Abstract
Following a lateralized spinal cord injury (SCI) in humans, substantial walking recovery occurs; however, deficits persist in adaptive features of locomotion critical for community ambulation, including obstacle negotiation. Normal obstacle negotiation is accomplished by an increase in flexion during swing. If an object is unanticipated or supraspinal input is absent, obstacle negotiation may involve the spinally organized stumbling corrective response. How these voluntary and reflex components are affected following partial SCI is not well studied. This study is the first to characterize recovery of obstacle negotiation following low-thoracic spinal hemisection in the cat. Cats were trained pre- and post-injury to cross a runway with an obstacle. Assessments focused on the hindlimb ipsilateral to the lesion. Pre-injury, cats efficiently cleared an obstacle by increasing knee flexion during swing. Post-injury, obstacle clearance permanently changed. At 2 weeks, when basic overground walking ability been recovered, the hindlimb was dragged over the obstacle (∼90%). Surprisingly, the stumbling corrective response was not elicited until after 2 weeks. Despite a notable increase, between 4 and 8 weeks, in the ability to modify limb trajectory when approaching an obstacle, limb lift during obstacle approach was insufficient during ∼50% of encounters and continued to evoke the stumbling corrective response even at 16 weeks. A post-injury lead limb bias identified during negotiations with complete clearance, suggests a potential training strategy to increase the number of successful clearances. Therefore, following complete severing of half of the spinal cord, the ability to modify ipsilateral hindlimb trajectory shows significant recovery and by 16 weeks permits effective clearing of an obstacle, without contact, ∼50% of the time. Although this suggests plasticity of supporting circuitry, it is insufficient to support consistent clearance. This inconsistency, even at the most chronic time point assessed (16 weeks), is probably a contributing factor to falls reported for people with SCI.
Key words: locomotion, obstacle negotiation, recovery, SCI, stumbling corrective response
Introduction
Locomotor adaptations are essential for safe walking. Successful negotiation of environmental change requires modification in limb trajectories controlled by different levels of the neural axis. Specifically, visualization of an obstacle allows voluntary step cycle modifications, which are mediated by the integration of signals from descending supraspinal and intraspinal pathways (Mohagheghi et al., 2004; Patla and Greig, 2006; Wilkinson and Sherk, 2005). The corticospinal and rubrospinal tracts have been identified as critical descending motor pathways in the control of voluntary obstacle negotiation in the cat (Beloozerova and Sirota, 1993; Drew et al., 1996; Lavoie and Drew, 2002; Widajewicz et al., 1994). When an unanticipated obstacle with no preceding visual cue is encountered, changes in limb trajectory can occur as result of paw contact with the obstacle. Limb trajectory changes initiated by stimuli directly to the surface of the paw or hindlimb are referred to as the stumbling corrective response (Forssberg, 1979; McVea and Pearson, 2007; Schillings et al., 1996). In contrast to the voluntary response, work in the low spinally transected cat indicates that this response can be mediated by spinal circuitry (Forssberg et al., 1975).
Our work (Jefferson et al., 2011; Tester and Howland, 2008), as well as that of others (Basso et al., 1994; Eidelberg et al., 1986; Helgren and Goldberger, 1993; Kuhtz-Buschbeck et al., 1996), shows that substantial motor recovery occurs following thoracic hemisection in the cat. Despite this recovery, quantifiable deficits still exist. The hindlimb ipsilateral to the lesion is reintegrated during basic overgound locomotion, and kinematic patterns of hindlimb stepping are similar to those seen pre-injury. However, performance deficits are obvious when the cat is crossing a ladder or peg walkway, which requires a high level of limb accuracy (Helgren and Goldberger, 1993; Jefferson et al., 2011; Tester and Howland, 2008). Further, there is a difference in the time course of recovery between basic overground locomotion and locomotion requiring accurate paw placement. Therefore, more adaptive features of locomotion have a longer recovery time and recovery is less complete.
The impairments and pattern of recovery seen in the cat following a thoracic hemisection are similar to those observed in humans with Brown-Séquard syndrome (BSS) (McKinley et al., 2007). Individuals with BSS present with asymmetrical impairments in gross motor function, and in many cases recover the ability to walk (Little and Halar, 1985; van Hedel et al., 2005). Yet, deficits are observed when an ambulatory individual with an incomplete spinal cord injury (SCI) is challenged by an environment that requires alterations in basic movement patterns including discrete, precise changes (Amatachaya et al., 2010). A variety of obstacles are identified as features of the community environment that tend to be avoided by individuals with SCI because of their limited walking ability and the potential for falls (Musselman and Yang, 2007; Musselman et al., 2011). Even though obstacle negotiation is an important adaptive feature of walking necessary for community ambulation, relatively few experimental studies have investigated this behavior post-SCI. The effect of SCI on obstacle negotiation while stepping on a treadmill has been assessed only following bilateral cortico- and rubrospinal tract lesions in the cat thoracic spinal cord (Drew et al., 1996; reviewed by Drew et al., 2002). These limited white matter injuries were used to determine the specific roles these tracts play in obstacle negotiation. The hemisection lesion, on the other hand, more closely represents asymmetrical injuries seen in humans. Further, these prior studies have investigated the effects of obstacle negotiation following SCI only during treadmill walking. Examination of obstacle negotiation on overground runways more closely approximates the community environment individuals re-enter following SCI. The current study was designed to examine and characterize kinematic adaptations in limb trajectories necessary for effective negotiation of an overground obstacle in normal cats and cats with SCIs. Because the ipsilateral hindlimb is inconsistently integrated during ladder crossing (Tester and Howland, 2008) and rarely used during crossing of a peg walkway (Jefferson et al., 2011) following lateral hemisection, we hypothesized that ipsilateral limb clearance during obstacle negotiation would recover, but that deficits in efficiency and consistency of angular and vertical limb trajectories would be seen. Cats were trained pre- and post-SCI to cross a basic overground runway with and without an obstacle, for a food reward. This was done to promote maximal recovery and reduce motivational confounds. To permit time for long-term recovery, cats were evaluated for 16 weeks post-SCI.
Methods
All animal procedures were conducted in accordance with the National Institutes of Health guidelines for the care and use of experimental animals and were approved by the University of Florida and the Malcom Randall Veterans Affair's Institutional Animal Care and Use Committees.
Five, purpose-bred, specific-pathogen free, female, adult cats were used. Cats were spayed to remove potential hormone effects associated with the estrus cycle on behavior and lesion size (Sribnick et al., 2005, 2010; Stein, 2008).
Spinal hemisection surgery
All cats received a left spinal thoracic (T) 10 hemisection as described in our previous studies (Jefferson et al., 2010, 2011; Tester and Howland, 2008). In summary, animals received 0.1cc of atropine sulfate (0.04–0.06 mg/kg) and acetlypromazine (0.4–0.5 mg/kg) subcutaneously (SQ), were anesthetized in a gaseous chamber with an isoflurane and oxygen mixture (2–5% isoflurane, 1–2 L of oxygen), intubated, and maintained at a surgical plane of anesthesia with isoflurane (2–3%). Throughout the duration of the surgical procedure, temperature, electrocardiogram, respiratory rate, and expired carbon dioxide were monitored and maintained within normal physiological limits.
The spinal cord was exposed by bilateral laminectomies at the vertebral T9–T10 level. A left lateral hemisection was made at the spinal T10 level using iridectomy scissors. Any fibers adhering to the dura were gently lifted with suction using a pulled glass pipette, and cut. Once the hemisection was complete, the dura was sutured and durafilm (Codman-Shurtleff, Inc., Randolf, MA) and then gelfoam (Pharmacian and Upjohn, Inc., Peakpack, NJ) placed over the sutures. Muscle and skin were sutured in layers. Buprenorphine (0.01 mg/kg, SQ) was given every 6–12 h for the first 48 h after surgery.
Procedures used to maintain the general health of the animals followed those used previously (Jefferson et al., 2010, 2011; Tester and Howland, 2008). Cats were housed singly or in pairs on thick beds of shredded newspaper or egg crate foam. Credé's maneuver was used to assist with bladder emptying for the first few days following injury until voluntary voiding recovered. Each cat's food intake, weight, and behavior were monitored closely throughout the study.
Training and assessments
Pre- and post-injury, cats were conditioned to perform a variety of basic and skilled locomotor tasks for food rewards 5 times/week. These included crossing of a basic runway (0.36×4.6 m) with and without an obstacle (7.7 cm high×2.5 cm wide) placed at the midpoint. Training on the runway with the obstacle occurred during 2 or 3 of the 5 training days per week. Cats completed ∼20 crossings on the obstacle task during each of these sessions. During filming, ∼50 crossings were performed by each animal on the obstacle task to reliably obtain a sufficient number of crossings at appropriate speed(s) for analysis. Cats were trained to walk at their most comfortable fast walk/slow trot speed pre-injury. Post-injury, speeds were closely matched to pre-injury speeds for each cat. Preferred speeds did vary across cats and reflect normal biological variations including limb lengths. Post-injury, training resumed within 2–3 days. Initially, trainer assistance was given, if needed, but ceased as soon as cats' weight support and postural control were sufficient to allow independent crossing. On training days, cats were fed exclusively in the behavior room. On non-training days, cats were fed ad libitum in their cages. Water was provided ad libitum throughout the study. Performances were filmed pre-injury and at 2, 4, 8, and 16 weeks post-injury for quantitative assessments. The ipsilateral hindlimb showed the most obvious motor deficits following hemisection, and therefore evaluations were focused on this limb.
Response type
All encounters in which the ipsilateral hindimb faced the camera (16–24 obstacle encounters) were assessed at pre- and all post-injury time points for general responses. The range of crossings used reflects a fatigue factor during the early post-injury time points. Each obstacle encounter was identified as a 1) complete clearance, 2) stumbling corrective response, or 3) other. The following criteria were used. During a complete clearance the ipsilateral hindlimb clears the obstacle without contact. The stumbling corrective response is characterized by hindlimb contact followed by an immediate obvious flexion response. Other responses occur when hindlimb contact does not evoke an obvious flexion response. These responses were averaged across animals at each time point and compared across time points. To determine if the lead limb over the obstacle affected the performance of the ipsilateral hindlimb during negotiation, these crossings also were classified by which hindlimb, ipsilateral versus contralateral, initially crossed the obstacle.
Three-dimensional angular kinematics
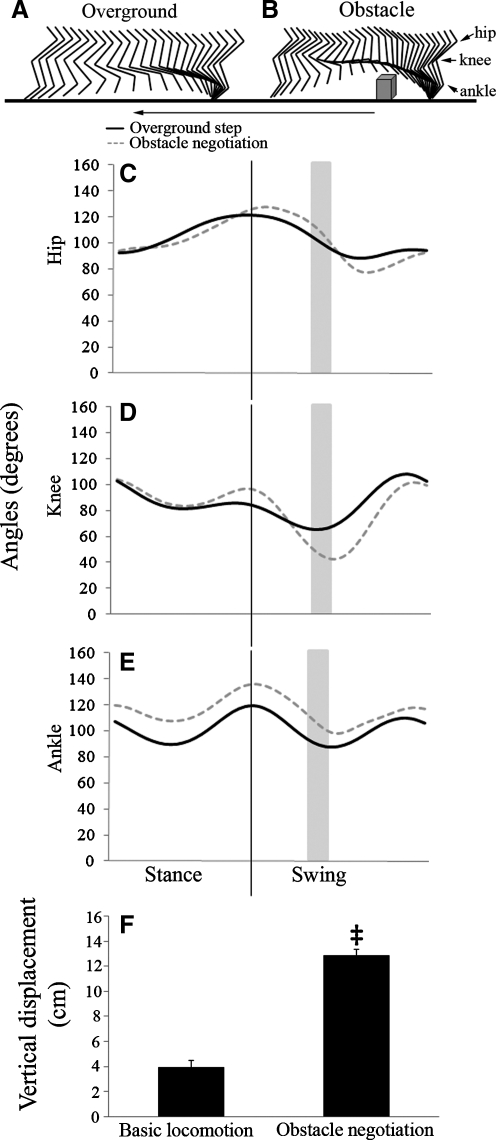
To illustrate normal hindlimb adjustments during obstacle negotiation, three-dimensional angular kinematics of 10 basic steps and 10 obstacle encounters for each cat were compared pre-injury. The 10 crossings that best matched pre-injury speed were chosen. To understand how injury impacts performance, angular kinematics of the 10 steps per time point during obstacle negotiation were compared across all time points. Each cat's post-injury performance always was compared to its pre-injury baseline, because cats, like humans, show individual variation. These variations in baseline may mask important changes post-injury if data are averaged across cats. To generate angles, reflective spheres were placed on four bony landmarks (iliac crest, greater trochanter, lateral malleolus, and base of the fifth metatarsal). A fifth sphere was placed on the fibula above the lateral malleolus as a vector mark, which, in combination with the length of the fibula, allowed for the automatic calculation of the knee joint using Motus software (Vicon Peak, Englewood, CO). Joint angles for the hip, knee, and ankle were calculated throughout the step cycle using the Peak Performance Analysis System (Vicon Peak, Englewood, CO). All data were analyzed using 60 images per sec. Representative steps were normalized to 51 frames in order to facilitate visual joint angle comparisons during different sub-phases for figure purposes only (Figs. 1 and 2).
FIG. 1.
Kinematic hindlimb data from runway crossings and obstacle negotiations pre-injury. Stick figures of one representative swing phase during basic locomotion (A) and obstacle negotiation (B) illustrate qualitative differences in hindlimb movement patterns between the two tasks. Arrow indicates direction of movement (A, B). Waveform graphs are of a single step or obstacle negotiation from a representative animal. Each is shown from paw down to paw down. The hip (C), knee (D), and ankle (E) joints are compared between tasks. The position of the obstacle is represented by the vertical thick gray line. Angular changes were seen at all joints, with the greatest occurring at the knee during clearance of the obstacle. Maximal vertical displacement of the limb was significantly greater (‡) during obstacle negotiation compared to basic locomotion (F). Error bars denote standard deviation (SD).
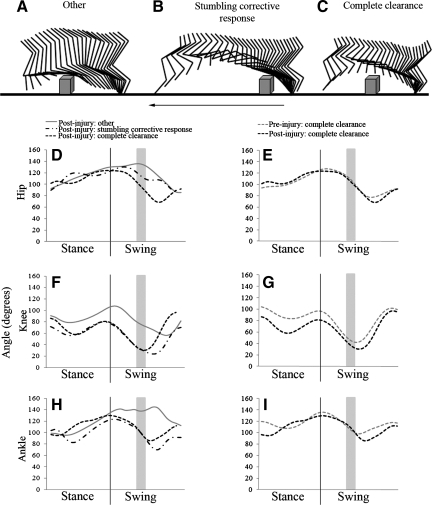
FIG. 2.
Kinematic data from the ipsilateral (left) hindlimb following injury during obstacle negotiation. Stick figures of one representative swing phase when the limb trajectory was not modified (A, other), during the stumbling corrective response (B), and during a complete clearance (C). Arrow indicates direction of movement (A–C). Waveform graphs depict post-injury changes in hip (D, E), knee (F, G), and ankle joints (H, I). Long-lasting changes in joint angles were seen even out to the most chronic time point (16 wks). The gray shaded area represents the position of the obstacle. The thin line indicates the division between stance and swing.
Approach and maximum heights
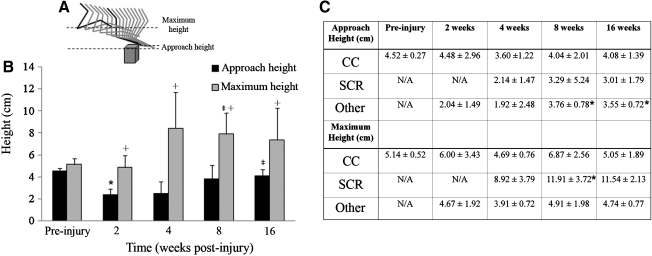
To examine the efficiency of limb lift during normal stepping and obstacle negotiation, the maximal vertical displacement was determined for both tasks pre-injury. The maximal vertical displacement was calculated by finding the overall range of movement (10 basic steps and 10 obstacle negotiations per cat) using the Y coordinates of the base of the fifth metatarsal marker. To determine negotiation efficiency, two height measurements were assessed. The height of the paw when it first reached the obstacle was measured in order to reflect limb trajectory adjustments in anticipation of clearing the obstacle. This was termed the approach height. The paw height also was measured at the maximum height it reached during the obstacle encounter. This was termed the maximum height. To achieve these measurements, the following was done. The Y coordinate of the runway surface was set equal to 0. The height of the obstacle was defined by the Y coordinate of its top edge and the height of the foot defined as the Y coordinate of the marker on the base of the fifth metatarsal. The maximum height was calculated by subtracting the obstacle height from the maximum value of the base of the fifth metatarsal (equal to the maximal vertical displacement of the paw). The approach height was defined as the Y coordinate of the base of the fifth metatarsal as it reached the vertical plane of the face of the obstacle minus the height of the obstacle. The maximal vertical displacement of the limb from the surface of the runway also was determined in order to compare limb displacements between basic locomotion and obstacle negotiation at the pre-injury time point. The most efficient movements were defined as those in which only a small movement was made in order to clear the walking or obstacle surface. A diagram illustrating maximum and approach heights is shown in Figure 4.
FIG. 4.
Ipsilateral hindlimb efficiency during obstacle negotiation. The stick figure of the left hindlimb over the obstacle depicts the distances used for the maximum and approach heights (A). The black indicates where the measurements were taken. Pre-injury, cats were very efficient in their limb lift and clearance during obstacle negotiation (B). At 2 weeks post-injury, there was a significant decrease in the approach height from pre-injury values (*), which by 16 weeks almost equaled pre-injury values (‡). The maximum height was significantly increased at 8 weeks from both pre-injury and 2 weeks post-injury (‡). Pre-injury approach and maximum heights were virtually equal, but at all post-injury time points the maximum height was significantly greater (+). When the responses were categorized it was evident that the contributions of particular response types to the approach and maximum height totals changed at each time point (C,* denotes significance, see text). Error bars denote SD.
Histology
Procedures used for basic lesion morphology were the same as those used in our previous studies (Jefferson et al., 2010, 2011; Tester and Howland, 2008). In brief, 5 months post-injury, all cats were anesthetized with an overdose of sodium pentobarbital (>40 mg/kg, intraperitoneal) and given 1.0 cc of both heparin (1000 U) and sodium nitrite (1%) intravenously 20 min apart. Immediately following sodium nitrite administration, cats were transcardially perfused with 0.9% saline followed by 4% paraformaldehyde in a 0.1 molar phosphate buffer (pH 7.4). The spinal cords were removed and the lesion segments were blocked and cryoprotected in 30% sucrose, 4% paraformaldehyde (pH 7.4). Tissue was sectioned at 25 μm using a cryostat. The first section of every 10 was mounted onto subbed slides and processed with cresyl violet (cresyl violet with acetate, Sigma-Aldrich, St. Louis, MO) and myelin (Eriochrome Cyanine R, Fluka, New York, NY) stains to view basic lesion morphology on the Nikon Eclipse E600 microscope.
Statistical analysis
Using SPSS software (Chicago, IL), nonparametric Kruskal–Wallis tests were conducted to identify significance. Once significance was determined, Mann–Whitney U tests were performed to isolate the significance using a p value of ≤0.05.
Results
Basic locomotion and obstacle negotiation
Movement patterns during basic locomotion and obstacle negotiation were compared pre-injury. The movement of the hindlimb during basic locomotion and obstacle negotiation was efficient with respect to surface clearance; however there were distinct differences in hindlimb movement patterns during each task. Qualitatively and quantitatively the most apparent change was the pronounced knee flexion that occurred as the hindlimb approached and cleared the obstacle (Fig.1A, B, and D). Less pronounced changes were observed in the hip (Fig. 1A–C). A larger range of hip movement was seen negotiating obstacles because of greater extension during the transition from stance to swing and more pronounced flexion in mid-late swing. In contrast to the hip and knee, the ankle joint was maintained in a more extended posture throughout obstacle clearance (Fig. 1C–E). Despite this, the range of angular excursion was similar to that seen during basic locomotion (Fig. 1C–E). These data are in agreement with Lavoie and colleagues (1995) who showed larger changes in the knee and ankle joint angles, and to a lesser extent at the hip, in cats as they stepped over an obstacle versus walking with no obstacle on an overground runway. To quantify differences between basic locomotion and obstacle negotiation, the maximum vertical displacement of the hindlimb was assessed. Specifically, the distance between the marker on the base of the fifth metatarsal and the walking surface was averaged across 50 step cycles (10 steps from 5 cats). During basic locomotion, the maximum vertical displacement averaged 3.98 cm whereas during obstacle negotiation the average was 12.84 cm (Fig. 1F). Because the length of the longest metatarsal was ∼3 cm, this indicated that the paw cleared the walking surface with ∼1cm, and the 7.7 cm high obstacle with ∼2 cm of space. The difference in the maximum vertical displacements between these two tasks showed that a significant increase in paw height occurred during obstacle negotiation (p=0.008). The vertical displacement of the paw also illustrates the efficiency of limb lift during both types of locomotion, albeit efficiency was greatest during basic overground locomotion, which is the less adaptive task.
Qualitative characterization of post-injury obstacle negotiation
Following a lateral hemisection, changes in the ipsilateral hindlimb's response to the obstacle were seen. Three general responses were characterized: other, stumbling corrective response, and complete clearance (Fig. 2A–C). The other response generally was characterized by lack of apparent alterations in ipsilateral hindlimb trajectory. Typically, during other responses, the ipsilateral hindlimb appeared to be dragged over the obstacle and in many instances was trapped between the body of the cat and the obstacle (Fig. 2A). All three joints were very extended, relative to other responses, while passing over the obstacle (Fig. 2D–H). After the limb was no longer in contact with the obstacle, one of two things occurred: either the limb dropped to the runway landing on the paw dorsum, or the knee flexed, allowing for plantigrade placement. In the latter instance the knee appeared to play a role in repositioning the limb underneath the body.
The stumbling corrective response was characterized by a notable increase in flexion evoked by contact of the dorsal surface of the paw with the obstacle (Fig. 2B). This response caused hypermetric knee, and sometimes ankle, flexion after obstacle contact (compare Fig. 2F and G; 2H and I). The observed hypermetric flexion of the knee is in agreement with electromyograph (EMG) recordings by others that show activation of knee flexors after stimulation of the dorsal paw (Buford and Smith, 1993; Forssberg et al., 1977 ; McVea and Pearson, 2007).
The complete clearance response was defined by any encounter with the obstacle during which the hindlimb did not make contact. Therefore, all responses seen pre-injury were categorized as complete (Fig. 1). The complete clearances seen post-injury, however, showed distinct differences. Comparison of pre-injury complete clearances with those seen 16 weeks post-injury (the most chronic time point) identified long-term changes in obstacle negotiation (Fig. 2E–I). Post-injury, the hip and ankle joints remained in a slightly more flexed position as the limb transitioned from swing to stance (Fig. 2E, I). The knee, which showed the greatest chronic changes, was characterized by increased flexion throughout the step over the obstacle (Fig. 2G). Therefore, complete clearances were seen post-injury, but the joint angles were altered.
Quantification of post-injury obstacle negotiation
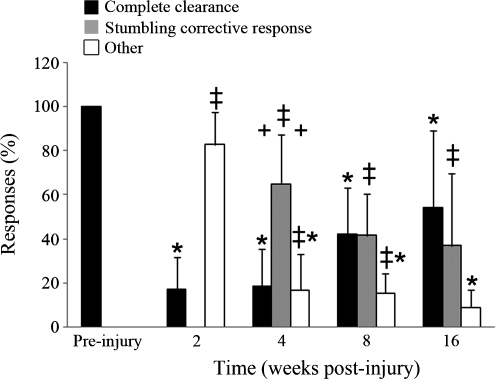
To evaluate recovery over time, the number of other, stumbling corrective responses, and complete clearances, were quantified. Pre-injury, hindlimb trajectories were modified and the obstacle consistently cleared (100% complete clearances; Fig. 3). The initial 2-week time point predominantly was characterized as “other” responses and therefore, there was a significant decrease in complete clearances (20%, p=0.008) and increase in other responses (71%, p=0.008). The stumbling corrective response did not emerge until the 4 weeks post-injury time point, when it became the predominant response (65%) and was significantly greater than the number of complete clearances (p=0.016) and other responses (p=0.016). Although the complete clearances remained significantly decreased throughout the time course of this study (p=0.008 at 4 and 8 weeks, p=0.032 at 16 weeks), there was a strong recovery trend with an increase in the number of complete clearances from 2 to 8 weeks (p=0.056). Because the stumbling corrective response was not present pre-injury or at 2 weeks post-injury, a significant increase in this response was seen from these time points out to the most chronic time point (p=0.008 at both 4 and 8 weeks, p=0.032 at 16 weeks), but no changes were seen between 4 and 16 weeks. The other responses were significantly increased from pre-injury out to 8 weeks post-injury (p=0.008 for all time points), but by 16 weeks there was no difference from the 0% pre-injury value (p=0.151). The significant decreases seen in the other response category between 2 weeks and all other time points (p=0.008) correspond with the recovery of complete clearance and the emergence of the stumbling corrective response. A plateau in recovery appeared between 8 and 16 weeks post-injury when the stumbling corrective response and complete clearances each characterized ∼50% of encounters.
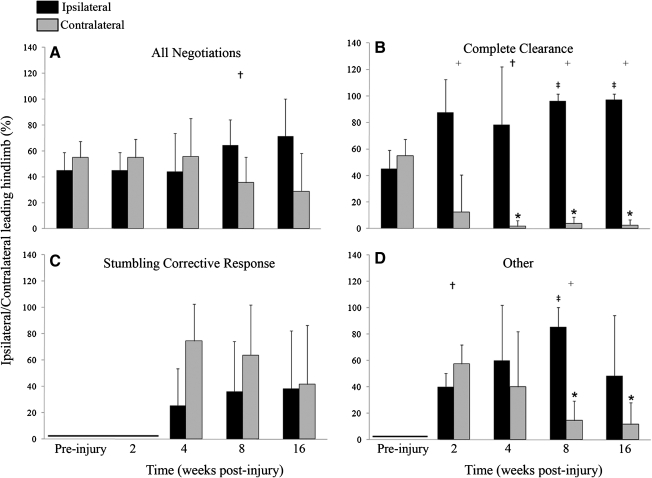
FIG. 3.
Recovery time course of ipsilateral hindlimb responses during obstacle negotiation. Complete clearances were significantly decreased at all post-injury time points (*) from pre-injury levels. The stumbling corrective response initially emerged as the predominant response at 4 weeks post-injury (+) and was significantly increased from pre-injury and 2 weeks out to 16 weeks (‡). Other responses were significantly increased at 2, 4, and 8 weeks post-injury from pre-injury values (‡), but 4, 8, and 16 week values were significantly decreased from 2 weeks (*). Error bars denote SD.
Efficiency of obstacle negotiation
In order to further understand the efficiency with which animals were able to negotiate the obstacle, the height of the paw was assessed at two points as the limb passed over the obstacle (Fig. 4). In contrast to the maximal vertical displacement (Fig. 1F), these displacements were calculated from the top surface of the obstacle. The approach height was defined as the height of the paw when it broke the vertical plane of the obstacle (Fig. 4A). The maximum height was the highest point that the paw reached during obstacle negotiation (Fig. 4A). The more similar the two values were, the more efficient the animal was at clearing the obstacle. Pre-injury, the approach height (4.52 cm) and the maximum height (5.14 cm) were not significantly different (Fig. 4B, C; p=0.095). Following injury, at 2 weeks there was a significant decrease in the approach height (2.36cm), because of the increase in crossings during which the paw simply dragged over the obstacle (Fig. 4B and Fig. 3; p=0.008). This also caused the maximum height to remain similar to pre-injury values (4.86 cm) because the width of the paw (∼3cm) filled the space between the obstacle and the marker on the base of the fifth metatarsal. As the ability to clear the obstacle recovered, the approach height also returned to values more similar to baseline. The approach height significantly increased from 2 weeks to 16 weeks post-injury (p=0.008), with no difference from pre-injury values by 16 weeks (p=0.151). On the other hand, the maximum height showed long lasting changes. At 4 weeks, characterized by the emergence of the stumbling corrective response (Fig. 3), the maximum height began to increase, becoming significant at 8 weeks from both normal and 2 weeks post-injury (p=0.008 from pre-injury, p=0.016 from 2 weeks). During many obstacle encounters, the maximum height occurred after the hindlimb had cleared the obstacle as a response to the initial paw contact. This also can be defined by significantly smaller approach heights at all post-injury time points (p=0.008 for all time points). Categorization of responses to the obstacle made it apparent that paw heights were correlated with response type (Fig. 4C). The largest approach heights were seen during the pre-injury complete clearance responses because animals were able to lift their limb to the anticipated height required to clear the obstacle without any alterations. By 16 weeks, the approach height (4.08cm) continued to be less then pre-injury values. Post-injury, encounters during which the paw contacted the obstacle (the other and the stumbling corrective responses) decreased the approach height. By 8 (3.76 cm) and 16 weeks (3.55 cm), however, the other response encounters were characterized by approach heights that were still smaller, yet very similar to complete clearance approach heights. These heights were significantly greater than at 2 weeks (p=0.008 for 8 weeks and p=0.036 for 16 weeks). When further examined, these responses had initial limb lift, and were just short of clearing the obstacle. Based upon this analysis, the 10% of other encounters present at 8 and 16 weeks post-injury (Fig. 3) would be more appropriately termed “complete clearance with toe drag”. During these encounters, the tip of the paw dragged over the obstacle, did not notably interrupt forward progression, and therefore did not evoke the stumbling corrective response. The increased flexion caused by the stumbling corrective response contributed to the significant increase in maximum height seen at 8 weeks compared to complete clearance (p=0.032) and other (p=0.016) responses (Fig. 4C).
Leading limb preference
Pre-injury, cats crossed the obstacle without any bias in the leading limb. The hindlimbs were equally efficient, independent of which limb led (Fig. 5A, B). Following injury, a general preference to lead with the ipsilateral hindlimb showed a strong trend (p=0.056) at 8 weeks, but overall no change was seen over time (Fig. 5A).
FIG. 5.
Leading limb preference during obstacle negotiation. Overall, animals had no bias, although there was a strong trend to a left hindlimb preference at 8 weeks post-injury (†; A). During complete clearances (B), however, the ipsilateral hindlimb was the dominant leading limb at all post-injury time points (+,†) as crossings that were led with the contralateral hindlimb were significantly decreased (*). No hindlimb bias was present during stumbling corrective responses (C). During other responses, the contralateral hindlimb showed a trend toward being the dominant leading limb at 2 weeks post-injury (D,†). After 2 weeks, however, a bias for leading with the ipsilateral hindlimb emerged. By 8 weeks there were significantly more encounters that were led with the ipsilateral hindlimb than with the contralateral one (‡,+). Overall, the number of other response crossings was significantly decreased from 2 weeks (*). Error bars denote SD.
When the hindlimb responses to the obstacle were categorized, a distinct bias with respect to the leading limb during effective obstacle clearances was seen. As mentioned previously, cats had no bias toward the leading hindlimb during pre-injury complete clearance responses (Fig. 5A, B). Post-injury, however, during complete clearance responses there was a strong bias toward crossing the obstacle with the ipsilateral hindlimb first (Fig. 5B). The complete negotiations in which the ipsilateral hindlimb led were significantly or strongly increased compared to crossings in which the contralateral hindlimb led (2 weeks p=0.016, 4 weeks p=0.056, 8 weeks p=0.008, 16weeks p=0.008, Fig. 5B). In contrast, the stumbling corrective response, which emerged at 4 weeks post-injury, appeared to be associated with a contralateral hindlimb bias although there were no significant differences over time or between the ipsilateral and contralateral hindlimbs (Fig. 5C). The crossings that were classified as “other responses of the ipsilateral hindlimb” (Fig. 5D) had an initial trend toward a contralateral leading hindlimb bias (p=0.056), but switched to an ipsilateral hindlimb bias at the 8-week time point (p=0.008). This switch at the later time points is similar to the bias that was seen during the complete clearance encounters. This may be because the few other responses that occurred at 8 and 16 weeks could be categorized as “complete clearances with the presence of toe drag” as indicated previously. Therefore, the most successful post-injury responses occurred when the ipsilateral hindlimb encountered the obstacle first. This placed the contralateral hindlimb in an advantageous support position on the runway.
Discussion
Pre-injury, all cats showed efficient, smooth adjustments in limb trajectories, which allowed effective clearance of an obstacle during basic overground walking. Although substantial locomotor recovery occurred post-hemisection, deficits in adaptive skills necessary for obstacle negotiation were seen even at 4 months post-injury. For at least 2 weeks post-injury, although basic walking ability was present, modifications in the trajectory of the ipsilateral hindlimb in response to, anticipation of, or contact with, an obstacle were rare. Surprisingly, the spinally mediated stumbling corrective response did not emerge until between 2 and 4 weeks post-injury. Despite a notable increase in ability to modify limb trajectory between 4 and 8 weeks, insufficient initial limb lift during 40–50% of the obstacle encounters continued to evoke the stumbling corrective response at 8 and 16 weeks. Therefore, efficiency of paw lift and clearance never fully recovered, and smooth negotiation of an obstacle occurred in only half of all events. Even though complete clearances were greatest when the ipsilateral limb crossed the obstacle first, cats did not show a preference for this strategy post-injury.
Model relevance
BSS and Brown-Séquard-plus syndrome (BSPS), specific types of motor incomplete SCIs, are the result of asymmetrical lesions characterized by both ipsilateral motor and proprioceptive impairments as well as contralateral loss in pain and temperature sensitivity. To our knowledge, there are no reports that specifically evaluate obstacle negotiation or movements requiring skilled adaptation of the lower extremities in a defined group of patients with BSS or BSPS. However, there is evidence that ambulatory individuals with American Spinal Injury Association (ASIA) impairment scale (AIS) D injuries (the least severe injury category) fail to adequately clear obstacles (Amatachaya et al., 2010). The limited information available is probably, at least in part, the result of the fact that clinical walking tests typically are not designed to detect higher level gait adaptations. (Amatachaya et al., 2010; van Hedel et al., 2005) The asymmetrical impairment and rapid, early recovery of basic walking, seen in these individuals (Little and Halar, 1985), is similar to that seen following a lateral hemisection in the cat (Basso et al., 1994; Eidelberg et al., 1986; Helgren and Goldberger, 1993; Jefferson et al., 2011; Tester and Howland, 2008). The cat is an advantageous translational model to use for analysis of locomotor control because of its well-defined step cycle as well as its larger size, challenging the system to reorganize and/or regenerate across distances that are more similar to humans than are rodents. In the current study, specific changes in joint flexion and extension patterns, as well as loss of clearance efficiency, defined important features of post-injury obstacle negotiation, which can be used to improve current clinical tests and in the development of new treatment approaches.
Pathways involved in functional recovery
In the cat, voluntary obstacle negotiation requires the cortico- and rubrospinal tracts (Beloozerova and Sirota, 1993; Beloozerova et al., 2010; Drew et al., 1996, 2002; Lavoie and Drew, 2002; Widajewicz et al., 1994). During the first 2 weeks post-injury, obstacle negotiation typically was characterized by a seemingly unresponsive ipsilateral hindlimb that was dragged over the obstacle. In a few instances (∼15%) the limb cleared the obstacle without contact. These early successful clearances may have been mediated by spared tissue. In particular, the preserved corticospinal tract collaterals that cross in the commissura along the length of the spinal cord in the cat (Satomi et al., 1991) and monkey (Lacroix et al., 2004) are obvious candidates. After 2 weeks post-injury, the increasing recovery seen in the ability to completely clear the obstacle, potentially reflecting voluntary control of the limb, suggests that mechanisms other than spared substrates may be involved at these later time points. In other words, the contralateral, spared substrate alone is probably not sufficient to mediate the recovery that we described.
A number of studies suggest that plasticity of spared descending systems occurs following SCI. Following incomplete SCIs in rodents, substantial increases in collateral sprouting of the corticospinal tract have been reported during the first 2 months post-injury (Bareyre et al., 2004; Li et al., 1994). Although some aspects of recovery are reported to occur fairly rapidly, activation of denervated muscles with cortical stimulation is delayed until after 4 weeks post-injury, correlating this aspect of recovery with corticospinal plasticity (Bareyre et al., 2004). In the current study, complete clearance of the ipsilateral hindlimb during obstacle negotiation showed substantial recovery beginning 4 weeks after injury. This time point of recovery is consistent with a dependence upon new connections, which could include plasticity of supraspinal systems (Bareyre et al., 2004; Jefferson et al., 2011). Following injury, direct connections with the caudal spinal cord may not be necessary to mediate corticospinal input. Alternatively, supraspinal input may be relayed through plasticity of other systems. Likely relay candidates include the propriospinal systems. Corticospinal axons axotomized in the rat thoracic spinal cord have been shown to sprout collaterals at the cervical level, which synapse onto long propriospinal neurons. These long propriospinal neurons, already connected with the caudal cord, serve as an indirect pathway capable of mediating function (Bareyre et al., 2004). Short propriospinal neurons in the thoracic spinal cord of the mouse also have been reported to serve as a bridge and support basic stepping activity (Courtine et al., 2008, 2009). Further, studies in which training alters the stepping performance of low spinal cats suggests that plasticity of spinal interneurons also occurs in the caudal spinal cord below the lesion and may contribute to hindlimb function (de Leon et al., 1998, 1999; Lovely et al., 1986). This is supported by one study from another group showing direct anatomical evidence of interneuronal sprouting in the cat (Fenrich and Rose, 2009). The data illustrate that the spared substrate alone is not able to mediate full recovery. Understanding the endogenous plastic capability of specific populations of neurons over time on behavioral recovery may provide insight into new therapeutic targets aimed at maximizing recovery. As a result, tract tracing is being pursued in additional studies.
Suppression and recovery of the stumbling corrective response
In the normal cat, cutaneous afferents are responsible for triggering the stumbling corrective response (Forssberg, 1979; Prochazka et al., 1978; Wand et al., 1980). Therefore, it is classified as a contact/tactile placing response that is mediated by cortical and cerebellar connections to the rubrospinal tract and spinal circuitry (Amassian and Batson, 1988; Batson and Amassian, 1986; Fleshman et al., 1988). Consistent with our findings, others have reported that this response is abolished following hemisection (Helgren and Goldberger, 1993; Murray and Goldberger, 1974). However, in the current study, recovery of a stumbling corrective response was seen beginning ∼ 4 weeks post-injury. Although the exact amount of force was not measured, the observed limb displacement that occurred prior to eliciting the stumbling corrective response far exceeded contact (Forssberg, 1979; McVea and Pearson, 2007). Prochazka and colleagues (1978) suggest that the stumbling corrective response has an underlying proprioceptive stretch reflex in the ankle flexors, which surfaces when cutaneous sensory input to the dorsum of the paw is blocked. Our results are consistent with a proprioceptive response, and its delayed onset is probably caused by a change in the main afferent control. Further, the lack of a cutaneous response is consistent with the disruption of the dorsal columns. Immediately following spinal transection or interruption of descending monoaminergic pathways in the rodent, persistent inward currents (PICs) associated with the dendrites of motoneurons are greatly decreased. This reduces motoneuron excitability and leads to complete or partial disruption of reflex activity for > 1 month (Bennett et al., 2001; Li and Bennett, 2003). Motoneurons recover the ability to generate large PICs causing motoneuron hyperexcitability in the chronically injured spinal cord (reviewed by Bennett et al., 2001; Heckman et al., 2005; Li and Bennett, 2003). PICs have been studied primarily with respect to reflex responses and have not been assessed during voluntary walking. Although hemisection does not completely disrupt the descending monoaminergic input, partial disruption may be sufficient to alter PIC levels and contribute to the suppression of the stumbling corrective response seen post-injury in the current study. Recovery of overground locomotion, which occurs before the stumbling corrective response is elicited, suggests that responses mediated by descending systems are less affected by a lateral hemisection than are behaviors mediated by sensory afferent input below the lesion. Further, the post-injury change in threshold (tactile to proprioceptive) for the stumbling corrective response is consistent with altered motoneuron excitation thresholds associated with changes in PICs.
Adaptive strategies
Pre-injury, cats exhibited no leading limb bias. Post-injury, however, negotiation of the obstacle was most effective when the ipsilateral hindlimb led over the obstacle. This approach increased the cats' stability by leaving the contralateral hindlimb in an effective weight- supporting position as the more motor-impaired ipsilateral hindlimb negotiated the obstacle. Postural adjustments such as this are extremely important during locomotion, in which the number of supporting limbs and their positions constantly change. Postural control requires the integration of spinal mechanisms controlled by somatosensory input and descending systems including the corticospinal and rubrospinal tracts (reviewed by Deliagina et al., 2008). In both the rabbit (Lyalka et al., 2005) and cat (Helgren and Goldberger, 1993; Tester and Howland, 2008), sufficient postural control of the hindquarters to allow independent walking recovers within the first 2 weeks following a thoracic lateral hemisection. In contrast, the more challenging tasks of beam and ladder crossing, which require greater balance and precision, take longer to show some recovery (Helgren and Goldberger, 1993; Tester and Howland, 2008). Obstacle negotiation also requires adaptations in limb trajectory, posture, and balance. Therefore, its incomplete and delayed recovery compared to that of flat surface walking is consistent with the concept that tasks requiring greater adaptation have a longer recovery period. Although cats did not show an overall significant preference to lead with the limb that resulted in more successful negotiations, an effective rehabilitative approach could involve training to lead with a particular limb. In the human population, this simple strategy might promote improved postural control and balance among highly functional individuals in situations requiring changes in foot trajectory, thereby decreasing the likelihood of falls.
Conclusions
This article provides the first detailed characterization of the temporal recovery of obstacle negotiation following spinal hemisection in the cat. The data from this study show substantial, yet incomplete recovery of obstacle negotiation during overground walking following low thoracic hemisection. Interestingly, initially post-injury there was a depression of the spinally mediated stumbling corrective response, which when recovered resembled a proprioceptive response rather than a contact-mediated response. It is unlikely that the improvements in hindlimb clearance responses that occur over multiple weeks are dependent only upon the descending contralateral spared substrate. More likely, plasticity of descending and/or spinal circuitry, in combination with spared substrates, plays a significant role in the changes seen.
These kinematic studies will provide the basis and impetus for future work. It will be important to understand the specific muscle contributions and the timing of their activation through the use of EMGs. Such studies will allow us to begin to understand mechanisms underlying voluntary control of successful obstacle clearances and what fails (i.e. timing, sufficient activation) when the obstacle is not cleared. Establishing the role of behavioral training and the most effective type(s) of training required for recovery will contribute to the development of new clinical rehabilitative strategies that do not exist for individuals with BSS or other types of incomplete injuries, who recover basic walking skills but have difficulty adapting limb trajectories to meet environmental demands. Identifying the combination of underlying neuronal substrates supporting obstacle negotiation post-injury will be important in providing further insight regarding potential therapeutic targets beyond spared substrates, to enhance plasticity and increase recovery.
Acknowledgments
This research was supported by National Institute of Health (NIH) National Institute of Neurological Disorders and Stroke (NINDS) RO1 NS050699, T32 HD043730, the Daniel Heumann Fund, the Department of Veterans Affairs Offices of Rehabilitation Research and Development (RR&D) and Basic Laboratory Research and Development (BLR&D) Services, RR&D Center Support, and 2009 Brooks Rehabilitation Research Grant. We thank Wilbur O'Steen (Laboratory Manager) who assisted with data collection and animal procedures throughout the study.
The contents of this article do not represent the views of the Department of Veterans Affairs or the United States government.
Author Disclosure Statement
No conflicting financial interests exist.
References
- Amassian V. Batson D. Long loop participation of red nucleus in contact placing in the adult cat with faciliation by tactile input at the spinal level. Behav. Brain Res. 1988;28:225–232. doi: 10.1016/0166-4328(88)90100-3. [DOI] [PubMed] [Google Scholar]
- Amatachaya S. Thaweewannakij T. Adirek–udomrat J. Siritaratiwat W. Factors related to obstacle crossing in independent ambulatory patients with spinal cord injury. J. Spinal Cord Med. 2010;33:144–149. doi: 10.1080/10790268.2010.11689689. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bareyre F.M. Kerschensteiner M. Raineteau O. Mettenleiter T.C. Weinmann O. Schwab M.E. The injured spinal cord spontaneously forms a new intraspinal circuit in adult rats. Nat. Neurosci. 2004;7:269–277. doi: 10.1038/nn1195. [DOI] [PubMed] [Google Scholar]
- Basso D. Murray M. Goldberger M. Differential recovery of bipedal and overground locomotion following complete spinal cord hemisection in cats. Restor. Neurol. Neurosci. 1994;7:95–110. doi: 10.3233/RNN-1994-7205. [DOI] [PubMed] [Google Scholar]
- Batson D. Amassian V. A dynamic role of rubral neurons in contact placing by the adult cat. J. Neurophysiol. 1986;56:835–856. doi: 10.1152/jn.1986.56.3.835. [DOI] [PubMed] [Google Scholar]
- Beloozerova I.N. Sirota M.G. The role of the motor cortex in the control of accuracy of locomotor movements in the cat. J. Physiol. 1993;461:1–25. doi: 10.1113/jphysiol.1993.sp019498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beloozerova I.N. Farrell B.J. Sirota M.G. Prilutsky B.I. Differences in movement mechanics, electromyographic, and motor cortex activity between accurate and non-accurate stepping. J. Neurophysiol. 2010;103:2285–2300. doi: 10.1152/jn.00360.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bennett D.J. Li Y. Harvey P.J. Gorassini M. Evidence for plateau potentials in tail motoneurons of awake chronic spinal rats with spasticity. J. Neurophysiol. 2001;86:1972–1982. doi: 10.1152/jn.2001.86.4.1972. [DOI] [PubMed] [Google Scholar]
- Buford J.A. Smith J.L. Adaptive control for backward quadrupedal walking. III. Stumbling corrective reactions and cutaneous reflex sensitivity. J. Neurophysiol. 1993;70:1102–1114. doi: 10.1152/jn.1993.70.3.1102. [DOI] [PubMed] [Google Scholar]
- Courtine G. Gerasimenko Y. van den Brand R. Yew A. Musienko P. Zhong H. Song B. Ao Y. Ichiyama R.M. Lavrov I. Roy R.R. Sofroniew M.V. Edgerton V.R. Transformation of nonfunctional spinal circuits into functional states after the loss of brain input. Nat. Neurosci. 2009;12:1333–1342. doi: 10.1038/nn.2401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Courtine G. Song B. Roy R.R. Zhong H. Herrmann J.E. Ao Y. Qi J. Edgerton V.R. Sofroniew M.V. Recovery of supraspinal control of stepping via indirect propriospinal relay connections after spinal cord injury. Nat. Med. 2008;14:69–74. doi: 10.1038/nm1682. [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Leon R.D. Hodgson J.A. Roy R.R. Edgerton V.R. Full weight-bearing hindlimb standing following stand training in the adult spinal cat. J. Neurophysiol. 1998;80:83–91. doi: 10.1152/jn.1998.80.1.83. [DOI] [PubMed] [Google Scholar]
- de Leon R.D. Tamaki H. Hodgson J.A. Roy R.R. Edgerton V.R. Hindlimb locomotor and postural training modulates glycinergic inhibition in the spinal cord of the adult spinal cat. J. Neurophysiol. 1999;82:359–369. doi: 10.1152/jn.1999.82.1.359. [DOI] [PubMed] [Google Scholar]
- Deliagina T.G. Beloozerova I.N. Zelenin P.V. Orlovksy G.N. Spinal and supraspinal postural networks. Brain Res. Rev. 2008;57:212–221. doi: 10.1016/j.brainresrev.2007.06.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Drew T. Jiang W. Widajewicz W. Contributions of the motor cortex to the control of the hindlimbs during locomotion in the cat. Brain Res. Rev. 2002;40:178–191. doi: 10.1016/s0165-0173(02)00200-x. [DOI] [PubMed] [Google Scholar]
- Drew T. Jiang W. Kably B. Lavoie S. Role of the motor cortex in the control of visually triggered gait modifications. Can. J. Physiol. Pharmacol. 1996;74:426–442. [PubMed] [Google Scholar]
- Eidelberg E. Nguyen L.H. Deza L.D. Recovery of locomotor function after hemisection of the spinal cord in cats. Brain Res. Bull. 1986;16:507–515. doi: 10.1016/0361-9230(86)90180-2. [DOI] [PubMed] [Google Scholar]
- Fenrich K.K. Rose P.K. Spinal interneuron axons spontaneously regenerate after spinal cord injury in the adult feline. J. Neurosci. 2009;29:12,145–12,158. doi: 10.1523/JNEUROSCI.0897-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fleshman J. Rudomin P. Burke R. Supraspinal control of a short-latency cutaneous pathway to hindlimb motoneurons. Exp. Brain Res. 1988;69:449–459. doi: 10.1007/BF00247299. [DOI] [PubMed] [Google Scholar]
- Forssberg H. Stumbling corrective reaction: a phase-dependent compensatory reaction during locomotion. J. Neurophysiol. 1979;42:936–953. doi: 10.1152/jn.1979.42.4.936. [DOI] [PubMed] [Google Scholar]
- Forssberg H. Grillner S. Rossignol S. Phase dependent reflex reversal during walking in chronic spinal cats. Brain Res. 1975;85:103–107. doi: 10.1016/0006-8993(75)91013-6. [DOI] [PubMed] [Google Scholar]
- Heckman C.J. Gorassini M.A. Bennett D.J. Persistent inward currents in the motoneuron dendrites: Implications for motor output. Muscle Nerve. 2005;31:135–156. doi: 10.1002/mus.20261. [DOI] [PubMed] [Google Scholar]
- Helgren M.E. Goldberger M.E. The recovery of postural reflexes and locomotion following low thoracic hemisection in adult cats involves compensation by undamaged primary afferent pathways. Exp. Neurol. 1993;123:17–34. doi: 10.1006/exnr.1993.1137. [DOI] [PubMed] [Google Scholar]
- Jefferson S.C. Tester N.J. Howland D.R. Chondroitinase ABC promotes recovery of adaptive limb movements and plasticity of the rubrospinal tract. J. Neurosci. 2011;31:5710–5720. doi: 10.1523/JNEUROSCI.4459-10.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jefferson S.C. Tester N.J. Rose M. Blum A.E. Howland B.G. Bolser D.C. Howland D.R. Cough following low thoracic hemisection in the cat. Exp. Neurol. 2010;222:165–170. doi: 10.1016/j.expneurol.2009.12.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kuhtz–Buschbeck J.P. Boczek–Funcke A. Mautes A. Nacimiento W. Weinhardt C. Recovery of locomotion after spinal cord hemisection: an X-Ray study of the cat hindlimb. Exp. Neurol. 1996;137:212–224. doi: 10.1006/exnr.1996.0020. [DOI] [PubMed] [Google Scholar]
- Lacroix S. Havton L.A. McKay H. Yang H. Brant A. Roberts J. Tuszynski M.H. Bilateral corticospinal projections arise from each motor cortex in the macaque monkey: a quantitative study. J. Comp. Neurol. 2004;473:147–161. doi: 10.1002/cne.20051. [DOI] [PubMed] [Google Scholar]
- Lavoie S. Drew T. Discharge characteristics of neurons in the red nucleus during voluntary gait modifications: a comparison with the motor cortex. J. Neurophysiol. 2002;88:1791–1814. doi: 10.1152/jn.2002.88.4.1791. [DOI] [PubMed] [Google Scholar]
- Lavoie S. McFayden B. Drew T. A kinematic and kinetic analysis of locomotion during voluntary gait modifications in the cat. Exp. Brain Res. 1995;106:39–59. doi: 10.1007/BF00241355. [DOI] [PubMed] [Google Scholar]
- Li W.W.Y. Yew D.T.W. Chuah M.I. Leung P.C. Tsang D.S.C. Axonal sprouting in the hemisected adult rat spinal cord. Neuroscience. 1994;61:133–139. doi: 10.1016/0306-4522(94)90066-3. [DOI] [PubMed] [Google Scholar]
- Li Y. Bennett D.J. Persistent sodium and calcium currents cause plateau potentials in motoneurons of chronic spinal rats. J. Neurophysiol. 2003;90:857–869. doi: 10.1152/jn.00236.2003. [DOI] [PubMed] [Google Scholar]
- Little J.W. Halar E. Temporal course of motor recovery after Brown–Sequard spinal cord injuries. Paraplegia. 1985;23:39–46. doi: 10.1038/sc.1985.7. [DOI] [PubMed] [Google Scholar]
- Lovely R.G. Gregor R.J. Roy R.R. Edgerton V.R. Effects of training on the recovery of full-weight-bearing stepping in the adult spinal cat. Exp. Neurol. 1986;92:421–435. doi: 10.1016/0014-4886(86)90094-4. [DOI] [PubMed] [Google Scholar]
- Lyalka V.F. Zelenin P.V. Karayannidou A. Orlovsky G.N. Grillner S. Deliagina T.G. Impairment and recovery of postural control in rabbits with spinal cord lesions. J. Neurophysiol. 2005;94:3677–3690. doi: 10.1152/jn.00538.2005. [DOI] [PubMed] [Google Scholar]
- McKinley W. Santos K. Meade M. Brooke K. Incidence and outcomes of spinal cord injury clinical syndromes. J. Spinal Cord Med. 2007;30:215–224. doi: 10.1080/10790268.2007.11753929. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McVea D.A. Pearson K.G. Long-lasting, Context-dependent modification of stepping in the cat after repeated stumbling-corrective responses. J. Neurophysiol. 2007;97:659–669. doi: 10.1152/jn.00921.2006. [DOI] [PubMed] [Google Scholar]
- Mohagheghi A.A. Mortaes R. Patla A.E. The effects of distant and on-line visual information on the control of approach phase and step over an obstacle during locomotion. Exp. Brain Res. 2004;155:459–468. doi: 10.1007/s00221-003-1751-7. [DOI] [PubMed] [Google Scholar]
- Murray M. Goldberger M.E. Restitution of function and collateral sprouting in the cat spinal cord: the partially hemisected animal. J. Comp. Neurol. 1974;158:19–36. doi: 10.1002/cne.901580103. [DOI] [PubMed] [Google Scholar]
- Musselman K. Brunton K. Lam T. Yang J. Spinal cord injury functional ambulation profile: a new measure of walking ability. Neurorehabil. Neural Repair. 2011;25:285–293. doi: 10.1177/1545968310381250. [DOI] [PubMed] [Google Scholar]
- Musselman K.E. Yang J.F. Walking tasks encountered by urban-dwelling adults and persons with incomplete spinal cord injury. J. Rehabil. Med. 2007;39:567–574. doi: 10.2340/16501977-0090. [DOI] [PubMed] [Google Scholar]
- Patla A.E. Greig M. Any way you look at it, successful obstacle negotiation needs visually guided on-line foot placement regulation during the approach phase. Neurosci. Lett. 2006;397:110–114. doi: 10.1016/j.neulet.2005.12.016. [DOI] [PubMed] [Google Scholar]
- Prochazka A. Sontag K.-H. Wand P. Motor reactions to perturbations of gait: proprioceptive and somesthetic involvement. Neurosci. Lett. 1978;7:35–39. doi: 10.1016/0304-3940(78)90109-x. [DOI] [PubMed] [Google Scholar]
- Satomi H. Takahashi K. Mizuguchi A. Aoki M. An observation on hitherto unknown corticospinal fibers that descend between the tractus corticosinalis lateralis and ventralis in the cat. Neurosci. Lett. 1991;129:168–172. doi: 10.1016/0304-3940(91)90453-z. [DOI] [PubMed] [Google Scholar]
- Schillings A.M. Van Wezel B.M.H. Duysens J. Mechanically induced stumbling during human treadmill walking. J. Neurosci. Methods. 1996;67:11–17. doi: 10.1016/0165-0270(95)00149-2. [DOI] [PubMed] [Google Scholar]
- Sribnick E. Wingrave J. Matzelle D. Wilford G. Ray S. Banik N. Estrogen attenuated markers of inflammation and decreased lesion volume in acute spinal cord injury in rats. J. Neurosci. Res. 2005;82:283–293. doi: 10.1002/jnr.20622. [DOI] [PubMed] [Google Scholar]
- Sribnick E.A. Samantaray S. Das A. Smith J. Matzelle D.D. Ray S.K. Banik N.L. Postinjury estrogen treatment of chronic spinal cord injury improves locomotor function in rats. J. Neurosci. Res. 2010;88:1738–1750. doi: 10.1002/jnr.22337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stein D.G. Progesterone exerts neuroprotective effects after brain injury. Brain Res. Rev. 2008;57:386–397. doi: 10.1016/j.brainresrev.2007.06.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tester N.J. Howland D.R. Chondroitinase ABC improves basic and skilled locomotion in spinal cord injured cats. Exp. Neurol. 2008;209:483–496. doi: 10.1016/j.expneurol.2007.07.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van Hedel H. Wirth B. Dietz V. Limits of locomotor ability in subjects with a spinal cord injury. Spinal Cord. 2005;43:593–603. doi: 10.1038/sj.sc.3101768. [DOI] [PubMed] [Google Scholar]
- Wand P. Prochazka A. Sontag K.-H. Neuromuscular responses to gait perurbations in freely moving cats. Exp. Brain Res. 1980;38:109–114. doi: 10.1007/BF00237937. [DOI] [PubMed] [Google Scholar]
- Widajewicz W. Kably B. Drew T. Motor cortical activity during voluntary gait modifications in the cat. II. Cells related to the hindlimbs. J. Neurophysiol. 1994;72:2070–2089. doi: 10.1152/jn.1994.72.5.2070. [DOI] [PubMed] [Google Scholar]
- Wilkinson E.J. Sherk H.A. The use of visual information for planning accurate steps in a cluttered environment. Behav. Brain Res. 2005;164:270–274. doi: 10.1016/j.bbr.2005.06.023. [DOI] [PubMed] [Google Scholar]