Abstract
Parkinson's disease (PD) is a neurodegenerative disorder characterized by the loss of nigrostriatal dopaminergic neurons and the accumulation of alpha-synuclein. Both traumatic brain injury (TBI) and pesticides are risk factors for PD, but whether TBI causes nigrostriatal dopaminergic cell loss in experimental models and whether it acts synergistically with pesticides is unknown. We have examined the acute and long-term effects of TBI and exposure to low doses of the pesticide paraquat, separately and in combination, on nigrostriatal dopaminergic neurons in adult male rats. In an acute study, rats received moderate TBI by lateral fluid percussion (LFP) injury, were injected with saline or paraquat (10 mg/kg IP) 3 and 6 days after LFP, were sacrificed 5 days later, and their brains processed for immunohistochemistry. TBI alone increased microglial activation in the substantia nigra, and caused a 15% loss of dopaminergic neurons ipsilaterally. Paraquat increased the TBI effect, causing a 30% bilateral loss of dopaminergic neurons, reduced striatal tyrosine hydroxylase (TH) immunoreactivity more than TBI alone, and induced alpha-synuclein accumulation in the substantia nigra pars compacta. In a long-term study, rats received moderate LFP, were injected with saline or paraquat at 21 and 22 weeks post-injury, and were sacrificed 4 weeks later. At 26 weeks post injury, TBI alone induced a 30% bilateral loss of dopaminergic neurons that was not exacerbated by paraquat. These data suggest that TBI is sufficient to induce a progressive degeneration of nigrostriatal dopaminergic neurons. Furthermore, TBI and pesticide exposure, when occurring within a defined time frame, could combine to increase the PD risk.
Key words: alpha-synuclein, lateral fluid percussion, microglia, paraquat, Parkinson's disease, traumatic brain injury
Introduction
Parkinson's disease (PD) is an age-related neurological disorder of mixed etiology that affects more than 1% of the population over 65. PD is characterized by the degeneration of nigrostriatal dopaminergic neurons and the widespread accumulation of alpha-synuclein (Braak et al., 2002). The loss of nigrostriatal dopaminergic neurons results in the cardinal motor symptoms observed in PD patients, primarily akinesia, postural tremor, and rigidity. Thus although PD affects many neuronal systems and results in a wide range of symptoms, the particular vulnerability of nigrostriatal dopaminergic neurons to the disease process remains a defining aspect of the disorder. Risk factors associated with sporadic forms of PD include age, genetic polymorphisms, pesticide exposure, and according to some studies, traumatic brain injury (TBI) Bower et al., 1999; Braak et al., 2002; Costello et al., 2009; Farrer et al., 2001; Goldman et al., 2006; Polymeropoulos et al., 1997).
Each year some 1.4 million Americans suffer from TBI, resulting in over 200,000 hospitalizations (Langlois et al., 2006). In humans and laboratory animals, TBI can induce the accumulation of alpha-synuclein (Knoblach and Faden, 1998; Thompson et al., 2005a; Uryu et al., 2003), and a neuroinflammatory response (Csuka et al., 1999; Holmin and Hojeberg, 2004; Holmin et al., 1995), similar to what is observed in PD (Braak et al., 2002,2006; Gerhard et al., 2006; Imamura et al., 2003; McGeer et al., 1988). Lateral fluid percussion (LFP) injury, a model of experimental TBI (McIntosh et al., 1989; Thompson et al., 2005a), induces the presence of silver-stained and lipofuschin-containing neurons in the substantia nigra (SN) of adult rats (Hicks et al., 1996). However, the phenotype of the injured neurons was not determined, and no stereological evaluation of the number of nigrostriatal neurons was performed (Hicks et al., 1996). Therefore, although TBI has been linked to alterations of dopamine levels in the striatum (Bales et al., 2009), the effect of TBI on the cell bodies of the nigrostriatal dopaminergic neurons that are affected in PD remains unclear.
Recently, Goldman and associates (2006) linked TBI with an increased risk for PD in twins. However, not all epidemiological studies have been able to demonstrate a statistically significant link between TBI and PD; some studies show a strong correlation (Bower et al., 2003; Goldman et al., 2006; Taylor et al., 1999), while others do not (Bharucha et al., 1986; Rugbjerg et al., 2008; Werneck and Alvarenga, 1999). These observations suggest that the link between TBI and PD may be contingent upon exposure to other risk factors, with TBI being one element sensitizing neurons to other risk factors, as suggested in the “multiple hits” hypothesis of PD (Sulzer, 2007). Indeed, TBI has been linked to increased neuronal vulnerability to a subsequent insult (Bramlett et al., 1999; Jenkins et al., 1989).
Factors that may synergize with TBI to induce the risk of PD could include exposure to environmental pesticides, for example the herbicide paraquat (1,1′-dimethyl-4,4′-bipyridinium). Indeed, epidemiological studies have shown that paraquat exposure is a risk factor for PD (Hertzman et al., 1990; Liou et al., 1997; Semchuk et al., 1992). Furthermore, repeated administration of paraquat can induce nigrostriatal cell death in both mice and rats (Cicchetti et al., 2005; Fernagut et al., 2007; McCormack et al., 2002; Ossowska et al., 2005).
A first goal of this study was to test the hypothesis that mild TBI induced by LFP causes progressive degeneration of nigrostriatal dopaminergic neurons, and induces the neuropathological anomalies observed in PD, such as neuroinflammation and the accumulation of alpha-synuclein. A second goal was to determine whether LFP-induced TBI could sensitize nigrostriatal dopaminergic neurons to a sub-threshold dose of paraquat.
Methods
Animals
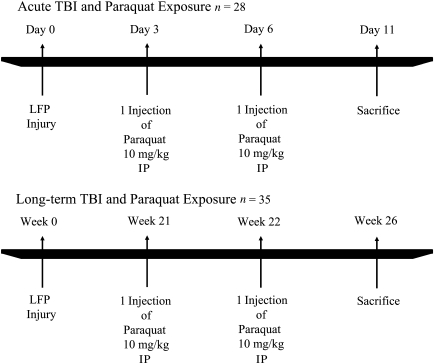
Sixty-three male Sprague Dawley rats 2–3 months of age were obtained from Charles River (San Diego, CA), and weighed 300–350 g at the time of LFP injury. Animals were separated into 2 cohorts or 4 groups each: naive-saline, TBI-saline, naive-paraquat, and TBI-paraquat. The first cohort was sacrificed 11 days post-injury, and the second cohort 26 weeks post injury (Fig. 1). Animal care was conducted in accordance with the United States Public Health Service Guide for the Care and Use of Laboratory Animals, and procedures were approved by the University of California–Los Angeles Institutional Animal Care and Use Committee.
FIG. 1.
Experimental time line of traumatic brain injury (TBI) and paraquat exposure (LFP, lateral fluid percussion).
Moderate lateral fluid percussion
The animals were anesthetized with 1.5–2.0 mL/min of isofluorane in 100% oxygen, placed in a stereotaxic frame, and prepped in a sterile fashion. Body temperature was kept constant at 36.6–38.0°C with a thermostatically controlled heating pad. A midline incision was made in the scalp, the temporalis muscle was retracted, and the cranium was exposed. A craniotomy 3.0 mm in diameter 3.0 mm posterior to the bregma and 6.0 mm lateral (left) of the midline was performed with a high-speed drill. The injury cap was secured with silicone adhesive, cyanoacrylate glue, and finally dental cement. After hardening, the cap was filled with 0.9% saline. The animals were removed from anesthesia and attached to the fluid percussion device. Once the animals responded to a toe pinch, the fluid pulse was administered. The fluid percussion device delivers a transient (21–23 msec) fluid pulse into the epidural space causing diffuse biomechanical deformation. Apnea and time of unconsciousness (as measured by withdrawal response to toe pinch assessed at 15-sec intervals until the response recovered) were recorded, and the injured animals were returned to anesthesia for removal of the cap and suturing of the scalp. The wounds were treated with 0.25% bupivacaine and the animals were removed from anesthesia and placed in a heated cage until recovery. All animals were monitored for up to 3 h after injury and returned to their home cages when stable. The severity of TBI was determined by direct physiological measures: the time of loss of consciousness (LOC) and time of apnea. These physiological measures have been shown to correlate with the fluid pulse pressure (atm) and severity (Giza et al., 2002; Prins et al., 1996).
The typical sham surgery for the LFP injury model of TBI is a 3-mm craniotomy, a calvarial defect. The sham surgery is identical to calvarial defect models employed to evaluate bone growth and wound healing (Aghaloo et al., 2006). Peripheral inflammation is well documented in bone defect models and plays an integral role in the bone healing process (Gerstenfeld et al., 2003; Giannoudis et al., 1999; Kalfas, 2001; Tsuji et al., 2006). Most recently Stokely and Orr (2008), in an effort to model non-penetrating head injuries, revealed that a parietal craniotomy or even scoring of the parietal bone surface resulted in an inflammatory response characterized by mast cell activation, increased cortical histamine, and the breakdown of the blood–brain barrier under the site of injury. These data suggest that the sham surgery is not an appropriate control due to its ability to injure the brain and induce inflammation. Consequently we chose to use naive animals as controls.
Intraperitoneal administration of paraquat
For the acute TBI study the paraquat regimen consisted of 2 IP injections of paraquat (10 mg/kg dissolved in 0.9% saline). Animals received the first paraquat injection 3 days post-injury, and the second injection 6 days post-injury, and were sacrificed 5 days following the last paraquat injection, 11 days post-injury (Fig. 1). This paraquat dose is one-fourth of that previously described to cause nigrostriatal cell death in adult rats (Cicchetti et al., 2005). Our objective was to use a paraquat regimen that alone would not induce nigrostriatal cell death, to assess the ability of experimental TBI to sensitize nigrostriatal dopaminergic neurons to a sub-threshold dose of the pesticide. This paraquat regimen did not result in mortality and the animals had normal weight gain.
For the long-term study, the paraquat injections were spaced by 7 rather than 3 days because older animals are more susceptible to the systemic toxic effects and/or nigrostriatal dopaminergic degeneration induced by paraquat (McCormack et al., 2002; Petrovic et al., 1986; Saint-Pierre et al., 2006; Thiruchelvam et al., 2003). Our goal was to maintain a subthreshold dose of paraquat to determine if experimental TBI could induce the long-term sensitization of nigrostriatal dopaminergic neurons to paraquat. Thus, at 21 weeks post-injury (8 months of age), the animals were exposed to paraquat (10 mg/kg in 0.9% saline) once a week for 2 weeks, and were sacrificed 4 weeks following the last paraquat injection (Fig. 1).
Tissue preparation
The animals were anesthetized with pentobarbital, transcardially perfused with phosphate-buffered saline (PBS), and then with 4% paraformaldehyde in PBS. Their brains were removed, cryoprotected in 30% sucrose at 4°C overnight, and stored at −80°C.
Immunohistochemistry with diaminobenzidine
Tissue sections received three 5-min washes in 0.1 M PBS to remove the cryoprotectant. To quench endogenous peroxidases, the sections were incubated for 15 min in 0.1 M PBS containing 0.5% H2O2, followed by three 5-min washes in 0.1 M PBS. Sections were then incubated for 1 h in a blocking solution containing 10% goat serum and 0.5% Triton-X 100 in 0.1 M PBS. The sections were incubated overnight with the primary antibody in 0.1 M PBS containing 2% goat serum and 0.5% Triton X-100 (anti-TH 1:600) (Pel Freeze, Rodgers, Arkansas); anti-alpha-synuclein 1:250 (BD Biosciences, San Jose, California); or anti-CD11b 1:200 (AbDSerotec, Raleigh, North Carolina). After incubation in the primary antibody, the sections received three 5-min washes in 0.1 M PBS to remove the primary antibody, followed by a 2-h incubation with the secondary antibody in 0.1 M PBS containing 2% goat serum and 0.5% Triton X-100 (anti-rabbit 1:1000, anti-mouse 1:250, anti-rat 1:500; all from MP Biomedicals, Solon, OH). The sections were then rinsed in three 5-min washes of 0.1 M PBS, and subsequently incubated in avidin-biotin complex for 45 min. The sections were washed again with three 5-min washes in 0.05 M Tris-buffered saline (TBS), followed by an incubation in 0.05 M TBS containing 3-3′-diaminobenzidine (DAB) and 0.3% H2O2 to reveal staining. The sections were washed with 0.1 M PBS to stop the DAB reaction, and then mounted onto gelatin-coated slides and cover-slipped with Eukitt mounting medium (Calibrated Instruments, Inc., Hawthorne, NY).
TH immunofluorescence
The sections received three 5-min washes in 0.1 M PBS to remove the cryoprotectant and were then incubated for 1 h in a blocking solution containing 10% goat serum and 0.5% Triton X-100 in 0.1 M PBS. The sections were then incubated overnight with the primary antibody in 0.1 M PBS containing 2% goat serum and 0.5% Triton X-100 (anti-TH 1:600). A 0.1 M PBS wash to remove the primary antibody was followed by 2 h of incubation in the secondary antibody in 0.1 M PBS containing 2% goat serum and 0.5% Triton X-100 (cy3 anti-rabbit 1:500; Chemicon, Temecula, CA). The sections were then washed in 0.1 M PBS to remove the secondary antibody. A small brush was used to quickly wash the individual sections in ddH2O prior to mounting on slides in order to remove excess salt. The sections were then air-dried for 24 h and stored at −20°C.
Quantification of TH immunofluorescence
The slides were scanned using an Agilent DNA microarray scanner (Agilent Techonologies Inc., Santa Clara, CA) at 10-μm resolution and analyzed with ImageJ software (version 1.38). TH immunofluorescence was quantified in the striatum at three levels: rostral (bregma 1.60), medial (bregma 0.20), and caudal (bregma −1.30; Lu et al., 2009).
Stereological analysis of nigrostriatal dopaminergic neurons and qualitative assessment of microglial activation
The absolute number of neurons in the substantia nigra pars compacta (SNpc) was estimated using the optical fractionator method. This unbiased sampling technique uses a stack of optical sections to determine the number of objects in a known fraction of a defined reference space (Mouton et al., 2002). In this study, the local neuronal estimate in each section was scaled to the total span of the SNpc. This method does not require reference volume estimates and is not affected by artifact changes during immunostaining. The SNpc was sampled every eighth section from its first appearance near the caudal portions of the mammillary bodies, past the region of the oculomotor nerve to its disappearance in the caudal SN. Following delineation of the SNpc with a 5× objective to ensure anatomical accuracy and an adequate number of sampling sites in the whole SNpc and subdivisions, counting was performed with a 40× objective. The dissector height was set at 16 μm, and guard zones of 2 μm ensured the exclusion of lost profiles on the top and bottom of the section sampled. The estimated total number of neurons in the substantia nigra was calculated based on the following formula: N=Q−×1/ssf×1/asf×t/h (West, 2002), where N is the estimate of the total number of cells, Q− is the number of objects counted, ssf is the section sampling fraction, asf is the area sampling fraction, and t/h is the actual section thickness divided by the height of the dissector. Two to three hundred objects (the whole SNc) were counted to generate the stereological estimates. Gundersen coefficients of error were less than 0.1.
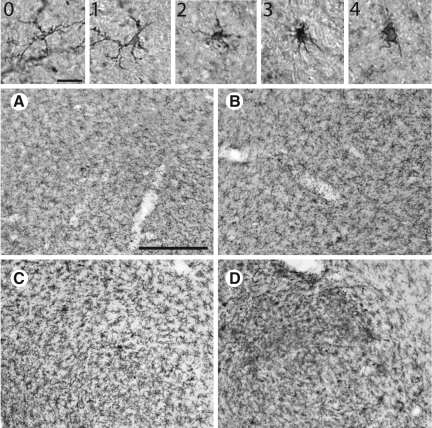
The quantification of microglia was performed with the fractionator method of unbiased stereology in three sections per animal containing the substantia nigra, at −4.40, −5.20, and −6.00 mm bregma. The fractionator method of unbiased stereology provides a method of systematic random sampling to generate population estimates within an area of interest. Unlike the optical fractionator method of unbiased stereology, the fractionator method does not estimate the absolute number of objects in a 3D structure of interest. After delineation of the SN with the 5× objective to ensure anatomical accuracy, counting of activated microglia was performed with a 40× objective. Stages of microglial activation were scored on a scale of 1–4, based on morphological parameters including cell body diameter and changes in the length of the processes: 1 corresponds to the early stages of microglial activation with retraction of processes, 2 to the thickening and further retraction process, 3 to the increase in cell body diameter and appearance of amoeboid morphology, and 4 to full amoeboid microglia.
In both cases, stereological sampling was performed with StereoInvestigator software (MicroBrightField, Colchester, VT) and a Leica DM-LB microscope with a Ludl XYZ motorized stage and z-axis microcator (MT12; Heidenheim, Traunreut, Germany).
Data analysis
Histopathological data were analyzed using a three-way analysis of variance (ANOVA), with injury, drug, and side as independent variables. Post-hoc analysis was performed with Fisher's least significant difference (LSD) test. Statistical analysis was performed with GB-STAT v8.0 (Dynamic Microsystems, Silver Springs, MD). For all statistical tests, the level of significance was set at p<0.05. Data are presented as mean±standard error of the mean (SEM).
Results
Acute TBI with or without paraquat administration
Moderate LFP was used to model a diffuse concussive injury in adult rats. As previously defined, a moderate LFP-induced TBI causes a loss of consciousness of 30–170 sec in adult rats (Giza et al., 2002; Prins et al., 1996). The mean time of LOC for all animals (n=35) was 80.29±5.45 sec (±SEM).
Acute TBI induces a loss of nigrostriatal dopaminergic neurons that is increased by exposure to paraquat
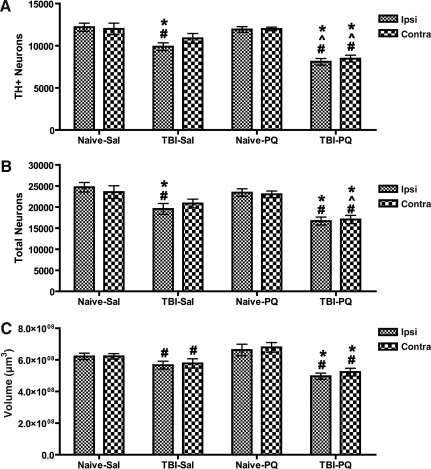
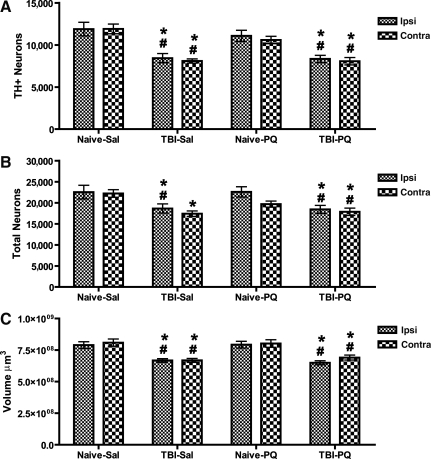
In order to determine whether this diffuse injury model of TBI induced rapid damage in dopaminergic neurons of the SNpc, which are progressively lost in PD, we first examined TH-positive (TH+) neurons in the SNpc 11 days after injury (Fig. 2A–D). Although visual examination did not reveal obvious damage to the substantia nigra after single or combined treatment, unbiased stereology, the most accurate method of estimating a neuronal population in a three-dimensional structure, revealed significant changes in the total number of TH+ nigrostriatal dopaminergic neurons and the total number of neurons (TH+ and TH−/Nissl+) in the SNpc following moderate TBI with or without paraquat exposure. The number of neurons in the SNpc was quantified both ipsilateral and contralateral to the side of injury. For the number of TH+ neurons in the SNc (Fig. 3A), we observed a main effect of injury [F(1,56)=69.32, p<0.0001], a main effect of drug [F(1,56)=12.10, p=0.0011], no effect of side [F(1,56)=0.90, p=0.3], and an injury×drug interaction [F(1,56)=9.26, p=0.0038]. Post-hoc analysis revealed that TBI alone resulted in a 15% reduction of TH+ neurons ipsilateral to the side of injury compared to naive animals with or without paraquat injection. Contralateral to the side of injury, TBI did not significantly reduce TH+ neurons. The combination of acute TBI and paraquat exposure resulted in a synergistic effect on the loss of nigrostriatal dopaminergic neurons, leading to a bilateral 30% loss of TH+ neurons ipsilateral and contralateral to the side of injury, a significant difference compared to the other groups, including TBI alone. As expected, the low dose of paraquat used in this study did not induce a loss of TH+ neurons in naive animals. Notably, the duration of apnea or unconsciousness was not correlated with TH+ cell loss (data not shown).
FIG. 2.
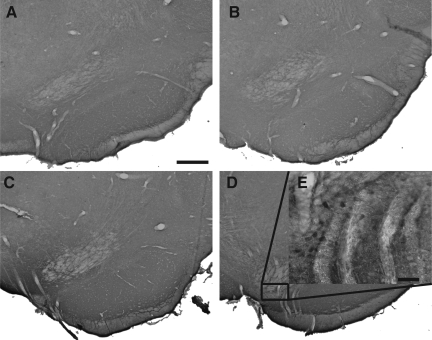
Photomicrographs of tyrosine hydroxylase immunostaining in the substantia nigra pars compacta. (A–D) Acute traumatic brain injury (TBI): naive (A and B) and lateral fluid percussion (LFP)-induced TBI (C and D) animals were sacrificed 5 days after the second of two vehicle (Sal, saline; A and C) or paraquat (PQ) injections (B and D). (E–H) Long-term TBI: naive (E and F) and LFP-induced TBI (G and H) animals were sacrificed 4 weeks after the second of two vehicle (E and G) or paraquat injections (F and H; scale bar in A=1 mm).
FIG. 3.
Unbiased stereology of the substantia nigra pars compacta (SNpc) after acute traumatic brain injury (TBI) and paraquat exposure. TBI and paraquat have a synergistic affect on the loss of nigrostriatal dopaminergic neurons. Animals were sacrificed 11 days after lateral fluid percussion (LFP)-induced TBI, and 5 days after the second of two vehicle or paraquat injections. (A) Unbiased stereology of tyrosine hydroxylase-positive (TH+) neurons. Analysis of variance (ANOVA) revealed a main effect of surgery, treatment and surgery×treatment interaction. (B) Total numbers of neurons (TH+ and TH−/Nissl+). ANOVA revealed a main effect of surgery and treatment. (C) SNpc volume. ANOVA revealed a main effect of surgery and surgery×treatment interaction (Naïve-Sal, naïve and vehicle injection; Naïve-PQ, naive and paraquat injections; TBI-Sal, LFP-induced TBI and vehicle injections; TBI-PQ, LFP-induced TBI and paraquat injections. p<0.05; *Naïve-Sal; ^TBI-Sal; #Naïve-PQ, Fisher's LSD; n=6–8 rats per group; Ipsi, ipsilateral; Contra, contralateral).
In order to determine whether TBI and paraquat induced a loss of a TH-phenotype or a loss of dopaminergic neurons, we estimated the total number of neurons (TH+ and Nissl+/TH−) in the SNpc. A three-way ANOVA indicated a main effect of injury [F(1,56)=45.98, p<0.0001], a main effect of drug [F(1,56)=7.7651, p=0.0076], and no effect of side [F(1,56)=0.0006, p=0.9]. The post-hoc analysis confirmed a unilateral loss after TBI alone, and a bilateral loss of neurons after combined TBI and paraquat (Fig. 3B). These data confirm that both TBI alone and TBI in combination with paraquat exposure induced the degeneration of nigrostriatal dopaminergic neurons rather than simply a loss of the TH phenotype. These are the first data revealing that the LFP model of experimental TBI can induce the degeneration of nigrostriatal dopaminergic neurons in adult rats, and that this insult sensitizes the neurons to paraquat toxicity.
Unbiased stereology also generates volume data for the three-dimensional structure of interest, as volume is one of the parameters used in determining stereological population estimates. For the total volume of the SNpc (Fig. 3C), statistical analysis revealed a main effect of injury [F(1,56)=31.90, p<0.0001], and an injury×drug interaction [F(1,56)=8.89, p=0.0045]. Although TBI resulted in a unilateral loss of neurons in the SNpc, it induced a bilateral loss of volume of the SNpc when compared to naive animals exposed to paraquat. When combined with paraquat, TBI also induced a reduction in volume both ipsilateral and contralateral to the side of injury, compared to both naive groups.
Acute TBI reduced TH immunoreactivity in the striatum
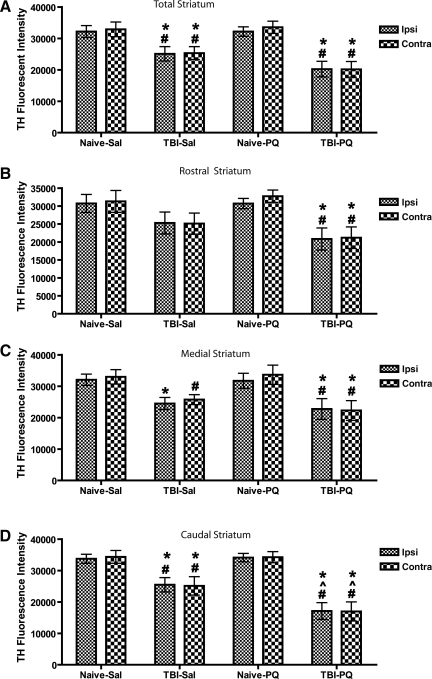
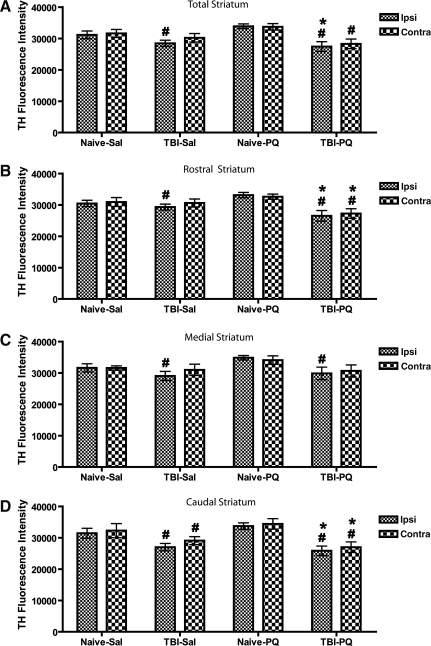
Injuries to the nigrostriatal dopaminergic neurons or to their nerve terminals can result in the loss of TH immunoreactivity in the striatum. We used a microarray scanner to evaluate alterations in TH fluorescence intensity in the rostral, medial, caudal, and whole striatum, a method more sensitive and reliable than traditional optical density measurements. For TH fluorescence intensity in the whole striatum (Fig. 4A), statistical analysis revealed a main effect of injury [F(1,36)=40.17, p<0.0001]. Animals with either TBI or TBI and paraquat displayed a bilateral reduction in TH fluorescence intensity in the striatum compared to naive animals with or without paraquat, whereas paraquat alone did not alter TH fluorescence intensity in the striatum of naive animals.
FIG. 4.
Tyrosine hydroxylase (TH) fluorescence intensity in the striatum after acute traumatic brain injury (TBI) and paraquat exposure. TBI induced a bilateral reduction of TH fluorescence intensity in the striatum, with the largest effect seen in the caudal striatum. (A) Total striatum. (B) Rostral striatum. (C) Medial striatum. (D) Caudal striatum. Analysis of variance revealed a main effect of surgery in A–C, and a main effect of surgery, treatment, and a treatment×surgery interaction in D (Naïve-Sal, naïve and vehicle injection; Naïve-PQ, naive and paraquat injections; TBI-Sal, LFP-induced TBI and vehicle injections; TBI-PQ, LFP-induced TBI and paraquat injections. p<0.05; *Naïve-Sal; ^TBI-Sal; #Naïve-PQ, Fisher's LSD; n=4–5 rats per group; Ipsi, ipsilateral; Contra, contralateral).
TH fluorescence intensity exhibits a marked caudal>medial>rostral gradient, and the effect of each treatment was examined in these striatal subregions. In the rostral striatum (Fig. 4B) there was a main effect of injury [F(1,36)=17.42, p=0.0003], although the decrease in TH fluorescence intensity induced by TBI alone did not reach significance. However, paraquat combined with TBI induced a significant bilateral reduction in TH fluorescence intensity compared to both naive groups.
In the medial striatum (Fig. 4C) there was a main effect of injury [F(1,36)=23.28, p<0.0001], and TBI induced a significant reduction in TH fluorescence intensity compared to naive animals on the side of injury, and compared to paraquat-injected naive animals on the contralateral side. In contrast, paraquat-treated TBI animals showed a bilateral reduction in TH fluorescence intensity compared to both naive groups.
The largest loss of TH fluorescence intensity was seen in the caudal striatum (Fig. 4D), with a main effect of injury [F(1,36)=56.68, p<0.0001], drug [F(1,36)=5.58, p=0.0253], and an injury×drug interaction [F(1,36)=5.94, p=0.0214]. TBI induced a significant reduction in TH fluorescence intensity, ipsilateral and contralateral to the side of injury, compared to both naive groups, and this bilateral decrease was greater in TBI animals treated with paraquat. Thus reductions in TH fluorescence intensity varied by subregions in a caudal>medial>rostral pattern, with the greatest reduction of TH fluorescence intensity close to the site where the LFP device delivered the injury.
Effects of acute TBI with or without paraquat on microglial activation in the SN
To determine whether TBI induced an inflammatory response in the SN, we used the fractionator method of unbiased stereology to quantify the number of activated CD11b-immunoreactive microglia ipsilateral and contralateral to the side of injury following TBI. Activated microglia were defined by their morphology rather than the intensity of staining, using a scale of 1–4 based on cell body size and changes in the length of processes to characterize the different stages of microglial activation (Fig. 5). Microglial activation was present in the SN at 11 days following TBI and/or paraquat exposure. For the total number of activated microglia (stages 1–4) seen following acute TBI with or without paraquat exposure, a three-way ANOVA revealed a main effect of injury [F(1,50)=17.14, p=0.0002; Table 1). Fisher's LSD indicated a significant increase in the total number of activated microglia in TBI compared to naive animals ispilateral but not contralateral to the side of injury. In TBI animals treated with paraquat, the increase in microglial activation was present not only on the ipsilateral side (compared to both naive and paraquat-treated rats), but also on the contralateral side, compared to naive animals.
FIG. 5.
Stages of microglial activation following acute traumatic brain injury (TBI) and paraquat exposure (0=represents a resting microglia, not active). Images 1–4 represent the stages of microglial activation (1=early stage of microglial activation with the retraction of small processes; 2=thickening and further retraction of processes; 3=increase in cell body diameter and appearance of amoeboid morphology; 4=a fully amoeboid microglia; scale bar shown in 0=20 μm). Representative images of animals treated as in Figure 3. (A) Naive and vehicle injections. (B) Naive and paraquat injections. (C) TBI and vehicle injections. (D) TBI and paraquat injections (scale bar shown in A≈500 μm).
Table 1.
Quantification of Microglial Activation in the Substantia Nigra following TBI and Paraquat Exposure
| Grade | Side | Naive-Sal n=6 | Acute TBI-Sal n=6 | Naive-PQ n=6 | Acute TBI-PQ n=7 |
|---|---|---|---|---|---|
| 1 | Ipsilateral | 16±10.12 | 106.67±32.44* | 16±5.84 | 128±37.44*# |
| Contralateral | 16±8.26 | 88±45.40 | 32±16 | 121.14±67.60* | |
| 2 | Ipsilateral | 2.67±2.67 | 32±18.93 | 2.67±2.67 | 36.57±11.95*# |
| Contralateral | 0±0 | 21.33±15.82 | 2.67±2.67 | 22.86±9.79 | |
| 3 | Ipsilateral | 0±0 | 13.33±10.46 | 2.67±2.67 | 25.14±9.14# |
| Contralateral | 2.67±2.67 | 21.33±14.11 | 0±0 | 18.29±6.47 | |
| 4 | Ipsilateral | 0±0 | 13.33±7.64*#+ | 2.67±2.67 | 20.57±2.95*#+ |
| Contralateral | 0±0 | 0±0.09 | 2.67±2.67 | 6.86±3.23 | |
| Total | Ipsilateral | 18.67±9.61 | 165.33±65.41* | 24±9.0 | 210.289±48.56*# |
| Contralateral | 18.67±9.61 | 130.67±71.48 | 37.33±17.36 | 169.14±74.96* |
Three-way ANOVA followed by Fisher's LSD.
p<0.05: *Naive-Sal; #Naive-PQ, +within group. Mean±SEM.
Analysis of the different stages of microglial activation revealed that the overall effect is due to an increase in all stages of microglia, although the magnitude of the effect was much larger for stage 1, mildly-activated microglia. Also, this analysis revealed that more advanced stages of activation (stages 2 and 3, but not stage 4) were only observed on the ipsilateral side of TBI animals also treated with paraquat, indicating that paraquat did increase the inflammatory response observed with TBI alone (Table 1). Indeed, analysis of activated microglia with a score of 1 confirmed a main effect of injury [F(1,50)=12.07, p=0.0012], and an increase in TBI compared to naive animals ipsilateral to the side of injury only. Also similarly to the global measures, TBI animals treated with paraquat showed an increase in stage 1 microglia on the side of the lesion, compared to both naive and paraquat-treated animals, and on the contralateral side, compared to naive rats. For microglia with a score of 2 there was main effect of injury [F(1,50)=12.39, p=0.0011], but post-hoc tests only revealed an increase in stage 2 microglia ipsilateral to the side of injury in paraquat-treated TBI animals, compared to naive and paraquat-treated rats (p<0.05). A main effect of injury [F(1,50)=11.56, p=0.0015] was also found for stage 3 microglia, with an increase limited to TBI and paraquat-treated animals on the side of injury compared to naive and paraquat-only rats. Surprisingly, the evaluation of microglia with a score of 4 found a main effect of injury [F(1,50)=13.75, p=0.006], drug [F(1,50)=4.13, p=0.048], side [F(1,50)=8.01, p=0.007], and an injury×side interaction [F(1,50)=8.01, p=0.007]. The increase in stage 4 microglia was found ipsilateral to the side of injury in TBI animals compared to naive and paraquat-treated rats, and also compared to the contralateral side of injury. This ipsilateral increase was also seen in TBI animals treated with paraquat compared to naive and paraquat-treated rats, and to the side contralateral to injury. However, the number of these highly-activated microglia remained small and a minor fraction of the overall pool of activated microglia.
Acute TBI in combination with paraquat exposure induced the accumulation of alpha-synuclein in the SN
PD is characterized by the pathological accumulation of alpha-synuclein in specific brain regions, including the SN (Braak et al., 2002). Very low, diffuse alpha-synuclein immunoreactivity was detected in the brains of naive animals and of animals with TBI or paraquat treatment alone (Fig. 6A–C). However, animals with combined acute TBI and paraquat exposure displayed accumulation of alpha-synuclein in neurons within the medial part of the SNpc compared to the age-matched controls, as shown in the box in Figure 6D and E. This increased alpha-synuclein staining was restricted to this region of the SNpc on the side ipsilateral to the lesion, and was not found in other regions of the SNpc, or the side contralateral to the lesion. Thus shortly after combined exposure to TBI and paraquat, some of the remaining neurons of the SNpc display a pathological feature associated with aging (Cruz-Muros et al., 2007), and PD (Braak et al., 2002).
FIG. 6.
Alpha-synuclein immunoreactivity in the substantia nigra after acute traumatic brain injury (TBI) and paraquat exposure. Representative images of animals treated as in Figure 3. (A) Naive and vehicle injections. (B) Naive and paraquat injections. (C) TBI and vehicle injections. (D) TBI and paraquat injections. TBI in combination with paraquat induced the pathological accumulation of alpha-synuclein ipsilateral to the side of injury in the medial substantia nigra pars compacta as shown in panels D and E. (A–D, scale bar shown in A≈500 μm; E, scale bar≈100 μm).
Long-Term TBI with or without Paraquat Administration
Progressive bilateral degeneration of nigrostriatal dopaminergic neurons after TBI
Having shown that TBI alone induced a significant loss of dopaminergic neurons in the ipsilateral SNpc only 11 days after injury, we asked whether this neuronal loss was sustained and/or progressive at later time points after injury. Rats were treated similarly to the first cohort, but were sacrificed 26 weeks post-injury, and TH+ neurons in both the ipsilateral and contralateral SNpc were analyzed by stereology as described above. Naive and TBI rats were treated with either vehicle or paraquat over a 2-week period, 20 weeks after injury, such that animals were killed 4 weeks after the last paraquat or vehicle injection (Fig. 1).
As with acute TBI, visual examination of immunostaining of the SNpc did not reveal a marked alteration in TH+ staining in any of the experimental conditions (Fig. 2E–G). However, statistical analysis of the number of TH+ neurons measured with unbiased stereological methods (Fig. 7A) revealed a main effect of injury [F(1,70)=70.38238, p<0.0001]. Animals with TBI alone or with TBI and paraquat exhibited a 30% bilateral reduction of TH+ neurons compared to naive animals with or without paraquat treatment. In contrast to the acute situation, paraquat exposure did not enhance the reduction of TH+ neurons. For the total neuron count (TH+/Nissl+ and TH−/Nissl+; Fig. 7B), there was a main effect of injury [F(1,70)=24.68, p<0.0001]. Fisher's LSD confirmed that ipsilateral to the side of injury, TBI animals had a significant reduction in the total number of neurons compared to naive animals with or without paraquat treatment. Contralateral to the side of injury, TBI animals also displayed a reduction in the total number of neurons compared to naive rats. Animals with TBI and paraquat also exhibited a significant decline in the total number of neurons ipsilateral and contralateral to the side of injury, compared to naive animals with or without paraquat treatment. Thus, 5 months after a single TBI episode, the loss of dopaminergic neurons has progressed and now involves the contralateral side; however, this effect is no longer potentiated by delayed exposure to paraquat.
FIG. 7.
Unbiased stereology of the substantia nigra pars compacta (SNpc) after long-term traumatic brain injury (TBI) and paraquat exposure. TBI alone results in the bilateral degeneration of nigrostriatal dopaminergic neurons. Animals were sacrificed 26 weeks after lateral fluid percussion (LFP)-induced TBI, and 4 weeks after the second of two vehicle or paraquat injections. (A) Unbiased stereology tyrosine hydroxylase-positive (TH+) neurons. (B) Total number of neurons (TH+ and TH−/Nissl+). (C) Volume of SNpc. Analysis of variance revealed a main effect of surgery in A–C (Naïve-Sal, naïve and vehicle injection; Naïve-PQ, naive and paraquat injections; TBI-Sal, LFP-induced TBI and vehicle injections; TBI-PQ, LFP-induced TBI and paraquat injections. p<0.05; *Naïve-Sal; ^TBI-Sal; #Naïve-PQ, Fisher's LSD; n=7–10 rats per group; Ipsi, ipsilateral; Contra, contralateral).
In agreement with the observed loss of neurons, there was a main effect of injury on SNpc volume after TBI [F(1,70)=70.90, p<0.0001]. TBI-alone and TBI-paraquat animals displayed a significant reduction in volume ipsilateral and contralateral to the side of injury compared to naive animals with or without paraquat treatment (Fig. 7C). Similarly to neuronal loss, paraquat exposure at 20 weeks post-injury did not augment the TBI-induced loss of volume.
TH immunoreactivity in the striatum of long-term TBI animals
To determine whether the TBI-induced loss of dopaminergic neurons results in a loss of TH+ terminals in the striatum, we measured TH fluorescence intensity in the whole striatum (Fig. 8A), and at different levels throughout the region. A three-way ANOVA revealed a main effect of injury [F(1,52)=19.55, p<0.0001], and an injury×drug interaction [F(1,52)=4.65, p=0.036]. Post-hoc analysis revealed a significant reduction in TH fluorescence in TBI animals ipsilateral to the side of injury, compared to naive animals treated with paraquat. In animals with both TBI and paraquat, that had no greater loss of dopaminergic neurons than those with TBI alone, TH fluorescence was decreased compared to both naive and naive plus paraquat rats on the side ipsilateral to the injury. Thus in the presence of paraquat, the TBI-induced loss of striatal TH was more widespread compared to naive animals, but it was not significantly different than after TBI alone.
FIG. 8.
Tyrosine hydroxylase (TH) fluorescence intensity in the striatum after long-term traumatic brain injury (TBI) and paraquat exposure. TBI decreased TH-fluorescent intensity in the striatum. (A) Total striatum. (B) rostral striatum. (C) Medial striatum. (D) Caudal striatum. Analysis of variance revealed a main effect of surgery and a surgery×treatment interaction in A and B, and a main effect of surgery in C and D (Naïve-Sal, naïve and vehicle injection; Naïve-PQ, naive and paraquat injections; TBI-Sal, LFP-induced TBI and vehicle injections; TBI-PQ, LFP-induced TBI and paraquat injections. p<0.05; *Naïve-Sal; ^TBI-Sal; #Naïve-PQ, Fisher's LSD; n=6–7 rats per group; Ipsi, ipsilateral; Contra, contralateral).
Similar changes in TH immunoreactivity were observed in the rostral striatum (Fig. 8B), ipsilateral and contralateral to the side of injury, with a main effect of injury [F(1,52)=13.99, p=0.0005], and an injury×drug interaction [F(1,52)=8.51, p=0.0055]. Ipsilateral to the side of injury, TBI animals displayed a reduction in TH fluorescence compared to naive-paraquat only animals. TBI and paraquat-treated animals displayed a significant reduction in TH fluorescence compared to both naive and paraquat-treated rats, ipsilateral and contralateral to the side of injury.
A main effect of injury [F(1,52)=7.04753, p=0.011] was observed in the medial striatum (Fig. 8C), with a significant reduction in TH fluorescence in TBI animals compared to naive paraquat-treated rats, ipsilateral and contralateral to the side of injury. In animals with TBI and paraquat, a decrease in TH fluorescence was seen contralateral to the side of injury, compared to naive animals treated with paraquat.
In the caudal striatum (Fig. 8D), there was a main effect of injury [F(1,52)=27.13, p<0.0001]. TBI only significantly decreased TH fluorescence ipsilateral and contralateral to the side of injury, compared to naive paraquat animals. With paraquat treatment, TBI animals showed reduced TH fluorescence compared to naive and naive paraquat-treated animals, both ipsilateral and contralateral to the side of injury. Thus delayed paraquat administration did not increase either the TBI-induced loss of TH-positive neurons in the substantia nigra, or of TH-positive fibers in the striatum.
Microglial activation and alpha-synuclein staining after long-term TBI
In contrast to the earlier time point, no microglial activation could be observed in either the saline or paraquat-treated TBI animals at 26 weeks post-injury (data not shown). Similarly, we did not observe any accumulation of alpha-synuclein in these animals (data not shown).
Discussion
PD pathology is widespread and patients experience a broad range of symptoms that are not related to nigrostriatal dopaminergic cell loss (Braak et al., 2006). Nevertheless, nigrostriatal dopaminergic neurons exhibit a distinct vulnerability to the disease process, and understanding the factors that lead to their demise in the context of PD remains a high priority to help design effective neuroprotective strategies. Epidemiological studies have consistently implicated pesticide exposure as a risk factor in the development of PD, and experimental studies have demonstrated the ability of systemic administration of pesticides, in particular paraquat, to kill nigrostriatal dopaminergic neurons in rats and mice (Cicchetti et al., 2005; Costello et al., 2009; Fernagut et al., 2007; Hertzman et al., 1990; Liou et al., 1997; McCormack et al., 2002; Ossowska et al., 2005; Semchuk et al., 1992). In contrast, the role of TBI in the etiology of PD has been inconsistently documented (Bharucha et al., 1986; Bower et al., 2003; Goldman et al., 2006; Rugbjerg et al., 2008; Taylor et al., 1999; Werneck and Alvarenga, 1999) and, although it is known that TBI alters dopamine levels (Bales et al., 2009), and causes the appearance of degenerating neurons in the substantia nigra (Hicks et al., 1996), no previous research has demonstrated the ability of experimental TBI to induce a loss of nigrostriatal dopaminergic neurons similar to that observed in PD.
A main result from the present study is the demonstration that TBI induced by moderate LFP resulted in a 15% unilateral loss of nigrostriatal dopaminergic neurons in the SNpc as early as 11 days post-injury in adult rats. Furthermore, this unilateral nigrostriatal lesion progressed to a 30% bilateral loss of neurons by 26 weeks post-injury. In addition, TBI-induced reductions in the volume of the substantia nigra even in the absence of cell loss, which could be due to a loss of cell types not counted, a reduction in cell size (early signs of delayed cell death), and/or a reduction in extracellular space.
A second main finding is that LFP transiently induced an increased vulnerability of nigrostriatal dopaminergic neurons to a subsequent exposure to a low dose of paraquat that by itself did not kill dopaminergic neurons. Indeed, when administered shortly after TBI, paraquat had a synergistic effect with TBI on nigrostriatal dopaminergic neurons, resulting in a 30% bilateral loss of dopaminergic neurons at 11 days post-injury. The combination of acute TBI and paraquat exposure also induced the accumulation of alpha-synuclein in the medial part of the SNpc, a pathological feature observed in PD, and not induced by paraquat or TBI alone. In the striatum, acute TBI induced a bilateral reduction in TH fluorescence intensity in a caudal>medial>rostral pattern. Paraquat exposure exacerbated striatal pathology induced by acute TBI, while maintaining the caudal>medial>rostral loss of TH fluorescence intensity.
The augmentation of the detrimental effect of TBI on dopaminergic neurons was not observed when paraquat was administered 21 weeks after injury. This suggests that the increased vulnerability to paraquat after TBI was limited in time. However, paraquat injections were more widely spaced in older rats, a modification that was necessary to avoid excessive mortality from paraquat toxicity in these older animals. In mice a single paraquat injection causes a priming effect, leading to significant nigrostriatal cell loss after the second injection (Purisai et al., 2007). In rats, however, at least 5 injections of paraquat at the same dose (10 mg/kg) are necessary to induce cell death (Cicchetti et al., 2005; Kuter et al., 2007). Therefore the lack of potentiation after paraquat administration seen in older rats is unlikely to be due to differences in the priming effect of the first injection in our rat model, but may due to the longer time period between the two insults, as suggested for other factors that modify the effects of TBI (Bramlett et al., 1999; Jenkins et al., 1989). It is possible, however, that paraquat and TBI both specifically affect a particularly vulnerable population of nigrostriatal dopaminergic neurons, and that the increased loss induced by TBI at the later time point masks any effect of paraquat at that later age (Fernagut et al., 2007; McCormack et al., 2002; Purisai et al., 2007).
The data presented here support epidemiological data indicating that exposure to the pesticide paraquat and TBI are both risk factors in the development of sporadic PD (Bower et al., 2003; Costello et al., 2009; Goldman et al., 2006; Liou et al., 1997; Taylor et al., 1999). The 30% loss induced by TBI and paraquat exposure in this study may not be sufficient to produce the neurological symptoms of PD that may require a 60% loss (Ma et al., 1995; Pakkenberg et al., 1991; Rudow et al., 2008). Nevertheless, our data expand on the concept of increased neuronal vulnerability following TBI (Bramlett et al., 1999; Jenkins et al., 1989), and support the “multiple hits” hypothesis of PD (Sulzer, 2007), by suggesting that TBI and pesticide exposure could act in concert to increase the risk of PD when they occur within a defined time frame, with TBI acting as a “primary hit” that sensitizes dopaminergic neurons to paraquat exposure, the “secondary hit.” Furthermore, it is possible that even in the absence of a secondary pesticide exposure, the decreased number of DA neurons seen following a mild concussion could predispose an individual to PD, because the age-related decline in dopaminergic neurons would reach the threshold for expression of motor symptoms at an earlier age (Langston, 1990).
The effect of TBI on nigrostriatal dopaminergic neurons
Controlled cortical impact (CCI) in rats has been reported to cause changes in subcortical dopamine neurotransmission. Specifically, the kinetic parameters associated with dopamine release are altered following CCI, and striatal DAT protein expression is reduced. This has led to the suggestion that TBI can cause functional hypodopaminergia (Wagner et al., 2005). For an extensive review of the literature regarding the changes in dopaminergic functioning after TBI, see Bales et al., 2009. The present study, although based on TBI induced by LFP (extensively reviewed by Thompson et al., 2005b) suggests that at least some of these changes can be due to TBI-induced nigrostriatal dopaminergic degeneration. The underlying mechanisms of this effect, however, have yet to be determined. LFP is characterized by both focal and diffuse pathology, including cortical damage without brainstem compression (Thompson et al., 2005b). However, previous work by Soares and associates, evaluating the integrity of the blood–brain barrier, the authors observed “small focused hemorrhagic regions in the substantia nigra” following moderate LFP in adult rats (Soares et al., 1995). It is possible that disruptions to local blood flow in the SN may create small and discrete ischemic regions, resulting in nigrostriatal dopaminergic cell death. Additionally, small intraparenchymal hemorrhages increase interstitial pressure, injuring surrounding tissue that may include nigrostriatal dopaminergic neurons. Hicks and colleagues (1996), characterizing neuronal injury after moderate LFP in rats, identified injured neurons in the SNr and SNc up to 7 days post-injury by silver staining of degenerating neurons, but did not identify these cells as dopaminergic neurons, nor did they document their loss. Hicks and associates have suggested that the vulnerability of this region derives from its anatomical characteristics, specifically the density of fibers that traverse the SN (Hicks et al., 1996). The shearing forces that occur with rapid acceleration and/or deceleration of the brain during TBI (Li and Feng, 2009; Maxwell et al., 1997) have the greatest effect at the interface of two regions with different physical characteristics (Chan et al., 2003; Meythaler et al., 2001; Povlishock, 1993), such as the white and gray matter interface present in the SN. Indeed, fluid percussion in the rodent subjects brainstem structures to biomechanical forces that vary depending on the positioning of the injury cap. Consequently, cell loss in the rhombencephalon and/or at the junction between the medulla and the cervical region of the spinal cord after LFP, could be due to direct damage produced by the movement of the brain in relationship to the cervical region of the spinal cord (Dixon et al., 1987,1988).
One of the attributes of the LFP model of TBI is its ability to model axonal injury (Graham et al., 2000; Pierce et al., 1998), a common consequence of TBI (Maxwell et al., 1997). Nigrostriatal dopaminergic neurons are projection neurons whose axons and axon collaterals provide extensive innervation of the striatum (Oorschot, 1996; Prensa and Parent, 2001; Roberts et al., 2002), and would be particularly susceptible to the forces induced by TBI. Interestingly, LFP is documented to induce chronic axonal degeneration, characterized by axonal swellings and protein accumulation within the striatum (Pierce et al., 1998). However, Pierce and associates did not specifically identify nigrostriatal dopaminergic axons or axon collaterals in their report. In addition, TBI results in a number of cellular events that are related to excitotoxic cell death, apoptosis, and the induction of autophagy (Clark et al., 2008; Conti et al., 1998; Dikranian et al., 2008; Fiskum, 2000; Hutchison et al., 2001; Raghupathi, 2004). TBI-induced axonal injury results in the release and unregulated movement of intracellular and extracellular substances, including ions, neurotransmitters, proteins, and lipids. The unfettered release of glutamate and aspartate could lead to excitotoxicity in nigrostriatal dopaminergic neurons, as they express both N-methyl-D-aspartate (NMDA) and glutamate receptors (Albers et al., 1999; Kosinski et al., 1998). The unregulated release of dopamine can directly injure nigrostriatal dopaminergic neurons via oxidization of dopamine, creating reactive oxygen species (ROS), and dopamine quinones that damage lipid membranes, proteins, DNA, and mitochondria (Berman and Hastings, 1999; Graham, 1978; Miyazaki and Asanuma, 2009; Stokes et al., 1999). Nigrostriatal dopaminergic neurons have increased sensitivity to ROS due to their reduced antioxidant capacity (Greenamyre et al., 1999), making them characteristically sensitive to these types of insults. An increased influx of calcium across the damaged axon disrupts mitochondrial function, decreasing energy production while simultaneously activating caspases that can induce apoptosis (Kim et al., 2003; Lifshitz et al., 2003; Mazzeo et al., 2009). Mitochondrial dysfunction is believed to play a prominent role in the etiology of PD, as patients with sporadic PD display systemic mitochondrial complex-1 dysfunction (Mizuno et al., 1989; Nicklas et al., 1985; Parker et al., 1989; Schapira et al., 1989). Furthermore, animal models of PD based exclusively on mitochondrial inhibition have revealed that nigrostriatal dopaminergic neurons are highly susceptible to the effects of mitochondrial dysfunction (Betarbet et al., 2000; German et al., 1992; Liang et al., 1996; Meurers et al., 2009; Sherer et al., 2002,2003).
Chronic central nervous system (CNS) inflammation is a consequence of TBI in humans, and has been observed in animal models of TBI (Gentleman et al., 2004; Nonaka et al., 1999; Rodriguez-Paez et al., 2005; Smith et al., 1997; Stokely and Orr, 2008). Several lines of evidence suggest that CNS inflammation plays a role in nigrostriatal degeneration in PD. PD is marked by a CNS inflammatory response, characterized by microglial activation and significant increases in inflammatory factors throughout the nigrostriatal dopaminergic system (Imamura et al., 2003; McGeer et al., 1988). Previous research based on the administration of lipopolysaccharide (LPS) to experimentally induce microglial activation, both in vivo and in vitro, has shown that inflammation alone can induce delayed and progressive nigrostriatal dopaminergic degeneration (Arimoto and Bing, 2003; Carvey et al., 2003; Gayle et al., 2002; Kim et al., 2000; Qin et al., 2004,2007). Furthermore, attenuation of LPS-induced microglial activation led to the survival of dopaminergic neurons (Li et al., 2004; Liu et al., 2000,2003; Wang et al., 2004). In the present study we found that LFP-induced TBI stimulated microglial activation in the SNpc shortly after injury, suggesting that microglial activation may play a role in the degeneration of nigrostriatal dopaminergic neurons. This is compatible with evidence that TBI in laboratory animals results in long-term microglial activation and progressive degeneration up to at least 1 year post-injury (Bramlett and Dietrich, 2002; Nonaka et al., 1999; Pierce et al., 1998; Rodriguez-Paez et al., 2005; Smith et al., 1997). Thus, multiple factors known to contribute to PD could contribute to the loss of dopaminergic neurons observed after TBI in this study.
Synergistic effects of TBI and paraquat on nigrostriatal dopaminergic neurons
The concept of TBI-induced cellular vulnerability has been clinically recognized for many years (Doberstein et al., 1993), and has been demonstrated experimentally after LFP brain injury in initial experiments demonstrating that following LFP, reductions in cerebral blood flow that normally do not cause cell damage result in extensive cell death (Jenkins, 1986; Jenkins et al., 1989). This type of injury-induced vulnerability has also been well described in models of post-traumatic exposure to cellular activation (Maeda et al., 2005; Zanier et al., 2003), and/or physiological challenge (Bramlett et al., 1999; Church and Andrew, 2005; Dietrich et al., 1996; Griesbach, 2002; Hartings et al., 2009; Ip et al., 2003; Law et al., 1996; Suzuki et al., 2004; Taya, 2010; Van Putten et al., 2005). However, this induced vulnerability is expressed temporarily, and depending on the type of secondary challenge, can last from hours to days after injury in rodents. The mechanisms behind this TBI-induced vulnerability have been thought to be related to the post-injury neurochemical and metabolic cascade that compromises the ability of surviving cells to function due to pronounced ionic fluxes that are associated with mitochondrial dysfunction and energy crisis (Giza, 2000; Giza and Hovda, 2001,; Lifshitz et al., 2004), which spontaneously resolves over the first 2 weeks after experimental TBI.
Previous studies in mice have shown that three successive injections, once a week for 3 weeks, of paraquat (10 mg/kg IP) are necessary to induce a 25% loss of nigrostriatal dopaminergic neurons in mice (Fernagut et al., 2007;McCormack et al., 2002). In rats, Cicchetti and associates injected paraquat (10 mg/kg IP) twice a week for 4 weeks in order to induce a 15% loss of nigrostriatal dopaminergic neurons (Cicchetti et al., 2005). In the present study, two injections of paraquat (10 mg/kg IP) alone in adult rats did not induce dopaminergic cell losses, confirming that this dose by itself does not reach the threshold of toxicity necessary to induce nigrostriatal dopaminergic cell death. When administered shortly (3 and 6 days) after a moderate LFP, however, this regimen of paraquat increased the effects of TBI alone, suggesting that the LFP had sensitized dopaminergic neurons to paraquat toxicity, as previously shown for other secondary insults.
Previous work has shown that LPS-induced microglial activation can sensitize nigrostriatal dopaminergic neurons to a sub-threshold dose of paraquat in mice (Purisai et al., 2007). LPS-induced inflammation is also known to exacerbate the effects of other dopaminergic toxins, such as MPTP and rotenone, in laboratory animals (Gao et al., 2003a,2003b; Goralski and Renton, 2004). Recent data suggest that paraquat exerts its toxicity on dopaminergic neurons through its ability to generate ROS (Bonneh-Barkay et al., 2005a,2005b; Cocheme and Murphy, 2008; Purisai et al., 2007; Wu et al., 2005). Unlike 6-OHDA or MPTP, paraquat does not selectively target nigrostriatal dopaminergic neurons through a specific uptake mechanism. Rather, the vulnerability of nigrostriatal dopaminergic neurons to paraquat may be due to their reduced antioxidant capacity (Greenamyre et al., 1999). Thus, paraquat's effect on dopaminergic neurons may be indirect, related to its ability to redox cycled by NOS and NADPH in activated microglia; this would then generate ROS that eventually result in the degeneration of dopaminergic neurons (Bonneh-Barkay et al., 2005a,2005b; Purisai et al., 2007; Wu et al., 2005). Based on these previous findings, it is possible that TBI-induced inflammation is the underlying mechanism that sensitizes nigrostriatal dopaminergic neurons to paraquat toxicity. This hypothesis remains to be tested by preventing inflammatory responses after TBI in our model. Interestingly, converging epidemiological studies indicate that genetic polymorphisms in inflammatory genes increase the risk of PD (Wahner et al., 2007b), and long-term use of nonsteroidal anti-inflammatory agents is protective against PD (Chen et al., 2003; Wahner et al., 2007a), thus suggesting that inflammatory processes could contribute to PD risk induced by a variety of factors.
As part of the normal aging process, male Sprague-Dawley rats display a natural accumulation of alpha-synuclein in the cell bodies of nigrostriatal dopaminergic neurons after 18 months of age (Cruz-Muros et al., 2007). However, TBI-PQ animals displayed accumulation of alpha-synuclein in neurons within the medial portion of the SNpc, both ipsilateral and contralateral to the side of injury, at less than 4 months of age when paraquat was administered shortly after injury. The restricted pattern of increased alpha-synuclein accumulation after short-term TBI followed by paraquat is reminiscent of the location of the silver-stained neurons reported by Hicks and associates (1996), in the region of the SNpc immediately adjacent to the ventral tegmental area. Increased levels of alpha-synuclein have been unequivocally linked to an increased risk of PD and the transient increase in alpha-synuclein staining seen after TBI, also previously observed in the olfactory bulb (Uryu et al., 2003), another region vulnerable in PD. This may contribute to the increased neuronal loss seen after combined TBI and paraquat.
Conclusion
The present data show for the first time that a mild TBI alone can cause progressive loss of nigrostriatal dopaminergic neurons in an animal model, and that the effect of TBI can be potentiated by subsequent exposure to a widely used environmental pesticide, paraquat. This supports epidemiological studies indicating that TBI and pesticide exposure are risk factors for PD, and provides evidence that they may act synergistically to cause dopaminergic cell loss and alpha-synuclein accumulation in laboratory animals. These observations suggest that greater attention should be given to the long-term risk of PD after TBI, and that the epidemiology of both risk factors should be evaluated in combination.
Acknowledgments
This work was supported by a UC TSR&TP student fellowship, and 1 F31 NS51163-011 (C.B.H.); an APA minority fellowship (C.R.L.); grants U54 ES12078, P01 ES016732, and P50 NS38367 (M.F.C.); grant NS027544 (D.H.); grants NS057420 and NS02197 (C.C.G.); The Michael J. Fox Foundation for Parkinson's Research (F.M.); and UCLA BIRC.
Author Disclosure Statement
No financial conflicts of interests exist.
References
- Aghaloo T. Cowan C.M. Chou Y.F. Zhang X. Lee H. Miao S. Hong N. Kuroda S. Wu B. Ting K. Soo C. Nell-1-induced bone regeneration in calvarial defects. Am. J. Pathol. 2006;169:903–915. doi: 10.2353/ajpath.2006.051210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Albers D.S. Weiss S.W. Iadarola M.J. Standaert D.G. Immunohistochemical localization of N-methyl-D-aspartate and alpha-amino-3-hydroxy-5-methyl-4-isoxazolepropionate receptor subunits in the substantia nigra pars compacta of the rat. Neuroscience. 1999;89:209–220. doi: 10.1016/s0306-4522(98)00328-5. [DOI] [PubMed] [Google Scholar]
- Arimoto T. Bing G. Up-regulation of inducible nitric oxide synthase in the substantia nigra by lipopolysaccharide causes microglial activation and neurodegeneration. Neurobiol. Dis. 2003;12:35–45. doi: 10.1016/s0969-9961(02)00017-7. [DOI] [PubMed] [Google Scholar]
- Bales J.W. Wagner A.K. Kline A.E. Dixon C.E. Persistent cognitive dysfunction after traumatic brain injury: A dopamine hypothesis. Neurosci. Biobehav. Rev. 2009;33:981–1003. doi: 10.1016/j.neubiorev.2009.03.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berman S.B. Hastings T.G. Dopamine oxidation alters mitochondrial respiration and induces permeability transition in brain mitochondria: implications for Parkinson's disease. J. Neurochem. 1999;73:1127–1137. doi: 10.1046/j.1471-4159.1999.0731127.x. [DOI] [PubMed] [Google Scholar]
- Betarbet R. Sherer T.B. MacKenzie G. Garcia-Osuna M. Panov A.V. Greenamyre J.T. Chronic systemic pesticide exposure reproduces features of Parkinson's disease. Nat. Neurosci. 2000;3:1301–1306. doi: 10.1038/81834. [DOI] [PubMed] [Google Scholar]
- Bharucha N.E. Stokes L. Schoenberg B.S. Ward C. Ince S. Nutt J.G. Eldridge R. Calne D.B. Mantel N. Duvoisin R. A case-control study of twin pairs discordant for Parkinson's disease: a search for environmental risk factors. Neurology. 1986;36:284–288. doi: 10.1212/wnl.36.2.284. [DOI] [PubMed] [Google Scholar]
- Bonneh-Barkay D. Langston W.J. Di Monte D.A. Toxicity of redox cycling pesticides in primary mesencephalic cultures. Antioxid. Redox. Signal. 2005a;7:649–653. doi: 10.1089/ars.2005.7.649. [DOI] [PubMed] [Google Scholar]
- Bonneh-Barkay D. Reaney S.H. Langston W.J. Di Monte D.A. Redox cycling of the herbicide paraquat in microglial cultures. Brain Res. Mol. Brain Res. 2005b;134:52–56. doi: 10.1016/j.molbrainres.2004.11.005. [DOI] [PubMed] [Google Scholar]
- Bower J.H. Maraganore D.M. McDonnell S.K. Rocca W.A. Incidence and distribution of parkinsonism in Olmsted County, Minnesota, 1976–1990. Neurology. 1999;52:1214–1220. doi: 10.1212/wnl.52.6.1214. [DOI] [PubMed] [Google Scholar]
- Bower J.H. Maraganore D.M. Peterson B.J. McDonnell S.K. Ahlskog J.E. Rocca W.A. Head trauma preceding PD: a case-control study. Neurology. 2003;60:1610–1615. doi: 10.1212/01.wnl.0000068008.78394.2c. [DOI] [PubMed] [Google Scholar]
- Braak H. Bohl J.R. Muller C.M. Rub U. de Vos R.A. Del Tredici K. Stanley Fahn Lecture 2005: The staging procedure for the inclusion body pathology associated with sporadic Parkinson's disease reconsidered. Mov. Disord. 2006;21:2042–2051. doi: 10.1002/mds.21065. [DOI] [PubMed] [Google Scholar]
- Braak H. Del Tredici K. Bratzke H. Hamm-Clement J. Sandmann-Keil D. Rub U. Staging of the intracerebral inclusion body pathology associated with idiopathic Parkinson's disease (preclinical and clinical stages) J. Neurol. 2002;249(Suppl.3):III/1–5. doi: 10.1007/s00415-002-1301-4. [DOI] [PubMed] [Google Scholar]
- Bramlett H.M. Dietrich W.D. Green E.J. Secondary hypoxia following moderate fluid percussion brain injury in rats exacerbates sensorimotor and cognitive deficits. J. Neurotrauma. 1999;16:1035–1047. doi: 10.1089/neu.1999.16.1035. [DOI] [PubMed] [Google Scholar]
- Bramlett H.M. Dietrich W.D. Quantitative structural changes in white and gray matter 1 year following traumatic brain injury in rats. Acta Neuropathol. 2002;103:607–614. doi: 10.1007/s00401-001-0510-8. [DOI] [PubMed] [Google Scholar]
- Carvey P.M. Chang Q. Lipton J.W. Ling Z. Prenatal exposure to the bacteriotoxin lipopolysaccharide leads to long-term losses of dopamine neurons in offspring: a potential, new model of Parkinson's disease. Front. Biosci. 2003;8:s826–s837. doi: 10.2741/1158. [DOI] [PubMed] [Google Scholar]
- Chan J.H. Tsui E.Y. Peh W.C. Fong D. Fok K.F. Leung K.M. Yuen M.K. Fung K.K. Diffuse axonal injury: detection of changes in anisotropy of water diffusion by diffusion-weighted imaging. Neuroradiology. 2003;45:34–38. doi: 10.1007/s00234-002-0891-y. [DOI] [PubMed] [Google Scholar]
- Chen H. Zhang S.M. Hernan M.A. Schwarzschild M.A. Willett W.C. Colditz G.A. Speizer F.E. Ascherio A. Nonsteroidal anti-inflammatory drugs and the risk of Parkinson disease. Arch. Neurol. 2003;60:1059–1064. doi: 10.1001/archneur.60.8.1059. [DOI] [PubMed] [Google Scholar]
- Church A.J. Andrew R.D. Spreading depression expands traumatic injury in neocortical brain slices. J. Neurotrauma. 2005;22:277–290. doi: 10.1089/neu.2005.22.277. [DOI] [PubMed] [Google Scholar]
- Cicchetti F. Lapointe N. Roberge-Tremblay A. Saint-Pierre M. Jimenez L. Ficke B.W. Gross R.E. Systemic exposure to paraquat and maneb models early Parkinson's disease in young adult rats. Neurobiol. Dis. 2005;20:360–371. doi: 10.1016/j.nbd.2005.03.018. [DOI] [PubMed] [Google Scholar]
- Clark R.S. Bayir H. Chu C.T. Alber S.M. Kochanek P.M. Watkins S.C. Autophagy is increased in mice after traumatic brain injury and is detectable in human brain after trauma and critical illness. Autophagy. 2008;4:88–90. doi: 10.4161/auto.5173. [DOI] [PubMed] [Google Scholar]
- Cocheme H.M. Murphy M.P. Complex I is the major site of mitochondrial superoxide production by paraquat. J. Biol. Chem. 2008;283:1786–1798. doi: 10.1074/jbc.M708597200. [DOI] [PubMed] [Google Scholar]
- Conti A.C. Raghupathi R. Trojanowski J.Q. McIntosh T.K. Experimental brain injury induces regionally distinct apoptosis during the acute and delayed post-traumatic period. J. Neurosci. 1998;18:5663–5672. doi: 10.1523/JNEUROSCI.18-15-05663.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Costello S. Cockburn M. Bronstein J. Zhang X. Ritz B. Parkinson's disease and residential exposure to maneb and paraquat from agricultural applications in the Central Valley of California. Am. J. Epidemiol. 2009;169:919–926. doi: 10.1093/aje/kwp006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cruz-Muros I. Afonso-Oramas D. Abreu P. Barroso-Chinea P. Rodriguez M. Gonzalez M.C. Hernandez T.G. Aging of the rat mesostriatal system: differences between the nigrostriatal and the mesolimbic compartments. Exp. Neurol. 2007;204:147–161. doi: 10.1016/j.expneurol.2006.10.004. [DOI] [PubMed] [Google Scholar]
- Csuka E. Morganti-Kossmann M.C. Lenzlinger P.M. Joller H. Trentz O. Kossmann T. IL-10 levels in cerebrospinal fluid and serum of patients with severe traumatic brain injury: relationship to IL-6, TNF-alpha, TGF-beta1 and blood-brain barrier function. J. Neuroimmunol. 1999;101:211–221. doi: 10.1016/s0165-5728(99)00148-4. [DOI] [PubMed] [Google Scholar]
- Dietrich W.D. Alonso O. Halley M. Busto R. Delayed posttraumatic brain hyperthermia worsens outcome after fluid percussion brain injury: a light and electron microscopic study in rats. Neurosurgery. 1996;38:533–541. doi: 10.1097/00006123-199603000-00023. discussion 541. [DOI] [PubMed] [Google Scholar]
- Dikranian K. Cohen R. Mac Donald C. Pan Y. Brakefield D. Bayly P. Parsadanian A. Mild traumatic brain injury to the infant mouse causes robust white matter axonal degeneration which precedes apoptotic death of cortical and thalamic neurons. Exp. Neurol. 2008;211:551–560. doi: 10.1016/j.expneurol.2008.03.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dixon C.E. Lighthall J.W. Anderson T.E. Physiologic, histopathologic, and cineradiographic characterization of a new fluid-percussion model of experimental brain injury in the rat. J. Neurotrauma. 1988;5:91–104. doi: 10.1089/neu.1988.5.91. [DOI] [PubMed] [Google Scholar]
- Dixon C.E. Lyeth B.G. Povlishock J.T. Findling R.L. Hamm R.J. Marmarou A. Young H.F. Hayes R.L. A fluid percussion model of experimental brain injury in the rat. J. Neurosurg. 1987;67:110–119. doi: 10.3171/jns.1987.67.1.0110. [DOI] [PubMed] [Google Scholar]
- Doberstein C.E. Hovda D.A. Becker D.P. Clinical considerations in the reduction of secondary brain injury. Ann. Emerg. Med. 1993;22:993–997. doi: 10.1016/s0196-0644(05)82740-4. [DOI] [PubMed] [Google Scholar]
- Farrer M. Maraganore D.M. Lockhart P. Singleton A. Lesnick T.G. de Andrade M. West A. de Silva R. Hardy J. Hernandez D. Alpha-synuclein gene haplotypes are associated with Parkinson's disease. Hum. Mol. Genet. 2001;10:1847–1851. doi: 10.1093/hmg/10.17.1847. [DOI] [PubMed] [Google Scholar]
- Fernagut P.O. Hutson C.B. Fleming S.M. Tetreaut N.A. Salcedo J. Masliah E. Chesselet M.F. Behavioral and histopathological consequences of paraquat intoxication in mice: Effects of alpha-synuclein over-expression. Synapse. 2007;61:991–1001. doi: 10.1002/syn.20456. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fiskum G. Mitochondrial participation in ischemic and traumatic neural cell death. J. Neurotrauma. 2000;17:843–855. doi: 10.1089/neu.2000.17.843. [DOI] [PubMed] [Google Scholar]
- Gao H.M. Hong J.S. Zhang W. Liu B. Synergistic dopaminergic neurotoxicity of the pesticide rotenone and inflammogen lipopolysaccharide: relevance to the etiology of Parkinson's disease. J. Neurosci. 2003a;23:1228–1236. doi: 10.1523/JNEUROSCI.23-04-01228.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gao H.M. Liu B. Zhang W. Hong J.S. Synergistic dopaminergic neurotoxicity of MPTP and inflammogen lipopolysaccharide: relevance to the etiology of Parkinson's disease. FASEB J. 2003b;17:1957–1959. doi: 10.1096/fj.03-0203fje. [DOI] [PubMed] [Google Scholar]
- Gayle D.A. Ling Z. Tong C. Landers T. Lipton J.W. Carvey P.M. Lipopolysaccharide (LPS)-induced dopamine cell loss in culture: roles of tumor necrosis factor-alpha, interleukin-1beta, and nitric oxide. Brain Res. Dev. Brain Res. 2002;133:27–35. doi: 10.1016/s0165-3806(01)00315-7. [DOI] [PubMed] [Google Scholar]
- Gentleman S.M. Leclercq P.D. Moyes L. Graham D.I. Smith C. Griffin W.S. Nicoll J.A. Long-term intracerebral inflammatory response after traumatic brain injury. Forensic Sci. Int. 2004;146:97–104. doi: 10.1016/j.forsciint.2004.06.027. [DOI] [PubMed] [Google Scholar]
- Gerhard A. Pavese N. Hotton G. Turkheimer F. Es M. Hammers A. Eggert K. Oertel W. Banati R.B. Brooks D.J. In vivo imaging of microglial activation with [11C](R)-PK11195 PET in idiopathic Parkinson's disease. Neurobiol. Dis. 2006;21:404–412. doi: 10.1016/j.nbd.2005.08.002. [DOI] [PubMed] [Google Scholar]
- German D.C. Manaye K.F. Sonsalla P.K. Brooks B.A. Midbrain dopaminergic cell loss in Parkinson's disease and MPTP-induced parkinsonism: sparing of calbindin-D28k-containing cells. Ann. NY Acad. Sci. 1992;648:42–62. doi: 10.1111/j.1749-6632.1992.tb24523.x. [DOI] [PubMed] [Google Scholar]
- Gerstenfeld L.C. Cho T.J. Kon T. Aizawa T. Tsay A. Fitch J. Barnes G.L. Graves D.T. Einhorn T.A. Impaired fracture healing in the absence of TNF-alpha signaling: the role of TNF-alpha in endochondral cartilage resorption. J. Bone Miner Res. 2003;18:1584–1592. doi: 10.1359/jbmr.2003.18.9.1584. [DOI] [PubMed] [Google Scholar]
- Giannoudis P.V. Smith R.M. Bellamy M.C. Morrison J.F. Dickson R.A. Guillou P.J. Stimulation of the inflammatory system by reamed and unreamed nailing of femoral fractures. An analysis of the second hit. J. Bone Joint Surg. Br. 1999;81:356–361. doi: 10.1302/0301-620x.81b2.8988. [DOI] [PubMed] [Google Scholar]
- Giza C.C. Hovda D.A. The neurometabolic cascade of concussion. J. Athl. Train. 2001;36:228–235. [PMC free article] [PubMed] [Google Scholar]
- Giza C.C. Prins M.L. Hovda D.A. Herschman H.R. Feldman J.D. Genes preferentially induced by depolarization after concussive brain injury: effects of age and injury severity. J. Neurotrauma. 2002;19:387–402. doi: 10.1089/08977150252932352. [DOI] [PubMed] [Google Scholar]
- Giza R.C. Ionic and metabolic consequence of concussion. In: Cantu R.C., editor. Neurologic Athletic Head and Spine Injuries. WB Saunders; Philadelphia: 2000. pp. 80–100. [Google Scholar]
- Goldman S.M. Tanner C.M. Oakes D. Bhudhikanok G.S. Gupta A. Langston J.W. Head injury and Parkinson's disease risk in twins. Ann. Neurol. 2006;60:65–72. doi: 10.1002/ana.20882. [DOI] [PubMed] [Google Scholar]
- Goralski K.B. Renton K.W. Brain inflammation enhances 1-methyl-4-phenylpyridinium-evoked neurotoxicity in rats. Toxicol. Appl. Pharmacol. 2004;196:381–389. doi: 10.1016/j.taap.2003.12.022. [DOI] [PubMed] [Google Scholar]
- Graham D.G. Oxidative pathways for catecholamines in the genesis of neuromelanin and cytotoxic quinones. Mol. Pharmacol. 1978;14:633–643. [PubMed] [Google Scholar]
- Graham D.I. Raghupathi R. Saatman K.E. Meaney D. McIntosh T.K. Tissue tears in the white matter after lateral fluid percussion brain injury in the rat: relevance to human brain injury. Acta Neuropathol. 2000;99:117–124. doi: 10.1007/pl00007414. [DOI] [PubMed] [Google Scholar]
- Greenamyre J.T. MacKenzie G. Peng T.I. Stephans S.E. Mitochondrial dysfunction in Parkinson's disease. Biochem. Soc. Symp. 1999;66:85–97. doi: 10.1042/bss0660085. [DOI] [PubMed] [Google Scholar]
- Griesbach G.S. Molteni R. Gomez-Pinilla F. Hovda D.A. Voluntary exercise therapy after TBI: A critical window of opportunity. J. Neurotrauma. 2002;19:1276. [Google Scholar]
- Hartings J.A. Strong A.J. Fabricius M. Manning A. Bhatia R. Dreier J.P. Mazzeo A.T. Tortella F.C. Bullock M.R. Spreading depolarizations and late secondary insults after traumatic brain injury. J. Neurotrauma. 2009;26:1857–1866. doi: 10.1089/neu.2009.0961. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hertzman C. Wiens M. Bowering D. Snow B. Calne D. Parkinson's disease: a case-control study of occupational and environmental risk factors. Am. J. Ind. Med. 1990;17:349–355. doi: 10.1002/ajim.4700170307. [DOI] [PubMed] [Google Scholar]
- Hicks R. Soares H. Smith D. McIntosh T. Temporal and spatial characterization of neuronal injury following lateral fluid-percussion brain injury in the rat. Acta Neuropathol. 1996;91:236–246. doi: 10.1007/s004010050421. [DOI] [PubMed] [Google Scholar]
- Holmin S. Hojeberg B. In situ detection of intracerebral cytokine expression after human brain contusion. Neurosci. Lett. 2004;369:108–114. doi: 10.1016/j.neulet.2004.07.044. [DOI] [PubMed] [Google Scholar]
- Holmin S. Mathiesen T. Shetye J. Biberfeld P. Intracerebral inflammatory response to experimental brain contusion. Acta Neurochir. (Wien.) 1995;132:110–119. doi: 10.1007/BF01404857. [DOI] [PubMed] [Google Scholar]
- Hutchison J.S. Derrane R.E. Johnston D.L. Gendron N. Barnes D. Fliss H. King W.J. Rasquinha I. MacManus J. Robertson G.S. MacKenzie A.E. Neuronal apoptosis inhibitory protein expression after traumatic brain injury in the mouse. J. Neurotrauma. 2001;18:1333–1347. doi: 10.1089/08977150152725632. [DOI] [PubMed] [Google Scholar]
- Imamura K. Hishikawa N. Sawada M. Nagatsu T. Yoshida M. Hashizume Y. Distribution of major histocompatibility complex class II-positive microglia and cytokine profile of Parkinson's disease brains. Acta Neuropathol. (Berl.) 2003;106:518–526. doi: 10.1007/s00401-003-0766-2. [DOI] [PubMed] [Google Scholar]
- Ip E.Y. Zanier E.R. Moore A.H. Lee S.M. Hovda D.A. Metabolic, neurochemical, and histologic responses to vibrissa motor cortex stimulation after traumatic brain injury. J. Cereb. Blood Flow Metab. 2003;23:900–910. doi: 10.1097/01.WCB.0000076702.71231.F2. [DOI] [PubMed] [Google Scholar]
- Jenkins L.W. Marmarou A. Lewelt W. Becker D.P. Increased vulnerability of the traumatized brain to early ischemia. In: Baethmann A., editor; Go G.K., editor; Unterberg A., editor. Mechanisms of Secondary Brain Damage. Plenum; New York: 1986. pp. 273–282. [Google Scholar]
- Jenkins L.W. Moszynski K. Lyeth B.G. Lewelt W. DeWitt D.S. Allen A. Dixon C.E. Povlishock J.T. Majewski T.J. Clifton G.L. Young H.F. Becker D.P. Hayes R.L. Increased vulnerability of the mildly traumatized rat brain to cerebral ischemia: the use of controlled secondary ischemia as a research tool to identify common or different mechanisms contributing to mechanical and ischemic brain injury. Brain Res. 1989;477:211–224. doi: 10.1016/0006-8993(89)91409-1. [DOI] [PubMed] [Google Scholar]
- Kalfas I.H. Principles of bone healing. Neurosurg. Focus. 2001;10:E1. doi: 10.3171/foc.2001.10.4.2. [DOI] [PubMed] [Google Scholar]
- Kim J.S. He L. Lemasters J.J. Mitochondrial permeability transition: a common pathway to necrosis and apoptosis. Biochem. Biophys. Res. Commun. 2003;304:463–470. doi: 10.1016/s0006-291x(03)00618-1. [DOI] [PubMed] [Google Scholar]
- Kim W.G. Mohney R.P. Wilson B. Jeohn G.H. Liu B. Hong J.S. Regional difference in susceptibility to lipopolysaccharide-induced neurotoxicity in the rat brain: role of microglia. J. Neurosci. 2000;20:6309–6316. doi: 10.1523/JNEUROSCI.20-16-06309.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Knoblach S.M. Faden A.I. Interleukin-10 improves outcome and alters proinflammatory cytokine expression after experimental traumatic brain injury. Exp. Neurol. 1998;153:143–151. doi: 10.1006/exnr.1998.6877. [DOI] [PubMed] [Google Scholar]
- Kosinski C.M. Standaert D.G. Testa C.M. Penney J.B., Jr. Young A.B. Expression of metabotropic glutamate receptor 1 isoforms in the substantia nigra pars compacta of the rat. Neuroscience. 1998;86:783–798. doi: 10.1016/s0306-4522(97)00654-4. [DOI] [PubMed] [Google Scholar]
- Kuter K. Smialowska M. Wieronska J. Zieba B. Wardas J. Pietraszek M. Nowak P. Biedka J. Roczniak W. Konieczny J. Wolfarth S. Ossowska K. Toxic influence of subchoronic paraquat administration on dopaminergic neurons in rats. Brain Res. 2007;1155:196–207. doi: 10.1016/j.brainres.2007.04.018. [DOI] [PubMed] [Google Scholar]
- Langlois J.A. Rutland-Brown W. Thomas K.E. Traumatic Brain Injury in the United States: Emergency Department Visits, Hospitalizations, and Deaths. Centers for Disease Control and Prevention. Atlanta: National Center for Injury Prevention and Control; 2006. [Google Scholar]
- Langston J.W. Predicting Parkinson's disease. Neurology. 1990;40(Suppl.):70–74. [PubMed] [Google Scholar]
- Law M.M. Hovda D.A. Cryer H.G. Fluid-percussion brain injury adversely affects control of vascular tone during hemorrhagic shock. Shock. 1996;6:213–217. [PubMed] [Google Scholar]
- Liang C.L. Sinton C.M. Sonsalla P.K. German D.C. Midbrain dopaminergic neurons in the mouse that contain calbindin-D28k exhibit reduced vulnerability to MPTP-induced neurodegeneration. Neurodegeneration. 1996;5:313–318. doi: 10.1006/neur.1996.0042. [DOI] [PubMed] [Google Scholar]
- Li F.Q. Lu X.Z. Liang X.B. Zhou H.F. Xue B. Liu X.Y. Niu D.B. Han J.S. Wang X.M. Triptolide, a Chinese herbal extract, protects dopaminergic neurons from inflammation-mediated damage through inhibition of microglial activation. J. Neuroimmunol. 2004;148:24–31. doi: 10.1016/j.jneuroim.2003.10.054. [DOI] [PubMed] [Google Scholar]
- Lifshitz J. Friberg H. Neumar R.W. Raghupathi R. Welsh F.A. Janmey P. Saatman K.E. Wieloch T. Grady M.S. McIntosh T.K. Structural and functional damage sustained by mitochondria after traumatic brain injury in the rat: evidence for differentially sensitive populations in the cortex and hippocampus. J. Cereb. Blood Flow Metab. 2003;23:219–231. doi: 10.1097/01.WCB.0000040581.43808.03. [DOI] [PubMed] [Google Scholar]
- Lifshitz J. Sullivan P.G. Hovda D.A. Wieloch T. McIntosh T.K. Mitochondrial damage and dysfunction in traumatic brain injury. Mitochondrion. 2004;4:705–713. doi: 10.1016/j.mito.2004.07.021. [DOI] [PubMed] [Google Scholar]
- Li X.Y. Feng D.F. Diffuse axonal injury: novel insights into detection and treatment. J. Clin. Neurosci. 2009;16:614–619. doi: 10.1016/j.jocn.2008.08.005. [DOI] [PubMed] [Google Scholar]
- Liou H.H. Tsai M.C. Chen C.J. Jeng J.S. Chang Y.C. Chen S.Y. Chen R.C. Environmental risk factors and Parkinson's disease: a case-control study in Taiwan. Neurology. 1997;48:1583–1588. doi: 10.1212/wnl.48.6.1583. [DOI] [PubMed] [Google Scholar]
- Liu B. Jiang J.W. Wilson B.C. Du L. Yang S.N. Wang J.Y. Wu G.C. Cao X.D. Hong J.S. Systemic infusion of naloxone reduces degeneration of rat substantia nigral dopaminergic neurons induced by intranigral injection of lipopolysaccharide. J. Pharmacol. Exp. Ther. 2000;295:125–132. [PubMed] [Google Scholar]
- Liu Y. Qin L. Li G. Zhang W. An L. Liu B. Hong J.S. Dextromethorphan protects dopaminergic neurons against inflammation-mediated degeneration through inhibition of microglial activation. J. Pharmacol. Exp. Ther. 2003;305:212–218. doi: 10.1124/jpet.102.043166. [DOI] [PubMed] [Google Scholar]
- Lu X.H. Fleming S.M. Meurers B. Ackerson L.C. Mortazavi F. Lo V. Hernandez D. Sulzer D. Jackson G.R. Maidment N.T. Chesselet M.F. Yang X.W. Bacterial artificial chromosome transgenic mice expressing a truncated mutant parkin exhibit age-dependent hypokinetic motor deficits, dopaminergic neuron degeneration, and accumulation of proteinase K-resistant alpha-synuclein. J. Neurosci. 2009;29:1962–1976. doi: 10.1523/JNEUROSCI.5351-08.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ma S.Y. Roytta M. Rinne J.O. Collan Y. Rinne U.K. Single section and dissector counts in evaluating neuronal loss from the substantia nigra in patients with Parkinson's disease. Neuropathol. Appl. Neurobiol. 1995;21:341–343. doi: 10.1111/j.1365-2990.1995.tb01068.x. [DOI] [PubMed] [Google Scholar]
- Maeda T. Lee SM. Hovda DA. Restoration of cerebral vasoreactivity by an L-type calcium channel blocker following fluid percussion brain injury. J. Neurotrauma. 2005;22:763–771. doi: 10.1089/neu.2005.22.763. [DOI] [PubMed] [Google Scholar]
- Maxwell W.L. Povlishock J.T. Graham D.L. A mechanistic analysis of nondisruptive axonal injury: a review. J. Neurotrauma. 1997;14:419–440. doi: 10.1089/neu.1997.14.419. [DOI] [PubMed] [Google Scholar]
- Mazzeo A.T. Beat A. Singh A. Bullock M.R. The role of mitochondrial transition pore, and its modulation, in traumatic brain injury and delayed neurodegeneration after TBI. Exp. Neurol. 2009;218:363–370. doi: 10.1016/j.expneurol.2009.05.026. [DOI] [PubMed] [Google Scholar]
- McCormack A.L. Thiruchelvam M. Manning-Bog A.B. Thiffault C. Langston J.W. Cory-Slechta D.A. Di Monte D.A. Environmental risk factors and Parkinson's disease: selective degeneration of nigral dopaminergic neurons caused by the herbicide paraquat. Neurobiol. Dis. 2002;10:119–127. doi: 10.1006/nbdi.2002.0507. [DOI] [PubMed] [Google Scholar]
- McGeer P.L. Itagaki S. Boyes B.E. McGeer E.G. Reactive microglia are positive for HLA-DR in the substantia nigra of Parkinson's and Alzheimer's disease brains. Neurology. 1988;38:1285–1291. doi: 10.1212/wnl.38.8.1285. [DOI] [PubMed] [Google Scholar]
- McIntosh T.K. Vink R. Noble L. Yamakami I. Fernyak S. Soares H. Faden A.L. Traumatic brain injury in the rat: characterization of a lateral fluid-percussion model. Neuroscience. 1989;28:233–244. doi: 10.1016/0306-4522(89)90247-9. [DOI] [PubMed] [Google Scholar]
- Meurers B.H. Zhu C. Fernagut P.O. Richter F. Hsia Y.C. Fleming S.M. Oh M. Elashoff D. Dicarlo C.D. Seaman R.L. Chesselet M.F. Low dose rotenone treatment causes selective transcriptional activation of cell death related pathways in dopaminergic neurons in vivo. Neurobiol. Dis. 2009;33:182–192. doi: 10.1016/j.nbd.2008.10.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meythaler J.M. Peduzzi J.D. Eleftheriou E. Novack T.A. Current concepts: diffuse axonal injury-associated traumatic brain injury. Arch. Phys. Med. Rehabil. 2001;82:1461–1471. doi: 10.1053/apmr.2001.25137. [DOI] [PubMed] [Google Scholar]
- Miyazaki I. Asanuma M. Approaches to prevent dopamine quinone-induced neurotoxicity. Neurochem. Res. 2009;34:698–706. doi: 10.1007/s11064-008-9843-1. [DOI] [PubMed] [Google Scholar]
- Mizuno Y. Ohta S. Tanaka M. Takamiya S. Suzuki K. Sato T. Oya H. Ozawa T. Kagawa Y. Deficiencies in complex I subunits of the respiratory chain in Parkinson's disease. Biochem. Biophys. Res. Commun. 1989;163:1450–1455. doi: 10.1016/0006-291x(89)91141-8. [DOI] [PubMed] [Google Scholar]
- Mouton P.R. Gokhale A.M. Ward N.L. West M.J. Stereological length estimation using spherical probes. J. Microsc. 2002;206:54–64. doi: 10.1046/j.1365-2818.2002.01006.x. [DOI] [PubMed] [Google Scholar]
- Nicklas W.J. Vyas I. Heikkila R.E. Inhibition of NADH-linked oxidation in brain mitochondria by 1-methyl-4-phenyl-pyridine, a metabolite of the neurotoxin, 1-methyl-4-phenyl-1,2,5,6-tetrahydropyridine. Life Sci. 1985;36:2503–2508. doi: 10.1016/0024-3205(85)90146-8. [DOI] [PubMed] [Google Scholar]
- Nonaka M. Chen X. Pierce J.S. Leoni M.J. McIntoch T.K. Wolf J.A. Smith D.H. Prolonged activation of NF-kB following traumatic brain injury in rats. J. Neurotrauma. 1999;16:1023–1034. doi: 10.1089/neu.1999.16.1023. [DOI] [PubMed] [Google Scholar]
- Oorschot D.E. Total number of neurons in the neostriatal, pallidal, subthalamic, and substantia nigral nuclei of the rat basal ganglia: a stereological study using the cavalieri and optical dissector methods. J. Comp. Neurol. 1996;366:580–599. doi: 10.1002/(SICI)1096-9861(19960318)366:4<580::AID-CNE3>3.0.CO;2-0. [DOI] [PubMed] [Google Scholar]
- Ossowska K. Wardas J. Smialowska M. Kuter K. Lenda T. Wieronska J.M. Zieba B. Nowak P. Dabrowska J. Bortel A. Kwiecinski A. Wolfarth S. A slowly developing dysfunction of dopaminergic nigrostriatal neurons induced by long-term paraquat administration in rats: an animal model of preclinical stages of Parkinson's disease? Eur. J. Neurosci. 2005;22:1294–1304. doi: 10.1111/j.1460-9568.2005.04301.x. [DOI] [PubMed] [Google Scholar]
- Pakkenberg B. Moller A. Gundersen H.J. Mouritzen Dam A. Pakkenberg H. The absolute number of nerve cells in substantia nigra in normal subjects and in patients with Parkinson's disease estimated with an unbiased stereological method. J. Neurol. Neurosurg. Psychiatry. 1991;54:30–33. doi: 10.1136/jnnp.54.1.30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parker W.D., Jr. Boyson S.J. Parks J.K. Abnormalities of the electron transport chain in idiopathic Parkinson's disease. Ann. Neurol. 1989;26:719–723. doi: 10.1002/ana.410260606. [DOI] [PubMed] [Google Scholar]
- Petrovic V.M. Spasic M. Milic B. Saicic Age-dependent resistance to the toxic effects of paraquat in relation to superoxide dismutase activity in rat lung. Free Radical Research Commun. 1986;1:305–309. doi: 10.3109/10715768609080969. [DOI] [PubMed] [Google Scholar]
- Pierce J.E. Smith D.H. Trojanowski J.Q. McIntosh T.K. Enduring cognitive, neurobehavioral and histopathological changes persist for up to one year following severe experimental brain injury in rats. Neuroscience. 1998;87:359–369. doi: 10.1016/s0306-4522(98)00142-0. [DOI] [PubMed] [Google Scholar]
- Polymeropoulos M.H. Lavedan C. Leroy E. Ide S.E. Dehejia A. Dutra A. Pike B. Root H. Rubenstein J. Boyer R. Stenroos E.S. Chandrasekharappa S. Athanassiadou A. Papapetropoulos T. Johnson W.G. Lazzarini A.M. Duvoisin R.C. Di Iorio G. Golbe L.I. Nussbaum R.L. Mutation in the alpha-synuclein gene identified in families with Parkinson's disease. Science. 1997;276:2045–2047. doi: 10.1126/science.276.5321.2045. [DOI] [PubMed] [Google Scholar]
- Povlishock J.T. Pathobiology of traumatically induced axonal injury in animals and man. Ann. Emerg. Med. 1993;22:980–986. doi: 10.1016/s0196-0644(05)82738-6. [DOI] [PubMed] [Google Scholar]
- Prensa L. Parent A. The nigrostriatal pathway in the rat: A single-axon study of the relationship between dorsal and ventral tier nigral neurons and the striosome/matrix striatal compartments. J. Neurosci. 2001;21:7247–7260. doi: 10.1523/JNEUROSCI.21-18-07247.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Prins M.L. Lee S.M. Cheng C.L. Becker D.P. Hovda D.A. Fluid percussion brain injury in the developing and adult rat: a comparative study of mortality, morphology, intracranial pressure and mean arterial blood pressure. Brain Res. Dev. Brain Res. 1996;95:272–282. doi: 10.1016/0165-3806(96)00098-3. [DOI] [PubMed] [Google Scholar]
- Purisai M.G. McCormack A.L. Cumine S. Li J. Isla M.Z. Di Monte D.A. Microglial activation as a priming event leading to paraquat-induced dopaminergic cell degeneration. Neurobiol. Dis. 2007;25:392–400. doi: 10.1016/j.nbd.2006.10.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Qin L. Liu Y. Wang T. Wei S.J. Block M.L. Wilson B. Liu B. Hong J.S. NADPH oxidase mediates lipopolysaccharide-induced neurotoxicity and proinflammatory gene expression in activated microglia. J. Biol. Chem. 2004;279:1415–1421. doi: 10.1074/jbc.M307657200. [DOI] [PubMed] [Google Scholar]
- Qin L. Wu X. Block M.L. Liu Y. Breese G.R. Hong J.S. Knapp D.J. Crews F.T. Systemic LPS causes chronic neuroinflammation and progressive neurodegeneration. Glia. 2007;55:453–462. doi: 10.1002/glia.20467. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Raghupathi R. Cell death mechanisms following traumatic brain injury. Brain Pathol. 2004;14:215–222. doi: 10.1111/j.1750-3639.2004.tb00056.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roberts R.C. Force M. Kung L. Dopaminergic synapses in the matrix of the ventrolateral striatum after chronic haloperidol treatment. Synapse. 2002;45:78–85. doi: 10.1002/syn.10081. [DOI] [PubMed] [Google Scholar]
- Rodriguez-Paez A.C. Brunschwig J.P. Bramlett H.M. Light and electron microscopic assessment of progressive atrophy following moderate traumatic brain injury in the rat. Acta Neuropathol. 2005;109:603–616. doi: 10.1007/s00401-005-1010-z. [DOI] [PubMed] [Google Scholar]
- Rudow G. O'Brien R. Savonenko A.V. Resnick S.M. Zonderman A.B. Pletnikova O. Marsh L. Dawson T.M. Crain B.J. West M.J. Troncoso J.C. Morphometry of the human substantia nigra in ageing and Parkinson's disease. Acta Neuropathol. 2008;115:461–470. doi: 10.1007/s00401-008-0352-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rugbjerg K. Ritz B. Korbo L. Martinussen N. Olsen J.H. Risk of Parkinson's disease after hospital contact for head injury: population based case-control study. BMJ. 2008;337:a2494. doi: 10.1136/bmj.a2494. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saint-Pierre M. Tremblay M.E. Sik A. Gross R.E. Cicchetti F. Temporal effects of paraquat/maneb on microglial activation and dopamine neuronal loss in older rats. J. Neurochem. 2006;98:760–772. doi: 10.1111/j.1471-4159.2006.03923.x. [DOI] [PubMed] [Google Scholar]
- Schapira A.H. Cooper J.M. Dexter D. Jenner P. Clark J.B. Marsden C.D. Mitochondrial complex I deficiency in Parkinson's disease. Lancet. 1989;1:1269. doi: 10.1016/s0140-6736(89)92366-0. [DOI] [PubMed] [Google Scholar]
- Semchuk K.M. Love E.J. Lee R.G. Parkinson's disease and exposure to agricultural work and pesticide chemicals. Neurology. 1992;42:1328–1335. doi: 10.1212/wnl.42.7.1328. [DOI] [PubMed] [Google Scholar]
- Sherer T.B. Betarbet R. Stout A.K. Lund S. Baptista M. Panov A.V. Cookson M.R. Greenamyre J.T. An in vitro model of Parkinson's disease: linking mitochondrial impairment to altered alpha-synuclein metabolism and oxidative damage. J. Neurosci. 2002;22:7006–7015. doi: 10.1523/JNEUROSCI.22-16-07006.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sherer T.B. Kim J.H. Betarbet R. Greenamyre J.T. Subcutaneous rotenone exposure causes highly selective dopaminergic degeneration and alpha-synuclein aggregation. Exp. Neurol. 2003;179:9–16. doi: 10.1006/exnr.2002.8072. [DOI] [PubMed] [Google Scholar]
- Smith D.H. Chen X.H. Pierce J.E. Wolf J.A. Trojanowski J.Q. Graham D.I. McIntosh T.K. Progressive atrophy and neuron death for one year following brain trauma in the rat. J. Neurotrauma. 1997;14:715–727. doi: 10.1089/neu.1997.14.715. [DOI] [PubMed] [Google Scholar]
- Soares H.D. Hicks R.R. Smith D. McIntosh T.K. Inflammatory leukocytic recruitment and diffuse neuronal degeneration are separate pathological processes resulting from traumatic brain injury. J. Neurosci. 1995;15:8223–8233. doi: 10.1523/JNEUROSCI.15-12-08223.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stokely M.E. Orr E.L. Acute effects of calvarial damage on dural mast cells, pial vascular permeability, and cerebral cortical histamine levels in rats and mice. J. Neurotrauma. 2008;25:52–61. doi: 10.1089/neu.2007.0397. [DOI] [PubMed] [Google Scholar]
- Stokes A.H. Hastings T.G. Vrana K.E. Cytotoxic and genotoxic potential of dopamine. J. Neurosci. Res. 1999;55:659–665. doi: 10.1002/(SICI)1097-4547(19990315)55:6<659::AID-JNR1>3.0.CO;2-C. [DOI] [PubMed] [Google Scholar]
- Sulzer D. Multiple hit hypotheses for dopamine neuron loss in Parkinson's disease. Trends Neurosci. 2007;30:244–250. doi: 10.1016/j.tins.2007.03.009. [DOI] [PubMed] [Google Scholar]
- Suzuki T. Bramlett H.M. Ruenes G. Dietrich W.D. The effects of early post-traumatic hyperthermia in female and ovariectomized rats. J. Neurotrauma. 2004;21:842–853. doi: 10.1089/0897715041526186. [DOI] [PubMed] [Google Scholar]
- Taya K. Marmarou C.R. Okuno K. Prieto R. Marmarou A. Effect of secondary insults upon aquaporin-4 water channels following experimental cortical contusion in rats. J. Neurotrauma. 2010;27:229–239. doi: 10.1089/neu.2009.0933. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taylor C.A. Saint-Hilaire M.H. Cupples L.A. Thomas C.A. Burchard A.E. Feldman R.G. Myers R.H. Environmental, medical, and family history risk factors for Parkinson's disease: a New England-based case control study. Am. J. Med. Genet. 1999;88:742–749. [PubMed] [Google Scholar]
- Thiruchelvam M. McCormack A. Richfield E.K. Baggs R.B. Tank A.W. Di Monte D.A. Cory-Slechta D.A. Age-related irreversible progressive nigrostriatal dopaminergic neurotoxicity in the paraquat and maneb model of the Parkinson's disease phenotype. Eur. J. Neurosci. 2003;18:589–600. doi: 10.1046/j.1460-9568.2003.02781.x. [DOI] [PubMed] [Google Scholar]
- Thompson H.J. Hoover R.C. Tkacs N.C. Saatman K.E. McIntosh T.K. Development of posttraumatic hyperthermia after traumatic brain injury in rats is associated with increased periventricular inflammation. J. Cereb. Blood Flow Metab. 2005a;25:163–176. doi: 10.1038/sj.jcbfm.9600008. [DOI] [PubMed] [Google Scholar]
- Thompson H.J. Lifshitz J. Marklund N. Grady M.S. Graham D.I. Hovda D.A. McIntosh T.K. Lateral fluid percussion brain injury: a 15-year review and evaluation. J. Neurotrauma. 2005b;22:42–75. doi: 10.1089/neu.2005.22.42. [DOI] [PubMed] [Google Scholar]
- Tsuji K. Bandyopadhyay A. Harfe B.D. Cox K. Kakar S. Gerstenfeld L. Einhorn T. Tabin C.J. Rosen V. BMP2 activity, although dispensable for bone formation, is required for the initiation of fracture healing. Nat. Genet. 2006;38:1424–1429. doi: 10.1038/ng1916. [DOI] [PubMed] [Google Scholar]
- Uryu K. Giasson B.I. Longhi L. Martinez D. Murray I. Conte V. Nakamura M. Saatman K. Talbot K. Horiguchi T. McIntosh T. Lee V.M. Trojanowski J.Q. Age-dependent synuclein pathology following traumatic brain injury in mice. Exp. Neurol. 2003;184:214–224. doi: 10.1016/s0014-4886(03)00245-0. [DOI] [PubMed] [Google Scholar]
- Van Putten H.P. Bouwhuis M.G. Muizelaar J.P. Lyeth B.G. Berman R.F. Diffusion-weighted imaging of edema following traumatic brain injury in rats: effects of secondary hypoxia. J. Neurotrauma. 2005;22:857–872. doi: 10.1089/neu.2005.22.857. [DOI] [PubMed] [Google Scholar]
- Wagner A.K. Sokoloski J.E. Ren D. Chen X. Khan A.S. Zafonte R.D. Michael A.C. Dixon C.E. Controlled cortical impact injury affects dopaminergic transmission in the rat striatum. J. Neurochem. 2005;95:457–465. doi: 10.1111/j.1471-4159.2005.03382.x. [DOI] [PubMed] [Google Scholar]
- Wahner A.D. Bronstein J.M. Bordelon Y.M. Ritz B. Nonsteroidal anti-inflammatory drugs may protect against Parkinson disease. Neurology. 2007a;69:1836–1842. doi: 10.1212/01.wnl.0000279519.99344.ad. [DOI] [PubMed] [Google Scholar]
- Wahner A.D. Sinsheimer J.S. Bronstein J.M. Ritz B. Inflammatory cytokine gene polymorphisms and increased risk of Parkinson disease. Arch. Neurol. 2007b;64:836–840. doi: 10.1001/archneur.64.6.836. [DOI] [PubMed] [Google Scholar]
- Wang T. Liu B. Zhang W. Wilson B. Hong J.S. Andrographolide reduces inflammation-mediated dopaminergic neurodegeneration in mesencephalic neuron-glia cultures by inhibiting microglial activation. J. Pharmacol. Exp. Ther. 2004;308:975–983. doi: 10.1124/jpet.103.059683. [DOI] [PubMed] [Google Scholar]
- Werneck A.L. Alvarenga H. Genetics, drugs and environmental factors in Parkinson's disease. A case-control study. Arq. Neuropsiquiatr. 1999;57:347–355. doi: 10.1590/s0004-282x1999000300001. [DOI] [PubMed] [Google Scholar]
- West M.J. Design-based stereological methods for counting neurons. Prog. Brain Res. 2002;135:43–51. doi: 10.1016/S0079-6123(02)35006-4. [DOI] [PubMed] [Google Scholar]
- Wu X.F. Block M.L. Zhang W. Qin L. Wilson B. Zhang W.Q. Veronesi B. Hong J.S. The role of microglia in paraquat-induced dopaminergic neurotoxicity. Antioxid. Redox. Signal. 2005;7:654–661. doi: 10.1089/ars.2005.7.654. [DOI] [PubMed] [Google Scholar]
- Zanier E.R. Lee S.M. Vespa P.M. Giza C.C. Hovda D.A. Increased hippocampal CA3 vulnerability to low-level kainic acid following lateral fluid percussion injury. J. Neurotrauma. 2003;20:409–420. doi: 10.1089/089771503765355496. [DOI] [PubMed] [Google Scholar]