Abstract
Although no specific role has been demonstrated for ethylene during Arbuscular Mycorrhizal (AM) symbiosis, recent results suggest its participation in the regulation of the AM. Analysis of arbuscular mycorrhizal colonization in the abscisic acid (ABA)-deficient tomato sitiens mutant has shown that ABA deficiency induced ethylene production. It has also been suggested that one of the mechanisms used by ABA to determine susceptibility to fungal infection is negative modulation of the ethylene pathway. In this study, we describe the pattern of mycorrhization in mutant plants with altered ethylene biosynthesis and/or perception pathways. Epinastic (epi) plants with increased ethylene response were unaffected in terms of mycorrhizal frequency, although this mutation had a considerable negative impact on the intensity of mycorrhizal root colonization. The negative impact of the mutation in epi plants on the intensity of mycorrhizal root colonization was associated with a transitory increase in the transcript level of the LeETR6 ethylene receptor gene. On the other hand, ripenenig-inhibitor (rin) tomato mutant plants were positively affected in relation to all the mycorrhizal colonization parameters measured, suggesting that, at least in tomato plants, the regulation of AM formation is mediated by the RIN pathway.
Key words: arbuscular mycorrhiza, ethylene, ethylene mutants
The establishment of an arbuscular mycorrhizal (AM) symbiotic interaction is a successful strategy to improve the nutritional status of both plants and AM fungi1 and requires the host root cells to undergo significant structural and functional modifications, leading to reciprocal beneficial effects. A combination of genetic, molecular and cellular studies actually shows that functional symbiosis appears to occur at the end of a series of plant-controlled checkpoints, where different classes of plant hormones play an essential role.2 In recent years, through a combination of hormonal mutant/transgenic plant and transcriptomic analyses, much progress has been made in explaining the role of plant hormones during the interaction of plants with AM fungi. In relation to tomato plants, functional mycorrhization is associated with the regulation of genes directly involved in the metabolism of important isoprenoid plant hormones such as abscisic acid (ABA) and strigolactones.3,4 Analysis of AM colonization in the ABA-deficient tomato sitiens mutant has shown that ABA is necessary in order to complete the arbuscule formation process.5,6 ABA deficiency induced ethylene production, suggesting that the negative modulation of the ethylene pathway is one of the mechanisms used by ABA to determine susceptibility to fungal infection.7 However, no specific role has been demonstrated for ethylene during AM symbiosis, although recent findings suggest that it is involved in regulating AM.8–10 In this study, we describe the pattern of mycorrhization in mutant plants with altered ethylene biosynthesis and/or perception pathways. The results of histochemical analysis of AM formation combined with molecular biology techniques to analyze tomato ethylene receptor gene expression clearly show that the intensity of mycorrhizal root colonization is mediated by the ethylene pathway.
Mycorrhizal Colonization under High Ethylene Production Conditions
Previously, it was shown that the colonization by Glomus clarum was inhibited in the epinastic (epi) ethylene overproducer tomato mutant, when compared with control Micro-Tom.10 The epi mutant was isolated on the basis of severe leaf epinasty; it exhibits an enhanced triple response in the absence of ethylene and has elevated ethylene levels in some tissues.11 To data the molecular defect in the epi mutant has not been reported. To examine the extent and significance of this factor, we determined different mycorrhizal parameters in a time course experiment during mycorrhization in both the epi mutant and its isogenic wild-type line VNF8 (both plant lines were obtained from the Tomato Genetic Research Center, University of California, Davis). Forty-four and fifty-six days after inoculation with G. intraradices, stained roots were observed using a light microscope, and the intensity of root cortex colonization by AM fungus was determined using MYCOCALC software (www.dijon.inra.fr/mychintec/Mycocalc-prg/download.html). The parameters measured were frequency of colonization (F), intensity of colonization (M) as well as arbuscular abundance (a) in mycorrhizal root fragments (Fig. 1). Epinastic plants with increased ethylene response were unaffected in terms of mycorrhizal frequency (%F), and only a slight reduction in arbuscular abundance (%a) was observed 56 days after inoculation. Nevertheless, epi plants recorded a sharp reduction in the intensity of the colonization parameter (%M) at the two harvest points examined. This suggests that an early stage in the signal dialogue necessary for fungal colonization was functional in epi plants since they showed frequency values similar to those for wild-type plants. We also found a similar capacity to form arbuscules in the mycorrhizal root zones (measured as %a) regardless of the plant's ethylene sensitivity, suggesting that regulation of arbuscule abundance in the mycorrhizal zones of the roots is not mediated by the ethylene pathway. By contrast, our results clearly show that the mutation in epi plants has a considerable negative impact on the intensity of mycorrhizal root colonization, suggesting that this parameter is mediated by the ethylene pathway.
Figure 1.
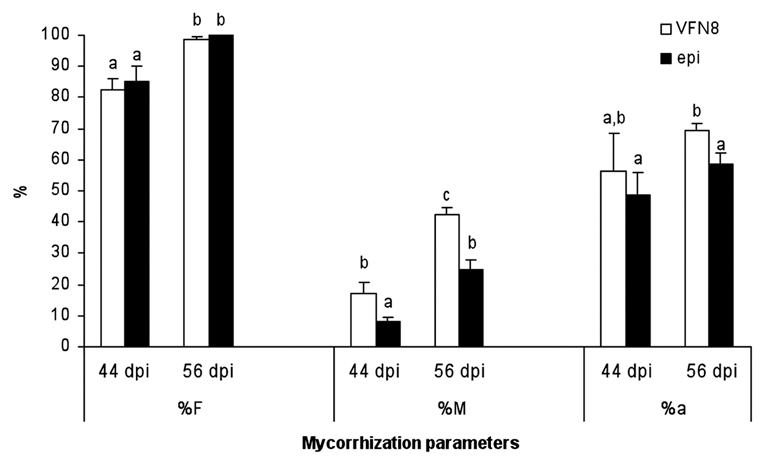
Mycorrhization parameters in the roots of VFN8 wild-type and epi mutant plants 44 and 56 days after inoculation with G. intraradices. Mycorrhization parameters of frequency (%F), intensity (%M) and arbuscular abundance (%a) in mycorrhizal zones of the root were calculated using MYCOCALC software. Values correspond to means ± SE. For each mycorrhization parameter, bars with similar letters do not significantly differ (p = 0.05) according to Duncan's multiple range test.
Mycorrhizal Colonization in ripenenig-inhibitor Tomato Mutants
Since ethylene overproduction in tomato significantly affects intraradical arbuscular mycorrhizal colonization, it would be interesting to investigate whether changes in ethylene content/sensitivity in opposite directions lead to a contrary and/or similar effect on AM formation. To do this, we carried out mycorrhization experiments on the rin mutant tomato plant line similar to those described above for the epi mutant. The rin mutation is a recessive mutation that effectively blocks the ripening process and results in mutant fruit that fail to produce high levels of ethylene and to repining in response to exogenous ethylene.12 Rin plants were positively affected in all the mycorrhizal colonization parameters measured (Fig. 2). An increase in mycorrhizal frequency (%F) was observed only at initial harvest time and was less pronounced than the increases recorded in the parameters of mycorrhizal intensity (%M) and arbuscular abundance in the mycorrhizal root zones (%a), which improved significantly in the roots of mycorrhizal rin plants usually forty-four days after inoculation. Our results clearly show that the rin mutation had a positive impact on the mycorrhizal colonization capacity of tomatoes, suggesting that, at least in tomato plants, the regulation of AM formation is mediated by the RIN pathway. The ripening inhibitor (RIN) is a MADS-box type transcription factor associated with crucial ripening activities (including ethylene production) and has some ethylene-independent functions.12 Its mediation with respect to climacteric and non-climacteric ripening physiologies linked to its role in AM formation suggests that the RIN MADS protein could play an effective role in regulating physiological phenomena conserved among fruit ripening and AM root colonization, such as carotenoid accumulation, plant cell wall softening and production of volatile compounds.
Figure 2.
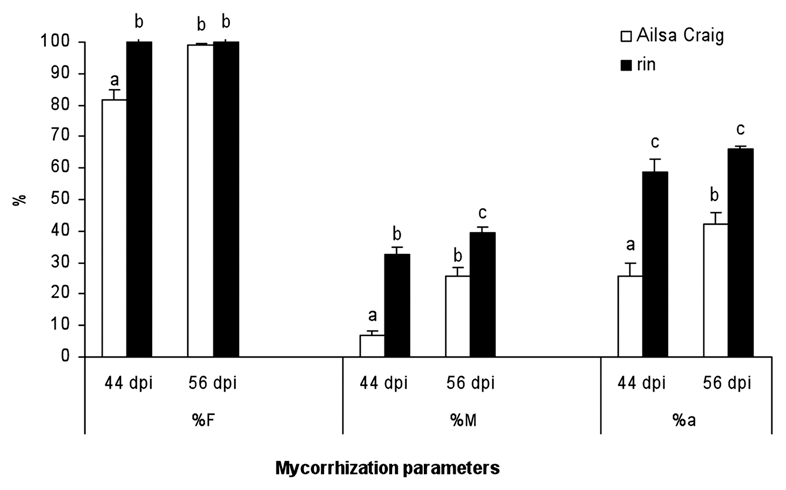
Mycorrhization parameters in the roots of Ailsa Craig wild-type and rin mutant plants 44 and 56 days after inoculation with G. intraradices. Mycorrhization parameters of frequency (%F), intensity (%M) and arbuscular abundance (%a) in mycorrhizal zones of the root were calculated using MYCOCALC software. Values correspond to means ± SE. For each mycorrhization parameter, bars with similar letters do not significantly differ (p = 0.05) according to Duncan's multiple range test.
Differential Expression of LeETR4-6 Ethylene-Receptor Genes in Mycorrhizal Tomato Ethylene Mutants
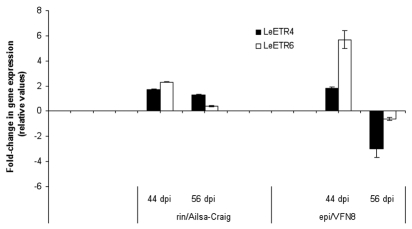
Expression analysis using real-time qPCR was carried out to check the expression of tomato genes encoding LeETR4 and LeETR6 tomato ethylene receptor proteins. We used these ethylene-inducible13 genes as molecular markers of the ethylene pathway during AM formation in wild-type and mutant plants with varying degrees of ethylene sensitivity. The expression for each gene in the different mutants is given relative to the expression of the same gene in the corresponding wild-type plant (Fig. 3). Rin and epi mycorrhizal plants showed different patterns of LeETR4 and LeETR6 ethylene receptor transcript accumulation. In rin plants as compared to wild-type plants, G. intraradices infection had only a slightly positive effect on the accumulation of both LeETR4 and LeETR6 transcripts 44 and 56 days after inoculation. In the epi mutant, while LeETR4 transcript accumulation slightly increased, the accumulation of LeETR6 transcript clearly increased 44 days after inoculation (nearly six times that for wild-type plants). At 56 days after inoculation, both LeETR4, and more significantly, LeETR6 gene expression decreased in the mycorrhizal epi mutant with respect to mycorrhizal wild-type plants. Our results clearly suggest that the negative impact of the mutation in epi plants on the intensity of mycorrhizal root colonization was associated with a transitory increase in the transcript level of the ethylene receptor LeETR6 gene, reinforcing the idea that this negative effect is mediated by the ethylene pathway. Conversely, the improvement in the mycorrhization parameters for the rin mutants was not associated with a reduction in the transcript levels of LeETR4 and LeETR6, suggesting that the regulation of AM formation mediated by the RIN pathway occurs independently of ethylene.
Figure 3.
Gene expression analysis using real-time quantitative PCR (qRT-PCR) of the tomato ethylene receptor genes LeETR4 and LeETR6 in mycorrhizal tomato roots 44 and 56 days after inoculation with G. intraradices. Real-time qPCR was based on Ct values that were normalized using the household gene LeEF-1α (X14449). The expression for each gene in the different mutants is given relative to the expression of the same gene in the corresponding wild-type plant. Bars represent mean values ±SE of three independent biological replicates.
Acknowledgements
This study was supported by grants from the Spanish Ministry of Science and Innovation (MICINN-AGL2008-00742), the Carolina foundation (awarded to R. Torres de los Santos) and the Junta de Andalucía (group BIO 260).
References
- 1.Smith SE, Read DJ. Mycorrhizal symbiosis. San Diego: Academic Press; 2008. p. 787. [Google Scholar]
- 2.Ludwig-Müller J. Hormonal responses in host plants triggered by arbuscular mycorrhizal fungi. In: Koltai, Kapulnik, editors. Arbuscular Mycorrhizas: physiology and function. Springer; 2010. pp. 169–190. [Google Scholar]
- 3.García Garrido JM, León Morcillo R, Martín Rodríguez JA, Ocampo Bote JA. Variations in the mycorrhization characteristics in roots of wild-type and ABA-deficient tomato are accompanied by specific transcriptomic alterations. Mol Plant-Microbe Interact. 2010;23:651–664. doi: 10.1094/MPMI-23-5-0651. [DOI] [PubMed] [Google Scholar]
- 4.López-Ráez JA, Kohlen W, Charnikhova T, Mulder P, Undas AK, Sergeant MJ, et al. Does abscisic acid affect strigolactone biosynthesis? New Phytol. 2010;187:343–354. doi: 10.1111/j.1469-8137.2010.03291.x. [DOI] [PubMed] [Google Scholar]
- 5.Herrera-Medina MJ, Steinkellner S, Vierheilig H, Ocampo JA, García-Garrido JM. Abscisic acid determinates arbuscule development and functionality in the tomato arbuscular mycorrhiza. New Phytol. 2007;175:554–564. doi: 10.1111/j.1469-8137.2007.02107.x. [DOI] [PubMed] [Google Scholar]
- 6.Martín Rodriguez JA, León Morcillo R, Vierheilig H, Ocampo Bote JA, Ludwig-Müller J, García-Garrido JM. Mycorrhization of the notabilis and sitiens tomato mutants in relation to abscisic acid and ethylene contents. J Plant Physiol. 2010;167:606–613. doi: 10.1016/j.jplph.2009.11.014. [DOI] [PubMed] [Google Scholar]
- 7.Martín Rodríguez JA, León-Morcillo R, Vierheilig H, Ocampo JA, Ludwig-Müller J, García-Garrido JM. Ethylene-dependent/ethylene-independent ABA regulation of tomato plants colonized by arbuscular mycorrhiza fungi. New Phytol. 2011;190:193–205. doi: 10.1111/j.1469-8137.2010.03610.x. [DOI] [PubMed] [Google Scholar]
- 8.Riedel T, Groten K, Baldwin I. Symbiosis between Nicotiana attenuate and Glomus intraradices: ethylene plays a role, jasmonic acid does not. Plant Cell Environm. 2008;31:1203–1213. doi: 10.1111/j.1365-3040.2008.01827.x. [DOI] [PubMed] [Google Scholar]
- 9.Penmetsa R, Uribe P, Anderson J, Lichtenzveig J, Gish J, Nam Y, Ocamp JA. The Medicago truncatula ortholog of Arabidopsis EIN2, sickle, is a negative regulator of symbiotic and pathogenic microbial associations. Plant Cell. 2008;55:580–595. doi: 10.1111/j.1365-313X.2008.03531.x. [DOI] [PubMed] [Google Scholar]
- 10.Zsögön A, Lambais M, Benedito V, Vargas A, Pereira Peres L. Reduced arbuscular mycorrhizal colonization in tomato ethylene mutants. Scientia Agricola (Piracicaba, Braz.) 2008:65259–65267. [Google Scholar]
- 11.Barry CS, Fox EA, Yen H, Lee S, Ying T, Grierson D, Giovannoni JJ. Analysis of the ethylene response in the epinastic mutant of tomato. Plant Physiol. 2001;127:58–66. doi: 10.1104/pp.127.1.58. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Vrebalov J, Ruezinsky D, Padmanabhan V, White R, Medrano D, Drake R, Ocamp JA. A MADS-Box Gene Necessary for Fruit Ripening at the Tomato Ripening-Inhibitor (Rin) Locus. Science. 2002;296:343–346. doi: 10.1126/science.1068181. [DOI] [PubMed] [Google Scholar]
- 13.Kevany BM, Tieman DM, Taylor MG, Dal Cin V, Klee HJ. Ethylene receptor degradation controls the timing of ripening in tomato fruit. Plant J. 2007;51:458–467. doi: 10.1111/j.1365-313X.2007.03170.x. [DOI] [PubMed] [Google Scholar]