Abstract
Mitochondrial dysfunction is known to play a pivotal role in cell death mechanisms following traumatic brain injury (TBI). N-methyl-4-isoleucine-cyclosporin (NIM811), a non-immunosuppressive cyclosporin A (CsA) analog, inhibits the mitochondrial permeability transition pore (mPTP) and has been shown to be neuroprotective following TBI in mice. However, the translation of the neuroprotective effects of mPTP inhibitors, including CsA and NIM811, into improved cognitive end points has yet to be fully investigated. Therefore, to build upon these results, a severe unilateral controlled cortical impact model of TBI was used in the present study to establish a dose–response curve for NIM811 in rats. The findings demonstrate that the neuroprotection afforded by NIM811 is dose dependent, with the 10 mg/kg dose being the most effective dose. Once the dose response was established, we evaluated the effect of the optimal dose of NIM811 on behavior, mitochondrial bioenergetics, and mitochondrial oxidative damage following TBI. For behavioral studies, rats were administered NIM811 at 15 min and 24 h post-injury, with cognitive testing beginning 10 days post-injury. Mitochondrial studies involved a single injection of NIM811 at 15 min post-injury followed by mitochondrial isolation at 6 h post-injury. The results revealed that the optimal dose of NIM811 improves cognition, improves mitochondrial functioning, and reduces oxidative damage following TBI.
Key words: cognitive function, mitochondria, neurodegeneration, neuroprotection, TBI
Introduction
Traumatic brain injury (TBI) is characterized by significant neurological dysfunction, which results from a primary rapid necrosis of tissue at the site of injury followed by a delayed secondary injury. Mechanisms believed to play a role in cell death following TBI are numerous and include excitotoxicity, inflammation, mitochondrial dysfunction, oxidative stress, calpain activation, and caspase activation (Deng et al., 2007; Deng-Bryant et al., 2008; Hall et al., 2004; Kelley et al., 2007; Lifshitz et al., 2003, 2004; McGinn et al., 2009; Morganti-Kossmann et al., 2007; Saatman et al., 2010; Singh et al., 2006). Following TBI, excessive activation of N-methyl-d-aspartate (NMDA) receptors by excitatory amino acids results in an elevation in cytoplasmic Ca2+(Hinzman et al., 2010). Under normal conditions, mitochondria primarily act to regulate energy metabolism and as high-capacity Ca2+ sinks (Giacomello et al., 2007). However, excessive mitochondrial Ca2+ loading following TBI may result in formation of the mitochondrial permeability transition pore (mPTP) (Sullivan et al., 2005). A consequence of mPTP formation is a loss of membrane potential, which supports the uncoupling of electron transport from adenosine triphosphate (ATP) production. The release of pro-apoptotic molecules (i.e., cytochrome c, SMAC/DIABLO, and apoptosis-inducing factor) from the mitochondria is, in part, orchestrated by mPTP and leads to the activation of cell death pathways (Jordan et al., 2003). An additional consequence of mPTP formation is the production of reactive oxygen species (ROS), which contribute to cellular damage by oxidizing cellular proteins and lipids (Mazzeo et al., 2009).
Well-known components of the mPTP include the adenine nucleotide translocase (ANT) of the inner membrane, the voltage-dependent anion channel (VDAC or porin) of the outer membrane, and the matrix-specific peptidyl-prolyl cis–trans isomerase cyclophilin D (Cyp-D). Cyp-D translocates from the matrix to the mPTP where it interacts with ANT and promotes pore formation (Lemasters et al., 2009). Cyclosporin A (CsA) and N-methyl-4-isoleucine-cyclosporin (NIM811), inhibit the opening of mPTP by binding Cyp-D, which prevents the binding of Cyp-D to ANT (Nicolli et al., 1996).
Abundant evidence demonstrates the neuroprotective efficacy of mPTP inhibition following TBI (Cook et al., 2009; Vink et al., 2001). Administration of CsA following TBI significantly improves mitochondrial function and subsequently decreases neuronal damage (Buki et al., 1999; Okonkwo and Povlishock, 1999; Scheff and Sullivan, 1999; Sullivan et al., 1999, 2000 a,b). However, studies investigating the effects of CsA administration on cognition following TBI are limited and contradictory. In one study, CsA's neuroprotection directly translated into improved cognition (Alessandri et al., 2002). In contrast, another study reported that CsA administration did not result in improved cognition following experimental TBI (Riess et al., 2001). Therefore, the absence of cognitive data supporting the use of CsA following TBI warrants further investigation of mPTP inhibitors.
The non-immunosuppressive CsA analog NIM811 lacks the ability to inhibit calcineurin, and therefore can be administered in vivo at much higher doses than CsA (Rosenwirth et al., 1994; Waldmeier et al., 2002). NIM811 has been shown to have a favorable pharmacokinetic profile with oral bioavailability similar to that of CsA (Rosenwirth et al., 1994). The toxicity of NIM811 is significantly less than that of CsA, as is indicated by its inducing fewer nephrotoxic effects. Recently, we demonstrated that a 10 mg/kg dose of NIM811 was as effective as a 20 mg/kg dose of CsA in attenuating mitochondrial dysfunction and neurodegeneration, and improving motor function following experimental controlled cortical impact (CCI) TBI in mice (Mbye et al., 2008, 2009).
In the present study, we used the CCI TBI model to assess whether or not the effects of NIM811 administration to rats would duplicate the neuroprotective effects of NIM811 previously observed with mice, and to determine if these effects would translate into improved cognition. First, we determined the dose–response of NIM811 for increased tissue sparing. Second, we determined the effect of the optimal dose of NIM811 for improved cognitive performance using the Morris Water Maze (MWM). Third, we assessed the effect of the optimal dose of NIM811 for improving mitochondrial function and decreasing mitochondrial oxidative damage. The results demonstrate that NIM811 administration is capable of both conferring neuroprotection and improving cognition following TBI.
Methods
Animals, injury, and experimental design
All experimental animal procedures were approved by the Animal Care and Use Committee at the University of Kentucky. Adult male Sprague-Dawley rats weighing ∼300–350 g were subjected to a severe (2.0 mm) unilateral controlled cortical contusion TBI or sham operation (n=66). Surgical procedures were performed as previously described under 2% isoflurane (Davis et al., 2008). For tissue sparing assessment studies (n=25), animals were administered vehicle (100% DMSO) or NIM811 (Novartis Pharma Ltd., Basal, Switzerland) (5, 10, 20, or 40 mg/kg) at 15 min and 24 h post-injury depending upon group designation (n=5/group). Animals for behavioral studies (n=31) were administered vehicle or NIM811 (10 mg/kg) at 15 min and 24 h post-injury. Animals designated for mitochondrial studies (n=10) were administered either vehicle or NIM811 (10 mg/kg) at 15 min post-injury (n=5/group).
Tissue processing and measurements of lesion volume
At 15 days post-injury, animals were anesthetized by an overdose of pentobarbital and transcardially perfused with physiological saline followed by 4% paraformaldehyde. After removal, the brains were placed in 4% paraformaldehyde-sucrose (15%) for an additional 24 h. Coronal sections (50 μm) were then cut using a freezing microtome, throughout the rostrocaudal extent of the brain, extending through the septal area to the most posterior extent of the hippocampus.
The extent of tissue sparing following TBI was assessed blindly with respect to treatment group. The assessment used an unbiased stereological protocol, the Cavalieri method (Michel and Cruz-Orive, 1988). A systematic random subset of sections from the region of interest (minimum of 12), separated by a known distance, was then selected for analysis. The distance (d) was known because it was the mean measured section thickness multiplied by the number of sections between the sampled sections. Lesion volumes were measured with ImageJ (NIH) software. On each section, the total cortical area, defined as the dorsal aspect of lamina I to the dorsal aspect of the corpus callosum, was determined for the entire hemisphere. Each area was multiplied by d to calculate a sub-volume. All of the sub-volumes were summed to yield the total volume. The volume of the contralateral hemisphere was also determined in an identical fashion using the Cavalieri method. The volume of ipsilateral tissue spared was compared with the volume of tissue spared contralateral to the injury, and the results were expressed as the percentage tissue spared [(ipsilateral/contralateral) × 100].
Cognitive assessment
Two separate cohorts of animals were used for MWM testing. Cohort I involved NIM811-treated and vehicle-treated rats (n=5/group) and only involved training. Cohort II included sham (n=5), NIM811-treated (n=8), and vehicle-treated (n=8) rats and involved training and a probe trial. Beginning 10 days after the injury, spatial memory was assessed using an adapted MWM paradigm (Morris et al., 1984) as previously described (Guseva et al., 2008). The MWM was divided into four quadrants, with the platform submerged in one of the quadrants. Visual cues located throughout the testing room aided in spatial orientation. Swimming during the “transfer” trials was videotaped and processed using a video motion analyzer (Columbus Instruments, Columbus, OH). Briefly, for each trial quadrant, entry was randomized for different starting positions, and animals were allowed to swim until they found the platform, where they remained for 15 sec after the trial. If the platform was not located within the allotted 60-sec trial, the animal was placed on the platform for 15 sec. Each day consisted of four acquisition trials separated by 5-min intervals. Over 5 days a total of 20 acquisition trials were administered for each animal. The average time to reach the platform each day was calculated for individual groups. For cohort II, training was followed by one 15-sec probe trial. The platform was removed from the pool for the probe trial. The number of times each animal crossed the platform was recorded.
Mitochondrial isolation, mitochondrial calcium buffering capacity, and measurement of mitochondrial function
All steps of the mitochondrial isolation protocol were performed on ice. At 6 h post-injury rats were asphyxiated with CO2 until unconscious, decapitated, and the brains were rapidly removed and placed in isolation buffer (215 mM mannitol, 75 mM sucrose, 0.1% BSA, 20 mM HEPES, and 1 mM EGTA; pH 7.2). The cortices were dissected with a 5 mm diameter punch centered on the site of impact. The cortical tissue punch contained tissue from the site of the impact and the surrounding penumbra. The tissue punches were homogenized and isolated by differential centrifugation as previously described (Singh et al., 2006). Briefly, the homogenate was centrifuged twice at 1300×g for 3 min. The pellet was discarded, and the supernatant was further centrifuged at 13,000×g for 10 min. In order to release synaptic mitochondria, the crude mitochondrial pellet was subsequently subjected to nitrogen decompression using a nitrogen cell disruption bomb (1200 psi, 10 min). After nitrogen disruption, the mitochondria were suspended in 3.5 mL of 15% Percoll™ and placed on a Percoll gradient consisting of 3.5 mL of 24% Percoll and 3.5 mL of 40% Percoll in 13-mL ultraclear tubes. The gradient was centrifuged in a fixed-angle rotor at 30,400×g for 10 min at 4°C. The fraction accumulated at the interphase of the 40% and 24% Percoll layers was carefully removed and diluted with isolation buffer without EGTA, then centrifuged at 12,300×g for 10 min at 4°C. The supernatants were carefully removed, and the pellet was re-suspended in isolation buffer without EGTA and centrifuged at 13,000×g for 10 min at 4°C. In order to wash out any remaining Percol, the mitochondrial pellets were re-centrifuged at 10,000×g for 5 min. The final mitochondrial pellet was re-suspended in isolation buffer without EGTA to yield a concentration of ∼10 mg/mL. The protein concentration was determined using a bicinchoninic acid protein assay kit (Pierce, Rockford, IL).
Mitochondria isolated from naïve animals were used to assess the effect of in vitro NIM811 treatment (1 μM) on mitochondrial Ca2+ buffering capacity and mPTP. Mitochondrial Ca2+ buffering capacity was assessed using a fluorescent spectrofluorophotometer assay as previously described (Brown et al., 2006). CaG5N (100 nM, excitation, 506 nm, emission, 532 nm) was used to measure extramitochondrial Ca2+ in a Shimadzu RF-5301PC spectrofluorophotometer (Kyoto, Japan). Ca2+ was infused using a KD Scientific model 310 series infusion syringe pump (Holliston, MA) (160 nmol of Ca2+/mg of protein/min).
Mitochondrial respiration was assessed using a miniature Clark-type oxygen electrode (Hansatech Instruments, Norfolk, UK) in a sealed, thermostatically controlled chamber at 37°C as described previously (Patel et al., 2010; Sullivan et al., 2004). Mitochondrial respiration was assessed using standard polarographic methods with pyruvate and malate (5 and 2.5 mM, respectively) as oxidative substrates (Sullivan et al., 2003). Following the addition of pyruvate and malate (state II respiration), 120 nmol adenosine diphosphate (ADP) was added to the chamber to induce state III respiration; this was followed by the addition of 1 μm oligomycin to block ATP synthesis (state IV respiration). The respiratory control ratio (RCR) was used as an overall metric of mitochondrial functionality. The RCR was calculated by dividing the slope of the response of isolated mitochondria to state III respiration by the slope of the response to state IV respiration.
Mitochondrial oxidative damage assessment
Mitochondrial lipid peroxidation was assessed by western blot, using an antibody directed against 4-hydroxynonenonal (4-HNE) (Sullivan et al., 2002, Sullivan et al., 2004). Mitochondrial protein oxidation was assessed by measuring protein carbonyls using the OxyBlot Protein Oxidation Detection Kit (OxyBlot; Intergen Company, Purchase, NY) as previously described (Pandya et al., 2007).The films were scanned and the densitometric measurements were quantified by outlining the reactive bands to obtain average pixel intensities, using NIH Image software (Sullivan et al., 2002, 2004).
Statistical analysis
For all statistical comparisons, significance was set at p≤0.05. Tissue sparing assessment data were analyzed using a one-factor analysis of variance (ANOVA) followed by post-hoc analysis (Fisher Projected Least Significant Difference test). MWM training data were analyzed using a two-factor repeated-measure ANOVA, with the day of training as the repeated measure, followed by Bonferroni post-hoc analysis. MWM probe data was analyzed by one-factor ANOVA followed by Student–Newman–Keuls (SNK) post-hoc analysis. Mitochondrial RCR and oxidative damage data were analyzed using unpaired t tests.
Results
Dose–response analysis of NIM811 on cortical tissue sparing after TBI
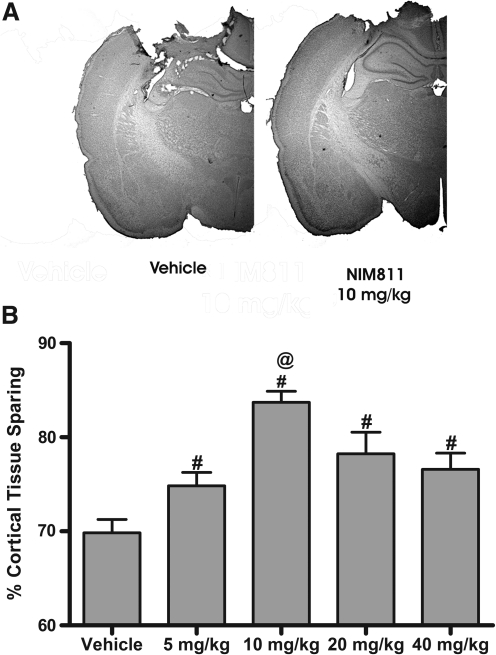
To establish a dose–response curve for NIM811, adult rats received a severe unilateral 2-mm cortical contusion and were administered various doses (5, 10, 20, or 40 mg/kg) of NIM811 or vehicle at 15 min post-injury with a subsequent injection 24 h later. In these experiments, cortical tissue sparing was determined at 15 days post-injury using the Cavalieri method. An ANOVA revealed a significant group effect in the percentage of the cortex damaged following TBI [F (4, 20)=9.359; p<.0002] (Fig. 1). Post-hoc comparisons indicated that all groups administered NIM811 demonstrated significantly greater tissue sparing (p<0.05) than did vehicle-treated animals. However, animals receiving a 10 mg/kg dose of NIM811 demonstrated a significant (p<0.0001) increase in tissue sparing compared to those receiving all other doses of NIM811.
FIG. 1.
NIM811 administration increases tissue sparing following TBI. Tissue sparing was differentially affected depending upon the dose of NIM811 administered following a severe (2 mm) CCI TBI. Adult rats were administered various dosages of NIM811 (5, 10, 20, or 40 mg/kg) or vehicle at 15 min and 24 h post-injury, with subsequent tissue sparing assessment at 15 days post-injury. (A) Representative sections from a vehicle-treated animal and a 10 mg/kg NIM811-treated animal. (B) Quantitative assessment of tissue sparing revealed that all animals receiving NIM811 demonstrated a significant increase in tissue sparing when compared to vehicle-treated animals. Data points represent group means±SEM. #p<0.05 vs. vehicle; @p<0.0001 vs. all other doses of NIM811.
NIM811 improves behavioral outcome after TBI
Experimental CCI TBI produces significant deficits in both acquisition and retention of the MWM spatial memory task (Scheff et al., 1997). Spatial memory was assessed beginning 10 days post-injury using the MWM. In this experiment, the effect of the 10 mg/kg dose of NIM811 on MWM performance was compared to the effect of vehicle. NIM811 administration was associated with significant improvement in the acquisition phase of the MWM. ANOVA results revealed significant effects of the day of testing [F(4, 28)=23.95; p<0.0001], treatment [F(2, 28)=4.766; p=0.0165] and a day X treatment interaction [F(8, 28)=2.51; p=0.0152] (Fig. 2A). On day 13, post-hoc analysis of daily latencies indicated that NIM811-treated animals had significantly improved MWM performance compared to vehicle-treated animals (p<0.01). Also, on day 14, vehicle-treated rats demonstrated significantly increased escape latency compared to sham animals (p<0.01). During the probe trial, NIM811-treated rats crossed the platform significantly more times than vehicle-treated animals [F(2, 20)=3.555; p=0.050] (Fig. 2B). No significant group differences in swim speed were present during the acquisition phase of testing (data not shown).
FIG. 2.
NIM811 treatment improves MWM performance following TBI. (A) Animals that received 10 mg/kg of NIM811 (15 min and 24 h post-injury) (triangles) found the submerged platform significantly faster on day 13 than did vehicle-treated animals (squares). (B) Number of platform crossings over the 15-sec memory retention test. Vehicle-treated animals found the platform significantly fewer times than did sham animals; NIM811 treatment attenuated this deficit in memory retention. Data points represent group means±SEM. *p<0.01 vs. vehicle and NIM811; #p<0.01 vs. vehicle, @p<0.05 vs. vehicle.
NIM811 attenuates post-traumatic mitochondrial dysfunction and oxidative damage
The ability of NIM811 to inhibit mPTP in isolated mitochondria from naïve animals was assessed using a calcium infusion assay. In vitro treatment of naïve mitochondria with NIM811 indicated that NIM811 increases the Ca2+ buffering capacity of isolated mitochondria and delays the onset of mPTP compared to vehicle treatment (Fig. 3A). In order to elucidate the effects of NIM811 administration on mitochondrial bioenergetic function, mitochondria were isolated at 6 h post-injury from the ipsilateral hemisphere of animals administered NIM811 (10 mg/kg) or vehicle at 15 min post-injury. Mitochondrial respiration was measured using a Clark-type oxygen electrode. RCRs were used as a metric for overall mitochondrial functionality. RCR values (state III/state IV) represent the extent of coupling between proton transport and ATP production. As expected, vehicle-treated rats showed significantly lower RCR values (3.06±0.18) than did NIM811-treated rats (5.65±0.27) (p<0.0001) following TBI (Fig. 3B).
FIG. 3.
NIM811 is a potent inhibitor of mPTP and NIM811 treatment attenuates injury-induced impairments in mitochondrial bioenergetics following TBI. (A) Representative Ca2+ infusion traces from isolated naïve mitochondria in the presence and absence of NIM811 (1 μM). In the presence of NIM811, mitochondria buffered more of the infused Ca2+ than in the absence of NIM811. Animals were subjected to a severe CCI TBI and administered the optimal dose of NIM811 (10 mg/kg) or vehicle at 15 min post-injury. (B) At 6 h post-injury, NIM811-treated rats demonstrated significantly higher respiratory control ratios than did vehicle-treated rats, indicating that coupling of the electron transport system to oxidative phosphorylation was maintained. Bars are group means±SEM. @p<0.0001 vs. vehicle.
Mitochondrial oxidative stress and deficits in mitochondrial functioning have been shown to occur simultaneously following experimental TBI (Opii et al., 2007; Singh et al., 2006). In order to determine the effects of NIM811 treatment on mitochondrial oxidative damage, a portion of the mitochondria isolated from the ipsilateral cortex were used for quantitative measurement of 4-HNE, as an index of lipid peroxidation, and protein carbonyls, as an index of protein oxidation, using slot blots and OxyBlots, respectively. Analysis of oxidative damage markers at 6 h post-injury demonstrated that mitochondria isolated from NIM811-treated rats had significantly lower levels of 4-HNE (p<0.01) and protein carbonyls (p<0.02) (Fig. 4). These data indicate that treatment with NIM811 attenuates TBI-induced mitochondrial oxidative damage.
FIG. 4.
Administration of NIM811 (10 mg/kg) reduces mitochondrial oxidative damage following TBI. NIM811 administration significantly decreased levels of mitochondrial (A) lipid peroxidation and (B) protein carbonyls at 6 h post-injury. Bars are group means±SEM. #p<0.05 vs. vehicle.
Discussion
Following TBI, mitochondrial dysfunction precedes secondary cell death (Robertson, 2004). The overall hypothesis for the current study was that attenuation of this dysfunction with NIM811 would result in neuroprotection. In the present study, NIM811, which inhibits mPTP without affecting calcineurin, significantly increased tissue sparing when compared to vehicle. The dose–response of NIM811 is an inverted U-shaped curve, with 10 mg/kg as the most effective dose. The 10 mg/kg dose of NIM811 was selected as the optimal dose based on these results and is in line with a previous report of Mbye and colleagues (2008). Human TBI results in significant memory dysfunction and potential TBI therapeutics should be aimed at attenuating this common clinical observation. The neuroprotective efficacy of NIM811 has been recently demonstrated following severe TBI. However, prior to the current study, the neuroprotective effects of NIM811 had yet to be translated into improved cognitive end points. In the present study we determined if the neuroprotective effects of NIM811 would translate into significant improvements in MWM performance. Indeed the neuroprotection provided by the 10 mg/kg dose of NIM811 translated into a significant reduction in brain injury-induced spatial learning deficits in the acquisition phase and memory phase of the MWM. Whereas there was a general trend of NIM811 improving memory on every day of training, only on day 13 did NIM81 treatment significantly decrease escape latency compared to vehicle treatment. Also, on day 14 of training, vehicle-treated rats had significantly increased escape latencies compared to sham animals (on day 14 NIM811-treated animals were not significantly different than sham animals). During the probe trial, on average ∼3 out 4 NIM811-treated animals crossed the platform at least once, whereas only 1 in 4 vehicle-treated animals crossed the platform.
Because mitochondrial dysfunction occurs as early as 15–30 min post-injury, it has been suggested that therapeutic interventions targeting mitochondria must be initiated early after TBI (Gilmer et al., 2009; Singh et al., 2006; Sullivan et al., 1998). In previous studies, mice showed a significant improvement in mitochondrial function following a single intraperitoneal injection of NIM811 administered 15 min post-injury. Based on these findings, it was determined that a single injection at 15 min post-injury may be sufficient to attenuate the rapid mitochondrial dysfunction occurring following TBI. Based on our dose–response studies, we decided to investigate the effects of treatment with the 10 mg/kg dose of NIM811 on mitochondrial respiration compared to vehicle. We chose to evaluate the effect of NIM811 or vehicle at 6 h post-injury based on previous studies showing that mitochondrial dysfunction peaks between 6 and 12 h post-injury (Singh et al., 2006; Xiong et al., 1997). Results from our mitochondrial studies indicated that 10 mg/kg of NIM811 was effective in attenuating trauma-induced mitochondrial dysfunction compared to vehicle treatment. This improvement in mitochondrial function most likely represents maintained mitochondrial electron transport as a result of NIM811-mediated inhibition of mPTP formation (Sullivan et al., 1999).
Although the mechanisms of secondary injury are not completely understood, increased free radical generation post-TBI and subsequent lipid peroxidation and protein nitration appear to play a role in the progressive neuropathology associated with TBI (Deng et al., 2007; Deng-Bryant et al., 2008; Hall, 1989; McCall et al., 1987; Readnower et al., 2010; Singh et al., 2006, 2007). Specifically, mitochondrial proteins involved in mitochondrial bioenergetics, including pyruvate dehydrogenase and the ATP synthase, have been shown to be oxidatively modified following TBI (Opii et al., 2007). mPTP formation and mitochondrial oxidative damage occur simultaneously following TBI (Sullivan et al., 2005). Here, we demonstrated that a 10 mg/kg dose of NIM811 significantly reduced levels of protein oxidation and lipid peroxidation in rats following TBI. These findings are in line with the results observed in mice by Mbye and colleagues (2008). Because NIM811 and CsA lack the chemical structure to act as direct antioxidants or free radical scavengers, the reduction in oxidative damage is most likely an indirect effect of maintained mitochondrial homeostasis, which would result in fewer free radicals and less oxidative damage (Mbye et al., 2008).
It is well established that Ca2+ increases mitochondrial ROS production; however, the mechanism through which Ca2+ induces ROS formation is largely unknown (Komary et al., 2010; Votyakova and Reynolds, 2005). Because mPTP decreases mitochondrial Ca2+ levels, it may be hypothesized that inhibition of mPTP would increase mitochondrial ROS production. But here, we show that mPTP inhibition actually decreases mitochondrial oxidative stress. This finding may be explained by two possible mechanisms: 1) mPTP formation results in inner membrane structural alterations leading to increased ROS production or 2) mPTP results in decreased clearance of ROS caused by impairment of antioxidant systems (Crompton, 1999; Kowaltowski et al., 1995). In isolated mitochondria, mPTP formation increases mitochondrial ROS production, and inhibition of mPTP with CsA decreases ROS production (Maciel et al., 2001). These findings suggest that the mechanism by which mitochondrial Ca2+ uptake promotes ROS production is actually mediated through mPTP formation. This notion is supported by the findings that Ca2+ does not increase ROS production without mPTP formation (Hansson et al., 2008).
To date, the efficacy of NIM811 has been demonstrated in multiple models of neurodegeneration, including transient focal cerebral ischemia, spinal cord injury, and TBI (Korde et al., 2007; Mbye et al., 2008, 2009; McEwen et al., 2007; Ravikumar et al., 2007). Despite the proven efficacy of CsA, NIM811 may have better potential as a TBI therapeutic because of its reduced toxicity and specificity as an mPTP inhibitor relative to CsA (Korde et al., 2007; Mazzeo et al., 2009). The substitution of isoleucine for leucine as the fourth amino acid of CsA allows NIM811 to bind Cyp-D and cyclophilin A (Cyp-A) but prevents NIM811 from inhibiting calcineurin. Similarly to other cyclophilins, Cyp-A exhibits peptidylprolyl isomerase (PPIase) activity, which is associated with chaperone-like actions that aid in de novo protein folding and protein repair/refolding following stress (Andreeva et al., 1999). This PPIase activity may be especially important following brain injury and may aid in recovery. One recent study demonstrated that Cyp-A protein expression was increased following CCI TBI and that exogenous administration of Cyp-A following a stab wound decreased blood–brain barrier permeability and reduced tissue damage (Redell et al., 2007). Our observed U-shaped dose–response could reflect this; although inhibition of mPTP with 10 mg/kg of NIM811 was protective, perhaps the higher doses became cytotoxic as a result of further inhibition of other cyclophilin family members, which indicates that isoform-specific cyclophilin inhibitors may provide even greater neuroprotection than either CsA or NIM811. Nevertheless, the current data support the involvement of the mPTP in TBI neuropathology and validate mPTP inhibition as a potential neuroprotective target for TBI.
In summary, 10 mg/kg of NIM811 is the most effective dose for achieving neuroprotection following TBI. NIM811 treatment is capable of improving mitochondrial function and reducing mitochondrial oxidative stress when compared to vehicle treatment following TBI. This is one of the first studies demonstrating that mPTP inhibition following TBI can be directly translated into improved cognitive behavior. These data highlight the importance of maintaining mitochondrial homeostasis following TBI. Because NIM811 is less toxic than CsA, it may be administered at higher doses, which may confer a greater degree of neuroprotection following trauma.
Acknowledgments
This research was supported by grants from the National Institutes of Health, United States Public Health Service grants R01 NS48191 (P.G.S.), R01 NS062993 (J.W.G, P.G.S.), P30 NS051220 (E.D.H), T32 DA022738 (E.D.H), and funding from the Kentucky Spinal Cord and Head Injury Research Trust.
Author Disclosure Statement
No competing financial interests exist.
References
- Alessandri B. Rice A.C. Levasseur J. DeFord M. Hamm R.J. Bullock M.R. Cyclosporin A improves brain tissue oxygen consumption and learning/memory performance after lateral fluid percussion injury in rats. J. Neurotrauma. 2002;19:829–841. doi: 10.1089/08977150260190429. [DOI] [PubMed] [Google Scholar]
- Andreeva L. Heads R. Green C. J. Cyclophilins and their possible role in the stress response. Int. J. Exp. Pathol. 1999;80:305–315. doi: 10.1046/j.1365-2613.1999.00128.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brown M.R. Sullivan P.G. Geddes J.W. Synaptic mitochondria are more susceptible to Ca2+overload than nonsynaptic mitochondria. J. Biol. Chem. 2006;281:11,658–11,668. doi: 10.1074/jbc.M510303200. [DOI] [PubMed] [Google Scholar]
- Buki A. Okonkwo D.O. Povlishock J.T. Postinjury cyclosporin A administration limits axonal damage and disconnection in traumatic brain injury. J. Neurotrauma. 1999;16:511–521. doi: 10.1089/neu.1999.16.511. [DOI] [PubMed] [Google Scholar]
- Cook A.M. Whitlow J. Hatton J. Young B. Cyclosporine A for neuroprotection: establishing dosing guidelines for safe and effective use. Expert Opin. Drug. Saf. 2009;8:411–419. doi: 10.1517/14740330903066742. [DOI] [PubMed] [Google Scholar]
- Crompton M. The mitochondrial permeability transition pore and its role in cell death. Biochem. J. 1999;341(Pt 2):233–249. [PMC free article] [PubMed] [Google Scholar]
- Davis L.M. Pauly J.R. Readnower R.D. Rho J.M. Sullivan P.G. Fasting is neuroprotective following traumatic brain injury. J. Neurosci. Res. 2008;86:1812–1822. doi: 10.1002/jnr.21628. [DOI] [PubMed] [Google Scholar]
- Deng–Bryant Y. Singh I.N. Carrico K.M. Hall E.D. Neuroprotective effects of tempol, a catalytic scavenger of peroxynitrite-derived free radicals, in a mouse traumatic brain injury model. J. Cereb. Blood Flow Metab. 2008;28:1114–1126. doi: 10.1038/jcbfm.2008.10. [DOI] [PubMed] [Google Scholar]
- Deng Y. Thompson B.M. Gao X. Hall E.D. Temporal relationship of peroxynitrite-induced oxidative damage, calpain-mediated cytoskeletal degradation and neurodegeneration after traumatic brain injury. Exp. Neurol. 2007;205:154–165. doi: 10.1016/j.expneurol.2007.01.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Giacomello M. Drago I. Pizzo P. Pozzan T. Mitochondrial Ca2+as a key regulator of cell life and death. Cell Death Differ. 2007;14:1267–1274. doi: 10.1038/sj.cdd.4402147. [DOI] [PubMed] [Google Scholar]
- Gilmer L.K. Roberts K.N. Sullivan P.G. Miller K. Scheff S. Early mitochondrial dysfunction following cortical contusion injury. J. Neurotrauma. 2009;26:1271–1280. doi: 10.1089/neu.2008.0857. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guseva M.V. Hopkins D.M. Schett S.W. Pauly J.R. Dietary choline supplementation improves behavioral, histological, and neurochemical outcomes in a rat model of traumatic brain injury. J. Neurotrauma. 2008;25:975–983. doi: 10.1089/neu.2008.0516. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hall E.D. Free radicals and CNS injury. Crit. Care Clin. 1989;5:793–805. [PubMed] [Google Scholar]
- Hall E.D. Detloff M.R. Johnson K. Kupina N.C. Peroxynitrite-mediated protein nitration and lipid peroxidation in a mouse model of traumatic brain injury. J. Neurotrauma. 2004;21:9–20. doi: 10.1089/089771504772695904. [DOI] [PubMed] [Google Scholar]
- Hansson M.J. Mansson R. Morota S. Uchino H. Kallur T. Sumi T. Ishii N. Shimazu M. Keep M.F. Jegorov A. Elmer E. Calcium-induced generation of reactive oxygen species in brain mitochondria is mediated by permeability transition. Free Radic. Biol. Med. 2008;45:284–294. doi: 10.1016/j.freeradbiomed.2008.04.021. [DOI] [PubMed] [Google Scholar]
- Hinzman J.M. Thomas T.C. Burmeister J.J. Quintero J.E. Huettl P. Pomerleau F. Gerhardt G.A. Lifshitz J. Diffuse brain injury elevates tonic glutamate levels and potassium-evoked glutamate release in discrete brain regions at two days post-injury: an enzyme-based microelectrode array study. J. Neurotrauma. 2010;27:889–899. doi: 10.1089/neu.2009.1238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jordan J. Cena V. Prehn J.H. Mitochondrial control of neuron death and its role in neurodegenerative disorders. J. Physiol. Biochem. 2003;59:129–141. doi: 10.1007/BF03179878. [DOI] [PubMed] [Google Scholar]
- Kelley B.J. Lifshitz J. Povlishock J.T. Neuroinflammatory responses after experimental diffuse traumatic brain injury. J. Neuropathol. Exp. Neurol. 2007;66:989–1001. doi: 10.1097/NEN.0b013e3181588245. [DOI] [PubMed] [Google Scholar]
- Komary Z. Tretter L. Adam–Vizi V. Membrane potential-related effect of calcium on reactive oxygen species generation in isolated brain mitochondria. Biochim. Biophys. Acta. 2010;1797:922–928. doi: 10.1016/j.bbabio.2010.03.010. [DOI] [PubMed] [Google Scholar]
- Korde A.S. Pettigrew L.C. Craddock S.D. Pocernich C.B. Waldmeier P.C. Maragos W.F. Protective effects of NIM811 in transient focal cerebral ischemia suggest involvement of the mitochondrial permeability transition. J. Neurotrauma. 2007;24:895–908. doi: 10.1089/neu.2006.0122. [DOI] [PubMed] [Google Scholar]
- Kowaltowski A.J. Castilho R.F. Vercesi A.E. Ca(2+)-induced mitochondrial membrane permeabilization: role of coenzyme Q redox state. Am. J. Physiol. 1995;269:C141–147. doi: 10.1152/ajpcell.1995.269.1.C141. [DOI] [PubMed] [Google Scholar]
- Lemasters J.J. Theruvath T.P. Zhong Z. Nieminen A.L. Mitochondrial calcium and the permeability transition in cell death. Biochim. Biophys. Acta. 2009;1787:1395–1401. doi: 10.1016/j.bbabio.2009.06.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lifshitz J. Friberg H. Neumar R. W. Raghupathi R. Welsh F. A. Janmey P. Saatman K.E. Wieloch T. Grady M.S. McIntosh T.K. Structural and functional damage sustained by mitochondria after traumatic brain injury in the rat: evidence for differentially sensitive populations in the cortex and hippocampus. J. Cereb. Blood Flow Metab. 2003;23:219–231. doi: 10.1097/01.WCB.0000040581.43808.03. [DOI] [PubMed] [Google Scholar]
- Lifshitz J. Sullivan P.G. Hovda D.A. Wieloch T. McIntosh T. K. Mitochondrial damage and dysfunction in traumatic brain injury. Mitochondrion. 2004;4:705–713. doi: 10.1016/j.mito.2004.07.021. [DOI] [PubMed] [Google Scholar]
- Maciel E.N. Vercesi A.E. Castilho R.F. Oxidative stress in Ca(2+)-induced membrane permeability transition in brain mitochondria. J. Neurochem. 2001;79:1237–1245. doi: 10.1046/j.1471-4159.2001.00670.x. [DOI] [PubMed] [Google Scholar]
- Mazzeo A.T. Beat A. Singh A. Bullock M.R. The role of mitochondrial transition pore, and its modulation, in traumatic brain injury and delayed neurodegeneration after TBI. Exp. Neurol. 2009;218:363–370. doi: 10.1016/j.expneurol.2009.05.026. [DOI] [PubMed] [Google Scholar]
- Mbye L.H. Singh I.N. Carrico K.M. Saatman K.E. Hall E.D. Comparative neuroprotective effects of cyclosporin A and NIM811, a nonimmunosuppressive cyclosporin A analog, following traumatic brain injury. J. Cereb. Blood Flow Metab. 2009;29:87–97. doi: 10.1038/jcbfm.2008.93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mbye L.H. Singh I.N. Sullivan P.G. Springer J.E. Hall E.D. Attenuation of acute mitochondrial dysfunction after traumatic brain injury in mice by NIM811, a non-immunosuppressive cyclosporin A analog. Exp. Neurol. 2008;209:243–253. doi: 10.1016/j.expneurol.2007.09.025. [DOI] [PubMed] [Google Scholar]
- McCall J.M. Braughler J.M. Hall E.D. Lipid peroxidation and the role of oxygen radicals in CNS injury. Acta Anaesthesiol. Belg. 1987;38:373–379. [PubMed] [Google Scholar]
- McEwen M.L. Sullivan P.G. Springer J.E. Pretreatment with the cyclosporin derivative, NIM811, improves the function of synaptic mitochondria following spinal cord contusion in rats. J. Neurotrauma. 2007;24:613–624. doi: 10.1089/neu.2006.9969. [DOI] [PubMed] [Google Scholar]
- McGinn M.J. Kelley B.J. Akinyi L. Oli M.W. Liu M.C. Hayes R.L. Wang K.K. Povlishock J.T. Biochemical, structural, and biomarker evidence for calpain-mediated cytoskeletal change after diffuse brain injury uncomplicated by contusion. J. Neuropathol. Exp. Neurol. 2009;68:241–249. doi: 10.1097/NEN.0b013e3181996bfe. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Michel R.P. Cruz–Orive L.M. Application of the Cavalieri principle and vertical sections method to lung: estimation of volume and pleural surface area. J. Microsc. 1988;150:117–136. doi: 10.1111/j.1365-2818.1988.tb04603.x. [DOI] [PubMed] [Google Scholar]
- Morganti–Kossmann M.C. Satgunaseelan L. Bye N. Kossmann T. Modulation of immune response by head injury. Injury. 2007;38:1392–1400. doi: 10.1016/j.injury.2007.10.005. [DOI] [PubMed] [Google Scholar]
- Nicolli A. Basso E. Petronilli V. Wenger R.M. Bernardi P. Interactions of cyclophilin with the mitochondrial inner membrane and regulation of the permeability transition pore, and cyclosporin A-sensitive channel. J. Biol. Chem. 1996;271:2185–2192. doi: 10.1074/jbc.271.4.2185. [DOI] [PubMed] [Google Scholar]
- Okonkwo D.O. Povlishock J.T. An intrathecal bolus of cyclosporin A before injury preserves mitochondrial integrity and attenuates axonal disruption in traumatic brain injury. J. Cereb. Blood Flow Metab. 1999;19:443–451. doi: 10.1097/00004647-199904000-00010. [DOI] [PubMed] [Google Scholar]
- Opii W.O. Nukala V.N. Sultana R. Pandya J.D. Day K.M. Merchant M.L. Klein J.B. Sullivan P.G. Butterfield D.A. Proteomic identification of oxidized mitochondrial proteins following experimental traumatic brain injury. J. Neurotrauma. 2007;24:772–789. doi: 10.1089/neu.2006.0229. [DOI] [PubMed] [Google Scholar]
- Pandya J.D. Pauly J.R. Nukala V.N. Sebastian A.H. Day K.M. Korde A.S. Maragos W.F. Hall E.D. Sullivan P.G. Post-injury administration of mitochondrial uncouplers increases tissue sparing and improves behavioral outcome following traumatic brain injury in rodents. J. Neurotrauma. 2007;24:798–811. doi: 10.1089/neu.2006.3673. [DOI] [PubMed] [Google Scholar]
- Patel S.P. Sullivan P.G. Lyttle T.S. Rabchevsky A.G. Acetyl-L–carnitine ameliorates mitochondrial dysfunction following contusion spinal cord injury. J. Neurochem. 2010;114:291–301. doi: 10.1111/j.1471-4159.2010.06764.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ravikumar R. McEwen M.L. Springer J.E. Post-treatment with the cyclosporin derivative, NIM811, reduced indices of cell death and increased the volume of spared tissue in the acute period following spinal cord contusion. J. Neurotrauma. 2007;24:1618–1630. doi: 10.1089/neu.2007.0329. [DOI] [PubMed] [Google Scholar]
- Readnower R.D. Chavko M. Adeeb S. Conroy M.D. Pauly J.R. McCarron R.M. Sullivan P.G. Increase in blood brain barrier permeability, oxidative stress, and activated microglia in a rat model of blast induced traumatic brain injury. J. Neurosci. Res. 2010;88:3530–3539. doi: 10.1002/jnr.22510. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Redell J.B. Zhao J. Dash P.K. Acutely increased cyclophilin a expression after brain injury: a role in blood–brain barrier function and tissue preservation. J. Neurosci. Res. 2007;85:1980–1988. doi: 10.1002/jnr.21324. [DOI] [PubMed] [Google Scholar]
- Riess P. Bareyre F.M. Saatman K.E. Cheney J.A. Lifshitz J. Raghupathi R. Grady M.S. Neugebauer E. McIntosh T.K. Effects of chronic, post-injury Cyclosporin A administration on motor and sensorimotor function following severe, experimental traumatic brain injury. Restor. Neurol. Neurosci. 2001;18:1–8. [PubMed] [Google Scholar]
- Robertson C.L. Mitochondrial dysfunction contributes to cell death following traumatic brain injury in adult and immature animals. J. Bioenerg. Biomembr. 2004;36:363–368. doi: 10.1023/B:JOBB.0000041769.06954.e4. [DOI] [PubMed] [Google Scholar]
- Rosenwirth B. Billich A. Datema R. Donatsch P. Hammerschmid F. Harrison R. Hiestand P. Jaksche H. Mayer P. Peichl P. Quesniaux V. Schatz F. Schuurman H. Traber R. Wenger R. Wolff B. Zenke G. Zurini M. Inhibition of human immunodeficiency virus type 1 replication by SDZ NIM 811, a nonimmunosuppressive cyclosporine analog. Antimicrob. Agents Chemother. 1994;38:1763–1772. doi: 10.1128/aac.38.8.1763. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saatman K.E. Creed J. Raghupathi R. Calpain as a therapeutic target in traumatic brain injury. Neurotherapeutics. 2010;7:31–42. doi: 10.1016/j.nurt.2009.11.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scheff S.W. Baldwin S.A. Brown R.W. Kraemer P.J. Morris water maze deficits in rats following traumatic brain injury: lateral controlled cortical impact. J. Neurotrauma. 1997;14:615–627. doi: 10.1089/neu.1997.14.615. [DOI] [PubMed] [Google Scholar]
- Scheff S.W. Sullivan P.G. Cyclosporin A significantly ameliorates cortical damage following experimental traumatic brain injury in rodents. J. Neurotrauma. 1999;16:783–792. doi: 10.1089/neu.1999.16.783. [DOI] [PubMed] [Google Scholar]
- Singh I.N. Sullivan P.G. Deng Y. Mbye L.H. Hall E.D. Time course of post-traumatic mitochondrial oxidative damage and dysfunction in a mouse model of focal traumatic brain injury: implications for neuroprotective therapy. J. Cereb. Blood Flow Metab. 2006;26:1407–1418. doi: 10.1038/sj.jcbfm.9600297. [DOI] [PubMed] [Google Scholar]
- Singh I.N. Sullivan P.G. Hall E.D. Peroxynitrite-mediated oxidative damage to brain mitochondria: Protective effects of peroxynitrite scavengers. J. Neurosci. Res. 2007;85:2216–2223. doi: 10.1002/jnr.21360. [DOI] [PubMed] [Google Scholar]
- Sullivan P.G. Dube C. Dorenbos K. Steward O. Baram T.Z. Mitochondrial uncoupling protein-2 protects the immature brain from excitotoxic neuronal death. Ann. Neurol. 2003;53:711–717. doi: 10.1002/ana.10543. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sullivan P.G. Keller J.N. Bussen W.L. Scheff S.W. Cytochrome c release and caspase activation after traumatic brain injury. Brain Res. 2002;949:88–96. doi: 10.1016/s0006-8993(02)02968-2. [DOI] [PubMed] [Google Scholar]
- Sullivan P.G. Keller J.N. Mattson M.P. Scheff S.W. Traumatic brain injury alters synaptic homeostasis: implications for impaired mitochondrial and transport function. J. Neurotrauma. 1998;15:789–798. doi: 10.1089/neu.1998.15.789. [DOI] [PubMed] [Google Scholar]
- Sullivan P.G. Rabchevsky A.G. Hicks R.R. Gibson T.R. Fletcher–Turner A. Scheff S. W. Dose–response curve and optimal dosing regimen of cyclosporin A after traumatic brain injury in rats. Neuroscience. 2000a;101:289–295. doi: 10.1016/s0306-4522(00)00380-8. [DOI] [PubMed] [Google Scholar]
- Sullivan P.G. Rabchevsky A.G. Keller J.N. Lovell M. Sodhi A. Hart R.P. Scheff Sullivan P.G. Rabchevsky A.G. Waldmeier P.C. Springer J.E. Mitochondrial permeability transition in CNS trauma: cause or effect of neuronal cell death? J. Neurosci. Res. 2005;79:231–239. doi: 10.1002/jnr.20292. [DOI] [PubMed] [Google Scholar]
- Sullivan P.G. Rippy N.A. Dorenbos K. Concepcion R.C. Agarwal A.K. Rho J.M. The ketogenic diet increases mitochondrial uncoupling protein levels and activity. Ann. Neurol. 2004;55:576–580. doi: 10.1002/ana.20062. [DOI] [PubMed] [Google Scholar]
- Sullivan P.G. Thompson M.B. Scheff S.W. Cyclosporin A attenuates acute mitochondrial dysfunction following traumatic brain injury. Exp. Neurol. 1999;160:226–234. doi: 10.1006/exnr.1999.7197. [DOI] [PubMed] [Google Scholar]
- Sullivan P.G. Thompson M. Scheff S.W. Continuous infusion of cyclosporin A postinjury significantly ameliorates cortical damage following traumatic brain injury. Exp. Neurol. 2000b;161:631–637. doi: 10.1006/exnr.1999.7282. [DOI] [PubMed] [Google Scholar]
- Vink R. Nimmo A.J. Cernak I. An overview of new and novel pharmacotherapies for use in traumatic brain injury. Clin. Exp. Pharmacol. Physiol. 2001;28:919–921. doi: 10.1046/j.1440-1681.2001.03548.x. [DOI] [PubMed] [Google Scholar]
- Votyakova T.V. Reynolds I.J. Ca2+-induced permeabilization promotes free radical release from rat brain mitochondria with partially inhibited complex I. J. Neurochem. 2005;93:526–537. doi: 10.1111/j.1471-4159.2005.03042.x. [DOI] [PubMed] [Google Scholar]
- Waldmeier P.C. Feldtrauer J.J. Qian T. Lemasters J.J. Inhibition of the mitochondrial permeability transition by the nonimmunosuppressive cyclosporin derivative NIM811. Mol. Pharmacol. 2002;62:22–29. doi: 10.1124/mol.62.1.22. [DOI] [PubMed] [Google Scholar]
- Xiong Y. Gu Q. Peterson P.L. Muizelaar J.P. Lee C.P. Mitochondrial dysfunction and calcium perturbation induced by traumatic brain injury. J. Neurotrauma. 1997;14:23–34. doi: 10.1089/neu.1997.14.23. [DOI] [PubMed] [Google Scholar]