Summary
Using a mouse model of ependymoma—a chemoresistant brain tumor—we combined multi-cell high-throughput screening (HTS), kinome-wide binding assays, and in vivo efficacy studies, to identify potential treatments with predicted toxicity against neural stem cells (NSC). We identified kinases within the insulin signaling pathway and centrosome cycle as regulators of ependymoma cell proliferation, and their corresponding inhibitors as potential therapies. FDA approved drugs not currently used to treat ependymoma were also identified that posses selective toxicity against ependymoma cells relative to normal NSCs both in vitro and in vivo e.g., 5-fluoruracil. Our comprehensive approach advances understanding of the biology and treatment of ependymoma including the discovery of several treatment leads for immediate clinical translation.
INTRODUCTION
Advances in cancer therapy have been made by testing empiric combinations of treatments in patients with histologically similar tumors. This clinical research has been supplemented by laboratory studies of human cancer cell lines and xenografts aimed at identifying therapies. Although this approach has reduced deaths from some common malignancies (Berry et al., 2005; Pui et al., 2004), it is inherently inefficient, failing to account for the different molecular subtypes that populate cancers, and providing little insight into the patients most likely to benefit from each therapy. This process is especially inadequate for developing treatments of rare cancers that have few patient numbers and limited pre-clinical tools.
Ependymomas are rare tumors of the brain and spinal cord that are incurable in up to 40% of cases (McGuire et al., 2009; Merchant et al., 2009). Treatment of this disease has changed little over the last 40 years and is essentially limited to surgery and radiation. Chemotherapy has not impacted survival from ependymoma, but the rarity of the disease and lack of preclinical models has precluded comprehensive drug testing (Bouffet and Foreman, 1999; Gilbert et al., 2010).
Histologic similarities between ependymomas from the different regions of the central nervous system (CNS) have led investigators to treat these tumors as a single entity. But these tumors include discrete subtypes that will likely require different therapies (Johnson et al., 2010; Taylor et al., 2005). To develop more rational treatments of ependymoma, we are generating mouse models of each disease subtype (Johnson et al., 2010). The first of these, mEPEphb2, faithfully recapitulates the histology and transcriptome of one form of human cerebral ependymoma (subtype-D), making it an attractive tool for developing treatments for this specific group of patients.
Recent evidence suggests that some cancers, including ependymomas, contain stem-like cancer cells that are both sufficient and required to propagate the disease (Johnson et al., 2010; Lapidot et al., 1994; Singh et al., 2004; Taylor et al., 2005). Therefore, the most effective treatments of these cancers are likely to include drugs that kill these cells (Zhou et al., 2009). However, ependymoma stem-like cells are remarkably similar to the normal NSCs from which they arise (Johnson et al., 2010): suggesting, such treatments may also be toxic to developing tissues. Developmental toxicities are a particular concern when treating children with cancer whose organ systems are immature (Kimura et al., 2008). Our ependymoma model system generates tumors from isolated NSCs, allowing the conduct of comparative drug toxicity studies. The goal of the current study was to employ this mouse model system in a multi-platform, in vitro and in vivo drug development approach to identify subtype-specific therapies with predicted stem cell toxicity.
RESULTS
High throughput screen (HTS) for predicting efficacy and toxicity
Cells isolated from mEPEphb2 ependymomas as well as distinct variants of mouse NSCs, maintain their functional and molecular identity when passaged clonally under conditions that promote stem cell growth (Johnson et al., 2010). As a first step to identify treatments of ependymoma, we adapted these cultures to perform in a HTS campaign that detected compound toxicity against normal and malignant stem cells with high reproducibility and sensitivity (Figure 1; Figure S1; Supplemental Experimental Procedures).
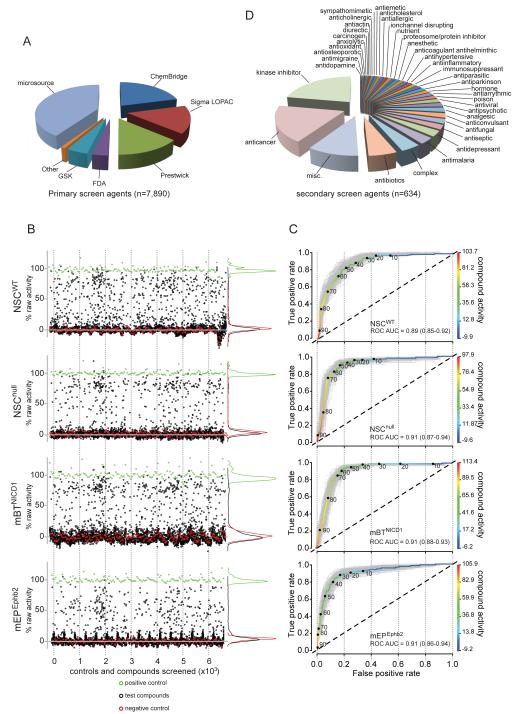
Figure 1. Compounds and quality control of the high throughput screen.
A. Sources of compounds used in the screen. B. Scatter plot of percent primary screen activity (relative to vehicle) of positive controls (green) negative controls (red) and test compounds (black) for all four cell types. C. ROC analyses showing the true (y-axis) versus false (x-axis) positive rates of percent drug activity correlating the primary and secondary screens. The ROC curves in gray are calculated from 200 bootstrap simulations. Percent activity is color-coded according to the right y-axis. D. Drug classes represented by the 634 compounds tested in secondary screens. See also Figure S1.
We screened a total of 7,890 (5,303 unique) compounds obtained from seven separate sources, divided among four libraries (Figure 1A): a ‘bioactive’ library that included 5,600 (3,161 unique) bioactive compounds, natural products, and known drugs; a ‘kinase-scaffold’ library composed of 1,648 compounds designed using kinase inhibitor pharmacophore models; GlaxoSmithKline’s Published Kinase Inhibitor Set (GSK-PKIS) composed of 367 kinase inhibitor tool compounds with good bioavailability and known specificity; and a ‘Food and Drug Administration (FDA)’ library that included 275 FDA approved compounds that were enriched for anti-cancer and neurologically active drugs.
We first screened all compounds in the bioactive library, each at 8 μM concentration. This primary screen was conducted against mEPEphb2 cells, as well as wild-type (NSCwt), and Ink4a/Arf−/− (NSCnull) mouse embryonic forebrain NSCs—the initiating cell of mEPEphB2 tumors (Johnson et al., 2010). To increase the probability of identifying ependymoma-specific therapies, we also screened a non-ependymoma mouse brain tumor that we generated by transducing NSCnull with the intracellular domain of NOTCH1 (mBTNICD1; Figure S1). The scatterplot of control and test compound activity in this primary screen demonstrated a good separation between signal and noise for each cell type (Figure 1B). Potency was then assessed in a 10-point concentration secondary screen for all compounds in the bioactive library that demonstrated ≥75% activity against any of the four cell types. This secondary screen was performed in triplicate using all four cell types. To assess the fidelity of the primary screen, we also included in the secondary screen an additional 254 compounds that demonstrated a range of primary screen activities <75%. Receiver operator characteristic (ROC) analysis of these data demonstrated good discriminatory power for each cell type, and indicated that a primary screen threshold of ≥50% activity would detect most true positives (Figure 1C). Having validated our primary and secondary screen using the ‘bioactive’ library, we completed HTS of the ‘kinase-scaffold’, ‘GSK-PKIS’, and ‘FDA’ libraries.
A total of 634 unique compounds across the four libraries, representing diverse drug classes, progressed from primary to secondary screening (Figure 1D). In all, 2.6% (n=140/5,303) of compounds displayed anti-mEPEphb2 secondary screen activity (Figure 2). Since our HTS strategy included non-ependymoma tumor cells and NSCs, we were able to refine our classification of compound activity (Table S1). To this end we determined for each cell type, the average ‘effective concentration’ of each compound that inhibited cell growth by 50% relative to control treatment (EC50). The data were then used to identify compounds with ≥2-fold activity against mEPEphb2 than other cells (0.08%, n=4/5,303; ‘mEPEphb2-selective’); equally active against mEPEphb2 and mBTNICD1 cells relative to NSCs (0.04%, n=2/5,303; ‘tumor-selective’); equally active against all four cell types (2.5%, n=134/5,303; ‘equipotent’); ≥2-fold more active against mBTNICD1 relative to all other cells (0.2%, n=13/5,303; ‘mBTNICD1-selective’); ≥2-fold more active against NSCs (NSCwt and/or NSCnull) relative to tumor cells (0.8%, n=42/5,303; ‘NSC-selective’); inactive against mEPEphb2 cells relative to all other cells (0.1%, n=7/5,303; ‘mEPEphb2-inactive’); and inactive against all four cell types (91%, n=4,809/5,303; ‘inactive’).
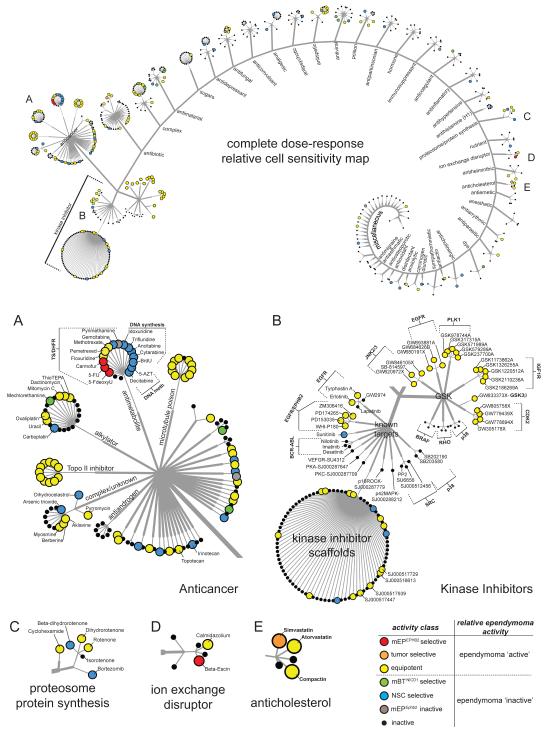
Figure 2. Drug class network tree of cell-type specific potencies of 634 compounds subjected to secondary screening.
Top figure shows compounds clustered according to major therapeutic indication and mechanism of action. A-E indicate the location of larger figures shown below. Activity was determined by dose response in secondary screening. Drug labels are colored according to cell-type activity as defined in the key (bottom right). See also Table S1.
HTS identifies cell-selective activity that varies with drug mechanism of action
As expected, compounds with activity against mEPEphb2 cells (‘mEPEphb2-selective’, ‘tumor-selective’ and ‘equipotent’) were significantly enriched for anti-cancer drugs (Fishers Exact P=1.9 × 10−7: Bonferroni correction threshold, p=0.0016); but they displayed patterns of cell-selective activity that varied according to their mechanism of action (Figures 2 and 3). Almost all topoisomerase II inhibitors (P=7.6 ×10−09) and microtubule poisons (P=4.0 × 10−07) were ‘equipotent’ against the four cell types. In contrast, dichotomous activity was observed among antimetabolite anticancer compounds. Antimetabolites that incorporate into DNA and disrupt normal DNA synthesis or methylation e.g., decitabine, were all ‘NSC-selective’ (P=5.0 × 10−09); while inhibitors of thymidylate synthase (TYMS) and/or dihydrofolate reductase (DHFR) e.g., 5-fluorouracil (5-FU), were enriched for ‘ependymoma-selective’ compounds (P=5.0 × 10−09). Indeed, three of the four ‘ependymoma-selective’ compounds detected in the entire HTS were in this class: 5-FU, the 5-FU precursor 5-Fluoro-5′-deoxyuridine and the 5-FU derivative carmofur.
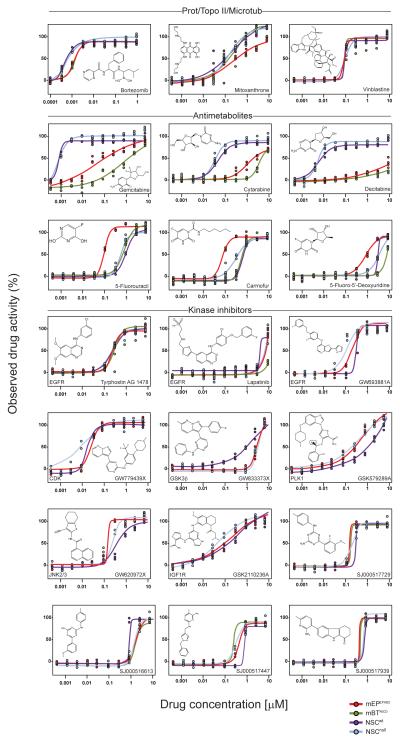
Figure 3. Patterns of drug class sensitivity among cells.
Dose response curves reveal distinct patterns of relative drug sensitivity among normal NSCs and tumor cells. The structure of the corresponding agent (and where appropriate molecular target) is shown in each graph.
The fourth ‘ependymoma selective’ agent identified in our HTS—the ionophore beta-escin—disrupts normal membrane ion exchange. Interestingly, potassium ionophores were recently identified as breast cancer stem cell selective inhibitors, suggesting this drug class might have broad activity against malignant stem cell populations (Gupta et al., 2009). Proteosome or protein synthesis inhibitors included drugs that were ‘NSC-selective’ e.g., bortezomib, while anticholesterol drugs were ‘equipotent’ or ‘tumor-selective’ (Figures 2 and 3).
Integration of HTS and kinome-wide binding assays unmasks pathways important for maintaining ependymoma cells and NSCs
Active compounds detected by HTS were also enriched for inhibitors of specific kinases (Figures 2 and 3), including inhibitors of: Epidermal Growth Factor Receptor (EGFR, n=9/11 inhibitors screened; P=1.0 ×10−08); Insulin Growth Factor 1 Receptor (IGF1R, n=5/5; P=7.3 ×10−07); Polo-like kinase 1 (PLK1, n=5/5; P=7.3 ×10−07); Cyclin dependent kinase 2 (CDK2, n=4/4; P=1.0 ×10−05); and Jun NH(2)-terminal kinase 2 and 3(JNK2/3, n=3/3; P=0.0001). A single Glycogen Synthase Kinase 3β (GSK3β) inhibitor also proved active in secondary screening. The ‘equipotent’ activity of EGFR inhibitors is compatible with reports that these receptors conduct critical signals in ependymoma and normal NSCs (Aguirre et al., 2010; Geoerger et al., 2008; Gilbertson et al., 2002; Mendrzyk et al., 2006); however, none of the other active kinase inhibitors identified, or their target molecules, have been suggested previously as treatments and drivers of ependymoma, respectively.
The finding that IGF1R and PLK1 inhibitors disrupt mEpEphb2 cell and NSC proliferation is especially noteworthy. IGF1R is expressed at the apical surface of forebrain NSCs—the cell-of-origin of cerebral ependymoma (Johnson et al., 2010)—and actives NSC proliferation following binding of its ligand IGF2 that is contained within the cerebrospinal fluid (CSF) (Lehtinen et al., 2011). Furthermore, elevated levels of IGF2 sufficient to activate aberrant NSC proliferation are found in the CSF of patients with brain tumors (Lehtinen et al., 2011). The proliferation of NSCs and ependymoma stem cells occurs via a process termed asymmetric division by which the parent stem cell self-renews: this process is critically dependent on cell polarity, cell cycle timing and mitotic spindle formation that are each regulated by PLK1 (Johnson et al., 2010; Noatynska et al., 2010; Wang et al., 2009). Thus, IGF1R and PLK1 inhibitors, of which more than 18 are currently in Phase I or II clinical trial, are attractive candidates for ependymoma clinical trials (Lens et al., 2010; Neal and Sequist, 2010). Importantly, several other kinase inhibitors currently under clinical development were inactive against mEPEphb2 e.g., BRAF inhibitors and BCR-ABL inhibitors, suggesting these drugs should given a lower priority for clinical trial in ependymoma.
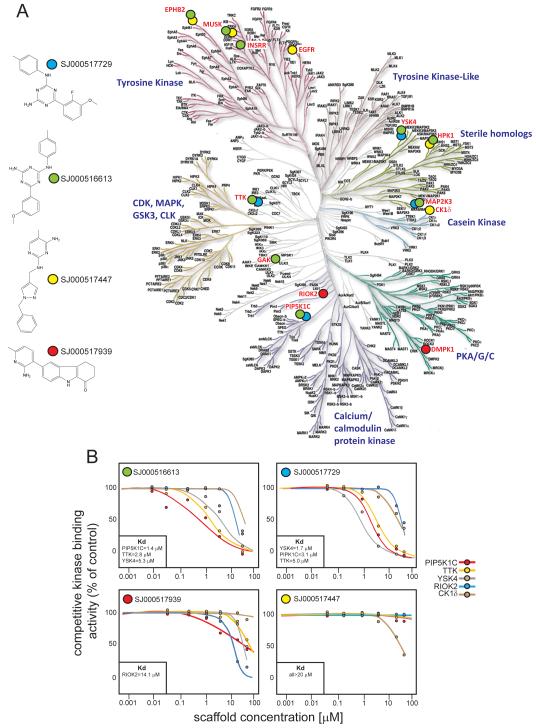
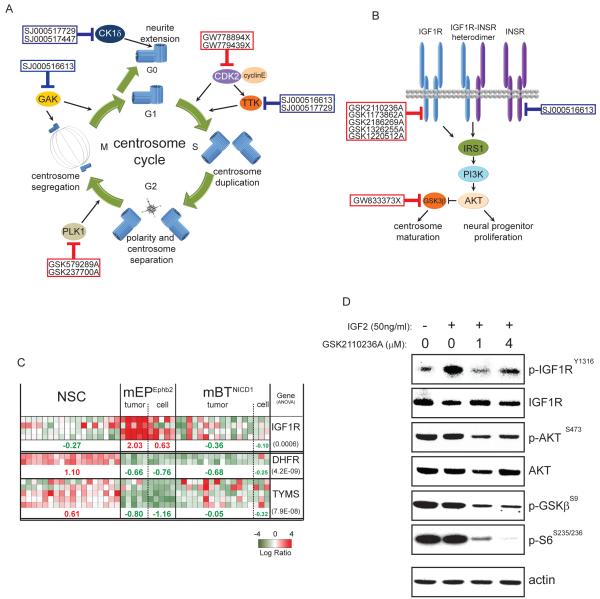
In addition to inhibitors of known kinases, we also identified 13 ‘equipotent’ orphan-kinase inhibitor scaffolds for which the molecular target is not known (Figures 2 and 3). Identifying the kinases targeted by these compounds could pinpoint further cell signals critical for ependymoma cell survival, as well as therapeutic approaches. Therefore, we subjected the four most active kinase scaffolds (SJ000516613, SJ000517447, SJ000517729, and SJ000517939; Figure 3) to competitive binding assays against 442 human kinases (see Supplemental Methods). Kinase scaffolds were first screened at a single fixed concentration (2.5μM) against all 442 kinases (Figure 4A). One or more kinase scaffolds bound 13 kinases with >50% activity relative to controls. In keeping with our HTS data, these included EGFR, Insulin Receptor-Related Receptor (INSRR), and EPHB2: the latter transforms NSCs in the mEPEphb2 model. To validate additional kinases as potential drivers and drug targets of cerebral ependymoma, we performed a series of six-point dose response binding assays against kinases most tightly bound in the single concentration assay. These data identified PIP5K1C, TTK, and YSK4 as kinase targets of SJ000516613 and SJ000517729, with binding Kds similar to their EC50s in whole cell proliferation assays (Figures 3 and 4B). RIOK2 and CK1δ were bound by SJ000517939 and SJ000517447, respectively, albeit at much higher concentrations.
Figure 4. Kinome-wide binding assay of ‘equipotent’ kinase inhibitor scaffolds.
A. Left, four kinase inhibitor scaffolds with ‘equipotent’ activity in the HTS were subject to a single concentration (2.5 μM) competitive binding assay against 442 kinases in the human kinome (Right). Kinases bound with more than 50% activity relative to controls are marked with labels colored according to scaffold. B. Six point dose response Kd binding assays of scaffolds against selected kinases. Boxes show the Kd values (≤20 μM) for each kinase.
Integration of our HTS and kinome binding data showed that 57% (n=25/44) of kinase inhibitors and scaffolds with activity against ependymoma cells and NSCs, target critical components of just three major cell pathways: the EGF, IGF and centrosome cycle pathways (Figures 3 to 5). Nine inhibitors targeted components of the centrosome cycle (Figure 5A), including PLK1 (discussed above), and CDK2 and its direct substrate TTK, that together regulate centrosome duplication (Cowan and Hyman, 2006; Kasbek et al., 2007). Seven inhibitors target the IGF pathway; including five direct inhibitors of IGF1R (Figure 5B and discussed above). Notably, gene expression profile analysis showed that IGF1R expression is significantly upregulated in mEPEphb2 cells relative to parental NSCs or mBTNICD1 cells (Figure 5C). Further, GSK2110236A, a potent inhibitor of mEPEphb2 proliferation (Figure 3) and known IGF1R kinase inhibitor within the ‘GSK-PKIS’ library, blocked IGF1R and its downstream signal in mEPEphb2 cells stimulated with IGF2 (Figure 5D). Together, these data identify the EGF, IGF and centrosome cycle pathways as key signals for maintaining cerebral ependymoma and their corresponding inhibitors as lead candidate targets of therapies. However, the ‘equipotent’ activity of these compounds in HTS should alert investigators to the possibility of NSC-toxicities in clinical trials.
Figure 5. Kinase inhibitors active against mEPEphb2 target the centrosome cycle and insulin growth factor signaling pathway.
Schematics of the centrosome cycle (A) and insulin growth factor pathway (B) marked with members of the ‘kinase inhibitor scaffold’ (blue boxes) and ‘GSK-PKIS’ (red boxes) libraries adjacent to their target molecules. C. Heatmaps reporting gene expression patterns in mouse cells and tumors (data from Johnson et al., 2010) of IGF1R, DHFR and TYMS. Figures below report the median Log Ratio of expression for each cell or tumor type and the number in parenthesis to the right the p-value for the ANOVA of this distribution. D. Western blot analysis of phosphorylated and total IGF1R, AKT, and phosphorylated GSK3β and S6 species in mEPEphb2 cells treated with the IGF1R kinase inhibitor GSK2110236A (or vehicle) followed by IGF2 stimulation.
5-FU and bortezomib are active against mEPEphb2 ependymoma in vivo
To allow the most rapid translation of treatments from our HTS to the clinic, we selected two FDA approved anticancer drugs for our first sets of in vivo studies. 5-FU is a well established chemotherapeutic for the treatment of certain cancers, e.g., colorectal cancer (Douillard et al., 2000; IMPACT, 1995), but this drug has not been tested formally in patients with ependymoma. The ‘ependymoma-selective’ activity of 5-FU in our HTS and relatively low expression of TYMS (Figure 5C)–the principal cellular target of 5-FU—in mEPEphb2 cells, suggest this drug as a rational treatment of cerebral ependymoma (Longley et al., 2003). Similarly, bortezomib—a reversible inhibitor of the 26S proteasome—has not been tested against ependymoma in the clinic but was highly potent against mEPEphb2 cells, and has activity against a variety of cancers including brain tumors (Adams, 2004; Phuphanich et al., 2010; Richardson et al., 2003; Taniguchi et al., 2009).
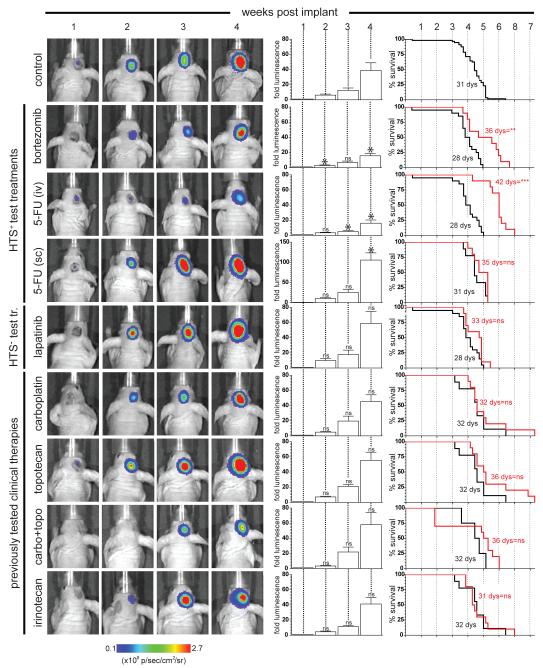
First, we tested the in vivo efficacy of 5-FU and bortezomib using a robust orthotopic allograft model of mEPEphb2 ependymoma. 1.5×106 mEPEphb2 cells transduced with luciferin (hereon, mEPEphb2-LUC) were injected stereotactically into the brains of immunocompromised mice. Bioluminescence detected brain tumors in these mice that conferred a median survival of 31 days (n=56 ‘control’ mice) and displayed a histology and gene expression profile indistinguishable from that of mEPEphb2 (Figure 6, Figure S2). Mice bearing mEPEphb2-LUC tumors were treated with eight different therapeutic regimens (n≥10 mice per cohort; Table 1). 5-FU and bortezomib were given intravenously at doses reported previously to be efficacious in cancer bearing mice (Houghton et al., 1996; Williamson et al., 2009). 5-FU is usually given as a prolonged infusion in the clinic; therefore to simulate this we also administered 5-FU via subcutaneous Alzet pumps (Kamano et al., 1997). For comparative purposes we also treated cohorts of mice with carboplatin, topotecan, irinotecan, or carboplatin plus topotecan that have produced clinical, but not durable, responses against ependymoma (Bomgaars et al., 2006; Bouffet and Foreman, 1999; Kadota et al., 1999). Neither carboplatin nor irinotecan were active against mEPEphb2 in our HTS, while topotecan was moderately equipotent against all cells (EC50=0.2-0.7 μM; Figure 2). As an additional test of the power of our HTS to predict in vivo efficacy, we treated a cohort of mice with laptinib that was inactive in our HTS, but is currently undergoing clinical trials in ependymoma. Each of these chemotherapies were administered according to existing preclinical regimens (Gorlick et al., 2009; Stewart et al., 2004; Thompson et al., 1997; Tonda et al., 1996; Wagner et al., 2010) or schedules designed to generate plasma levels in mice that are equivalent to those active in children with brain tumors (Table 1). Each ‘protocol’ group was paired with a ‘control’ group of ≥5 mice that were implanted with mEPEphb2-LUC cells, treated with vehicle alone, and imaged in parallel at weekly intervals by bioluminescence (Figure 6).
Figure 6. In vivo efficacy against mEPEphb2 ependymomas of drugs displaying different activities in the HTS.
Panels to the left show serial weekly bioluminescence scans of a single representative animal treated with the indicated drug(s). Central bar graphs report for the entire cohort of mice treated with the corresponding drug(s) the mean (±SD) weekly fold change in tumor bioluminescence relative to levels immediately following implantation. Asterisks in bar graphs of drug treated mice report whether the fold tumor bioluminescence at that time point differs from that observed in the control group. Graphs to the right report the survival of drug (red line) versus control (black line) treated mice in each cohort. In both graphs *, p=0.05; **, p=0.005; ***, p=0.0005 for the corresponding statistic. See also Figure S2.
Table 1.
Pre-clinical drug protocols used to treat mice harboring mEPEphb2 orthotpic xenografts
| Drug | Dose (per kg) |
Schedule | Reference |
|---|---|---|---|
| 5-FU | 75 mg | i.v*, once weekly |
Houghton et al., 1996 |
| 5-FU | up to to15mg | s.c., implants |
Kamano et al., 1997 |
| Bortezomib | 0.8mg | i.v, twice weekly |
Williamson, et al., 2009 |
| Lapatinib | 160 mg | p.o, twice daily 5/7dys |
Gorlick et al, 2009 |
| Irinotecan | 10 mg, | i.p, once daily × 5dys, recur |
Thompson et al., 1997 |
| Topotecan | 1 mg | i.p, once daily × 5dys, recur |
Stewart et al., 2004 |
| Carboplatin | 90 mg | i.v, once every 21dys |
Tonda et al., 1996 |
| Topotecan + Carboplatin | as above | as above | as above |
intravenous (i.v.), subcutaneous (s.c.), intraperitoneal (i.p.).
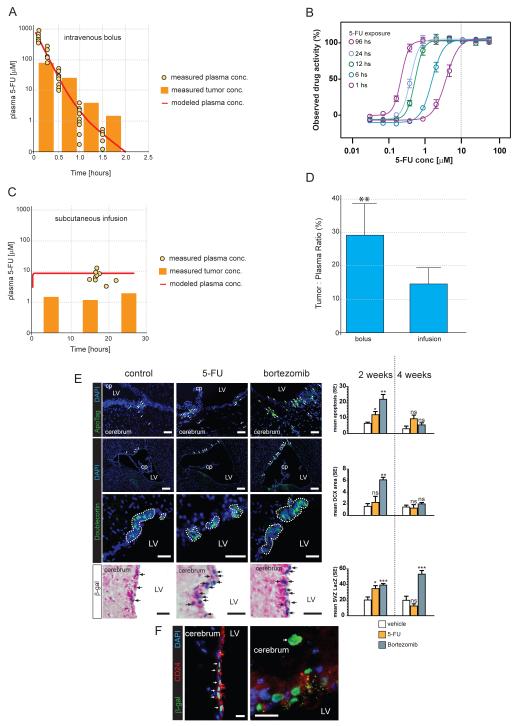
In keeping with the relative resistance of ependymoma to chemotherapies and the results of our HTS, carboplatin, topotecan, irinotecan and lapatinib were all inactive against mEPEphb2-LUC brain tumors (Figure 6). In stark contrast, both bortezomib and intravenous 5-FU halved the rate of mEPEphb2-LUC tumor growth and significantly prolonged the survival of mice harbouring these tumors. These data support our HTS as a relatively accurate tool for predicting in vivo drug efficacy. In contrast to intravenous 5-FU, this drug was inactive when delivered subcutaneously (Figure 6). To test if pharmacokinetic differences might account for the variation in efficacy between intravenous and subcutaneous administered 5-FU, we allowed mEPEphb2-LUC tumors to form around microdialysis catheters in the brains of recipient mice and measured simultaneously the concentration of 5-FU in the plasma and tumor following intravenous or subcutaneous drug administration (Supplemental Experimental Procedure). Intravenous 5-FU produced a concentration in brain tumors that peaked at 800 μM ±79S.D., and remained above 10μM for approximately 1 hour: an exposure profile shown by in vitro wash-out experiments to kill 90% of mEPEphb2 cells (Figures 7 A and B). In contrast, subcutaneous infusions of 5-FU at our ‘trial’ dose of 1.03 mg/kg/hour failed to generate detectable tumor drug levels (data not shown). Experiments using the maximum amount of 5-FU deliverable via Alzet pumps (13 mg/kg/hour) maintained 5-FU tumor concentrations of ~10 μM for over 24 hours, but this schedule was unacceptably toxic (≥20% weight loss) precluding assessment of in vivo therapeutic efficacy. Together these data identify bortezomib and 5-FU as potential treatments of ependymoma and indicate that the mode of administration of 5-FU should be given careful consideration when planning the treatment of ependymoma.
Figure 7. Pharmacokinetics and in vivo toxicity of 5-FU.
A. Concurrent measures of intratumoral and plasma 5-FU concentrations in 10 mice that each received a single intravenous injection of 75mg/kg of drug. B. In vitro washout studies of 5-FU activity against mEPEphb2 cells. Cells were incubated for the indicated time period with 5-FU and then to a total of 96 hours without drug. Dotted line denotes the concentration maintained in mEPEphb2 tumors in the brain for at least 1 hour following bolus administration. C. Concurrent measures of intratumoral and plasma 5-FU concentrations in 10 mice that received 13 mg/kg/hour of 5-FU via Alzet pumps. D. Percentage tumor:plasma AUC ratio of 5-FU delivered by 75mg/kg bolus injection and 13 mg/kg/hour infusion (**, p<0.005). E. Left: Top panel shows ApoTag stained apoptotic cells in the lateral SVZ of Prom1+/C-L mice following two weeks of vehicle control, 5-FU (75mg/kg bolus injection) or bortezomib treatment. Middle panels show low and high power images respectively of DCX+ neuroblast rests in the SVZ of the same mice. Bottom panel shows LacZ staining of the corresponding mice. Arrows indicate cells positive for the corresponding stain, dotted lines enclose DCX+ areas. Scale bar=50 μm, cp=choroid plexus, LV=lateral ventricle. Right: Graphs to the right report the patterns of for the corresponding stain in all three mice at each time point (*=p<0.05; ***=p<0.0005 relative to vehicle controls). F. Co-immunofluorescence of nuclear β-galactosidase expressed from the modified Prom1+/C-L locus and CD24. Left panel shows Prom1+/CD24+ ciliated ependymal cells. Panel to the right shows a Prom1+/CD24 cell (arrow) within the SVZ. Scale bar=20 μm.
HTS predicts patterns of 5-FU and bortezomib NSC toxicity in vivo
Our HTS was designed to predict the NSC toxicity of compounds as well as their efficacy against ependymoma. Since this information could prove extremely useful when prioritizing agents for clinical trials we sought to test in vivo the prediction from our HTS, that bortezomib would be relatively more toxic than 5-FU to NSCs (Figure 2 and 3).
Assessing the relative toxicity of drugs in vivo is complex because it is difficult to regulate drug levels in tissues, and tissue damage can involve multiple cell types. Nevertheless, we reasoned that the schedules of 5-FU and bortezomib that displayed equivalent efficacy against ependymomas in the brain (Figure 6, difference in median survival P=0.1 and no significant difference in tumor growth at anytime point) are clinically meaningful schedules for comparative studies of NSC toxicity. We treated non-tumor bearing mice with therapeutically equivalent schedules of 5-FU, bortezomib, or vehicle control, and after two or four weeks of therapy, measured the rate of apoptosis, and extent of doublecortin (DCX) positive neuroblast rests in the subventricular zone (SVZ; Figure 7E). The accumulation of apoptotic neuroblasts is an established feature of ischemic, chemotherapeutic, and genetic damage to the SVZ (Baker et al., 2006; Brown et al., 2003; Carlen et al., 2009; Dietrich et al., 2006; Jin et al., 2010). To provide an additional measure of the cellular response to drugs we used Prom1+/C-L mice in our studies: these mice express the LacZ reporter from the Prom1 locus in NSCs, neuroblasts, and ependymal cells (Coskun et al., 2008; Kriegstein and Alvarez-Buylla, 2009; Zhu et al., 2009).
Following two weeks of therapy, the number of apoptotic cells in the SVZ of 5-FU and bortezomib treated mice was double (12.0±1.7 SEM, P<0.05) and more than triple (22.2±5.1 SEM, P<0.005) that of controls (6.3±1.2 SEM), respectively (Figure 7E). Bortezomib also quadrupled the area of DCX+ neuroblast rests in the SVZ (6.0 μm2±0.6 per μm ventricular surface vs. control=1.5 μm2±0.6 per μm ventricular surface; P<0.005); but 5-FU therapy had no significant impact on DCX labelling (2.2 μm2±1.1 per μm ventricular surface). Both 5-FU (35.1%±3.3 SEM, P<0.05) and bortezomib (39.2%±2.8 SEM, P<0.005) increased the number of Prom1+ cells in the SVZ at two weeks relative to controls (20.9±2.4 SEM; Figure 7E). Following four weeks of therapy, apoptotic and DCX+ labelling in the SVZ of both 5-FU and bortezomib treated mice returned to control levels (Figure 7E); however, Prom1+ cell numbers continued to rise in the SVZ of bortezomib treated mice, reaching >2.5 times that of controls by four weeks (57.8%±4.0 SEM vs. control=19.4%±3.1 SEM, P<0.0005; Figure 7E). These cells included a mix of Prom1+/CD24+ ependymal cells, as well as a large number of Prom1+/CD24− cells that likely represent NSCs and neuroblasts (Figure 7F). These data strongly suggest that bortezomib is more damaging to the SVZ niche than therapeutically equivalent doses of 5-FU, and provide preliminary evidence that our HTS might be useful for predicting the NSC toxicity of potential ependymoma therapies. Further work is required to fully characterize the expanded Prom1+ SVZ cell population in drug treated mice, and to determine the clinical significance of histologic SVZ damage.
DISCUSSION
The approach described here should significantly advance the efficiency and speed with which we discover and develop treatments for rare cancers and cancer subtypes. Studies of genetic mouse models have uncovered treatments for some brain tumors (Romer et al., 2004; Rudin et al., 2009); but a lack of ependymoma models has precluded similar efforts for this disease. We describe an integrated, multi-platform, in vitro and in vivo drug screen of an accurate mouse model of ependymoma (mEPEphb2); identifying the IGF, EGF and centrosome cycle pathways as key candidate regulators of subtype-D tumors, as well as several treatment leads for the disease. We believe that these treatments are significantly enriched for drugs that will ultimately prove effective in patients because mEPEphb2 tumors reproduce the histology, ultrastructure, and transcriptome of subtype-D ependymoma with remarkable fidelity (Johnson et al., 2010).
Ependymomas retain many of the biological properties of their parent NSCs (Johnson et al., 2010). Therefore, many potent anti-ependymoma drugs may also be neurotoxic. This notion is supported by the results of our HTS, in which the majority of active agents, including molecular targeted therapies, were ‘equipotent’ against ependymoma and NSCs. Although similarities between ependymoma and NSCs may mean that cancer-selective agents are rare, our HTS system can identify these drugs. This represents a significant advance for clinical trial design. For example, ependymoma-selective agents (e.g., 5-FU) may be paired with effective but less selective drugs, to maximize efficacy while minimizing overlapping toxicity. Preclinical optimization of appropriate drug combinations for clinical trial in rare cancers is especially important, since the small patient populations place severe constraints on the number of clinical studies that can be conducted.
It is important to note that mEPEphb2 tumors model just one (subtype-D) of nine possible subtypes of human ependymoma (Johnson et al., 2010). Therefore, some drugs identified in the current study may retain ‘subtype-D’ specificity in humans, rather than displaying broad anti-ependymoma activity; however this is a strength rather than a weakness of our approach. Although many human cancers have been carved up into distinct subtypes, these are not usually considered in the clinical trial design because we have lacked the means to predict their sensitivity to drugs. Consequently, clinical trials that fail to recruit adequate numbers of treatment-sensitive patients run the risk of rejecting useful drugs. Accurate mouse models of genomic subtypes of cancers present the exciting opportunity to model human cancer heterogeneity during pre-clinical drug development. To this end, we are using the same methodology employed to produce mEPEphb2 tumors to generate mouse models of the remaining eight ependymoma subtypes. Together with our integrated in vitro and in vivo screening approach, this battery of models should allow us to complete in months, numerous single and multi-drug pre-clinical trials that would take decades to conduct in the clinic. Drugs prioritized through this approach could then be passed to definitive clinical trials.
The active agents identified in our study not only provide therapeutic leads for the clinic but also insights into disease biology. Specifically, our combined HTS and kinome-wide binding assays of active kinase inhibitor scaffolds, have unmasked the IGF signaling and centrosome cycle pathways as regulators of subtype-D ependymoma. Further work will be required to define the aberrant function and clinical significance of these pathways, but studies of normal NSCs strongly suggest that these pathways are likely to be important in ependymoma. Lehtinen and colleagues showed recently that the CSF provides a proliferative niche for forebrain NSCs that includes IGF2 as a major constituent. Specifically, they demonstrated that IGF2 present in the CSF is bound by IGF1R expressed in the apical membranes of NSCs, stimulating cell proliferation (Lehtinen et al., 2011). The vast majority of ependymomas arise directly adjacent to the ventricular system, most likely from NSCs of the SVZ (Johnson et al., 2010; Kleihues et al., 2002; Taylor et al., 2005). Furthermore, we show here that IGF1R is upregulated in mEPEphb2 cells relative to their parental NSCs, and that IGF2-IGF1R signaling in these cells is blocked by drugs that inhibit their proliferation.
Considerable evidence also points to the centrosome cycle as a critical regulator of NSC polarity, self-renewal and proliferation (Lesage et al., 2010). In this regard, Wang and colleagues showed that the centrioles are preferentially inherited during the asymmetric division of mouse NSCs, such that the renewed daughter inherits the maternal centriole while the new centrosome is inherited by the differentiating daughter neuroblast (Wang et al., 2009). Many of the inhibitors the we identified as active against mEPEphb2 cells and NSCs, target kinases that regulate critical steps in this process including centrosome duplication e.g., CDK2 and TTK; spindle orientation and centrosome separation e.g., PLK1; and spindle maintenance e.g., GAK. Thus the centrosome cycle is intimately related to NSC fate and may well be disrupted and targetable in ependymoma. We are currently working to determine the roles of PIP5K1C and YSK4 kinases in NSCs and mEPEphb2 cells that were also identified in our kinome-wide binding assays but are less well understood. Notably, PIP5K1C that bound three of four kinase scaffolds in our screen, maps to a focal amplicon that we observed in human cerebral ependymomas (19p13.3), and has been reported to maintain stem cell proliferation and inhibit neuronal differentiation (Johnson et al., 2010; Wang et al., 2007; Yu et al., 2011). Thus this kinase is of particular interest as a potential therapeutic target in ependymoma.
Our study also emphasizes the value of HTS for drug retooling. 5-FU is active against glioblastoma but this drug has never been tested formally in patients with ependymoma (Grunda et al., 2010). Indeed, in the absence of the evidence provided in the current study, it is unlikely that an early generation chemotherapeutic like 5-FU would ever be selected for clinical trial against ependymoma. Therefore, HTS approaches provide us with the opportunity to rationally re-purpose cancer drugs. Well characterized and accurate models should improve the efficiency and the confidence with which we do this. When these studies are coupled with comprehensive pharmacokinetic studies, these models can also help determine the most appropriate mode of administration of re-purposed drugs. Concurrent measures of brain tumor and plasma 5-FU levels in our model suggest that bolus administration might be a more effective way to deliver the drug to ependymoma. Importantly, we are now using these preclinical response and pharmacokinetic models to test combinations of drugs identified as most active in our single agent HTS and in vivo studies. This includes combinations of centrosome cycle or IGF inhibitors, with conventional cytotoxic agents such as 5-FU. We envisage that continued pharmacokinetic and response assessments of these combination therapies will allow us to design the optimal schedule for translation to the clinic.
In summary, we describe an integrated, multi-platform in vitro and in vivo HTS of ependymoma that identifies a series of biological insights and potential therapies for clinical development. Our mouse model allows both testing of drug efficacy in a specific genomic subtype of the disease, and concurrent assessment of toxicity to the parental normal NSC. The development of additional mouse models of ependymoma subtypes should allow further comprehensive preclinical assessment of therapies for clinical trials tailored to all disease subtypes.
EXPERIMENTAL PROCEDURES
Isolation and culture of mouse NSCs and mouse ependymoma cells
mEPEphb2 cells, mBTNICD1 cells, mouse embryonic cerebral NSCwt and NSCnull were isolated and cultured in supplemented neurobasal medium exactly as described previously (Johnson et al., 2010). mEPEphb2 cells were tagged with luciferase by transduction with an MSCV-Luc-IRES-YFP retrovirus. All animal studies, were approved by the St Jude Children’s Research Hospital Animal Care and Usage Committee and performed in full compliance with regulatory standards.
HTS
The HTS approach is described in detail in Supplemental Experimental Procedures. Briefly, for primary screening cells were seeded in 30 μl of neurobasal medium in each well of 384-well plates (Corning) using an automated plate filler (Wellmate, Matrix). After 24 hours, 25 nL of solution containing appropriate compounds were pin transferred into the 384-well plates resulting in ~8.3 μM final drug concentration. Each plate also included DMSO only negative controls and cyclohexamide single point (0.5μM) and dose-response (0.5 μM-0.01 nM) positive controls. Cell number was determined in each well using the Cell Titer Glo reagent (Promega) and read in an automated Envision plate reader (Perkin-Elmer) after 96 hours incubation. Luminescence data were normalized by log10 transformation and the percentage inhibition = 100 × (sample result − negative control mean)/(positive control mean − negative control mean) calculated. Secondary screens were conducted in a similar manner although compounds were applied in a dilution series (8.3 μM-0.5nM final concentration) and repeated in triplicate. All data processing and visualization was performed using custom programs written in the Pipeline Pilot platform (Accelrys, v.7.0.1) and the R program. The R drc package was used to fit sigmoidal curves and ROC statistics computed using the R rocr package. The quality of the primary screening strategy was assessed by multiple methods including ROC analysis, Z-prime, and other screening quality metrics (see Supplemental Experimental Procedures).
Kinome-wide competitive binding assays
Kinome-wide binding assays were completed for kinase inhibitor scaffolds using KINOMEscan technology through DiscoveRx. Briefly, KINOMEscan™ is based on a competition binding assay that quantitatively measures the ability of a compound to compete with an immobilized, active-site directed ligand. The assay is performed by combining three components: DNA-tagged kinase; immobilized ligand; and a test compound. The ability of the test compound to compete with the immobilized ligand is measured via quantitative PCR of the DNA tag.
Ependymoma allografts
1.5 × 106 mEPEphb2-LUC cells resuspended in 5 μl of Matrigel (BD BioScience) were implanted into the cerebral cortex of 6-8 week old CD-1 nu/nu immunocompromised mice exactly as described (Johnson et al., 2010). Mice were subject to daily clinical assessment of neurological function and weekly bioluminescence. Briefly, animals were injected with 200μl of an aqueous solution of D-luciferin (15 mg/ml, Caliper Life Sciences) approximately 5 minutes prior to being imaged using an IVIS200 in vivo imaging system (Xenogen, Caliper Life Sciences). A pseudocolour image representing light intensity was generated and the relative light intensity (photons/second) from each mouse was quantified using LivingImage 4.0 software (Xenogen). For drug efficacy studies, agents were administered to mice beginning seven days post implantation when tumor luminescence ranged from 1.7-8.0×107 photons/sec. Mice displaying signs of excessive morbidity or toxicity (>20% weight loss) were euthanized.
Animals were treated with compounds as described in Table 1 and all drugs with the exception of lapatinib were acquired as patient preparations from the Pharmacy at St Jude Children’s Research Hospital. 5FU, topotecan and carboplatin were diluted to the required concentration in sterile water whilst bortezomib and irinotecan were diluted in sterile PBS. Lapatinib (LC labs) was suspended in a solution of 0.5% hydroxypropylmethylcellulose/0.1% Tween 80 and administered by gavage.
5FU pharmacokinetic studies
CD-1 nude mice aged ≥ 8 weeks (23 to 28 g) were studied. Mice received a bolus tail vein injection of 5-FU (75 mg/kg) and blood samples collected at 5, 15, 30, 60, and 90 min post injection. For infusion studies, mice received an infusion of 5-FU (13 mg/kg/hour) via subcutaneous Alzet pumps (model 2001D; Cupertino, CA) and blood samples were taken at 24, 48, and 72 hours post pump implantation. 5-FU levels were determined in blood samples using a modified previously described HPLC method (Alsarra and Alarifi, 2004). Penetration of 5-FU into orthotopically implanted mEPEphb2 brain tumors following intravenous bolus or subcutaneous infusion was assessed using a previously published microdialysis technique (Zhuang et al., 2006).
In vivo assessment of drug toxicity
Mice where Prom1 expressing cells are marked with LacZ (Zhu et al., 2009) were treated with 5FU or Bortezomib either chronically (4 weeks) or acutely (two doses). The brains of these mice were fixed for frozen sectioning and processed as follows by investigators blinded to treatment group. Sagittal sections spanning the entire thickness of each brain were reviewed and three sections selected from equivalent positions in each brain representing the medial, mid and lateral aspects of the lateral ventricle. The three sections from each of the three points in the lateral ventricle were stained for LacZ, ApoTag or DCX. LacZ staining was performed and counterstained with nuclear-fast red as described (Zhu et al., 2009). Apoptosis within the SVZ was measured using the Apoptag fluorescein in situ apoptosis detection kit (Millipore). DCX immunofluorescence was performed using standard methods. Tiled images of the entire lateral ventricle were generated using a Zeiss microscope (Axio Imager M2) and processed using AxioVision Rel 4.8. Antibodies used include DCX (Santa Cruz #sc-8066); CD24 (BD #557436) and LacZ (β-galactosidase, MP biomedicals #55976).
Supplementary Material
Significance.
Trial therapies for rare cancers are usually selected empirically and often fail. The poor efficiency of this process is limited further by a lack of accurate preclinical models, and small patient numbers that may include distinct subtypes. More rational, evidence-based approaches to select and prioritize trial treatments are needed. Using HTS, kinome-wide binding assays and in vivo efficacy studies we interrogated an accurate mouse model of a specific subtype of human ependymoma. This comprehensive approach identified kinases not known to maintain ependymoma, and several treatment leads with predicted NSC toxicity for immediate clinical translation. This approach holds significant promise to prioritize therapies for clinical trials among patients with specific subtypes of cancer.
HIGHLIGHTS.
In vitro and in vivo study to uncover cancer subtype specific therapies.
Neural stem cells and daughter cancer cells co-screened to predict toxicity.
Insulin and centrosome cycle pathways identified as ependymoma regulators.
Molecular targeted and cytotoxic therapies identified for rapid clinical translation.
ACKNOWLEDGEMENTS
R.J.G. holds the Howard C. Schott Research Chair from the Malia’s Cord Foundation. R.K.G holds the Robert J. Ullrich Chair in Chemical Biology and Therapeutics. This work was supported by grants from the National Institutes of Health (R.J.G., R01CA129541, P01CA96832 and P30CA021765), and the Collaborative Ependymoma Research Network (R.J.G., R.K.G., C.F.S., W.K.Y., W.P.) and by the American Lebanese Syrian Associated Charities. We are grateful to the staff of the Hartwell Center for Bioinformatics and Biotechnology, the AIC and the ARC at St Jude Children’s Research Hospital for technical assistance. Figure 4A kinome illustration reproduced courtesy of Cell Signaling Technology,inc. (www.cellsignal.com).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
REFERENCES
- Adams J. The proteasome: a suitable antineoplastic target. Nat Rev Cancer. 2004;4:349–360. doi: 10.1038/nrc1361. [DOI] [PubMed] [Google Scholar]
- Aguirre A, Rubio ME, Gallo V. Notch and EGFR pathway interaction regulates neural stem cell number and self-renewal. Nature. 2010;467:323–327. doi: 10.1038/nature09347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alsarra IA, Alarifi MN. Validated liquid chromatographic determination of 5-fluorouracil in human plasma. J Chromatogr B Analyt Technol Biomed Life Sci. 2004;804:435–439. doi: 10.1016/j.jchromb.2004.01.043. [DOI] [PubMed] [Google Scholar]
- Baker KL, Daniels SB, Lennington JB, Lardaro T, Czap A, Notti RQ, Cooper O, Isacson O, Frasca S, Conover JC. Neuroblast protuberances in the subventricular zone of the regenerative MRL/MpJ mouse. The Journal of Comparative Neurology. 2006;498:747–761. doi: 10.1002/cne.21090. [DOI] [PubMed] [Google Scholar]
- Berry DA, Cronin KA, Plevritis SK, Fryback DG, Clarke L, Zelen M, Mandelblatt JS, Yakovlev AY, Habbema JD, Feuer EJ. Effect of screening and adjuvant therapy on mortality from breast cancer. N Engl J Med. 2005;353:1784–1792. doi: 10.1056/NEJMoa050518. [DOI] [PubMed] [Google Scholar]
- Bomgaars L, Kerr J, Berg S, Kuttesch J, Klenke R, Blaney SM. A phase I study of irinotecan administered on a weekly schedule in pediatric patients. Pediatr Blood Cancer. 2006;46:50–55. doi: 10.1002/pbc.20355. [DOI] [PubMed] [Google Scholar]
- Bouffet E, Foreman N. Chemotherapy for intracranial ependymomas. Childs Nerv Syst. 1999;15:563–570. doi: 10.1007/s003810050544. [DOI] [PubMed] [Google Scholar]
- Brown JP, Couillard-Despres S, Cooper-Kuhn CM, Winkler J, Aigner L, Kuhn HG. Transient expression of doublecortin during adult neurogenesis. J Comp Neurol. 2003;467:1–10. doi: 10.1002/cne.10874. [DOI] [PubMed] [Google Scholar]
- Carlen M, Meletis K, Goritz C, Darsalia V, Evergren E, Tanigaki K, Amendola M, Barnabe-Heider F, Yeung MSY, Naldini L, et al. Forebrain ependymal cells are Notch-dependent and generate neuroblasts and astrocytes after stroke. Nat Neurosci. 2009;12:259–267. doi: 10.1038/nn.2268. [DOI] [PubMed] [Google Scholar]
- Coskun V, Wu H, Blanchi B, Tsao S, Kim K, Zhao J, Biancotti JC, Hutnick L, Krueger RC, Jr., Fan G, et al. CD133+ neural stem cells in the ependyma of mammalian postnatal forebrain. Proc Natl Acad Sci U S A. 2008;105:1026–1031. doi: 10.1073/pnas.0710000105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cowan CR, Hyman AA. Cyclin E-Cdk2 temporally regulates centrosome assembly and establishment of polarity in Caenorhabditis elegans embryos. Nat Cell Biol. 2006;8:1441–1447. doi: 10.1038/ncb1511. Epub 2006 Nov 1419. [DOI] [PubMed] [Google Scholar]
- Dietrich J, Han R, Yang Y, Mayer-Proschel M, Noble M. CNS progenitor cells and oligodendrocytes are targets of chemotherapeutic agents in vitro and in vivo. Journal of Biology. 2006;5:22. doi: 10.1186/jbiol50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Douillard JY, Cunningham D, Roth AD, Navarro M, James RD, Karasek P, Jandik P, Iveson T, Carmichael J, Alakl M, et al. Irinotecan combined with fluorouracil compared with fluorouracil alone as first-line treatment for metastatic colorectal cancer: a multicentre randomised trial. Lancet. 2000;355:1041–1047. doi: 10.1016/s0140-6736(00)02034-1. [DOI] [PubMed] [Google Scholar]
- Geoerger B, Gaspar N, Opolon P, Morizet J, Devanz P, Lecluse Y, Valent A, Lacroix L, Grill J, Vassal G. EGFR tyrosine kinase inhibition radiosensitizes and induces apoptosis in malignant glioma and childhood ependymoma xenografts. Int J Cancer. 2008;123:209–216. doi: 10.1002/ijc.23488. [DOI] [PubMed] [Google Scholar]
- Gilbert M, Ruda R, Soffietti R. Ependymomas in Adults. Current Neurology and Neuroscience Reports. 2010;10:240–247. doi: 10.1007/s11910-010-0109-3. [DOI] [PubMed] [Google Scholar]
- Gilbertson RJ, Bentley L, Hernan R, Junttila TT, Frank AJ, Haapasalo H, Connelly M, Wetmore C, Curran T, Elenius K, Ellison DW. ERBB Receptor Signaling Promotes Ependymoma Cell Proliferation and Represents a Potential Novel Therapeutic Target for This Disease. Clin Cancer Res. 2002;8:3054–3064. [PubMed] [Google Scholar]
- Gorlick R, Kolb EA, Houghton PJ, Morton CL, Phelps D, Schaiquevich P, Stewart C, Keir ST, Lock R, Carol H, et al. Initial testing (stage 1) of lapatinib by the pediatric preclinical testing program. Pediatr Blood Cancer. 2009;53:594–598. doi: 10.1002/pbc.21989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grunda JM, Fiveash J, Palmer CA, Cantor A, Fathallah-Shaykh HM, Nabors LB, Johnson MR. Rationally designed pharmacogenomic treatment using concurrent capecitabine and radiotherapy for glioblastoma; gene expression profiles associated with outcome. Clin Cancer Res. 2010;16:2890–2898. doi: 10.1158/1078-0432.CCR-09-3151. Epub 2010 May 2811. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gupta PB, Onder TT, Jiang G, Tao K, Kuperwasser C, Weinberg RA, Lander ES. Identification of selective inhibitors of cancer stem cells by high-throughput screening. Cell. 2009;138:645–659. doi: 10.1016/j.cell.2009.06.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Houghton JA, Cheshire PJ, Hallman JD, 2nd, Lutz L, Luo X, Li Y, Houghton PJ. Evaluation of irinotecan in combination with 5-fluorouracil or etoposide in xenograft models of colon adenocarcinoma and rhabdomyosarcoma. Clin Cancer Res. 1996;2:107–118. [PubMed] [Google Scholar]
- IMPACT. International Multicentre Pooled Analysis of Colon Cancer Trials (IMPACT) investigators Efficacy of adjuvant fluorouracil and folinic acid in colon cancer. Lancet. 1995;345:939–944. [PubMed] [Google Scholar]
- Jin K, Wang X, Xie L, Mao XO, Greenberg DA. Transgenic ablation of doublecortin-expressing cells suppresses adult neurogenesis and worsens stroke outcome in mice. Proc Natl Acad Sci U S A. 2010;107:7993–7998. doi: 10.1073/pnas.1000154107. Epub 2010 Apr 7912. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnson RA, Wright KD, Poppleton H, Mohankumar KM, Finkelstein D, Pounds SB, Rand V, Leary SE, White E, Eden C, et al. Cross-species genomics matches driver mutations and cell compartments to model ependymoma. Nature. 2010;466:632–636. doi: 10.1038/nature09173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kadota RP, Stewart CF, Horn M, Kuttesch JF, Jr., Burger PC, Kepner JL, Kun LE, Friedman HS, Heideman RL. Topotecan for the treatment of recurrent or progressive central nervous system tumors - a pediatric oncology group phase II study. J Neurooncol. 1999;43:43–47. doi: 10.1023/a:1006294102611. [DOI] [PubMed] [Google Scholar]
- Kamano T, Mikami Y, Shirasaka T. Continuous infusion of 5-fluorouracil plus low-dose cisplatin in tumor-bearing mice. Anticancer Drugs. 1997;8:632–636. doi: 10.1097/00001813-199707000-00012. [DOI] [PubMed] [Google Scholar]
- Kasbek C, Yang CH, Yusof AM, Chapman HM, Winey M, Fisk HA. Preventing the degradation of mps1 at centrosomes is sufficient to cause centrosome reduplication in human cells. Mol Biol Cell. 2007;18:4457–4469. doi: 10.1091/mbc.E07-03-0283. Epub 2007 Sep 4455. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kimura H, Ng JM, Curran T. Transient inhibition of the Hedgehog pathway in young mice causes permanent defects in bone structure. Cancer Cell. 2008;13:249–260. doi: 10.1016/j.ccr.2008.01.027. [DOI] [PubMed] [Google Scholar]
- Kleihues P, Louis DN, Scheithauer BW, Rorke LB, Reifenberger G, Burger PC, Cavenee WK. The WHO classification of tumors of the nervous system. J Neuropathol Exp Neurol. 2002;61:215–225. doi: 10.1093/jnen/61.3.215. discussion 226-219. [DOI] [PubMed] [Google Scholar]
- Kriegstein A, Alvarez-Buylla A. The Glial Nature of Embryonic and Adult Neural Stem Cells. Annu Rev Neurosci. 2009;32:149–184. doi: 10.1146/annurev.neuro.051508.135600. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lapidot T, Sirard C, Vormoor J, Murdoch B, Hoang T, Caceres-Cortes J, Minden M, Paterson B, Caligiuri MA, Dick JE. A cell initiating human acute myeloid leukaemia after transplantation into SCID mice. Nature. 1994;367:645–648. doi: 10.1038/367645a0. [DOI] [PubMed] [Google Scholar]
- Lehtinen MK, Zappaterra MW, Chen X, Yang YJ, Hill AD, Lun M, Maynard T, Gonzalez D, Kim S, Ye P, et al. The cerebrospinal fluid provides a proliferative niche for neural progenitor cells. Neuron. 2011;69:893–905. doi: 10.1016/j.neuron.2011.01.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lens SM, Voest EE, Medema RH. Shared and separate functions of polo-like kinases and aurora kinases in cancer. Nat Rev Cancer. 2010;10:825–841. doi: 10.1038/nrc2964. Epub 2010 Nov 2024. [DOI] [PubMed] [Google Scholar]
- Lesage B, Gutierrez I, Marti E, Gonzalez C. Neural stem cells: the need for a proper orientation. Curr Opin Genet Dev. 2010;20:438–442. doi: 10.1016/j.gde.2010.04.013. Epub 2010 May 2025. [DOI] [PubMed] [Google Scholar]
- Longley DB, Harkin DP, Johnston PG. 5-fluorouracil: mechanisms of action and clinical strategies. Nat Rev Cancer. 2003;3:330–338. doi: 10.1038/nrc1074. [DOI] [PubMed] [Google Scholar]
- McGuire CS, Sainani KL, Fisher PG. Incidence patterns for ependymoma: a surveillance, epidemiology, and end results study. J Neurosurg. 2009;110:725–729. doi: 10.3171/2008.9.JNS08117. [DOI] [PubMed] [Google Scholar]
- Mendrzyk F, Korshunov A, Benner A, Toedt G, Pfister S, Radlwimmer B, Lichter P. Identification of Gains on 1q and Epidermal Growth Factor Receptor Overexpression as Independent Prognostic Markers in Intracranial Ependymoma. Clin Cancer Res. 2006;12:2070–2079. doi: 10.1158/1078-0432.CCR-05-2363. [DOI] [PubMed] [Google Scholar]
- Merchant TE, Li C, Xiong X, Kun LE, Boop FA, Sanford RA. Conformal radiotherapy after surgery for paediatric ependymoma: a prospective study. Lancet Oncol. 2009;10:258–266. doi: 10.1016/S1470-2045(08)70342-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Neal J, Sequist L. Exciting New Targets in Lung Cancer Therapy: ALK, IGF-1R, HDAC, and Hh. Current Treatment Options in Oncology. 2010;11:36–44. doi: 10.1007/s11864-010-0120-6. [DOI] [PubMed] [Google Scholar]
- Noatynska A, Panbianco C, Gotta M. SPAT-1/Bora acts with Polo-like kinase 1 to regulate PAR polarity and cell cycle progression. Development. 2010;137:3315–3325. doi: 10.1242/dev.055293. [DOI] [PubMed] [Google Scholar]
- Phuphanich S, Supko J, Carson K, Grossman S, Burt Nabors L, Mikkelsen T, Lesser G, Rosenfeld S, Desideri S, Olson J. Phase 1 clinical trial of bortezomib in adults with recurrent malignant glioma. Journal of Neuro-Oncology. 2010;100:95–103. doi: 10.1007/s11060-010-0143-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pui C-H, Relling MV, Downing JR. Acute Lymphoblastic Leukemia. New England Journal of Medicine. 2004;350:1535–1548. doi: 10.1056/NEJMra023001. [DOI] [PubMed] [Google Scholar]
- Richardson PG, Barlogie B, Berenson J, Singhal S, Jagannath S, Irwin D, Rajkumar SV, Srkalovic G, Alsina M, Alexanian R, et al. A Phase 2 Study of Bortezomib in Relapsed, Refractory Myeloma. New England Journal of Medicine. 2003;348:2609–2617. doi: 10.1056/NEJMoa030288. [DOI] [PubMed] [Google Scholar]
- Romer JT, Kimura H, Magdaleno S, Sasai K, Fuller C, Baines H, Connelly M, Stewart CF, Gould S, Rubin LL, Curran T. Suppression of the Shh pathway using a small molecule inhibitor eliminates medulloblastoma in Ptc1(+/−)p53(−/−) mice. Cancer Cell. 2004;6:229–240. doi: 10.1016/j.ccr.2004.08.019. [DOI] [PubMed] [Google Scholar]
- Rudin CM, Hann CL, Laterra J, Yauch RL, Callahan CA, Fu L, Holcomb T, Stinson J, Gould SE, Coleman B, et al. Treatment of Medulloblastoma with Hedgehog Pathway Inhibitor GDC-0449. N Engl J Med. 2009;2:2. doi: 10.1056/NEJMoa0902903. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Singh SK, Hawkins C, Clarke ID, Squire JA, Bayani J, Hide T, Henkelman RM, Cusimano MD, Dirks PB. Identification of human brain tumour initiating cells. Nature. 2004;432:396–401. doi: 10.1038/nature03128. [DOI] [PubMed] [Google Scholar]
- Stewart CF, Iacono LC, Chintagumpala M, Kellie SJ, Ashley D, Zamboni WC, Kirstein MN, Fouladi M, Seele LG, Wallace D, et al. Results of a phase II upfront window of pharmacokinetically guided topotecan in high-risk medulloblastoma and supratentorial primitive neuroectodermal tumor. J Clin Oncol. 2004;22:3357–3365. doi: 10.1200/JCO.2004.10.103. [DOI] [PubMed] [Google Scholar]
- Taniguchi E, Cho MJ, Arenkiel BR, Hansen MS, Rivera OJ, McCleish AT, Qualman SJ, Guttridge DC, Scott MP, Capecchi MR, Keller C. Bortezomib reverses a post-translational mechanism of tumorigenesis for patched1 haploinsufficiency in medulloblastoma. Pediatric Blood & Cancer. 2009;53:136–144. doi: 10.1002/pbc.21968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taylor MD, Poppleton H, Fuller C, Su X, Liu Y, Jensen P, Magdaleno S, Dalton J, Calabrese C, Board J, et al. Radial glia cells are candidate stem cells of ependymoma. Cancer Cell. 2005;8:323–335. doi: 10.1016/j.ccr.2005.09.001. [DOI] [PubMed] [Google Scholar]
- Thompson J, Zamboni WC, Cheshire PJ, Lutz L, Luo X, Li Y, Houghton JA, Stewart CF, Houghton PJ. Efficacy of systemic administration of irinotecan against neuroblastoma xenografts. Clin Cancer Res. 1997;3:423–431. [PubMed] [Google Scholar]
- Tonda ME, Heideman RL, Petros WP, Friedman HS, Murry DJ, Rodman JH. Carboplatin pharmacokinetics in young children with brain tumors. Cancer Chemother Pharmacol. 1996;38:395–400. doi: 10.1007/s002800050502. [DOI] [PubMed] [Google Scholar]
- Wagner LM, Perentesis JP, Reid JM, Ames MM, Safgren SL, Nelson MD, Jr., Ingle AM, Blaney SM, Adamson PC. Phase I trial of two schedules of vincristine, oral irinotecan, and temozolomide (VOIT) for children with relapsed or refractory solid tumors: a Children’s Oncology Group phase I consortium study. Pediatr Blood Cancer. 2010;54:538–545. doi: 10.1002/pbc.22407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang X, Tsai JW, Imai JH, Lian WN, Vallee RB, Shi SH. Asymmetric centrosome inheritance maintains neural progenitors in the neocortex. Nature. 2009;461:947–955. doi: 10.1038/nature08435. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang Y, Lian L, Golden JA, Morrisey EE, Abrams CS. PIP5KI gamma is required for cardiovascular and neuronal development. Proc Natl Acad Sci U S A. 2007;104:11748–11753. doi: 10.1073/pnas.0700019104. Epub 12007 Jul 11743. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Williamson MJ, Silva MD, Terkelsen J, Robertson R, Yu L, Xia C, Hatsis P, Bannerman B, Babcock T, Cao Y, Kupperman E. The relationship among tumor architecture, pharmacokinetics, pharmacodynamics, and efficacy of bortezomib in mouse xenograft models. Mol Cancer Ther. 2009;8:3234–3243. doi: 10.1158/1535-7163.MCT-09-0239. [DOI] [PubMed] [Google Scholar]
- Yu Y-L, Chou R-H, Chen L-T, Shyu W-C, Hsieh S-C, Wu C-S, Zeng H-J, Yeh S-P, Yang D-M, Hung S-C, Hung M-C. EZH2 Regulates Neuronal Differentiation of Mesenchymal Stem Cells through PIP5K1C-dependent Calcium Signaling. Journal of Biological Chemistry. 2011;286:9657–9667. doi: 10.1074/jbc.M110.185124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou B-BS, Zhang H, Damelin M, Geles KG, Grindley JC, Dirks PB. Tumour-initiating cells: challenges and opportunities for anticancer drug discovery. Nat Rev Drug Discov. 2009;8:806–823. doi: 10.1038/nrd2137. [DOI] [PubMed] [Google Scholar]
- Zhu L, Gibson P, Currle DS, Tong Y, Richardson RJ, Bayazitov IT, Poppleton H, Zakharenko S, Ellison DW, Gilbertson RJ. Prominin 1 marks intestinal stem cells that are susceptible to neoplastic transformation. Nature. 2009;457:603–607. doi: 10.1038/nature07589. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhuang Y, Fraga CH, Hubbard KE, Hagedorn N, Panetta JC, Waters CM, Stewart CF. Topotecan Central Nervous System Penetration Is Altered by a Tyrosine Kinase Inhibitor. Cancer Research. 2006;66:11305–11313. doi: 10.1158/0008-5472.CAN-06-0929. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.