Abstract
Until recently, mild traumatic brain injury (mTBI) or “concussion” was generally ignored as a major health issue. However, emerging evidence suggests that this injury is by no means mild, considering it induces persisting neurocognitive dysfunction in many individuals. Although little is known about the pathophysiological aspects of mTBI, there is growing opinion that diffuse axonal injury (DAI) may play a key role. To explore this possibility, we adapted a model of head rotational acceleration in swine to produce mTBI by scaling the mechanical loading conditions based on available biomechanical data on concussion thresholds in humans. Using these input parameters, head rotational acceleration was induced in either the axial plane (transverse to the brainstem; n=3), causing a 10- to 35-min loss of consciousness, or coronal plane (circumferential to the brainstem; n=2), which did not produce a sustained loss of consciousness. Seven days following injury, immunohistochemical analyses of the brains revealed that both planes of head rotation induced extensive axonal pathology throughout the white matter, characterized as swollen axonal bulbs or varicosities that were immunoreactive for accumulating neurofilament protein. However, the distribution of the axonal pathology was different between planes of head rotation. In particular, more swollen axonal profiles were observed in the brainstems of animals injured in the axial plane, suggesting an anatomic substrate for prolonged loss of consciousness in mTBI. Overall, these data support DAI as an important pathological feature of mTBI, and demonstrate that surprisingly overt axonal pathology may be present, even in cases without a sustained loss of consciousness.
Key words: concussion, diffuse axonal injury, head rotational acceleration, head rotational velocity, mild traumatic brain injury, post-concussion syndrome, traumatic axonal injury
Introduction
Although mild traumatic brain injury (mTBI) affects over 1.4 million individuals each year in the U.S. (Faul et al., 2010; Langlois et al., 2004), until recently it has generally been ignored as a major health issue. Commonly referred to as “concussion,” mTBI is defined as a traumatically-induced disruption of brain function, manifested by either a short loss of consciousness, memory dysfunction, or a Glasgow Coma Scale (GCS) score of 13–15 (Kay et al., 1993; Teasdale and Jennett, 1974). mTBI patients evaluated in hospital emergency departments are typically discharged home after an unremarkable computed tomography scan or lack of progressing symptoms. However, mTBI is by no means mild, considering that it induces persisting cognitive dysfunction in many patients. While there is debate regarding the overall impact of mTBI on society, it has been reported that more than a third of prospective mTBI patients did not resume work by 3 months after injury (Boake et al., 2005), and persisting neurocognitive deficits have been found in approximately 15% of mTBI patients (Roe et al., 2009; Williams et al., 2010).
Over the past several years, there has been growing awareness of the toll of mTBI on society (McGarry et al., 2002; National Center for Injury Prevention and Control, 2003; Rockhill et al., 2010). In part, this has been due to the wide recognition that TBI is the “signature wound” of current military conflicts. In addition, there has been substantial recent media attention on the dangers of sports concussion in amateur and professional competitions, especially with regard to potentially enhanced vulnerability with repeat injuries, leading to long-term neuropathological changes (McKee et al., 2009). However, little is known about the potential anatomic substrates or pathophysiological changes in the brain that cause the clinical manifestations of mTBI, such as loss of consciousness and/or cognitive impairment.
For moderate to severe TBI, diffuse axonal injury (DAI) is well recognized as one of the most common and important pathologies (Adams et al., 1982,1989; Graham et al., 1988; Povlishock, 1992; Povlishock and Becker, 1985; Povlishock and Katz, 2005; Povlishock et al., 1983; Smith and Meaney, 2000a). However, by definition, mTBI alone is non-fatal, thereby severely restricting the opportunity for neuropathological examination. Nonetheless, DAI has become widely accepted as the main pathological substrate of mTBI, despite scant direct neuropathological evidence. Indeed, there has only been one brief previous report in 1994 by Blumbergs and colleagues addressing the neuropathology of mTBI (Blumbergs et al., 1994). The study was limited to five patients diagnosed with mTBI, four of whom were over 78 years old, and one was 58 years of age, with deaths due to causes not related to the TBI, including complications of polytrauma and infection. Immunostaining of the brains for amyloid precursor protein (APP) demonstrated multifocal APP accumulation in axonal swellings in the white matter, particularly in the fornices. Notably, however, potentially due to the small number of cases and the ages at the time of injury, the authors stopped short of describing the pathology as DAI.
More recently, non-invasive examination of potential DAI in the white matter of living mTBI patients has been described in multiple studies using advanced neuroimaging techniques (Bazarian et al., 2007; Huang et al., 2009; Inglese et al., 2005; Messe et al., 2010; Wilde et al., 2008). While these reports have helped support the concept that DAI is a prominent feature of mTBI, there has been no direct evidence that the signal changes seen in the images identified axonal pathology.
To examine pathological changes in mTBI, in the present study we modified a model of head rotational acceleration in swine to induce mechanical loading conditions to the brain akin to those found in mTBI in humans. In past work using rotational acceleration in this species, we focused on the relatively high mechanical loading conditions that induced prolonged coma in association with moderate-to-severe diffuse axonal injury and neuron degeneration. In this study, we use the available biomechanical data on concussion thresholds in humans, along with data from our earlier work that presented loading conditions for injury thresholds (Meaney et al., 1995), to scale the rotational accelerations accordingly for swine. Two different planes of head rotation were utilized to examine the predominant neuropathological changes in mTBI, and to explore how potential differences in the distribution of pathological changes may play a role in transient loss of consciousness.
Methods
All experiments were carried out in accordance with policies set forth by the University of Pennsylvania Institutional Animal Care and Use Committee, and in accordance with National Institutes of Health (NIH) guidelines for the humane and ethical treatment of animals.
Mechanical input parameters for concussion loading
Our first objective was to scale the loading conditions used in previous studies of rotational acceleration in miniature swine (Meaney et al., 1995; Smith et al., 1997, 1999a, 2000b) to estimate the rotational motions associated with concussive loading. We used a simplified scaling relationship (A.H.S. Holbourn [1956]; private communication to Dr. Sabina Stritch, October 13, 1956; Ommaya et al., 1967), and a typical brain mass of the miniature pig (80–90 g) and humans (1000–1400 g) to transfer the accelerations associated with concussion described in recent studies (5600–8000 rad/sec2; Duma et al., 2005; Frechede and McIntosh, 2009; Greenwald et al., 2008; Newman et al., 2000; Pellman et al., 2003) to an equivalent loading in the smaller miniature swine brain. We also included scaling of rotational velocity in our estimates, using a different scaling relationship (Ommaya et al., 1967). Based on these estimated scaling relationships and the range of brain mass for humans and miniature swine, we targeted coronal plane rotational accelerations of at least 28,000 rad/sec2, and no more than 59,000 rad/sec2. Axial plane accelerations in the miniature pig cause an enhancement of strain in the brainstem region (Miller et al., 1998) compared to coronal plane accelerations, and we therefore targeted a proportionally lower level of peak axial plane accelerations (14,000–30,000 rad/sec2).
Groups and preparation for injury
The design of this study was based on previous examinations at higher levels of head rotational acceleration. In prior studies, even relatively high levels of acceleration in the coronal plane (circumferential to the brainstem) did not induce prolonged loss of consciousness across a large number of animals (Chen et al., 1999; Smith et al., 1997, 1999a, 2000b). For axial plane rotation (transverse to the brainstem), high levels of acceleration in one study previously induced prolonged coma (Smith et al., 2000b). For the current study, we utilized these two planes of head rotation to examine outcomes at accelerations scaled to levels relevant to human mTBI.
Animals (n=6) were fasted for 12 h, after which anesthesia was induced via intramuscular injection of midazolam (0.4 mg/kg). Once the animals were sedated, they were endotracheally intubated, and anesthesia was maintained with 1.5% isoflurane/2L O2. Heart rate, respiratory rate, oxygen saturation, and temperature were monitored continuously. One animal serving as a sham received the same anesthesia but received no brain injury.
Head injury procedures
Brain trauma was induced via head rotational acceleration as previously described in detail (Smith et al., 1997, 1999a, 2000b). Briefly, the animals' heads were secured to a padded snout clamp, which in turn was attached to a pneumatic actuator that converts linear motion into angular motion (rotational acceleration). Triggered release of pressurized nitrogen drives the linkage assembly. For injury in the coronal (n=2) and axial (n=3) planes (Fig. 1), the actuator was set to deliver a velocity between 95 and 120 rad/sec (duration 20 msec). Immediately prior to induction of the injury, the anesthesia tubing was disconnected from the endotracheal tube. The animals' heads were released from the clamp following injury. Animals injured in the coronal plane began to show signs of waking (eyes open and responsive to ear pinch) within 5 min of injury, at which time anesthesia was reinstated. Animals injured in the axial plane remained unconscious for 10–35 min (lack of spontaneous eye opening, corneal reflex, and response to pain) before reinstatement of anesthesia. Upon completion of the injury and the acute post-injury monitoring phase, the animals were transported back to their cages and given access to food and water ad libitum. Daily health checks were made to ensure that there were no adverse events during recovery.
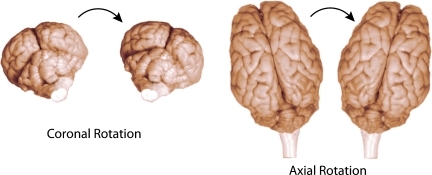
FIG. 1.
Idealized schematic demonstrating rotational acceleration in the coronal plane (left) and axial plane (right). The movement of the head was circumferential to the brainstem in the coronal plane and transverse to the brainstem in the axial plane. A pneumatic actuator was set to deliver approximate peak accelerations of 28,000–59,000 rad/sec2 in the coronal plane, and 14,000–30,000 rad/sec2 in the axial plane over 20 msec. Color image is available online at www.liebertonline.com/neu
Tissue preparation
All animals in both groups were euthanized 1 week after undergoing injury. Swine were fasted for 12 h prior to the procedure and prepared as described above. While under isoflurane anesthesia, the animals were transcardially perfused with heparinized saline followed by 4% paraformaldehyde and their brains were removed. Tissue was post-fixed in 4% paraformaldehyde for an additional 24 h and blocked into 2-mm-thick coronal sections. Intact brains, as well as blocked tissue, were evaluated for gross changes such as swelling, hemorrhage, and ventriculomegaly. Subsequently, the blocks were prepared for paraffin embedding and 10-micron-thick sections were cut on a rotary microtome. Twenty sections were cut from the face of each block and stained with cresyl violet, hematoxylin and eosin, or immunostain.
Evaluation of axonal pathology
Several slides from each of the frontal, parietal, hippocampal, occipital (including the midbrain), cerebellar, and brainstem regions were examined for axonal pathology using a mouse monoclonal antibody to NF200. The sections were deparaffinized and rehydrated, after which endogenous peroxidase activity was blocked using 5% H2O2 in methanol. Antigen unmasking was performed using high temperature epitope retrieval. Sections were then blocked in 2% normal horse serum and incubated in NF200 antibody (1:400; Sigma-Aldrich, St. Louis, MO) overnight at room temperature. The following day the sections were washed, incubated for 1 h in donkey anti-mouse HRP-conjugated secondary antibody (1:500; Jackson ImmunoResearch Labs, West Grove, PA), and washed again. Visualization of the antigen of interest was achieved after 5 min of exposure to diaminobenzidine (DAB; Vector Laboratories, Burlingame, CA).
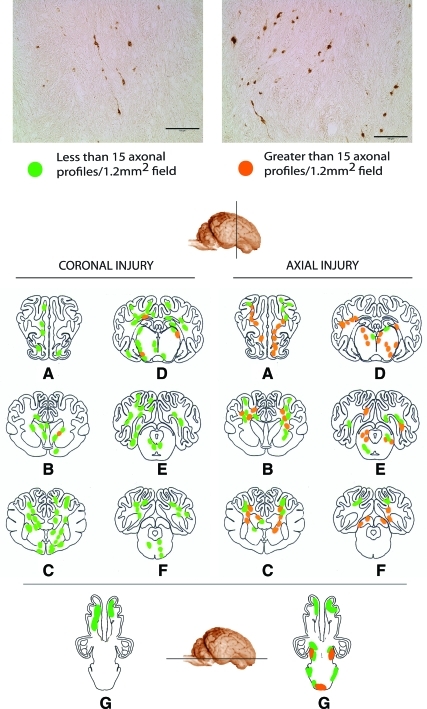
The slides were examined using a Nikon E600 Eclipse microscope equipped with a Canon G11 digital camera. We have previously developed a semi-quantitative sector-scoring technique to determine the number of profiles representing axonal injury (Smith et al., 1997). For each slide, scoring of axonal damage (swellings and/or bulbs) was performed by assigning a rating of mild (1–15 damaged axons/1.2 mm2 field) or moderate (greater than 15 damaged axons/1.2 mm2 field; Fig. 2). A schematic map of the injury pattern was created after an examiner blind to injury status evaluated all sections.
FIG. 2.
Photomicrographs showing detailed axonal pathology (top), and regionalized schematics (bottom), showing areas of mild (less than 15 profiles) or moderate (greater than 15 profiles) damage following mild traumatic brain injury in swine. Schematics represent coronal sections through the (A) frontal region, (B) anterior commisure level, (C) rostral thalamus, (D) caudal hippocampal level, (E) hippocampal/brainstem, (F) occipital/brainstem region. Schematic G is a transverse section through the ventral surface of the brain. Damaged axons presented as discrete bulbs or swellings. A higher number of moderate profiles were observed in the brains of animals injured in the axial plane, especially in the brainstem (lower profile). The reader is referred to the text for an explanation of axonal profiles (scale bars=100 μm). Color image is available online at www.liebertonline.com/neu
Results
Plane of head rotation and loss of consciousness
Our targeted applied loading conditions were intended to model the conditions associated with producing concussion in humans. Falling within our targeted conditions, peak rotational accelerations in the coronal plane ranged from 30,000–59,000 rad/sec2, while peak axial plane rotational accelerations ranged from 15,000–26,000 rad/sec2. Peak rotational velocities ranged from 112–146 rad/sec (coronal plane) and 99.6–115 rad/sec (axial plane). By comparison with previous studies using the swine model with injury in both planes of rotation, injury parameters included peak angular accelerations of approximately 105,000 rad/sec2 and peak angular velocities of approximately 290 rad/sec (Kimura et. al., 1996; Meaney et al., 1995; Smith et al., 1997, 2000b; Zhang et al., 2008). In these previous studies, both planes of head rotation produced extensive axonal pathology, while only axial plane rotation induced prolonged coma. By comparison to human data on the levels of head accelerations and velocities thought to induce concussion (Duma et al., 2005; Frechede and McIntosh, 2009; Greenwald et al., 2008; Newman et al., 2000; Pellman et al., 2003), we scaled our input accelerations to be 4–6 times higher. Based on our calculations, this increase was needed to produce the same extent of dynamic tissue deformation in an 80–90 g swine brain as occurs during concussive head accelerations for the human 1000–1400 g brain.
Through a series of previous examinations, it has been established that animals under anesthesia that do not receive injury begin to right themselves and attempt ambulation within 5 min following withdrawal of anesthesia. In this study, animals injured in the coronal plane opened their eyes and responded to stimuli within approximately 5 min of injury, a similar time interval to that of awakening for non-injured animals following withdrawal of anesthesia. No adverse effects on heart rate, respiratory rate, oxygen saturation, and temperature were noted, and the animals returned to eating, drinking, and normal behavior within 24 h of injury. In contrast, all 3 animals with head rotation in the axial plane remained in an unconscious state for 10–35 min, as determined by a lack of spontaneous movement and eye opening, lack of corneal reflex, and lack of response to stimuli. During this time period, no adverse effects on heart rate, respiratory rate, oxygen saturation, and temperature were noted. Following recovery of consciousness, both groups of animals receiving mild head rotational acceleration were slow-moving and had a sluggish response to stimuli over hours after injury. By comparison, through multiple previous studies, it was observed that non-injured swine recovering from anesthesia appeared bright, alert, and responsive to stimuli within 30 min. All injured animals returned to eating and drinking by the end of the day, and resumed normal behavior within 24 h of injury.
General pathology
Examination of the intact brain, as well as the dissected blocks of all animals, did not reveal any gross pathological changes, such as contusions or hemorrhages. Microscopically, a modest number of degenerating neurons (approximately 20–25 per entire coronal tissue section), as revealed by hematoxylin and eosin staining, were observed in random locations throughout the cortex and within the hippocampus of the brains extracted from the animals injured in the axial plane. In contrast, no degenerating neurons were observed in the hippocampi of animals injured in the coronal plane; however, some degenerating neurons (approximately 10–15 per tissue section) were observed in the cortex with no discernible pattern in their distribution. No evidence of microhemorrhages was observed in any of the animals in this study.
Axonal pathology
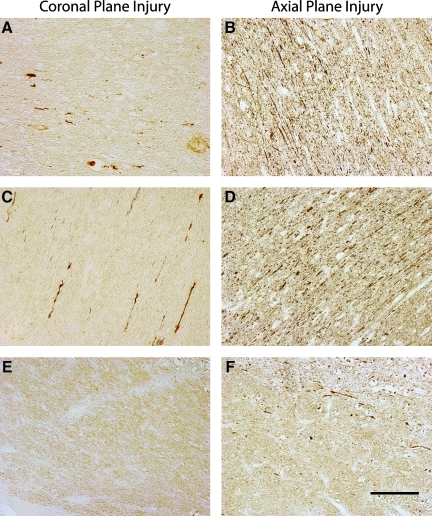
Axonal bulbs and swellings, as demonstrated by clearly morphologically swollen axon profiles immunoreactive for accumulating neurofilament protein (Fig. 3), were found throughout the white matter of all five injured animals. In both planes of injury, axonal pathology extended from the frontal lobe to the cerebellum, and was found in both the midbrain and brainstem (Fig. 2). Specifically, swellings in the form of discrete axonal bulbs and axonal varicosities were observed in the roots of the gyri, at the interface of the grey and white matter, and within the deep white matter. These profiles were observed in a wide range of shapes and sizes, identical to the character of traumatic axonal pathology found in humans with TBI (Adams et al., 1989; Chen et al., 2009; Grady et al., 1993; Povlishock et al., 1983; Uryu et al., 2007). While the anterior-posterior distribution of the pathology was similar between the two planes of injury, the density of profiles differed. By routine examination, it was clearly discernible that there were a far greater number of swollen axonal profiles in the brains of animals injured in the axial plane compared to the coronal plane, especially in the posterior regions such as the occipital lobe, cerebellum, and brainstem (Fig. 2).
FIG. 3.
Representative photomicrographs showing axonal damage in different regions of the swine brain following rotational injury in the coronal (left) or axial (right) plane. Damaged axons presented as discrete bulbs or swellings in the white matter of the frontal (A and B), parietal (C and D), and medulla (E and F) regions of the brain. Very few axonal profiles were noted in the brainstem region of the animals injured in the coronal plane. In contrast, axonal damage was prominent in the same region of animals injured in the axial plane (scale bar=100 μm). Color image is available online at www.liebertonline.com/neu
Discussion
By scaling real-world mechanical loading conditions of mTBI in humans, a mTBI model of head rotational acceleration in swine was developed. Since the swine has a smaller brain mass than humans, the levels of head rotational acceleration were increased to approximately 4–6 times the accelerations estimated to cause a similar extent of dynamic brain deformation found in human mTBI. Notably, however, the conditions associated with concussion in humans are under constant study, using both detailed reconstruction of sports events and field measurements of the exposures experienced by football players (Greenwald et al., 2008; Pellman et al., 2003). Thus, the mechanical loading conditions for swine mTBI can be sharpened in the future with advancing human data.
Two planes of head rotation were evaluated to explore a potential planar influence on functional and histopathological outcome. Notably, for animals without injury, the time interval to awakening after withdrawal of anesthesia was approximately 5 min. With anesthesia withdrawn just prior to head rotation circumferential to the brainstem in the coronal plane, the animals also attempted to right themselves within approximately 5 min after injury. Thus, while potential transient loss of consciousness due to the injury cannot be determined due to the residual anesthetic effects, no sustained loss of consciousness was observed. In contrast, head rotation transverse to the brainstem in the axial plane with the same loading conditions induced loss of consciousness lasting 10–35 min post-injury, substantially exceeding the effects of anesthesia alone. Importantly, this overall range in the duration of loss of consciousness across the two planes of head rotation in swine is similar to the range defined for mTBI in humans, from 0–30 min (Kay et al., 1993).
Upon histopathological examination, it was found that both planes of rotational injury resulted in DAI, characterized by widely disseminated multi-focal axonal pathology throughout the white matter, extending from the frontal lobe to the brainstem and cerebellum. This axonal pathology was identified as immunoreactive profiles of axonal swellings containing accumulated neurofilament protein, suggesting a local interruption of axonal transport. These profiles appeared in either the form of discrete bulb formations at the ends of disconnected axons, or as periodic swellings or varicosities along the length of an axon. While the morphology of the axonal pathology was the same between the two groups, injury via axial plane rotation induced a greater concentration of axonal swellings overall, especially in the brainstem.
The present data support the widely held notion that DAI is a key pathologic substrate of mTBI, and experimentally corroborate the observations by Blumbergs and colleagues of extensive axonal swellings immunoreactive for APP in the brains of 5 mTBI patients. In addition, these data demonstrate that DAI can occur even without a marked loss of consciousness, and that loss of consciousness in mTBI appears to be dependent on the density and/or distribution of axonal pathology in select regions, such as the brainstem.
DAI has long been recognized as the hallmark pathology of moderate and severe TBI in humans. Moreover, DAI has been shown to be responsible for the immediate loss of consciousness and prolonged coma seen in moderate and severe TBI, as demonstrated in seminal studies using a primate model of head rotational acceleration, and more recently in the swine model of head rotational acceleration as described above (Gennarelli and Thibault, 1982; Smith et al., 2000b). Thus it may appear somewhat anticipated that lower levels of head rotational acceleration in swine would induce a milder form of DAI. However, it was unknown whether levels of injury that induce only transient loss of consciousness in the swine would be accompanied by overt damage to axons manifested by swellings or only by subtle changes. Indeed, the severity and extent of overt axonal pathology observed in the mTBI swine model was quite surprising for animals with such rapid recoveries to apparently normal behavior. Since overt swellings due to transport interruption typically signal that the axons will go on to degenerate, these data demonstrate that even mild TBI induces permanent damage to the brain.
In a previous study, very high levels of head rotational acceleration in the coronal and axial planes induced far more severe axonal pathology than that found in the present study (Smith et al., 2000b). However, even with such extensive white matter damage, only the axial plane head rotation induced prolonged coma associated with greater brainstem injury compared with coronal plane injury. Collectively, the results from high and low level head rotational acceleration in swine suggest that while the length of unconsciousness may generally reflect the relative severity of axonal pathology, the reverse is not true. Specifically, even extensive axonal pathology throughout the hemispheric white matter may occur without a substantial loss of consciousness.
While the present data support the premise that axonal injury plays an important role in mTBI, the actual extent of the pathological changes seen under the “DAI” designation remains unknown. At all levels of TBI, large axonal swellings are considered to be only the extreme end of the range of pathologic changes seen under the heading of DAI. During TBI, all axons within a white matter tract are thought to suffer relatively similar dynamic deformations. Yet even in severe TBI, only a small percentage of axons within a given tract undergo transport interruption resulting in accumulation of transported cargoes in swellings. Unique to traumatic axonal injury, this immediate transport interruption may reflect mechanical disruption of the axonal cytoskeleton, such as breaking of microtubules due the dynamic aspect of the deformation (Smith et al., 1999b; Tang-Schomer et al., 2010). Ultimately, these axons may disconnect at distal points of swellings, which triggers degeneration within days after injury, as characterized by Povlishock and colleagues (Povlishock and Becker, 1985; Grady et al., 1993).
On the other hand, axons that undergo dynamic deformation but do not swell may nonetheless suffer subtle yet important pathophysiological changes, including an increase in intra-axonal calcium and sodium concentrations or mitochondrial dysfunction, as suggested by previous in vivo and in vitro studies (Iwata et al., 2004; Pettus and Povlishock, 1996; Pettus et al., 1994; Smith et al., 1999b; Staal et al., 2010; Wolf et al., 2001; Yuen et al., 2009). These processes are thought to contribute to physiological dysfunction of white matter pathways, such as the reduction in conduction velocity that has been observed in mTBI (Baker et al., 2002; Kumar et al., 2009; Nuwer et al., 2005). In addition, increased intra-axonal calcium concentrations may activate proteases that can initiate or further damage the axon cytoskeleton. Moreover, these pathological processes may induce persistent changes in many traumatically injured axons, as is suggested by the observation that axonal swelling and disconnection can occur even months and years after moderate and severe TBI (Chen et al., 2004,2009).
Although both overt and subtle pathological changes to axons appear to play roles in the immediate loss of consciousness and/or cognitive dysfunction that characterizes mTBI, the relative contributions of differing forms of axonal pathologies have yet to be determined. In addition, there may be many other subtle pathological changes not found in the present study that affect cognitive outcome, such as dendritic alterations, imbalances in neurotransmitters, or changes in brain metabolism, as previously suggested (Chen et al., 2010a,2010b; Monnerie et al., 2010; Schwarzbach et al., 2006). These considerations highlight the potential difficulties in using a “DAI” designation for the overall pathological changes seen in mTBI. With the growing interest in DAI, it may be important to have a consensus to define which pathological processes should be encompassed by the “DAI” designation.
Since DAI has been considered a “stealth” pathology in survivors of TBI, there has been great interest in advancing non-invasive techniques for its detection, especially for mTBI. Although found throughout the white matter, the microscopic nature of DAI renders it almost invisible to conventional neuroimaging techniques, and is often a diagnosis of exclusion for TBI patients. Nonetheless, several recent studies using advanced neuroimaging techniques, such as diffusion tensor imaging (DTI), have elucidated changes in the white matter of mTBI patients (Bazarian et al., 2007; Huang et al., 2009; Inglese et al., 2005; Messe et al., 2010; Wilde et al., 2008). These human studies have been supported by evidence in rodent models of TBI that the signal changes seen with DTI correspond with axonal pathology (Mac Donald et al., 2007). Therefore, the current data demonstrate the potential utility of the swine mTBI model of head rotational acceleration to further these investigations by providing direct comparisons of the distribution of axonal pathology with signal changes found with advanced neuroimaging techniques, an effort that is currently underway.
Overall, the present results support DAI as an important pathological feature of mTBI and demonstrate that surprisingly overt axonal pathology may be present, even in cases without a sustained loss of consciousness. Thus mTBI or concussion is only mild relative to severe TBI, and is by no means inconsequential, considering that it can induce permanent damage to the brain.
Acknowledgments
We would like to thank Robert F. Groff, Jun Zhang, and Andrew Eng for their excellent technical assistance. This study was conducted with support from NIH grants NS08803, NS038104, and NS056202.
Author Disclosure Statement
No competing financial interests exist.
References
- Adams J.H. Doyle D. Ford I. Gennarelli T.A. Graham D.I. McLellan D.R. Diffuse axonal injury in head injury: definition, diagnosis and grading. Histopathology. 1989;151:49–59. doi: 10.1111/j.1365-2559.1989.tb03040.x. [DOI] [PubMed] [Google Scholar]
- Adams J.H. Gennarelli T.A. Graham D.I. Brain damage in non missle head injury: observations in man and subhuman primates. In: Smith W.T., editor; Cavanaugh J.B., editor. Recent Advances in Neuropathology. Churchill Livingstone; Edinburgh: 1982. pp. 165–190. [Google Scholar]
- Baker A.J. Phan N. Moulton R.J. Fehlings M.G. Yucel Y. Zhao M. Liu E. Tian G.F. Attenuation of the electrophysiological function of the corpus callosum after fluid percussion injury in the rat. J. Neurotrauma. 2002;19:587–599. doi: 10.1089/089771502753754064. [DOI] [PubMed] [Google Scholar]
- Bazarian J.J. Zhong J. Blyth B. Zhu T. Kavcic V. Peterson D. Diffusion tensor imaging detects clinically important axonal damage after mild traumatic brain injury: a pilot study. J. Neurotrauma. 2007;24:1447–1459. doi: 10.1089/neu.2007.0241. [DOI] [PubMed] [Google Scholar]
- Blumbergs P.C. Scott G. Manavis J. Wainwright H. Simpson D.A. McLean A.J. Staining of amyloid precursor protein to study axonal damage in mild head injury. Lancet. 1994;344:1055–1056. doi: 10.1016/s0140-6736(94)91712-4. [DOI] [PubMed] [Google Scholar]
- Boake C. McCauley S.R. Pedroza C. Levin H.S. Brown S.A. Brundage S.I. Lost productive work time after mild to moderate traumatic brain injury with and without hospitalization. Neurosurgery. 2005;56:994–1003. discussion 1994–1003. [PubMed] [Google Scholar]
- Chen J.R. Wang T.J. Wang Y.J. Tseng G.F. The immediate large-scale dendritic plasticity of cortical pyramidal neurons subjected to acute epidural compression. Neuroscience. 2010a;167:414–427. doi: 10.1016/j.neuroscience.2010.02.028. [DOI] [PubMed] [Google Scholar]
- Chen L.J. Wang Y.J. Tseng G.F. Compression alters kinase and phosphatase activity and tau and MAP2 phosphorylation transiently while inducing the fast adaptive dendritic remodeling of underlying cortical neurons. J. Neurotrauma. 2010b;27:1657–1669. doi: 10.1089/neu.2010.1308. [DOI] [PubMed] [Google Scholar]
- Chen X.H. Johnson V.E. Uryu K. Trojanowski J.Q. Smith D.H. A lack of amyloid beta plaques despite persistent accumulation of amyloid beta in axons of long-term survivors of traumatic brain injury. Brain Pathol. 2009;19:214–223. doi: 10.1111/j.1750-3639.2008.00176.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen X.-H. Meaney D.F. Xu B.-N. Nonaka M. McIntosh T.K. Wolf J.A. Saatman K.E. annd Smith D.H. Evolution of neurofilament subtype accumulation in axons following diffuse brain injury in the pig. J. Neuropathol. Exp. Neurol. 1999;58:588–596. doi: 10.1097/00005072-199906000-00003. [DOI] [PubMed] [Google Scholar]
- Chen X.H. Siman R. Iwata A. Meaney D.F. Trojanowski J.Q. Smith D.H. Long-term accumulation of amyloid-beta, beta-secretase, presenilin-1, and caspase-3 in damaged axons following brain trauma. Am. J. Pathol. 2004;165:357–371. doi: 10.1016/s0002-9440(10)63303-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Duma S.M. Manoogian S.J. Bussone W.R. Brolinson P.G. Goforth M.W. Donnenwerth J.J. Greenwald R.M. Chu J.J. Crisco J.J. Analysis of real-time head accelerations in collegiate football players. Clin. J. Sport Med. 2005;15:3–8. doi: 10.1097/00042752-200501000-00002. [DOI] [PubMed] [Google Scholar]
- Faul M.X.L. Wald M.M. Coronado V.G. Traumatic Brain Injury in the United States: Emergency Department Visits, Hospitalizations and Deaths 2002–2006. Atlanta: Centers for Disease Control and Prevention; 2010. [Google Scholar]
- Frechede B. McIntosh A.S. Numerical reconstruction of real-life concussive football impacts. Med. Sci. Sports Exerc. 2009;41:390–398. doi: 10.1249/MSS.0b013e318186b1c5. [DOI] [PubMed] [Google Scholar]
- Gennarelli T.A. Thibault L.E. Experimental production of prolonged traumatic coma in the primate. In: Villani R., editor; Papo I., editor; Giovanelli M., editor; Gaini S.M., editor; Tomei G., editor. Advances in Neurotraumotology, 612. Excerpta Med; Amsterdam: 1982. pp. 31–33. [Google Scholar]
- Grady M.S. McLaughlin M.R. Christman C.W. Valadka A.B. Fligner C.L. Povlishock J.T. The use of antibodies targeted against the neurofilament subunits for the detection of diffuse axonal injury in humans. J. Neuropathol. Exp. Neurol. 1993;52:143–152. doi: 10.1097/00005072-199303000-00007. [DOI] [PubMed] [Google Scholar]
- Graham D.I. Adams J.H. Gennarelli T.A. Mechanisms of non-penetrating head injury. Prog. Clin. Biol. Res. 1988;234:159–168. [PubMed] [Google Scholar]
- Greenwald R.M. Gwin J.T. Chu J.J. Crisco J.J. Head impact severity measures for evaluating mild traumatic brain injury risk exposure. Neurosurgery. 2008;62:789–798. doi: 10.1227/01.neu.0000318162.67472.ad. discussion 798. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang M. Theilmann R.J. Robb A. Angeles A. Nichols S. Drake A. Dandrea J. Levy M. Holland M. Song T. Ge S. Hwang E. Yoo K. Cui L. Baker D.G. Trauner D. Coimbra R. Lee R.R. Integrated imaging approach with MEG and DTI to detect mild traumatic brain injury in military and civilian patients. J Neurotrauma. 2009;26:1213–1226. doi: 10.1089/neu.2008.0672. [DOI] [PubMed] [Google Scholar]
- Inglese M. Makani S. Johnson G. Cohen B.A. Silver J.A. Gonen O. Grossman R.I. Diffuse axonal injury in mild traumatic brain injury: a diffusion tensor imaging study. J. Neurosurg. 2005;103:298–303. doi: 10.3171/jns.2005.103.2.0298. [DOI] [PubMed] [Google Scholar]
- Iwata A. Stys P.K. Wolf J.A. Chen X.-H. Taylor A.G. Meaney D.F. Smith D.H. Traumatic axonal injury induces proteolytic cleavage of the voltage-gated sodium channels modulated by tetrodotoxin and protease inhibitors. J. Neurosci. 2004;24:4605–4613. doi: 10.1523/JNEUROSCI.0515-03.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kay T. Harrington D.E. Adams R. Anderson T. Berrol S. Cicerone K. Dahlberg C. Gerber D. Goka R. Harley P. Hilt J. Horn L. Lehmkuhl D. Malec J. Definition of mild traumatic brain injury. J. Head Trauma Rehabil. 1993;8:86–87. [Google Scholar]
- Kimura H. Meaney D.F. McGowan J.C. Grossman R.I. Lenkinski R.E. Ross D.T. McIntosh T.K. Gennarelli T.A. Smith D.H. Magnetization transfer imaging of diffuse axonal injury following experimental brain injury in the pig: Characterization by magnetization transfer ratio with histopathologic correlation. J. Comput. Assist. Tomography. 1996;20:540–546. doi: 10.1097/00004728-199607000-00007. [DOI] [PubMed] [Google Scholar]
- Kumar S. Rao S.L. Chandramouli B.A. Pillai S.V. Reduction of functional brain connectivity in mild traumatic brain injury during working memory. J. Neurotrauma. 2009;26:665–675. doi: 10.1089/neu.2008.0644. [DOI] [PubMed] [Google Scholar]
- Langlois J.A. Rutland-Brown W. Tomas K.E. Traumatic Brain Injury in the United States: Emergency Department Visits, Hospitalization, and Deaths. Atlanta: Centers for Disease Control and Prevention, National Center of Injury Prevention and Control; 2004. [Google Scholar]
- Mac Donald C.L. Dikranian K. Bayly P. Holtzman D. Brody D. Diffusion tensor imaging reliably detects experimental traumatic axonal injury and indicates approximate time of injury. J. Neurosci. 2007;27:11869–11876. doi: 10.1523/JNEUROSCI.3647-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McGarry L.J. Thompson D. Millham F.H. Cowell L. Snyder P.J. Lenderking W.R. Weinstein M.C. Outcomes and costs of acute treatment of traumatic brain injury. J. Trauma. 2002;53:1152–1159. doi: 10.1097/00005373-200212000-00020. [DOI] [PubMed] [Google Scholar]
- McKee A.C. Cantu R.C. Nowinski C.J. Hedley-Whyte E.T. Gavett B.E. Budson A.E. Santini V.E. Lee H.-Y. Kubilus C.A. Stern R.A. Chronic traumatic encephalopathy in athletes: Progressive tauopathy after repetitive head injury. J. Neuropath. Exp. Neurol. 2009;68:709–735. doi: 10.1097/NEN.0b013e3181a9d503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meaney D.F. Smith D.H. Shreiber D.I. Bain A.C. Miller R.T. Ross D.T. Gennarelli T.A. Biomechanical analysis of experimental diffuse axonal injury. J. Neurotrauma. 1995;12:689–694. doi: 10.1089/neu.1995.12.689. [DOI] [PubMed] [Google Scholar]
- Messe A. Caplain S. Paradot G. Garrigue D. Mineo J.F. Soto Ares G. Ducreux D. Vignaud F. Rozec G. Desal H. Pelegrini-Issac M. Montreuil M. Benali H. Lehericy S. Diffusion tensor imaging and white matter lesions at the subacute stage in mild traumatic brain injury with persistent neurobehavioral impairment. Hum. Brain Mapp. 2010;32:999–1011. doi: 10.1002/hbm.21092. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miller R.T. Margulies S.S. Leoni M. Nonaka M. Chen X.H. Smith D.H. Meaney D.F. Finite element modeling approaches for predicting injury in an experimental model of severe diffuse axonal injury; 42nd Stapp Car Crash Conference Proceedings. Nov.; 1998. [DOI] [Google Scholar]
- Monnerie H. Tang-Schomer M.D. Iwata A. Smith D.H. Kim H.A. Le Roux P.D. Dendritic alterations after dynamic axonal stretch injury in vitro. Exp. Neurol. 2010;224:415–423. doi: 10.1016/j.expneurol.2010.05.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- National Center for Injury Prevention and Control. Report to Congress on Mild Traumatic Brain Injury in the United States: Steps to Prevent a Serious Public Health Problem. Atlanta: Centers for Disease Control and Prevention; 2003. [Google Scholar]
- Newman J.A. Shewchenko N. Welbourne E. A proposed new biomechanical head injury assessment function—the maximum power index. Stapp Car Crash J. 2000;44:215–247. doi: 10.4271/2000-01-SC16. [DOI] [PubMed] [Google Scholar]
- Nuwer M.R. Hovda D.A. Schrader L.M. Vespa P.M. Routine and quantitative EEG in mild traumatic brain injury. Clin. Neurophysiol. 2005;116:2001–2025. doi: 10.1016/j.clinph.2005.05.008. [DOI] [PubMed] [Google Scholar]
- Ommaya A.K. Yarnell P. Hirsch A., et al. Scaling of experimental data on cerebral concussion in sub-human primates to concussive thresholds in man, in Proceedings of the 11th Stapp Car Crash Conference. Warrendale, PA: SAE; 1967. pp. 73–80. [Google Scholar]
- Pellman E.J. Viano D.C. Tucker A.M. Casson I.R. Waeckerle J.F. Concussion in professional football: reconstruction of game impacts and injuries. Neurosurgery. 2003;53:799–812. doi: 10.1093/neurosurgery/53.3.799. [DOI] [PubMed] [Google Scholar]
- Pettus E.H. Christman C.W. Giebel M.L. Povlishock J.T. Traumatically induced altered membrane permeability: its relationship to traumatically induced reactive axonal change. J. Neurotrauma. 1994;11:507–522. doi: 10.1089/neu.1994.11.507. [DOI] [PubMed] [Google Scholar]
- Pettus E.H. Povlishock J.T. Characterization of a distinct set of intra-axonal ultrastructural changes associated with traumatically induced alteration in axolemmal permeability. Brain Res. 1996;722:1–11. doi: 10.1016/0006-8993(96)00113-8. [DOI] [PubMed] [Google Scholar]
- Povlishock J.T. Becker D.P. Fate of reactive axonal swellings induced by head injury. Lab. Invest. 1985;52:540–552. [PubMed] [Google Scholar]
- Povlishock J.T. Becker D.P. Cheng C.L.Y. Vaughan G.W. Axonal change in minor head injury. J. Neuropathol. Exp. Neurol. 1983;42:225–242. doi: 10.1097/00005072-198305000-00002. [DOI] [PubMed] [Google Scholar]
- Povlishock J.T. Katz D.I. Update of neuropathology and neurological recovery after traumatic brain injury. J. Head Trauma Rehabil. 2005;20:76–94. doi: 10.1097/00001199-200501000-00008. [DOI] [PubMed] [Google Scholar]
- Povlishock J.T. Traumatically induced axonal injury: pathogenesis and pathobiological implications. Brain Pathol. 1992;2:1–12. [PubMed] [Google Scholar]
- Rockhill C.M. Fann J.R. Fan M.Y. Hollingworth W. Katon W.J. Healthcare costs associated with mild traumatic brain injury and psychological distress in children and adolescents. Brain Inj. 2010;24:1051–1060. doi: 10.3109/02699052.2010.494586. [DOI] [PubMed] [Google Scholar]
- Roe C. Sveen U. Alvsaker K. Bautz-Holter E. Post-concussion symptoms after mild traumatic brain injury: influence of demographic factors and injury severity in a 1-year cohort study. Disabil. Rehabil. 2009;31:1235–1243. doi: 10.1080/09638280802532720. [DOI] [PubMed] [Google Scholar]
- Schwarzbach E. Bonislawski D.P. Xiong G. Cohen A.S. Mechanisms underlying the inability to induce area CA1 LTP in the mouse after traumatic brain injury. Hippocampus. 2006;16:541–550. doi: 10.1002/hipo.20183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith D.H. Chen X.H. Nonaka M. Trojanowski J.Q. Lee V.M. Saatman K.E. Leoni M.J. Xu B.N. Wolf J.A. Meaney D.F. Accumulation of amyloid beta and tau and the formation of neurofilament inclusions following diffuse brain injury in the pig. J. Neuropathol. Exp. Neurol. 1999a;58:982–992. doi: 10.1097/00005072-199909000-00008. [DOI] [PubMed] [Google Scholar]
- Smith D.H. Chen X.H. Xu B.N. McIntosh T.K. Gennarelli T.A. Meaney D.F. Characterization of diffuse axonal pathology and selective hippocampal damage following inertial brain trauma in the pig. J. Neuropathol. Exp. Neurol. 1997;56:822–834. [PubMed] [Google Scholar]
- Smith D.H. Meaney D.F. Axonal damage in traumatic brain injury. Neuroscientist. 2000a;6:483–495. [Google Scholar]
- Smith D.H. Nonaka M. Miller R. Leoni M. Chen X.-H. Alsop D. Meaney D.F. Immediate coma following inertial brain injury dependent on pathological condition of axons in the brainstem. J. Neurosurg. 2000b;93:315–322. doi: 10.3171/jns.2000.93.2.0315. [DOI] [PubMed] [Google Scholar]
- Smith D.H. Wolf J.A. Lusardi T.A. Lee V.M.-Y. Meaney D.F. High tolerance and delayed elastic response of cultured axons to dynamic stretch injury. J. Neurosci. 1999b;19:4263–4269. doi: 10.1523/JNEUROSCI.19-11-04263.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Staal J.A. Dickson T.C. Gasperini R. Liu Y. Foa L. Vickers J.C. Initial calcium release from intracellular stores followed by calcium dysregulation is linked to secondary axotomy following transient axonal stretch injury. J. Neurochem. 2010;112:1147–1155. doi: 10.1111/j.1471-4159.2009.06531.x. [DOI] [PubMed] [Google Scholar]
- Tang-Schomer M.D. Patel A.R. Baas P.W. Smith D.H. Mechanical breaking of microtubules in axons during dynamic stretch injury underlies delayed elasticity, microtubule disassembly, and axon degeneration. FASEB J. 2010;24:1401–1410. doi: 10.1096/fj.09-142844. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Teasdale G. Jennett B. Assessment of coma and impaired consciousness. A practical scale. Lancet. 1974;2:81–84. doi: 10.1016/s0140-6736(74)91639-0. [DOI] [PubMed] [Google Scholar]
- Uryu K. Chen X.H. Martinez D. Browne K.D. Johnson V.E. Graham D.I. Lee V.M. Trojanowski J.Q. Smith D.H. Multiple proteins implicated in neurodegenerative diseases accumulate in axons after brain trauma in humans. Exp. Neurol. 2007;208:185–192. doi: 10.1016/j.expneurol.2007.06.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilde E.A. McCauley S.R. Hunter J.V. Bigler E.D. Chu Z. Wang Z.J. Hanten G.R. Troyanskaya M. Yallampalli R. Li X. Chia J. Levin H.S. Diffusion tensor imaging of acute mild traumatic brain injury in adolescents. Neurology. 2008;70:948–955. doi: 10.1212/01.wnl.0000305961.68029.54. [DOI] [PubMed] [Google Scholar]
- Williams W.H. Potter S. Ryland H. Mild traumatic brain injury and postconcussion syndrome: a neuropsychological perspective. J. Neurol. Neurosurg. Psychiatry. 2010;81:1116–1122. doi: 10.1136/jnnp.2008.171298. [DOI] [PubMed] [Google Scholar]
- Wolf J.A. Stys P.K. Lusardi T. Meaney D. Smith D.H. Traumatic axonal injury induces calcium influx modulated by tetrodotoxin-sensitive sodium channels. J. Neurosci. 2001;21:1923–1930. doi: 10.1523/JNEUROSCI.21-06-01923.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yuen T.J. Browne K.D. Iwata A. Smith D.H. Sodium channelopathy induced by mild axonal trauma worsens outcome after a repeat injury. J. Neurosci. Res. 2009;87:3620–3625. doi: 10.1002/jnr.22161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang J. Groff R.F., 4th Chen X.H. Browne K.D. Huang J. Schwartz E.D. Meaney D.F. Johnson V.E. Stein S.C. Rojkjaer R. Smith D.H. Hemostatic and neuroprotective effects of human recombinant activated factor VII therapy after traumatic brain injury in pigs. Exp. Neurol. 2008;210:645–655. doi: 10.1016/j.expneurol.2007.12.019. [DOI] [PMC free article] [PubMed] [Google Scholar]