Abstract
Piriformospora indica is an endophytic fungus that colonized monocot as well as dicot. P. indica has been termed as plant probiotic because of its plant growth promoting activity and its role in enhancement of the tolerance of the host plants against abiotic and biotic stresses. In our recent study, we have characterized a high affinity phosphate transporter (PiPT) and by using RNAi approach, we have demonstrated the involvement of PiPT in P transfer to the host plant. When knockdown strains of PiPT-P. indica was colonized with the host plant, it resulted in the impaired growth of the host plants. Here we have analyzed and discussed whether the growth promoting activity of P. indica is its intrinsic property or it is dependent on P availability. Our data explain the correlation between the availability of P and growth-promoting activity of P. indica.
Key words: Piriformospora indica, phosphate transport, plant growth promotion
Phosphorous (P) is one of the most essential mineral nutrients for plant growth and development. In the soil P is present mainly in the form of sparingly soluble complexes that are not directly accessible to plants. Thus, it is the nutrient that limits crop production throughout the world.1 Plants have therefore evolved a range of strategies to increase the availability of soil P, which include both morphological and biochemical changes at the soil-root interface. For example, increased root growth and branching, proliferation of root hairs, and release of root exudates can increase plant access to inorganic phosphate (Pi) from otherwise poorly available sources.2,3 Plant root possess two distinct modes of phosphate uptake, direct uptake by its own transporters and indirect uptake through mycorrhizal associations. In plants several high affinity P transporters specifically associated with the uptake of Pi from soil solution. Expression of these transporters is induced in response to P deficiency and enables Pi to be effectively taken up against the large concentration gradient that occurs between the soil solution and internal plant tissues.4 However, in arbuscular mycorrhizal associations (indirect uptake), plants acquire Pi from the extensive network of fine extra radical hyphae of fungus, that extend beyond root depletion zones to mine new regions of the soil.5 In the case of arbuscular mycorrhizal fungi (AMF), including Glomus versiforme and G. intraradices, the regulation of phosphate transporters that are expressed, typically upregulated under P deficiency but their role in P transfer to the host plant have not been characterized.5,6
P. indica was reported to be involved in high salt tolerance, disease resistance and strong growth-promoting activities leading to enhancement of host plant yield.7–9 Recently, we have shown the role of PiPT in the P transport to the host plant.10 Here we discuss the performance of P. indica (grown under P-rich and -deprived conditions and colonized with the host plant) and its involvement in the P transportation to, and the growth of the host plant.
Growth Promoting Activity of P. indica Depends on the P Availability
In the present study, we analyzed whether growth-promoting activity of P. indica is dependent on the available P in the soil or is a intrinsic property of the fungus. We have found no significant change in the biomass at seedling stage. However, at later stage, the difference in biomass between colonized and non-colonized plants was 2.5 times at deprived P and 1.2 times at rich P conditions respectively. These data suggest that P. indica enhances growth more effectively at low P. Using bicompartment assay for 32P transportation, P transfer by P. indica to the host plant was analyzed and a considerable amount of 32P was measured in maize plants, suggesting that hyphae were transporting P to host plant at P-deprived condition (Fig. 1A and B).10 On the other hand P. indica was found unable to transfer more P to the host plant as compared to the P-deprived condition (Fig. 1B). Interestingly we have found no difference in the colonization at both the conditions (Fig. 1Aa and c). RT-PCR analysis shows that PiPT gene was not expressed in P-rich condition as no band was observed and a very good band of PiPT was observed at P-deprived condition (Fig. 1C), which reveals that PiPT was not active at P-rich condition therefore a very low amount of P was transferred to the seedlings; however at P-deprived condition PiPT was expressed and therefore actively involved in transporting the comparatively higher amount of P to the seedlings. Additionally, we also conclude that PiPT expression is dependent on the P availability. Further, in a separate pot experiment we have observed that mP. indica enhances biomass more effectively at P-deprived condition than P-rich condition. It was also observed that growth of colonized plant at 40 µM phosphate was found equal to non-colonized plants grown at 400 µM. This shows that P. indica can mimic the growth even at ten-fold lower P supplement (Fig. 2). In case of AMF reports are available which shows the growth promotion activity is due to higher influx (2–5 times) of P in mycorrhizal roots than in non-mycorrhizal roots (at a rate of 10−11 molm−1s−1).11,12 However, this is the first report of P transportation to the host plant, which was checked by modulating the growth of P. indica at P-deprived and P-rich conditions. Further, this is a possible an additional explanation of growth promotion activity of the fungus. As far as the mechanism and P compound concerned for the transfer of P from P. indica to plant, it is unknown but Pi and organic P (such as polyphosphate) could be carried within the fungus by cytoplasmic streaming or by bulk flow to the plant root from external hyphae located in the soil. In case of mycorrhiza Pi is the major form effluxed by the fungus to the plant interface. In higher plants it is evidence that phosphocholine can be broken down outside cells to release Pi. We speculate that phosphocholine is also effluxed by the fungus to the plant; Pi would then be taken up by the plant via an H+ co-transporter, as in non-colonized roots. Since it is known that the phosphate transporter from Glomus versiforme (GvPT) is not expressed in fungal structures inside the plant and similarly in case of P. indica, PiPT expression was very low in intra-radical tissue of the plant, it cannot be a candidate for the fungal P efflux mechanism. Efflux of P must depend on a different transport system. In summary, P transfer by P. indica to host plant has significance effect on plant growth particularly at P-deprived condition therefore in our views helping the plant to overcome the P stress condition.
Figure 1.
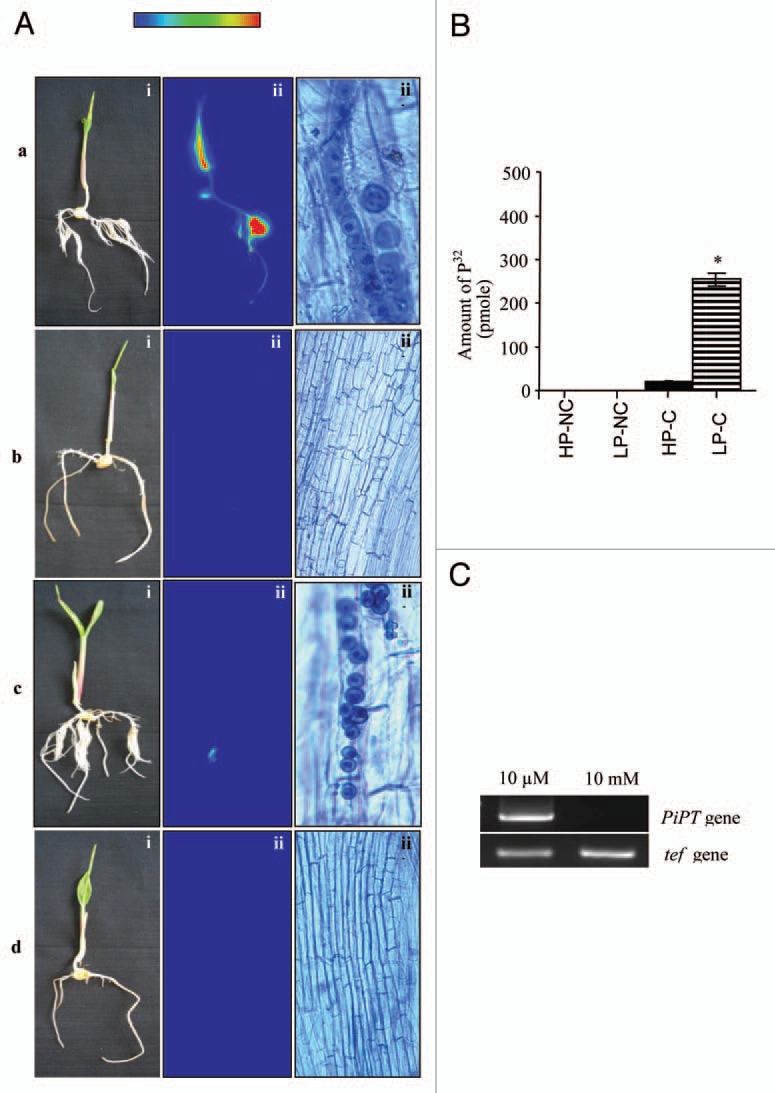
Transport of P to maize plants by P. indica was carried out in bi-compartment Petri dish culture system.10 (A) Radioactivity incorporated in plant was demonstrated by autoradiography. Radioactivity counts intensities are shown in false color code (vertical bar, low to high). (i) Whole maize plant before autoradiography. (ii) False-color autoradiograph of the maize plant obtained after 3 h of exposure of the maize plant. (iii) Microscopic view of a sample of plant root before autoradiography. (a) Maize plant colonized with P. indica grown at 10 µM P concentration referred as P-deprived condition (LP-C). (b) Non-colonized maize plant grown at 10 µM P concentration, (c) Maize plant colonized with P. indica grown at 10 mM P concentration referred as P-rich condition (HP-C). (d) Non-colonized maize plant grown at 10 mM P concentration. Transport assay was done at 100 mM with 1 mM of P32 (specific activity 200 mCi/mmol). (B) Amount of 32P transferred into the maize plant components by P. indica. Bar labeled with the (*) represents significance as compared with the (HP-C) (p < 0.05). (C) PiPT gene expression analysis during colonization at P-deprived and P-rich condition. RT-PCR analysis was performed to check the PiPT gene expression in P. indica grown at P-deprived and P-rich condition. Lane 1 shows amplified PiPT DNA fragment (1.6 kb) from P. indica grown at P-deprived condition (10 mM). Lane 2, no expression of PiPT was observed when P. indica was grown at P-rich condition (10 mM). tef gene (220 bp) of P. indica was used as a loading control. A color version of this image is available at www.landesbioscience.com/journals/psb/article/15106/.
Figure 2.

Effect of P. indica on plant growth at P-deprived and P-rich condition. PI 40, Plant colonized with P. indica supplied with 40 µM P. P 400, non colonized plants supplied with 400 µM P. Experiment was conducted for 28 days after inoculation (dai) in acid-washed sand supplied with Hoagland's solution containing 40 or 400 µM P, respectively.
References
- 1.Bieleski RL. Phosphate pools, phosphate transport and phosphate availability. Annu Rev Plant Physiol. 1973;24:225–252. [Google Scholar]
- 2.Raghothama KG. Phosphate acquisition. Annu Rev Plant Physiol Plant Mol Biol. 1999;50:665–693. doi: 10.1146/annurev.arplant.50.1.665. [DOI] [PubMed] [Google Scholar]
- 3.Vance CP, Uhde-Stone C, Allan DL. Phosphorus acquisition and use: Critical adaptations by plants for securing a nonrenewable resource. New Phytol. 2003;157:423–447. doi: 10.1046/j.1469-8137.2003.00695.x. [DOI] [PubMed] [Google Scholar]
- 4.Smith FW, Rae AL, Hawkesford MJ. Molecular mechanisms of phosphate and sulphate transport in plants. Biochim Biophys Acta. 2000;1465:236–245. doi: 10.1016/s0005-2736(00)00141-3. [DOI] [PubMed] [Google Scholar]
- 5.Harrison MJ, van Buuren ML. A phosphate transporter from the mycorrhizal fungus Glomus versiforme. Nature. 1995;378:626–629. doi: 10.1038/378626a0. [DOI] [PubMed] [Google Scholar]
- 6.Maldonado-Mendoza IE, Dewbre GR, Harrison MJ. A phosphate transporter gene from the extra-radical mycelium of an arbuscular mycorrhizal fungus Glomus intraradices is regulated in response to phosphate in the environment. Mol Plant Microbe Interact. 2001;14:1140–1148. doi: 10.1094/MPMI.2001.14.10.1140. [DOI] [PubMed] [Google Scholar]
- 7.Kumar M, Yadav V, Tuteja N, Johri AK. Antioxidant enzyme activities in plants colonized with Piriformospora indica. Microbiology. 2009;150:780–790. doi: 10.1099/mic.0.019869-0. [DOI] [PubMed] [Google Scholar]
- 8.Waller F, Achatz B, Baltruschat H, Fodor J, Becker K, Fischer M, et al. The endophytic fungus Piriformospora indica reprograms barley to salt-stress tolerance, disease resistance and higher yield. Proc Natl Acad Sci USA. 2005;102:13386–13391. doi: 10.1073/pnas.0504423102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Varma A, Verma S, Sudha, Sahay N, Butehorn B, Franken P. Piriformospora indica, a cultivable plant-growth-promoting root endophyte. Appl Env Microbiol. 1999;65:2741–2744. doi: 10.1128/aem.65.6.2741-2744.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Yadav V, Kumar M, Deep DK, Kumar H, Sharma R, Tripathy T, et al. A phosphate transporter from a root endophytic fungus Piriformospora indica plays a role in the phosphate transfer to the plants. J Biol Chem. 2010;285:26532–26544. doi: 10.1074/jbc.M110.111021. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 11.Bolan NS. A critical review on the role of mycorrhizal fungi in the uptake of phosphorus by plants. Plant Soil. 1991;134:189–207. [Google Scholar]
- 12.Smith SE, Read DJ. Mycorrhizal Symbiosis. San Diego: Academic Press; 1997. [Google Scholar]


