitutive” system of antioxidant defenses is activated as a consequence of different stresses is not true in many instances. The activities of different antioxidant enzymes have long been reported to decline greatly under severe excess-light stress, a condition to which plants are faced with, when suffering from the concomitant action of two or more stresses.21–23 Here (Fig. 1) we show that (1) the activity of ascorbate peroxidase (APX) declined steeply in L. vulgare leaves growing at 100% photosynthetic active radiation (PAR) when additionally exposed to UV-radiation, and particularly to root-zone salinity stress (in salt-treated plants the SOD activity also declined as compared with control plants), for more than two weeks; (2) the accumulation of quercetin 3-O-glycosides reached a maximum after three weeks of treatment.
Figure 1.
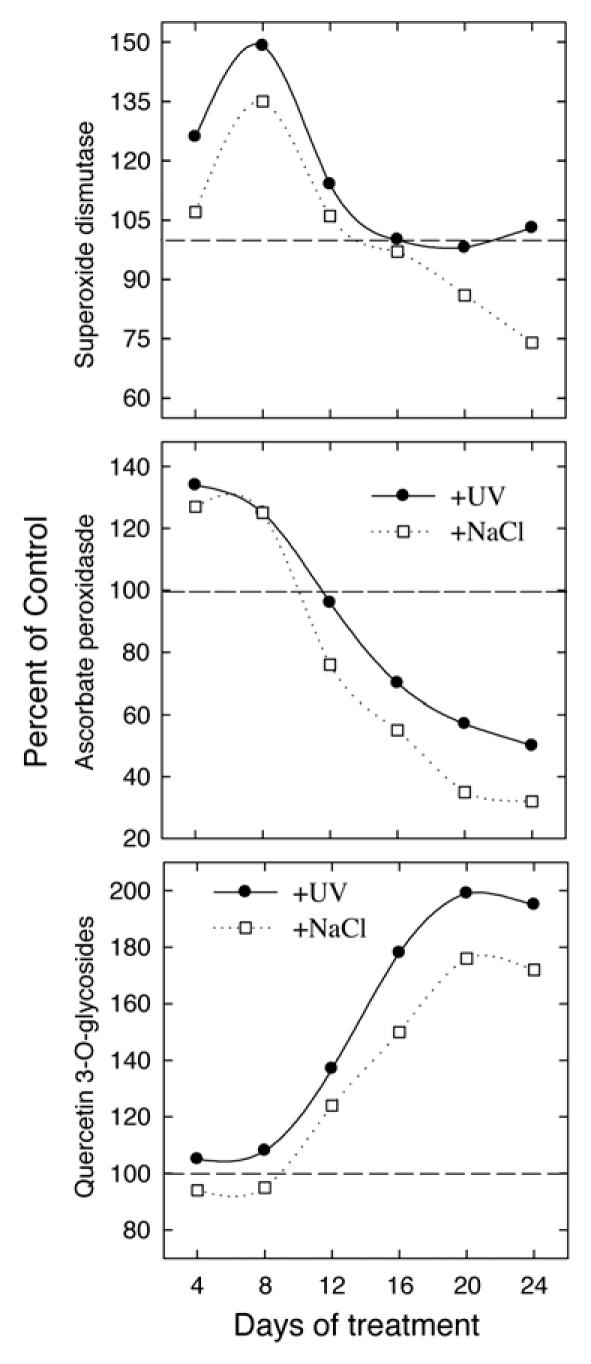
Time-course of SOD and CAT activities, and of quercetin 3-O-glycosides concentration in leaves of Ligustrum vulgare as affected by UV-radiation (UV-A: 803 and UV-B: 38.8 kJm−2, respectively) or root-zone salinity (125 mM NaCl). Control refers as to plants growing at full sunlight irradiance in the absence of UV-radiation.
It might be no a mere coincidence that the very conditions that lead to the inactivation of antioxidant enzymes can also induce the biosynthesis of antioxidant flavonoids (including anthocyanins).23 Excess PAR-irradiance led to a substantial decrease of SOD activity on a long-term basis,24 the reverse being observed for the accumulation of quercetin derivatives in epidermal cells.25 The biosynthesis of quercetin glycosides has been shown to be inversely correlated with the increase in SOD and CAT activities as a consequence of high light stress in two Oleaceae,26 and, consistently, the activity of antioxidant enzymes increased more in a soybean line with a lower flavonoid content in response to UV-B-induced oxidative damage.27
Antioxidant enzymes have long been proposed as representing the first line of defense against ROS generation, but their action needs to be complemented by that of other ROS scavenging systems during severe stress conditions.28 We suggest flavonoids as constituting a secondary ROS-scavenging system in plants suffering from severe excess excitation energy to the photosynthetic apparatus. Actually, excess excitation energy has been recently shown to specifically increase the biosynthesis of the antioxidant dihydroxy B-ring flavone derivatives.14 Fiorani et al. have reported PAL (phenylalanine ammonia lyase, the entry point in the phenylpropanoid metabolism) and CHS (the first committed step in the flavonoid biosynthetic branch-pathway) being strongly induced in plants overexpressing alternative oxidase (AOX, which is involved in stress-induced variations of the cellular REDOX state).30
Excess light may allow H2O2 to diffuse out of the chloroplast at considerable rates (as a consequence of APX depletion),21,31,32 and tonoplast intrinsic proteins may allow H2O2 to enter the vacuole,33 the storing site for flavonoids. Flavonoids are superb substrates for class III peroxidases to reducing H2O2, whereas ascorbic acid functions primarily to the recycling of flavonoid radicals to their reduced forms.34,35 There is intriguing evidence of a large redistribution of the ascorbate pool to the vacuolar compartment under excess light stress.36 It may be hypothesized that mesophyll flavonoids may effectively reduce H2O2 escaping from the chloroplast, when the pool of chloroplast antioxidants is depleted as a consequence of severe excess light. The unanticipated key role of the vacuole in the ROS homeostasis37 might be, therefore, mediated by flavonoids. There is a very narrow range of H2O2 concentration as a threat to the cell, including the programmed cell death, or as a signaling molecule responsible for increasing tolerance,37,38 and flavonoids may serve a key functional role to keeping the concentration of H2O2 at a sub-lethal level.
Acknowledgements
Work in the author's lab has been partially funded by Fondazione Ente Cassa di Risparmio di Firenze and Uniser Consortium Pistoia.
Abbreviations
- APX
ascorbate peroxidase
- CAT
catalase
- CHS
chalcone synthase
- F3′H
flavonoid 3′-hydroxylase
- FLS
flavonol synthase
- MAA
mycosporin-like aminoacid
- PAL
phenylalanine ammonia lyase
- ROS
reactive oxygen species
- SOD
superoxide dismutase
References
- 1.Winkel-Shirley B. Biosynthesis of flavonoids and effect on stress. Curr Opin Plant Biol. 2002;5:218–223. doi: 10.1016/s1369-5266(02)00256-x. [DOI] [PubMed] [Google Scholar]
- 2.Cockell CS, Knowland J. Ultraviolet radiation screening compounds. Biol Rev. 1999;74:311–345. doi: 10.1017/s0006323199005356. [DOI] [PubMed] [Google Scholar]
- 3.Agati G, Tattini M. Multiple functional roles of flavonoids in photoprotection. New Phytol. 2010;186:786–793. doi: 10.1111/j.1469-8137.2010.03269.x. [DOI] [PubMed] [Google Scholar]
- 4.Harborne JB, Williams CA. Advances in flavonoid research since 1992. Phytochemistry. 2000;55:481–504. doi: 10.1016/s0031-9422(00)00235-1. [DOI] [PubMed] [Google Scholar]
- 5.Kolb CA, Käser MA, Kopecky J, Zotz G, Riederer M, Pfündel EE. Effect of natural intensities of visible and ultraviolet radiation on epidermal ultraviolet screening and photosynthesis in grape leaves. Plant Physiol. 2001;127:863–875. [PMC free article] [PubMed] [Google Scholar]
- 6.Agati G, Stefano G, Biricolti S, Tattini M. Mesophyll distribution of antioxidant flavonoids in Ligustrum vulgare leaves under contrasting sunlight irradiance. Ann Bot. 2009;104:853–863. doi: 10.1093/aob/mcp177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Close DC, McArthur C. Rethinking the role of many plant phenolics—protection from photodamage, not herbivores? Oikos. 2002;99:166–172. [Google Scholar]
- 8.Tattini M, Galardi C, Pinelli P, Massai R, Remorini D, Agati G. Differential accumulation of flavonoids and hycroxycinnamates in leaves of Ligustrum vulgare under excess light and drought stress. New Phytol. 2004;163:547–561. doi: 10.1111/j.1469-8137.2004.01126.x. [DOI] [PubMed] [Google Scholar]
- 9.Agati G, Biricolti S, Guidi L, Ferrini F, Fini A, Tattini M. The biosynthesis of flavonoids is enhanced similarly by UV radiation and root zone salinity in L. vulgare leaves. J Plant Physiol. 2011;168:204–212. doi: 10.1016/j.jplph.2010.07.016. [DOI] [PubMed] [Google Scholar]
- 10.Walia H, Wilson C, Condamine P, Liu X, Ismail AM, Zeng L, et al. Comparative transcriptional profiling of two contrasting rice genotypes under salinity stress during the vegetative growth stage. Plant Physiol. 2005;139:822–835. doi: 10.1104/pp.105.065961. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Lillo C, Lea US, Ruoff P. Nutrient depletion as a key factor for manipulating gene expression and product formation in different branches of the flavonoid pathway. Plant Cell Environ. 2008;31:587–601. doi: 10.1111/j.1365-3040.2007.01748.x. [DOI] [PubMed] [Google Scholar]
- 12.Olsen KM, Slimestad R, Lea US, Brede C, Lovdal T, Ruoff P, et al. Temperature and nitrogen effects on regulators and products of the flavonoid pathway: Experimental and kinetic model studies. Plant Cell Environ. 2009;32:286–299. doi: 10.1111/j.1365-3040.2008.01920.x. [DOI] [PubMed] [Google Scholar]
- 13.Calvenzani V, Martinelli M, Lazzeri V, Giuntini D, Dall'Asta C, Galaverna G, et al. Response of wild-type and high pigment-1 tomato fruit to UV-B depletion: flavonoid profiling and gene expression. Planta. 2010;231:755–765. doi: 10.1007/s00425-009-1082-4. [DOI] [PubMed] [Google Scholar]
- 14.Akhtar TA, Lees HA, Lampi MA, Enstone D, Brain RA, Greenberg BM. Photosynthetic redox imbalance influences flavonoid biosynthesis in Lemma gibba. Plant Cell Environ. 2010;33:1205–1219. doi: 10.1111/j.1365-3040.2010.02140.x. [DOI] [PubMed] [Google Scholar]
- 15.Liu F, Xu W, Wei Q, Zhang Z, Xing Z, Tan L, et al. Gene expression profiles deciphering rice phenotypic variation between Nipponbare (Japonica) and 93-11 (Indica) during oxidative stress. PloS ONE. 2010;5:8632. doi: 10.1371/journal.pone.0008632. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Dubos C, Stracke R, Grotewold E, Weisshaar B, Martin C, Lepiniec L. MYB transcription factors in Arabidopsis. Trends Plant Sci. 2010;15:573–581. doi: 10.1016/j.tplants.2010.06.005. [DOI] [PubMed] [Google Scholar]
- 17.Agati G, Mattini P, Goti A, Tattini M. Chloroplast-located flavonoids can scavenge singlet oxygen. New Phytol. 2007;174:77–89. doi: 10.1111/j.1469-8137.2007.01986.x. [DOI] [PubMed] [Google Scholar]
- 18.Hernández I, Alegre J, van Bresugem F, Munné-Bosch S. How relevant are flavonoids as antioxidants in plants. Trends Plant Sci. 2009;14:125–132. doi: 10.1016/j.tplants.2008.12.003. [DOI] [PubMed] [Google Scholar]
- 19.Halliwell B. The wandering of a free radical. Free Radical Bio Med. 2009;46:531–542. doi: 10.1016/j.freeradbiomed.2008.11.008. [DOI] [PubMed] [Google Scholar]
- 20.Gould KS, McKelvie J, Markham KR. Do anthocyanins function as antioxidant in leaves? Imaging of H2O2 in red and green leaves after mechanical injury. Plant Cell Environ. 2002;25:1261–1269. [Google Scholar]
- 21.Karpinski S, Reynolds H, Karpinska B, Wingle G, Greissen G, Mullineaux P. Systemic signaling and acclimation in response to excess excitation energy in Arabidopsis. Science. 1999;284:654–657. doi: 10.1126/science.284.5414.654. [DOI] [PubMed] [Google Scholar]
- 22.Schwanz P, Polle A. Differential stress response of antioxidative systems to drought in pedunculate oak (Quercus robur) and maritime pine (Pinus pinaster) grown under high CO2 concentration. J Exp Bot. 2001;52:133–143. [PubMed] [Google Scholar]
- 23.Gould KS. Foliar anthocyanins as modulators of stress signals. J Theor Biol. 2008;253:625–627. doi: 10.1016/j.jtbi.2008.04.018. [DOI] [PubMed] [Google Scholar]
- 24.Guidi L, Degl'Innocenti E, Remorini D, Biricolti S, Fini A, Ferrini F, et al. The impact of UV-radiation on the physiology and biochemistry of Ligustrum vulgare leaves exposed to different visible-light irradiance. Environ Exp Bot. 2011;70:86–95. [Google Scholar]
- 25.Agati G, Cerovic Z, Pinelli P, Tattini M. Light-induced accumulation of ortho-dihydroxylated flavonoids as non-destructively monitored by chlorophyll fluorescence excitation techniques. Environ Exp Bot. 2011 doi: 10.1016/j.envexpbot.2010.10.002. [DOI] [Google Scholar]
- 26.Tattini M, Guidi L, Morassi-Bonzi L, Pinelli P, Remorini D, Degl'Innocenti E, et al. On the role of flavonoids in the integrated mechanisms of response of Ligustrum vulgare and Phillyrea latifolia exposed to excess light radiation. New Phytol. 2005;167:457–470. doi: 10.1111/j.1469-8137.2005.01442.x. [DOI] [PubMed] [Google Scholar]
- 27.Xu C, Sullivan JH, Garrett WM, Caperna TJ, Natarajan S. Impact of solar ultraviolet-B on the pro-tome in soybean lines differing in flavonoid contents. Phytochemistry. 2008;69:38–48. doi: 10.1016/j.phytochem.2007.06.010. [DOI] [PubMed] [Google Scholar]
- 28.Apel K, Hirt H. Reactive oxygen species: Metabolism, oxidative stress and signal transduction. Ann Rev Plant Biol. 2004;55:373–399. doi: 10.1146/annurev.arplant.55.031903.141701. [DOI] [PubMed] [Google Scholar]
- 29.Fiorani F, Umbach AL, Siedow JN. The alternative oxidase of plant mitochondria is involved in the acclimation of shoot growth at low temperature. A study of Arabidopsis AOX1a transgenic plants. Plant Physiol. 2005;139:1795–1805. doi: 10.1104/pp.105.070789. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Arnholdt-Schmitt B, Costa JH, de Melo DF. AOX—a functional marker for efficient reprogramming under stress? Trends Plant Sci. 2006;11:281–287. doi: 10.1016/j.tplants.2006.05.001. [DOI] [PubMed] [Google Scholar]
- 31.Polle A. Dissecting the superoxide dismutase-ascorbate-glutathione-pathway un chloroplasts by metabolic modeling. Computer simulations as a step towards flux analysis. Plant Physiol. 2001;126:445–462. doi: 10.1104/pp.126.1.445. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Mubarakshina MM, Ivanonv BN, Naydov IA, Hillier W, Badger MR, Krieger-Liszkay A. Production and diffusion of chloroplastic H2O2 and its implication to signaling. J Exp Bot. 2010;61:3577–3587. doi: 10.1093/jxb/erq171. [DOI] [PubMed] [Google Scholar]
- 33.Bienert GP, Møller ALB, Kristiansen KA, Møller IM, Schjoerring JK, Jahn TP. Specific aquaporins facilitate the diffusion of hydrogen peroxide across membranes. J Biol Chem. 2007;282:1183–1192. doi: 10.1074/jbc.M603761200. [DOI] [PubMed] [Google Scholar]
- 34.Yamasaki H, Sakihama Y, Ikehara N. Flavonoid-peroxidase reaction as a detoxification mechanism of plant cells against H2O2. Plant Physiol. 1997;115:1405–1412. doi: 10.1104/pp.115.4.1405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Sakihama Y, Mano J, Sano S, Asada K, Yamasaki H. Reduction of phenoxyl radicals mediated by monodehydroascorbate reductase. Biochem Biophys Res Comm. 2000;279:949–954. doi: 10.1006/bbrc.2000.4053. [DOI] [PubMed] [Google Scholar]
- 36.Zechmann B, Stumpe M, Mauch F. Immunocytochemical determination of the subcellular distribution of ascorbate in plants. Planta. 2011;133:1–12. doi: 10.1007/s00425-010-1275-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Mittler R, Vanderauwera S, Gollery M, van Breusegem F. Reactive oxygen gene network of plants. Trends Plant Sci. 2004;9:490–498. doi: 10.1016/j.tplants.2004.08.009. [DOI] [PubMed] [Google Scholar]
- 38.Van Breusegem F, Dat JF. Reactive oxygen species in plant cell death. Plant Physiol. 2006;141:384–390. doi: 10.1104/pp.106.078295. [DOI] [PMC free article] [PubMed] [Google Scholar]


