Abstract
Our previous data suggested that ongoing inflammation in the spinal cord 6 weeks following spinal cord injury was detrimental to locomotor function. Others have shown in the acute and sub-acute post-injury phase that microglial/macrophage activation and T regulatory cells are detrimental to recovery. Here, C57BL/6 mice with a moderately severe T9 contusion were injected intravenously daily with minocycline, which reduces microglial/macrophage activation, or with CD25 antibodies, which reduce T regulatory cell function, starting at 6 weeks after injury. Both anti-inflammatory drugs caused an improvement in hindlimb locomotor function over the 2-week treatment, as measured by the Basso Mouse Scale (BMS). The improvement was functionally important, with mice having problems with coordinated stepping (BMS ∼6) before treatment to walking essentially normally (BMS >7) at the end of the treatment. The effects diminished within 1 week after termination of the treatments, suggesting an ongoing and dynamic inflammatory process. The area of white matter or the inflammatory markers CD68 for activated microglia/macrophages and CD45 for leukocytes were not different between the groups. These data suggest that inflammation during the chronic phase following spinal cord injury reduces conduction through the epicenter, possibly by release of cytokines, and is amenable to treatment for improved neurological function.
Key words: CD25, contusion, inflammation, microglia, minocycline
Introduction
Inflammation at the injury epicenter following spinal cord injury has detrimental and beneficial components (Alexander and Popovich, 2009; Barrette et al., 2007; Blight, 1985, 1992; Donnelly and Popovich, 2008; Ghasemlou et al., 2007; Kigerl et al., 2009; Pineau and Lacroix, 2007; Popovich et al., 1999, 2002; Rabchevsky and Streit, 1997; Weaver et al., 2005; Yong and Rivest, 2009). Among others, anti-inflammatory treatments such as antibodies against CD11d integrin reducing neutrophil and macrophage infiltration (Gris et al., 2004; Weaver et al., 2005), depletion of macrophages (Blight, 1994; Popovich et al., 1999), and CXCL10 inhibition leading to reduced T cell infiltration (Gonzalez et al., 2007) have been used. Administered during the first week, these anti-inflammatory treatments are neuroprotective and promote functional recovery. Minocycline reduces activation of microglia and macrophages (Hains and Waxman, 2006) and has also been shown to have neuroprotective effects following acute spinal cord injury (Festoff et al., 2006; Lee et al., 2003, McPhail et al., 2004; Stirling et al., 2004, 2005; Teng et al., 2004; Wells et al., 2003; Yune et al., 2007). Regulatory T cells (CD4+/CD25+) seem to underlie a detrimental autoimmune response, and their removal leads to better outcomes after spinal cord injury in mice and in eye injury models (Kipnis et al., 2002, 2004). Besides the well-documented detrimental effects of early inflammatory responses, there are beneficial immune cells, including the recently identified M2 monocyte (Barrette et al., 2007; Kigerl et al., 2009). There are potentially important differences between rats and mice in terms of the waves of inflammatory cells entering the spinal cord. Therefore, whereas T cell infiltration into the spinal cord in rats is an early event, it is delayed in mice to the second week, after which T cells double in number up to 6 weeks post-injury (Sroga et al., 2003). By and large, the human inflammatory response to spinal cord injury is similar to that seen in rodents (Fleming et al., 2006; Norenberg et al., 2004). The rapid and transient expression of cytokines also seems to be similar (Streit et al., 1998; Yang et al., 2004).
Whereas much is known about the role of early inflammation in secondary degeneration following spinal cord injury, less is known about the effects of inflammation within the spinal cord during the chronic phase. Relatively few studies in the rodent spinal cord injury field have gone beyond 6 weeks post-injury. We (Han et al., 2010), like others (e.g., Kigerl et al., 2006; Rosenberg et al., 2005,), find inflammatory markers such as CD45 for total leukocytes and CD68 for activated microglia still present at 6 weeks post-injury in rats and mice. In humans, activated microglia/macrophages, as detectable by CD68 immunostaining, are also present for weeks to months following spinal cord injury (Fleming et al., 2006). It is unclear what the functional effects of these chronically activated or infiltrated cells are. Although we do not know the mechanism, mice treated with angiopoietin-1 plus an αvβ3 integrin agonist peptide during the first week after a T9 spinal cord contusion had better hindlimb locomotor function at 6 weeks than did mice treated with either drug alone, despite similar white matter sparing (Han et al., 2010). In the same mice, the CD45 and CD68 levels were decreased more than in the mice treated with either drug, suggesting that ongoing inflammation at the epicenter might reduce function of the pathways that course through spared white matter during the chronic post-injury phase.
Here, we set out to find proof-of-principle data for this idea by starting anti-inflammatory treatments, minocycline or CD25 antibodies, 6 weeks following a contusive spinal cord injury in C57BL/6 mice, and assessing their hindlimb locomotor function.
Methods
Animals
A total of 18 female C57BL/6 mice (8 weeks of age; 18–25 g; The Jackson Laboratory, Bar Harbor, ME) were used. Anesthesia was provided by an intraperitoneal injection of Avertin® (0.4 mg 2,2,2-tribromoethanol in 0.02 mL of 1.25% 2-methyl-2-butanol in saline per gram body weight; Sigma-Aldrich, St. Louis, MO). All experiments were performed according to University of Louisville Institutional Animal Care and Use Committee protocols and the guidelines of the National Institutes of Health. All animals were microchipped for identification.
Spinal cord injury
All 18 mice received a contusive injury at T9 spine level, on the same day, from an Infinite Horizons impactor (Precision Systems and Instrumentation) set at 50 kdyn with the spine stabilized using steel stabilizers inserted under the transverse processes one vertebra above and below the injury. Afterwards, the wound was sutured in layers, bacitracin ointment (Qualitest Pharmaceuticals, Huntsville, AL) was applied to the wound area, 0.1 mL of a 20 μg/mL stock of gentamicin (ButlerSchein, Dublin, OH) was injected subcutaneously, and the animals recovered on a water-circulating heating pad. They received 0.1 mL of a 15 μg/mL stock solution of buprenorphin (Reckitt Benckise, Hull, England) as an analgesic twice daily over the next 48 h, starting after they were awake, to prevent lethal interaction with Avertin. Bladders were manually expressed until automatic voiding returned spontaneously, which generally was within 7 days.
Basso Mouse Scale (BMS) analyses
The mice were tested weekly for hindlimb locomotor function to ensure that they reached a plateau in their recovery and to ensure that similar levels were used for the control and treatment group. The BMS values were stable from week 5 to week 6 post-injury. In short, mice were placed in a 47-inch diameter dry pool, where they were observed for 4 min by two people trained and certified by Dr. Michelle Basso at Ohio State University. The scores were on a scale of 0 – 9, where 0 is completely paralyzed; 1–2 represents slight (<90°) to extensive (>90°) ankle movement; 3–4 ranges from occasional to frequent dorsal stepping to occasional plantar stepping; 5 is defined as frequent to consistent plantar stepping and absence of or some coordination with rotated paw placement; 6–7 designates frequent to consistent stepping with some to mostly coordinated stepping with paw rotation and severe trunk instability; 8 indicates almost normal stepping with points taken off for trunk instability and whether the tail is up or down; and 9 represents normal coordination, paw placement, trunk stability, and tail up (Basso et al., 2006).
Anti-inflammatory injections
At 6 weeks after the injury, the mice were assigned to three groups, each having on average an approximately equal 6-week BMS score (6.3±0.4, 6.1±0.4, 6.5±0.4, standard error) IH impactor force (53±0.7, 51±0.4, 52±0.6), and spinal cord displacement (529±11, 535±61; 517±44) during injury. The latter is a better indicator of injury severity (Ghasemlou et al., 2005). Care was taken to perform the study in a blinded manner. The mice were only identifiable by the number of their randomly selected microchip. The BMS, treatment injections, and histological analyses were performed by different people who were blinded to each other's results. These three groups received intravenous injections (0.1 mL) through the tail veins with vehicle (saline, given daily), 200 μg minocycline (Cat.# M9511, Sigma-Aldrich, St. Louis, MO) in saline (given daily), or 100 μg rat monoclonal anti-mouse CD25 antibody (clone: PC61.5.3, BioXCell, West Lebanon, NH) in saline (given every third day). To reduce potential confounding effects on the tail, whose position is used in the BMS scoring, mice were not injected on the day before and, if treated on the day of BMS analyses, were injected only afterwards. We did not see any overt changes in tail function caused by the injections. The doses are informed by other mouse studies that used systemic intraperitoneal injections, where 1 mg/day minocycline had neuroprotective effects after spinal cord injury (Wells et al., 2003) and 0.5 mg CD25 antibody blocked function but not numbers of T regulatory cells in mouse cancer studies (Fecci et al., 2006; Kohm et al., 2006). This CD25 antibody is widely used to selectively target regulatory T cells, perhaps made possible by their particularly high expression of CD25. Our preferred route of administration is intravenous with a 10-fold lower volume than the body, translating into 10 times less reagent. Therefore, the 200 μg/day minocycline and 100 μg CD25 antibody used here are approximately twice that used in the systemic injection studies, possibly compensating for faster excretion. The treatments lasted 2 weeks and the locomotor function was assessed on a weekly basis using the BMS.
Histological analyses
One week after the treatments were terminated, to allow us to potentially see a washout effect, the mice were perfused with ice-cold phosphate buffered saline followed by 4% paraformaldehyde in phosphate buffer. Their spinal cords were collected and post-fixed overnight on a strip of dental wax to straighten them out and cryoprotected in 30% sucrose overnight, after which 20-μm transverse sections were cut on a cryostat from an 11-mm segment spanning at least 5 mm on each side of the epicenter. All the spinal cords of this study and a reference tissue to orient each slide were cast in a single block of Tissue Freezing Medium (TMF-5, Triangle Biomedical Sciences Inc., Durham, NC). Every other section was collected onto SuperFrost plus glass slides (VWR, Radnor, PA) to produce five series with each section in a series separated by 200 μm. One series of sections was processed for Eriochrome cyanine staining to detect white matter as described elsewhere (Han et al., 2010). The section with the least myelin for each mouse was viewed as the epicenter, and all additional staining and analyses used this as the epicenter reference. Adjacent sections spanning from 1 mm rostral to 1 mm caudal to the epicenter section were immunostained for CD45 or CD68 as described elsewhere (Han et al., 2010). Digital images of the entire transverse area of the spinal cord sections were taken on a DM 6000 Leica upright microscope at a 20×magnification using the automated stitching capability of Surveyer software (Objective Imaging, Cambridge, U.K.) combined with an Oasis Automation Controller (Objective Imaging, Cambridge, U.K.) driving the motorized stage. The total number of pixels of white matter, CD45, or CD68 was determined using the thresholding feature of ImageJ (Rasband, W.S., ImageJ, U. S. National Institutes of Health, Bethesda, MD http://imagej.nih.gov/ij/, 1997–2011) and calculated to total stained area. Area measurements are the least variable and most time-efficient method across different types of injuries and between users for quantifying activated microglia/macrophages (Donnelly et al., 2009). The white matter sparing was calculated from three sections spanning the epicenter. The CD45 and CD68 values were derived from three sections each at the epicenter and 1 mm caudal and rostral to it, and the nine values averaged for each mouse.
Statistical analysis
Statistical analyses were performed with the paired Student's t test to compare differences in BMS scores within each mouse over time, or ANOVA with post-hoc Tukey analyses to compare averages of the three injected groups at different time points. A value of p<0.05 was considered statistically significant.
Results
Anti-inflammatory treatments improve locomotor function after spinal cord injury
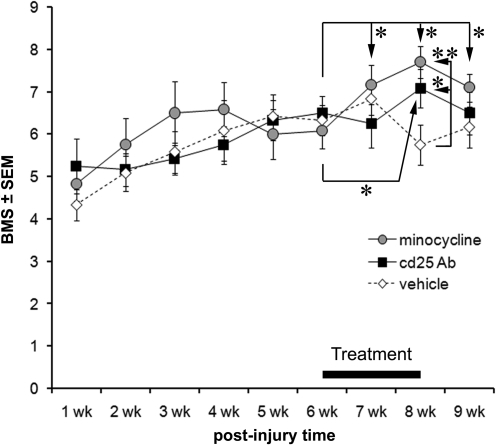
The average BMS scores of all groups were not significantly different over the first 6 weeks after the T9 contusion (Fig. 1). The mice were assigned to their treatment groups to achieve similar displacement and 6-week BMS scores and then treated daily for 2 weeks. Vehicle-treated mice showed no significant improvement in their BMS scores either over the 2-week period of injection or in the week afterwards (Fig. 1). In fact, the BMS score decreased from 6.8 to 5.8 between weeks 7 and 8 (p<0.05 by paired t test), similar to what can be seen in one of the few studies that also have used the BMS in mice beyond 7 weeks post-injury (Salazar et al., 2010). In the minocycline-treated group, BMS scores had increased from 6.1±0.4 to 7.2±0.5 by 1 week of treatment and to 7.7±0.4 by 2 (p<0.05 by paired t test; average±SEM). The minocycline-treated mice still had a higher BMS score 1 week after the treatment was terminated (7.1±0.3) than before the start of the treatment. In CD25 antibody treated mice, the BMS improved only after 2 weeks, from 6.5±0.4 to 7.1±0.5 (p<0.05 by paired t test), and 1 week following termination of the treatment, the BMS score (6.5±0.5) was not different from that seen at 6 weeks. At the maximal BMS levels, that is, with 2 weeks of treatment, the minocycline and CD25 antibody treated mice had significantly higher values than the vehicle-treated mice (p<0.01 and 0.05, respectively).
FIG. 1.
Anti-inflammatory treatment during the chronic phase following spinal cord injury improves hindlimb locomotor function. Mice received a 50 kdyn T9 injury and were assigned to treatment groups to achieve similar injury displacement and 6-week Basso Mouse Scale (BMS) values. The mice were injected intravenously daily over a 2-week period, starting at 6 weeks when BMS scores had stabilized, with vehicle, minocycline, or CD25 antibody (n=6 each). The anti-inflammatory treated mice improved over the 2-week period compared to the 6-week BMS values, and the effect was lost 1 week following termination of the treatment. Note that the vehicle-treated mice show a decrease in BMS between 7 and 8 weeks (p=0.034), which may indicate newly occurring detrimental processes. *p<0.05, **p<0.01.
Improved function is not caused by overt changes in white matter or presence of inflammatory cells
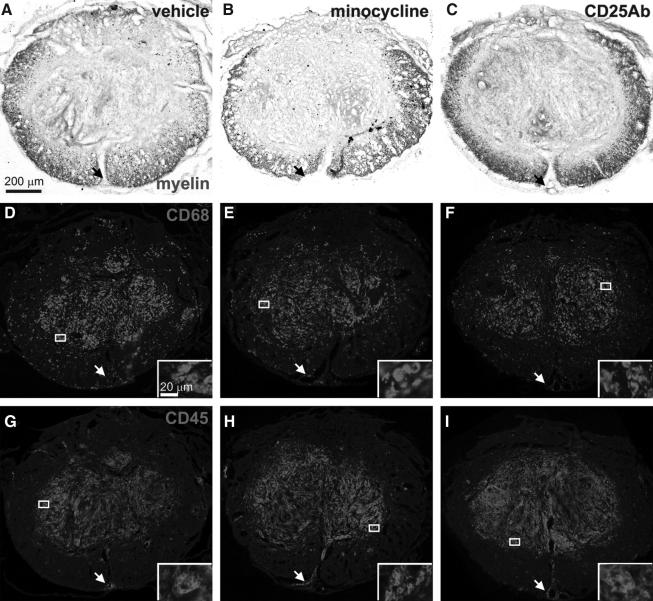
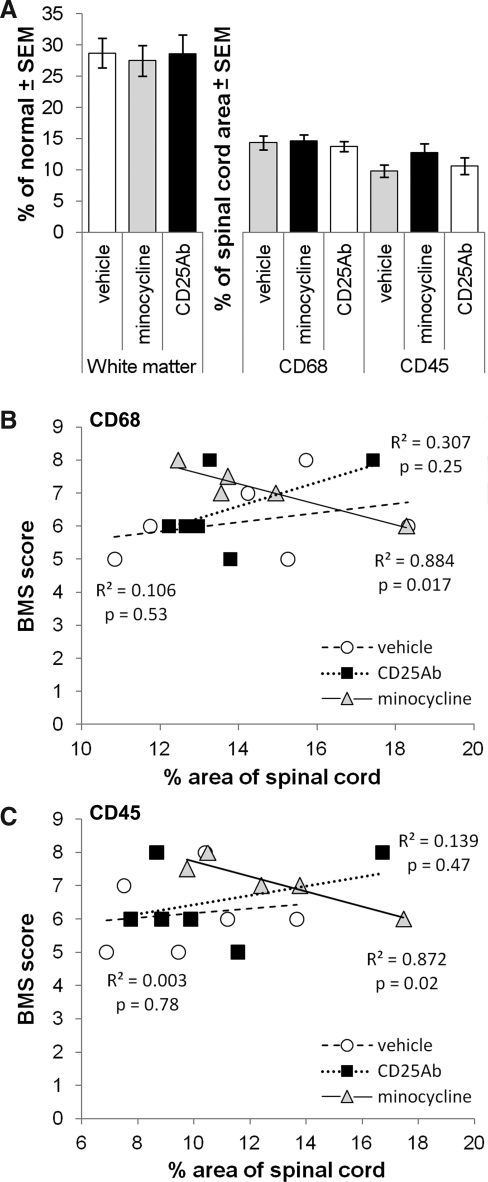
Histological analyses of the cords showed no difference in the white matter sparing among the three groups (Fig. 2A–C) with vehicle, minocycline, and CD25 antibody treated groups having average values of 29±2%, 27±2%, and 29±3% (Fig. 3A). CD68 and CD45 staining at the epicenter revealed an overall similar pattern and density in the minocycline (Fig. 2E,H) and CD25 antibody (Fig. 2F,I) treated groups compared to the vehicle-treated group (Fig. 2D,G). The core region of degeneration at the epicenter in mice is filled with mesenchymal cells and contained most of the CD68 and CD45 stained cells. In the white matter, CD68-stained cells appeared more numerous than CD45 stained cells, whereas around blood vessels, CD45 positive cells were more numerous, potentially representing infiltrating leukocytes. There were no obvious differences in these patterns seen among the treatment groups. Image analyses of the epicenter and at 1 mm distances from it confirmed that the average white matter sparing and CD68 and CD45 positive areas were not significantly different among the three groups (Fig. 3A). Therefore, the minocycline and CD25 antibody treatments do not appear to change microglia/macrophage activation or leukocyte infiltration, or the effect is lost within 1 week following termination of the treatment.
FIG. 2.
Anti-inflammatory treatment during the chronic phase had no overt effects on white matter or inflammatory markers. Adjacent transverse sections of mice representative of the groups (A,D,G=vehicle; B,E,H=minocycline; C,F,I=CD25 antibody) as defined by the average values in Figure 3, were stained for white matter (eriochrome cyanine; A–C), the activated microglia/macrophage marker CD68 (D–F), or the pan-leukocyte marker CD45 (G–I). Note that the CD68 immunostaining is more evident in the white matter rim than the CD45 immunostaining. The CD45-positive cells often could be seen around blood vessels (insets), including the large vessels in the ventral sulcus (arrows). Insets are higher magnifications of the boxes indicated in D–I and show the cellular nature of the staining. Scale bar in A is 200 μm for A–I. Scale bar in the inset of D is 20 μm for the insets in D–I.
FIG. 3.
Anti-inflammatory treatments did not affect average area of white matter or inflammatory markers: quantification. (A) The total area (stained pixels) was measured and recalculated as percentage of normal white matter or of the total area of the spinal cord (CD68, CD45). Neither minocycline nor CD25 antibody treatments affected the average area of white matter or of the inflammatory markers. Only minocycline-treated mice had a significant and substantial correlation between total CD68 (B) or CD45 (C) area and 9-week BMS locomotor function scores.
Inflammation during the acute phase generally correlates with functional deficits, most likely because of its role in tissue degeneration. Here, during the chronic post-injury phase, vehicle-injected mice showed no correlation between the area of inflammatory markers and the BMS score at 9 weeks (Fig. 3B,C). In minocycline-treated mice, both CD68 and CD45 correlated inversely with BMS scores, perhaps indicative of a treatment effect by reduced microglial/macrophage activation (Fig. 3B,C). No significant correlation was seen in CD25 antibody treated mice (Fig. 3B,C). Surprisingly, the amount of epicenter white matter did not correlate with the BMS scores at 5–9 weeks post-injury (data not shown), contrary what we and others have seen before at 6 weeks (Basso et al., 2006; Han et al., 2010).
Discussion
The main finding of this study is that treatment with anti-inflammatory drugs during the chronic phase following contusive T9 spinal cord injury in adult mice improves their locomotor function. This suggests that chronic inflammation is detrimental but is treatable. The decline in function seen in the vehicle-treated control mice between 7 and 8 weeks post-injury suggests that there are ongoing and dynamic changes in the chronic inflammatory process. This is also suggested by the rapid decline in function once the treatment was terminated. This dip in function can be seen in another study using the BMS in mice (Salazar et al., 2010). This late- occurring progression of dysfunction is also suggested by the current surprising finding that the amount of white matter did not correlate with the 9-week BMS scores, whereas it does at post-injury times of ≤6 weeks (Basso et al., 2006; Han et al., 2010). Our treatments coincided with this second progression of inflammation showing that this is very appropriate for the early chronic phase. Whether initiation of treatments at even longer post-injury times would also be effective remains to be seen, but would have important clinical implications. Therefore, one could foresee testing anti-inflammatory treatments in people long after the spinal cord injury, potentially improving their function or improving the efficacy of rehabilitation. The downside of our finding is that the beneficial effect appears to diminish after termination of the treatment. If chronic inflammation is a lifelong detrimental process, this would suggest that anti-inflammatory treatments might have to last longer, possibly requiring a lifelong low dose maintenance. On the other hand, it is unknown whether chronic inflammation resolves in rodents or humans after longer post-injury times. In humans, there is still evidence for ongoing inflammation in the form of foamy macrophages 1 year following spinal cord injury (Fleming et al., 2006).
The injury epicenter is the most likely location of the detrimental chronic inflammation that was reduced by our treatments. There was a striking presence of both CD45-positive perivascular infiltrates, indicating an ongoing progressive inflammatory process, and leukocytes within the parenchyma and white matter. The CD68-positive activated microglia and macrophages were also abundant and were also robustly present in the white matter. Although we did not see an effect of the anti-inflammatory treatments on the total area of the CD68 and CD45 markers this should be considered in light of the loss of the functional effects 1 week following termination of the treatment. Many other studies have shown that functionally beneficial effects of anti-inflammatory treatments given during the acute phase are related to reduced inflammation at the injury epicenter (Blight, 1994; Gris et al., 2004; Gonzalez et al., 2007; Kipnis et al., 2002, 2004; Popovich et al., 1999; Weaver et al., 2005). Moreover, the idea that a substantial proportion of the inflammatory cells at the injury epicenter are detrimental during the acute and sub-acute phase is widely accepted (Alexander and Popovich, 2009; Barrette et al., 2007; Blight, 1985, 1992; Donnelly and Popovich, 2008; Kigerl et al., 2009; Pineau and Lacroix, 2007; Popovich et al., 1999, 2002). During the chronic phase, B-cells have been shown to play a detrimental role, possibly by activating intra-spinal complement through release of antibodies (Ankeny et al., 2009). Astrocytes also play a role in inflammation, rapidly responding to spinal cord injury and pro-inflammatory chemokines, contributing to the initiation of the inflammatory cascade (Pineau and Lacroix, 2007; Pineau et al. 2010). This response continued to be elevated at the injury epicenter at 28 days post-injury (Pineau et al., 2010) and potentially continued to contribute to the detrimental effects of chronic inflammation. Neurons at the injury epicenter also contributed to the innate inflammatory response to spinal cord contusion through a caspase-associated inflammasome (de Rivero Vaccari et al., 2008). It will be important to assess whether it is still present during the chronic phase.
An alternative explanation for the functional benefits seen in this study is an effect on chronic systemic inflammation, which has been recognized as an ongoing and detrimental process (Popovich and McTigue, 2009; Riegger et al., 2007, 2009). However, the increase in the BMS scores represented an improvement of 6 to >7, which functionally means that the mice improved by achieving coordinated stepping and correct paw placement. This improvement is unlikely to represent decreases in systemic infection, although this is not impossible, as minocycline has bioactivity as a broad-spectrum antibiotic against various bacteria. However, our surgeries are performed under aseptic conditions; the mice are treated with topical bacitracin and systemic gentamicin postoperatively. Also, the minocycline treatment was started 6 weeks after spinal cord injury when cystitis, which occurs in some mice, would have resolved. In contrast, minocycline is known to affect activated microglia and macrophages, which are clearly prominent at the spinal cord injury epicenter. In addition, the inverse correlation between the 9-week BMS scores and the inflammatory markers at the injury site in the minocyline-treated group suggests that it acts primarily by reducing inflammation at that location.
The mechanisms at the injury epicenter by which the anti-inflammatory drugs might have improved locomotor function during the chronic post-injury phase remain to be determined. Our study was initiated based on our findings that mice with similar white matter sparing had much better BMS scores when treated with angiopoietin-1 plus an integrin agonist C16, and that this was accompanied by reduced inflammation at the epicenter (Han et al., 2010). This suggested that conduction of action potentials through the epicenter white matter might be reduced chronically after a contusive spinal cord injury. We also found that C16 reduces transmigration across an endothelial layer in vitro (Han et al., 2010). In the same study, C16 treatments between 28 and 34 days post-injury did not improve BMS scores. This suggests that the current functional improvements are not caused by reduced infiltration of leukocytes. The finding here that minocycline improved function despite a similar extent of CD68 at the epicenter could be consistent with a recurrence of microglia/macrophage activation 1 week following the termination of the treatment. Alternatively, minocyline may have reduced release of detrimental cytokines that otherwise reduce axonal function. For example, pro-inflammatory cytokines such as TNFα, IL-1, and IL-6 can reduce neural activity in normal dorsal roots of rats in vivo (Ozaktay et al., 2002) and TNFα can induce a central conduction deficit in guinea pig spinal cord white matter strips (Davies et al., 2006). The mechanisms underlying the beneficial effects of the CD25 antibody are not entirely clear. It is thought that administration of CD25 antibody causes a downregulation of the receptor and/or causes shedding of CD25 located on surface of T regulatory cells, rendering them nonfunctional by blocking their ability to bind IL-2, which is needed for activation of these cells (Kohm et al., 2006). The T regulatory cell function returns back to normal in 10–14 days after last injection of the CD25 antibody, explaining the loss of activity of our treatment after its termination. It has been proposed that suppression of CD25+ T regulatory cells enables beneficial autoimmunity to promote myelin clearance and protect neurons following spinal cord injury (Kipnis et al., 2002). However, those studies were during the acute and sub-acute injury phase, making such mechanisms less likely to be involved during the chronic phase, when myelin debris should have been cleared and neurons are no longer dying.
Finally, despite the exciting observation that chronic inflammation can be targeted to improve neurological function, much more refined immunological methods need to be utilized to pinpoint the underlying molecular mechanisms. That should help to develop more specific anti-inflammatory drug treatments for chronic spinal cord injury. It will also be important to replicate the current findings in rat and primate models before initiating more costly human clinical trials.
Acknowledgments
We thank Hillary Conway, Christine Yarberry, and Rollie Reid for their excellent technical assistance. Dr. David Magnuson is thanked for helpful discussions. This work was supported by National Institutes of Health grant NS45734, RR15576, Norton Healthcare, and the Commonwealth of Kentucky Challenge for Excellence.
Author Disclosure Statement
No competing financial interests exist.
References
- Alexander J.K. Popovich P.G. Neuroinflammation in spinal cord injury: therapeutic targets for neuroprotection and regeneration. Prog. Brain Res. 2009;175:125–137. doi: 10.1016/S0079-6123(09)17508-8. [DOI] [PubMed] [Google Scholar]
- Ankeny D.P. Guan Z. Popovich P.G. B cells produce pathogenic antibodies and impair recovery after spinal cord injury in mice. J. Clin. Invest. 2009;119:2990–2999. doi: 10.1172/JCI39780. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barrette B. Vallieres N. Dube M. Lacroix S. Expression profile of receptors for myelin-associated inhibitors of axonal regeneration in the intact and injured mouse central nervous system. Mol. Cell. Neurosci. 2007;34:519–538. doi: 10.1016/j.mcn.2006.12.004. [DOI] [PubMed] [Google Scholar]
- Basso D.M. Fisher L.C. Anderson A.J. Jakeman L.B. McTigue D.M. Popovich P.G. Basso Mouse Scale for locomotion detects differences in recovery after spinal cord injury in five common mouse strains. J. Neurotrauma. 2006;23:635–659. doi: 10.1089/neu.2006.23.635. [DOI] [PubMed] [Google Scholar]
- Blight A.R. Delayed demyelination and macrophage invasion: a candidate for secondary cell damage in spinal cord injury. Cent. Nerv. Syst. Trauma. 1985;2:299–315. doi: 10.1089/cns.1985.2.299. [DOI] [PubMed] [Google Scholar]
- Blight A.R. Macrophages and inflammatory damage in spinal cord injury. J. Neurotrauma. 1992;9(Suppl. 1):S83–S91. [PubMed] [Google Scholar]
- Blight A.R. Effects of silica on the outcome from experimental spinal cord injury: implication of macrophages in secondary tissue damage. Neuroscience. 1994;60:263–273. doi: 10.1016/0306-4522(94)90220-8. [DOI] [PubMed] [Google Scholar]
- Davies A.L. Hayes K.C. Shi R. Recombinant human TNFalpha induces concentration-dependent and reversible alterations in the electrophysiological properties of axons in mammalian spinal cord. J. Neurotrauma. 2006;23:1261–1273. doi: 10.1089/neu.2006.23.1261. [DOI] [PubMed] [Google Scholar]
- de Rivero Vaccari J.P. Lotocki G. Marcillo A.E. Dietrich W.D. Keane R.W. A molecular platform in neurons regulates inflammation after spinal cord injury. J. Neurosci. 2008;28:3404–3414. doi: 10.1523/JNEUROSCI.0157-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Donnelly D.J. Gensel J.C. Ankeny D.P. van R.N. Popovich P.G. An efficient and reproducible method for quantifying macrophages in different experimental models of central nervous system pathology. J. Neurosci. Methods. 2009;181:36–44. doi: 10.1016/j.jneumeth.2009.04.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Donnelly D.J. Popovich P.G. Inflammation and its role in neuroprotection, axonal regeneration and functional recovery after spinal cord injury. Exp. Neurol. 2008;209:378–388. doi: 10.1016/j.expneurol.2007.06.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fecci P.E. Sweeney A.E. Grossi P.M. Nair S.K. Learn C.A. Mitchell D.A. Cui X. Cummings T.J. Bigner D.D. Gilboa E. Sampson J.H. Systemic anti-CD25 monoclonal antibody administration safely enhances immunity in murine glioma without eliminating regulatory T cells. Clin. Cancer Res. 2006;12:4294–4305. doi: 10.1158/1078-0432.CCR-06-0053. [DOI] [PubMed] [Google Scholar]
- Festoff B.W. Ameenuddin S. Arnold P.M. Wong A. Santacruz K.S. Citron B.A. Minocycline neuroprotects, reduces microgliosis, and inhibits caspase protease expression early after spinal cord injury. J. Neurochem. 2006;97:1314–1326. doi: 10.1111/j.1471-4159.2006.03799.x. [DOI] [PubMed] [Google Scholar]
- Fleming J.C. Norenberg M.D. Ramsay D.A. Dekaban G.A. Marcillo A.E. Saenz A.D. Pasquale–Styles M. Dietrich W.D. Weaver L.C. The cellular inflammatory response in human spinal cords after injury. Brain. 2006;129:3249–3269. doi: 10.1093/brain/awl296. [DOI] [PubMed] [Google Scholar]
- Ghasemlou N. Jeong S.Y. Lacroix S. David S. T cells contribute to lysophosphatidylcholine-induced macrophage activation and demyelination in the CNS. Glia. 2007;55:294–302. doi: 10.1002/glia.20449. [DOI] [PubMed] [Google Scholar]
- Ghasemlou N. Kerr B.J. David S. Tissue displacement and impact force are important contributors to outcome after spinal cord contusion injury. Exp. Neurol. 2005;196:9–17. doi: 10.1016/j.expneurol.2005.05.017. [DOI] [PubMed] [Google Scholar]
- Gonzalez R. Hickey M.J. Espinosa J.M. Nistor G. Lane T.E. Keirstead H.S. Therapeutic neutralization of CXCL10 decreases secondary degeneration and functional deficit after spinal cord injury in mice. Regen. Med. 2007;2:771–783. doi: 10.2217/17460751.2.5.771. [DOI] [PubMed] [Google Scholar]
- Gris D. Marsh D.R. Oatway M.A. Chen Y. Hamilton E.F. Dekaban G.A. Weaver L.C. Transient blockade of the CD11d/CD18 integrin reduces secondary damage after spinal cord injury, improving sensory, autonomic, and motor function. J. Neurosci. 2004;24:4043–4051. doi: 10.1523/JNEUROSCI.5343-03.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hains B.C. Waxman S.G. Activated microglia contribute to the maintenance of chronic pain after spinal cord injury. J. Neurosci. 2006;26:4308–4317. doi: 10.1523/JNEUROSCI.0003-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Han S. Arnold S.A. Sithu S.D. Mahoney E.T. Geralds J.T. Tran P. Benton R.L. Maddie M.A. D'Souza S.E. Whittemore S.R. Hagg T. Rescuing vasculature with intravenous angiopoietin-1 and alpha v beta 3 integrin peptide is protective after spinal cord injury. Brain. 2010;133:1026–1042. doi: 10.1093/brain/awq034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kigerl K.A. Gensel J.C. Ankeny D.P. Alexander J.K. Donnelly D.J. Popovich P.G. Identification of two distinct macrophage subsets with divergent effects causing either neurotoxicity or regeneration in the injured mouse spinal cord. J. Neurosci. 2009;29(13):435–13,444. doi: 10.1523/JNEUROSCI.3257-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kigerl K.A. McGaughy V.M. Popovich P.G. Comparative analysis of lesion development and intraspinal inflammation in four strains of mice following spinal contusion injury. J. Comp. Neurol. 2006;494:578–594. doi: 10.1002/cne.20827. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kipnis J. Avidan H. Markovich Y. Mizrahi T. Hauben E. Prigozhina T.B. Slavin S. Schwartz M. Low-dose gamma-irradiation promotes survival of injured neurons in the central nervous system via homeostasis-driven proliferation of T cells. Eur. J. Neurosci. 2004;19:1191–1198. doi: 10.1111/j.1460-9568.2004.03207.x. [DOI] [PubMed] [Google Scholar]
- Kipnis J. Mizrahi T. Hauben E. Shaked I. Shevach E. Schwartz M. Neuroprotective autoimmunity: naturally occurring CD4+CD25+ regulatory T cells suppress the ability to withstand injury to the central nervous system. Proc. Natl. Acad. Sci. U.S.A. 2002;99(15):620–15,625. doi: 10.1073/pnas.232565399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kohm A.P. McMahon J.S. Podojil J.R. Begolka W.S. DeGutes M. Kasprowicz D.J. Ziegler S.F. Miller S.D. Cutting Edge: Anti-CD25 monoclonal antibody injection results in the functional inactivation, not depletion, of CD4+CD25+ T regulatory cells. J. Immunol. 2006;176:3301–3305. doi: 10.4049/jimmunol.176.6.3301. [DOI] [PubMed] [Google Scholar]
- Lee S.M. Yune T.Y. Kim S.J. Park D.W. Lee Y.K. Kim Y.C. Oh Y.J. Markelonis G.J. Oh T.H. Minocycline reduces cell death and improves functional recovery after traumatic spinal cord injury in the rat. J. Neurotrauma. 2003;20:1017–1027. doi: 10.1089/089771503770195867. [DOI] [PubMed] [Google Scholar]
- McPhail L.T. Stirling D.P. Tetzlaff W. Kwiecien J.M. Ramer M.S. The contribution of activated phagocytes and myelin degeneration to axonal retraction/dieback following spinal cord injury. Eur. J. Neurosci. 2004;20:1984–1994. doi: 10.1111/j.1460-9568.2004.03662.x. [DOI] [PubMed] [Google Scholar]
- Norenberg M.D. Smith J. Marcillo A. The pathology of human spinal cord injury: defining the problems. J. Neurotrauma. 2004;21:429–440. doi: 10.1089/089771504323004575. [DOI] [PubMed] [Google Scholar]
- Ozaktay A.C. Cavanaugh J.M. Asik I. DeLeo J.A. Weinstein J.N. Dorsal root sensitivity to interleukin-1 beta, interleukin-6 and tumor necrosis factor in rats. Eur. Spine J. 2002;11:467–475. doi: 10.1007/s00586-002-0430-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pineau I. Lacroix S. Proinflammatory cytokine synthesis in the injured mouse spinal cord: multiphasic expression pattern and identification of the cell types involved. J. Comp. Neurol. 2007;500:267–285. doi: 10.1002/cne.21149. [DOI] [PubMed] [Google Scholar]
- Pineau I. Sun L. Bastien D. Lacroix S. Astrocytes initiate inflammation in the injured mouse spinal cord by promoting the entry of neutrophils and inflammatory monocytes in an IL-1 receptor/MyD88-dependent fashion. Brain Behav. Immun. 2010;24:540–553. doi: 10.1016/j.bbi.2009.11.007. [DOI] [PubMed] [Google Scholar]
- Popovich P. McTigue D. Damage control in the nervous system: beware the immune system in spinal cord injury. Nat. Med. 2009;15:736–737. doi: 10.1038/nm0709-736. [DOI] [PubMed] [Google Scholar]
- Popovich P.G. Guan Z. McGaughy V. Fisher L. Hickey W.F. Basso D.M. The neuropathological and behavioral consequences of intraspinal microglial/macrophage activation. J. Neuropathol. Exp. Neurol. 2002;61:623–633. doi: 10.1093/jnen/61.7.623. [DOI] [PubMed] [Google Scholar]
- Popovich P.G. Guan Z. Wei P. Huitinga I. van R.N. Stokes B.T. Depletion of hematogenous macrophages promotes partial hindlimb recovery and neuroanatomical repair after experimental spinal cord injury. Exp. Neurol. 1999;158:351–365. doi: 10.1006/exnr.1999.7118. [DOI] [PubMed] [Google Scholar]
- Rabchevsky A.G. Streit W.J. Grafting of cultured microglial cells into the lesioned spinal cord of adult rats enhances neurite outgrowth. J. Neurosci. Res. 1997;47:34–48. [PubMed] [Google Scholar]
- Riegger T. Conrad S. Liu K. Schluesener H.J. Adibzahdeh M. Schwab J.M. Spinal cord injury-induced immune depression syndrome (SCI-IDS) Eur. J. Neurosci. 2007;25:1743–1747. doi: 10.1111/j.1460-9568.2007.05447.x. [DOI] [PubMed] [Google Scholar]
- Riegger T. Conrad S. Schluesener H.J. Kaps H.P. Badke A. Baron C. Gerstein J. Dietz K. Abdizahdeh M. Schwab J.M. Immune depression syndrome following human spinal cord injury (SCI): a pilot study. Neuroscience. 2009;158:1194–1199. doi: 10.1016/j.neuroscience.2008.08.021. [DOI] [PubMed] [Google Scholar]
- Rosenberg L.J. Zai L.J. Wrathall J.R. Chronic alterations in the cellular composition of spinal cord white matter following contusion injury. Glia. 2005;49:107–120. doi: 10.1002/glia.20096. [DOI] [PubMed] [Google Scholar]
- Salazar D.L. Uchida N. Hamers F.P. Cummings B.J. Anderson A.J. Human neural stem cells differentiate and promote locomotor recovery in an early chronic spinal cord injury NOD-scid mouse model. PLoS One. 2010;5:e12272. doi: 10.1371/journal.pone.0012272. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sroga J.M. Jones T.B. Kigerl K.A. McGaughy V.M. Popovich P.G. Rats and mice exhibit distinct inflammatory reactions after spinal cord injury. J. Comp. Neurol. 2003;462:223–240. doi: 10.1002/cne.10736. [DOI] [PubMed] [Google Scholar]
- Stirling D.P. Khodarahmi K. Liu J. McPhail L.T. McBride C.B. Steeves J.D. Ramer M.S. Tetzlaff W. Minocycline treatment reduces delayed oligodendrocyte death, attenuates axonal dieback, and improves functional outcome after spinal cord injury. J. Neurosci. 2004;24:2182–2190. doi: 10.1523/JNEUROSCI.5275-03.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stirling D.P. Koochesfahani K.M. Steeves J.D. Tetzlaff W. Minocycline as a neuroprotective agent. Neuroscientist. 2005;11:308–322. doi: 10.1177/1073858405275175. [DOI] [PubMed] [Google Scholar]
- Streit W.J. Semple–Rowland S.L. Hurley S.D. Miller R.C. Popovich P.G. Stokes B.T. Cytokine mRNA profiles in contused spinal cord and axotomized facial nucleus suggest a beneficial role for inflammation and gliosis. Exp. Neurol. 1998;152:74–87. doi: 10.1006/exnr.1998.6835. [DOI] [PubMed] [Google Scholar]
- Teng Y.D. Choi H. Onario R.C. Zhu S. Desilets F.C. Lan S. Woodard E.J. Snyder E.Y. Eichler M.E. Friedlander R.M. Minocycline inhibits contusion-triggered mitochondrial cytochrome c release and mitigates functional deficits after spinal cord injury. Proc. Natl. Acad. Sci. U.S.A. 2004;101:3071–3076. doi: 10.1073/pnas.0306239101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weaver L.C. Gris D. Saville L.R. Oatway M.A. Chen Y. Marsh D.R. Hamilton E.F. Dekaban G.A. Methylprednisolone causes minimal improvement after spinal cord injury in rats, contrasting with benefits of an anti-integrin treatment. J. Neurotrauma. 2005;22:1375–1387. doi: 10.1089/neu.2005.22.1375. [DOI] [PubMed] [Google Scholar]
- Wells J.E. Hurlbert R.J. Fehlings M.G. Yong V.W. Neuroprotection by minocycline facilitates significant recovery from spinal cord injury in mice. Brain. 2003;126:1628–1637. doi: 10.1093/brain/awg178. [DOI] [PubMed] [Google Scholar]
- Yang L. Blumbergs P.C. Jones N.R. Manavis J. Sarvestani G.T. Ghabriel M.N. Early expression and cellular localization of proinflammatory cytokines interleukin-1beta, interleukin-6, and tumor necrosis factor-alpha in human traumatic spinal cord injury. Spine (Phila Pa 1976) 2004;29:966–971. doi: 10.1097/00007632-200405010-00004. [DOI] [PubMed] [Google Scholar]
- Yong V.W. Rivest S. Taking advantage of the systemic immune system to cure brain diseases. Neuron. 2009;64:55–60. doi: 10.1016/j.neuron.2009.09.035. [DOI] [PubMed] [Google Scholar]
- Yune T.Y. Lee J.Y. Jung G.Y. Kim S.J. Jiang M.H. Kim Y.C. Oh Y.J. Markelonis G.J. Oh T.H. Minocycline alleviates death of oligodendrocytes by inhibiting pro-nerve growth factor production in microglia after spinal cord injury. J. Neurosci. 2007;27:7751–7761. doi: 10.1523/JNEUROSCI.1661-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]