Abstract
To adapt to waterlogging, maize (Zea mays) forms lysigenous aerenchyma in root cortex as a result of ethylene-promoted programmed cell death (PCD). Respiratory burst oxidase homolog (RBOH) gene encodes a homolog of gp91phox in NADPH oxidase, and has a role in the generation of reactive oxygen species (ROS). Recently, we found that during aerenchyma formation, RBOH was upregulated in all maize root tissues examined, whereas an ROS scavengingrelated metallothionein (MT) gene was downregulated specifically in cortical cells. Together these changes should lead to high accumulations of ROS in root cortex, thereby inducing PCD for aerenchyma formation. As further evidence of the involvement of ROS in root aerenchyma formation, the PCD was inhibited by diphenyleneiodonium (DPI), an NADPH oxidase inhibitor. Based on these results, we propose a model of cortical cell-specific PCD for root aerenchyma formation.
Key words: aerenchyma, ethylene, laser microdissection, maize (Zea mays), metallothionein, programmed cell death, reactive oxygen species, respiratory burst oxidase homolog
In both wetland and non-wetland plants, lysigenous aerenchyma is formed in roots by creating gas spaces as a result of death and subsequent lysis of some cortical cells, and allows internal transport of oxygen from shoots to roots under waterlogged soil conditions.1–3 In rice (Oryza sativa) and some other wetland plant species, lysigenous aerenchyma is constitutively formed under aerobic conditions, and is further enhanced under waterlogged conditions.4 On the other hand, in non-wetland plants, including maize (Zea mays), lysigenous aerenchyma does not normally form under well-drained soil conditions, but is induced by waterlogging.5 Ethylene is involved in lysigenous aerenchyma formation,1–3,6,7 but the molecular mechanisms are unclear.
We recently identified two reactive oxygen species (ROS)-related genes that were specifically regulated in maize root cortex by waterlogged conditions, but not in the presence of an ethylene perception inhibitor 1-methylcyclopropene (1-MCP).5 One was respiratory burst oxidase homolog (RBOH), which has a role in ROS generation and the other was metallothionein (MT), which has a role in ROS scavenging. These results suggest that ROS has a role in ethylene signaling in the PCD that occurs during lysigenous aerenchyma formation.
Cell Type-Specific Expression of Genes Related to ROS Generation and Scavenging
In maize, lysigenous aerenchyma is formed in root cortical cells under waterlogged conditions, but not under aerobic conditions. However, ethylene can induce its formation even under aerobic conditions (Fig. 1A).
Figure 1.
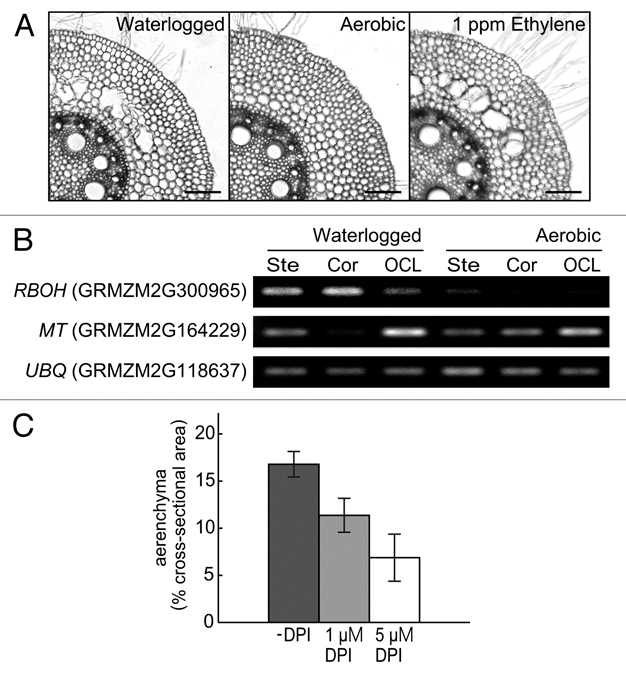
(A) Aerenchyma formation of maize primary roots under waterlogged conditions, aerobic conditions and aerobic conditions with 1 ppm ethylene. Tissue sections were prepared at 24 h after the start of treatments. Bars, 100 µm. (B) Cell type-specific expression analysis of the RBOH and the MT genes. Semi-quantitative reverse transcription-polymerase chain reaction (RT-PCR) was performed. The alphanumeric symbols in parentheses indicate the Gene IDs of the Maizesequence database. The Ubiquitin (UBQ) was used as a control. Ste, stelar cells; Cor, cortical cells; OCL, outer cell layers. (C) Aerenchyma formation in maize primary roots at 24 h grown under waterlogged conditions with or without DPI treatment. All values are means (n = 4) ±SD.
RBOH, a plant homolog of gp91phox in plasma membrane-associated NADPH oxidase of mammals, converts O2 to superoxide anion (O2.−), thereby leading to production of hydrogen peroxide (H2O2).8,9 In laser microdissection-isolated root tissues from waterlogged plants, expression of the RBOH gene is upregulated strongly in the cortical cells and slightly less strongly in the stelar cells and the outer cell layers (Fig. 1B).
MTs are low molecular weight, cysteine-rich metal-binding proteins that, in animals, seem to have roles in metal homeostasis, general stress responses and ROS scavenging.10,11 The MT gene was constitutively expressed in all of the laser microdissection-isolated root tissues under aerobic conditions, but interestingly, it was hardly expressed in the cortical cells under waterlogged conditions (Fig. 1B).
Involvement of ROS in Lysigenous Aerenchyma Formation in Maize Roots
To confirm the involvement of RBOH-produced ROS in lysigenous aerenchyma formation, we grew maize under waterlogged conditions with or without treatment of diphenyleneiodonium (DPI), an NADPH oxidase inhibitor. As shown in Figure 1C, aerenchyma formation of maize primary roots under waterlogged conditions was inhibited by the treatment with 1 µM or 5 µM DPI compared with no DPI treatment (-DPI), suggesting that the ROS generation mediated by NADPH oxidase (i.e., RBOH) under waterlogged conditions partly contributes to aerenchyma formation in maize roots.
ROS are key factors that transduce signals stimulated by environmental stresses or pathogen infections in plants. However, ROS, which are potentially toxic, can cause cellular damage, and thus their production must be tightly regulated.8,12 RBOHs are considered as the main producers of ROS for stress-stimulated signaling in plants.8,9,12 Several studies have shown that transcriptional activation of RBOH genes is accompanied by an oxidative burst,12–14 and the positive feedback regulation of the RBOH gene expression at transcriptional and post-transcriptional levels contributes to amplification of the ROS signals.12,15–17
There is increasing evidence that plant MTs regulate the accumulation of ROS: downregulation of the rice MT2b gene contributed to high H2O2 accumulation during defense signaling18 and ethylene-induced epidermal cell death,19 and H2O2 treatment and knockdown of MT2b gene promoted lysigenous aerenchyma formation in rice internodes.20 Biochemical analysis revealed that an animal Zn or Cd binding MT has more than 100 times higher antioxidant activity against hydroxyl radicals (OH.) than does reduced glutathione (GSH).10,21 Rice MT2b and cotton MT3a have higher antioxidative capacity against hydroxyl radicals than do the other antioxidants in vitro,18,22 and many cysteine residues in proteins are remarkably reactive to oxidizing agents.11,23 These results suggest that the MTs are responsible for reductions of H2O2 as well as other ROS in plant cells, although the antioxidative capacity of MTs against H2O2 has not been directly evaluated.
Sauter and colleagues19,20 proposed a model in which ethylene-promoted downregulation of MT2b gene expression enhances accumulation of H2O2 produced by NADPH oxidase, thereby inducing epidermal cell death in rice or aerenchyma formation in rice internodes. We propose a similar model for lysigenous aerenchyma formation in maize roots (Fig. 2). Waterlogging promotes biosynthesis and accumulation of ethylene, followed by induction of RBOH expression. The RBOH activity leads to the production and accumulation of O2.− at the apoplast, which is spontaneously or enzymatically converted to H2O2. H2O2 can easily diffuse into the cytosol through the plasma membrane.24 In the cytosol of stelar cells and cells in the outer cell layers, in which MT is constitutively expressed, H2O2 and other ROS are scavenged by MT. In contrast, in the cortical cells, the decreased MT expression prevents ROS scavenging, thereby leading to higher ROS accumulation, which activates the subsequent processes of PCD and lysis of the cortical cells (i.e., lysigenous aerenchyma formation) in maize roots.
Figure 2.
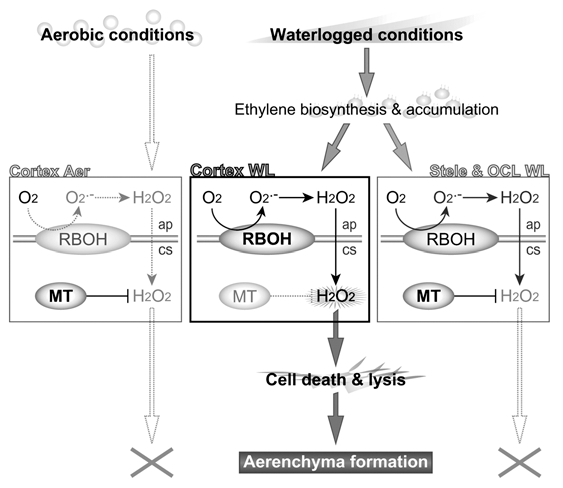
Model of lysigenous aerenchyma formation in maize root under waterlogged conditions. Waterlogging promotes biosynthesis and accumulation of ethylene. RBOH expression is upregulated in the cortical cells, the stelar cells and the outer cell layers, whereas MT expression is downregulated specifically in the root cortex. This leads to high accumulation of ROS (including H2O2), which initiates PCD and lysis of the cortical cells (i.e., aerenchyma formation). Note that direct scavenging of H2O2 by MT proteins remains to be elucidated, but some genetic studies support MT-mediated H2O2 reduction. Aer, aerobic conditions; WL, waterlogged conditions; ap, apoplast; cs, cytosol.
Conclusion and Perspectives
The preceding results suggest that both upregulation of RBOH gene expression and downregulation of MT gene expression in cortical cells contribute to cell type-dependent ROS accumulation in maize roots under waterlogged conditions. This results in accumulation of ROS in cortical cells, but not in the other root tissues, thereby inducing aerenchyma formation only in the root cortex. However, some cortical cells escape PCD and remain alive after completion of lysigenous aerenchyma formation. So far, it is unknown what factors determine which cells undergo PCD in the root cortex during aerenchyma formation in response to waterlogging stress. It would be of interest to examine whether cell fate is determined by differences in MT gene expression among the cortical cells.
Acknowledgements
We thank Drs. H. Takahashi, S. Nishiuchi, K. Shiono, R. Watanabe, A. Mliki, Y. Nagamura, N. Tsutsumi, N.K. Nishizawa, M. Sauter and B. Steffens for stimulating discussions. This work was partly supported by a grant from the Bio-oriented Technology Research Advancement Institution (Promotion of Basic Research Activities for Innovative Biosciences), a grant from Ministry of Agriculture, Forestry and Fisheries of Japan, and grants-in-aid from the Ministry of Education, Culture, Sports, Science and Technology of Japan.
References
- 1.Jackson MB, Armstrong W. Formation of aerenchyma and the processes of plant ventilation in relation to soil flooding and submergence. Plant Biol. 1999;1:274–287. [Google Scholar]
- 2.Colmer TD. Long-distance transport of gases in plants: A perspective on internal aeration and radial oxygen loss from roots. Plant Cell Environ. 2003;26:17–36. [Google Scholar]
- 3.Evans DE. Aerenchyma formation. New Phytol. 2003;161:35–49. [Google Scholar]
- 4.Shiono K, Ogawa S, Yamazaki S, Isoda H, Fujimura T, Nakazono M, et al. Contrasting dynamics of radial O2-loss barrier induction and aerenchyma formation in rice roots of two lengths. Ann Bot. 2011;107:89–99. doi: 10.1093/aob/mcq221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Rajhi I, Yamauchi T, Takahashi H, Nishiuchi S, Shiono K, Watanabe R, et al. Identification of genes expressed in maize root cortical cells during lysigenous aerenchyma formation using laser microdissection and microarray analyses. New Phytol. 2011;190:351–368. doi: 10.1111/j.1469-8137.2010.03535.x. [DOI] [PubMed] [Google Scholar]
- 6.Shiono K, Takahashi H, Colmer TD, Nakazono M. Role of ethylene in acclimations to promote oxygen transport in roots of plants in waterlogged soils. Plant Sci. 2008;175:52–58. [Google Scholar]
- 7.Drew MC, He CJ, Morgan PW. Programmed cell death and aerenchyma formation in roots. Trends Plant Sci. 2000;5:123–127. doi: 10.1016/s1360-1385(00)01570-3. [DOI] [PubMed] [Google Scholar]
- 8.Overmyer K, Brosché M, Kangasjärvi J. Reactive oxygen species and hormonal control of cell death. Trends Plant Sci. 2003;8:335–342. doi: 10.1016/S1360-1385(03)00135-3. [DOI] [PubMed] [Google Scholar]
- 9.Torres MA, Dangl JL. Functions of the respiratory burst oxidase in biotic interactions, abiotic stress and development. Curr Opin Plant Biol. 2005;8:397–403. doi: 10.1016/j.pbi.2005.05.014. [DOI] [PubMed] [Google Scholar]
- 10.Sato M, Bremner I. Oxygen free radicals and metallothionein. Free Radic Biol Med. 1993;14:325–337. doi: 10.1016/0891-5849(93)90029-t. [DOI] [PubMed] [Google Scholar]
- 11.Coyle P, Philcox JC, Carey LC, Rofe AM. Metallothionein: The multipurpose protein. Cell Mol Life Sci. 2002;59:627–647. doi: 10.1007/s00018-002-8454-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Mittler R, Vanderauwera S, Gollery M, Van Breusegem F. Reactive oxygen gene network of plants. Trends Plant Sci. 2004;9:490–498. doi: 10.1016/j.tplants.2004.08.009. [DOI] [PubMed] [Google Scholar]
- 13.Yoshioka H, Sugie K, Park HJ, Maeda H, Tsuda N, Kawakita K, et al. Induction of plant gp91 phox homolog by fungal cell wall, arachidonic acid and salicylic acid in potato. Mol Plant Microbe Interact. 2001;14:725–736. doi: 10.1094/MPMI.2001.14.6.725. [DOI] [PubMed] [Google Scholar]
- 14.Yoshioka H, Numata N, Nakajima K, Katou S, Kawakita K, Rowland O, et al. Nicotiana benthamiana gp91phox homologs NbrbohA and NbrbohB participate in H2O2 accumulation and resistance to Phytophthora infestans. Plant Cell. 2003;15:706–718. [Google Scholar]
- 15.Rizhsky L, Davletova S, Liang H, Mittler R. The zinc finger protein Zat12 is required for cytosolic ascorbate peroxidase 1 expression during oxidative stress in Arabidopsis. J Biol Chem. 2004;279:11736–11743. doi: 10.1074/jbc.M313350200. [DOI] [PubMed] [Google Scholar]
- 16.Takeda S, Gapper C, Kaya H, Bell E, Kuchitsu K, Dolan L. Local positive feed back regulation determines cell shape in root hair cells. Science. 2008;319:1241–1244. doi: 10.1126/science.1152505. [DOI] [PubMed] [Google Scholar]
- 17.Lin F, Ding H, Wang J, Zhang H, Zhang A, Zhang Y, et al. Positive feedback regulation of maize NADPH oxidase by mitogen-activated protein kinase cascade in abscisic acid signaling. J Exp Bot. 2009;60:3221–3238. doi: 10.1093/jxb/erp157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Wong HL, Sakamoto T, Kawasaki T, Umemura K, Shimamoto K. Downregulation of metallothionein, a reactive oxygen scavenger, by the small GTPase OsRac1 in rice. Plant Physiol. 2004;135:1447–1456. doi: 10.1104/pp.103.036384. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Steffens B, Sauter M. Epidermal cell death in rice is confined to cells with a distinct molecular identity and is mediated by ethylene and H2O2 through an autoamplified signal pathway. Plant Cell. 2009;21:184–196. doi: 10.1105/tpc.108.061887. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Steffens B, Geske T, Sauter M. Aerenchyma formation in the rice stem and its promotion by H2O2. New Phytol. 2011;190:369–378. doi: 10.1111/j.1469-8137.2010.03496.x. [DOI] [PubMed] [Google Scholar]
- 21.Thornalley PJ, Vasák M. Possible role for metallothionein in protection against radiation-induced oxidative stress. Kinetics and mechanism of its reaction with superoxide and hydroxyl radicals. Biochim Biophys Acta. 1985;827:36–44. doi: 10.1016/0167-4838(85)90098-6. [DOI] [PubMed] [Google Scholar]
- 22.Xue T, Li X, Zhu W, Wu C, Yang G, Zheng C. Cotton metallothionein GhMT3a, a reactive oxygen species scavenger, increased tolerance against abiotic stress in transgenic tobacco and yeast. J Exp Bot. 2009;60:339–349. doi: 10.1093/jxb/ern291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Chae HZ, Uhm TB, Rhee SG. Dimerization of thiolspecific antioxidant and the essential role of cysteine 47. Proc Natl Acad Sci USA. 1994;91:7022–7026. doi: 10.1073/pnas.91.15.7022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Miller G, Suzuki N, Ciftci-Yilmaz S, Mittler R. Reactive oxygen species homeostasis and signaling during drought and salinity stresses. Plant Cell Environ. 2010;33:453–467. doi: 10.1111/j.1365-3040.2009.02041.x. [DOI] [PubMed] [Google Scholar]


