Abstract
Rationale: There is increasing evidence that emphysema is associated with primary loss of pulmonary capillary endothelium. Plasma levels of endothelial microparticles (EMPs), small vesicles released from activated or apoptotic endothelial cells, are elevated in vascular-related disorders.
Objectives: To evaluate whether plasma EMP levels are elevated in smokers with early lung destruction as assessed by normal spirometry but reduced diffusing capacity of the lung for carbon monoxide (DlCO).
Methods: Lung health was assessed by pulmonary function tests (PFTs: spirometry, total lung capacity, DlCO) and chest X-ray; smoking status was assessed by urine nicotine and cotinine. EMP levels (CD42b−CD31+ microparticles) were quantified as activated or apoptotic. The initial cohort (n = 92) included healthy nonsmokers (normal PFTs), healthy smokers (normal PFTs), and smokers with early evidence of lung destruction (normal spirometry, low DlCO). Two prospective cohorts were then tested: a group similar to the initial cohort and an HIV1+ cohort.
Measurements and Main Results: Healthy smokers had mildly increased levels of EMPs. Strikingly, 95% of smokers with normal spirometry, low DlCO had increased EMPs, with reduced CD62+/CD31+ ratios (P < 10−4) and elevated CD42b−CD31+ annexin V+ EMPs (P < 10−4), suggesting derivation from endothelial apoptosis. Most elevated EMPs were angiotensin-converting enzyme positive, suggesting derivation from pulmonary capillaries. Both prospective cohorts confirmed the initial cohort data.
Conclusions: Plasma EMPs with apoptotic characteristics are elevated in smokers with normal spirometry but reduced DlCO, consistent with the concept that emphysema is associated, in part, with capillary endothelium apoptosis, suggesting that the early development of emphysema might be monitored with plasma EMP levels.
Keywords: endothelium, apoptosis, endothelium-derived factors, microcirculation, smoking
At a Glance Commentary
Scientific Knowledge on the Subject
Pulmonary endothelial apoptosis is a mechanism in emphysema development. Increased endothelial apoptosis occurs in the lungs of smokers with emphysema and alveolar destruction may be initiated, in part, by apoptosis of pulmonary capillaries.
What This Study Adds to the Field
Smokers with evidence of emphysema may have elevated plasma levels of endothelial microparticles, released from activated or apoptotic endothelial cells. This study may imply a plasma-based method to identify early onset of smoking-induced emphysema.
Gas exchange takes place in the alveoli, fragile structures that bring air and blood in close contact through the alveolar epithelium, interstitial connective tissue, and capillary endothelium (1). When put under the chronic stress of cigarette smoking, alveoli may be destroyed, resulting in emphysema (2–6). The pathogenesis of emphysema is complex and includes the balance of proteases and antiproteases in the lung, tilted toward an excess of unopposed proteases that destroy the connective tissue backbone of the lung parenchyma (2–7). There is increasing evidence, however, that loss of alveolar endothelial cells by apoptosis is also central to the pathogenesis of lung destruction (3, 8–14).
The physiologic correlate of emphysema is a reduction in the diffusion capacity of the lung of carbon monoxide (DlCO), a functional measure of the ability of the alveolar-capillary units to transfer gas from air to blood (15, 16). Eventually, as sufficient numbers of alveolar-capillary units are destroyed, the bronchial tree loses its supporting framework of surrounding alveoli, resulting in limitation to expiratory airflow (3, 17, 18). With this background, and in the context of the evidence that apoptosis of the pulmonary capillary endothelium participates in the pathogenesis of emphysema (8–13), we hypothesized that early in the process of lung destruction, smokers may have fragments of the endothelium in the circulation. This can be measured by quantifying circulating endothelial microparticles (EMPs), 0.1- to 1.5-μm vesicles, shed from the endothelium in response to cell activation, injury, and/or apoptosis (19–21). EMPs, quantified in plasma as particles that are CD31+ (the constitutive endothelial marker PECAM), but CD42b− (the constitutive platelet-specific glycoprotein Ib), are present in low levels in plasma of healthy individuals and reflecting normal endothelial turnover (19, 21, 22). EMP levels are increased in a variety of vascular-related disorders (21, 23–37). Using CD62 (E-selectin, an adhesion molecule expressed on activated endothelium), activation-induced EMPs have a high CD42b−CD62+/CD42b−CD31+ ratio, and apoptosis-induced EMPs have a low ratio (19–21, 34).
Based on these considerations, we assessed the levels of circulating EMPs in a cohort of 92 subjects, including healthy nonsmokers, healthy and symptomatic smokers with normal lung function, and healthy smokers with normal spirometry but low DlCO (i.e., smokers with early evidence of lung destruction before the development of expiratory airflow limitation). The data in this cohort, as well as in two prospective cohorts with similar physiologic findings, demonstrate that smokers with normal spirometry and normal DlCO have levels of circulating EMPs that are mildly elevated compared with healthy nonsmokers, but that smokers who are normal by the Global Initiative for Chronic Obstructive Lung Disease (GOLD) spirometric criteria for chronic obstructive pulmonary disease (COPD) (38), but have reduced DlCO (a parameter not part of the GOLD criteria), have marked increases in the levels of circulating EMPs. Most of these EMPs have a low CD42b−CD62+/CD42b−CD31+ ratio and elevated CD42b−CD31+ annexin V+ levels, suggesting these EMPs arise, at least in part, by apoptosis (19–21, 34). Finally, the majority of the EMPs in the low DlCO smokers are angiotensin-converting enzyme (ACE) positive, suggesting they are derived from the pulmonary capillary endothelium (39). Together, the data suggest that in the early stages of smoking-induced lung destruction, there is apoptosis-mediated loss of endothelium before any spirometric evidence of lung disease.
Some of these results have been previously reported in the form of an abstract (40).
Methods
Human Subjects and Clinical Phenotypes
All individuals were evaluated at the Weill Cornell National Institutes of Health Clinical and Translational Science Center (CTSC) and Department of Genetic Medicine Clinical Research Facility, under Institutional Review Board–approved clinical protocols. Written informed consent was obtained from each individual before enrollment. Screening included history, complete physical examination, blood studies, urinalysis, chest X-ray, electrocardiogram, and pulmonary function tests (PFTs), including FVC, FEV1, FEV1/FVC, total lung capacity (TLC), and DlCO, all performed under American Thoracic Society guidelines (41). If the FEV1 was less than 80% predicted and/or the FEV1/FVC less than 0.7, the spirometry was retested after standard bronchodilators (38, 42). Measurement of the DlCO was performed two to four times in all individuals; the average of the best two trials was used. The diameter of the main pulmonary artery was assessed by chest X-rays as a correlate to the pulmonary artery pressure. In all individuals, the PA diameter was less than 30 mm, indicating normal estimated pulmonary pressure. Percentage emphysema was evaluated with the EmphylxJ software application (EmphylxJ; Vancouver, BC, Canada) allowing automated quantitative analysis of transverse chest computed tomography (CT) scans. Emphysema was defined as greater than 3% lung volume with attenuation less than or equal to −950 Hounsfield units (HU) or greater than 16% lung volume with attenuation less than or equal to −910 HU, values derived from analyses of high-resolution CT (HRCT) in normal nonsmoking individuals with normal lung function. Current smokers were defined as self-reported current smokers with verification of current smoking status by urinary levels of nicotine and its derivative cotinine. The last cigarette was more than 12 hours before all testing. All individuals had normal α1-antitrypsin levels, normal C-reactive protein levels and all were HIV-1 negative (for full inclusion/exclusion criteria, see online supplement).
A total of 92 individuals were assessed as an initial study population (Table 1) using the following definitions: “healthy nonsmokers” (n = 32), lifelong never smokers with nondetectable urine nicotine (< 2 ng/ml) and cotinine (< 5 ng/ml), normal PFTs (spirometry, TLC, DlCO) and chest X-ray; “healthy smokers with normal spirometry and normal DlCO” (n = 41): asymptomatic active smokers with normal PFTs and chest X-ray (n = 32) and symptomatic smokers with normal PFTs and chest X-ray (n = 9), but with cough (0–4 scale [42]) and/or sputum production (0–4 scale [43]); and “healthy smokers with normal spirometry but low DlCO” (n = 19): active smokers with normal spirometry and TLC, but reduced DlCO.
TABLE 1.
INITIAL STUDY POPULATION
| Parameter | Group A: Healthy Nonsmokers with Normal Spirometry and Normal DlCO | Group B: Healthy Smokers with Normal Spirometry and Normal DlCO* | Group C: Healthy Smokers with Normal Spirometry but Low DlCO |
| n | 32 | 41 | 19 |
| Sex, male/female | 14/18 | 31/10 | 15/4 |
| Age, yr | 37 ± 15 | 40 ± 9 | 46 ± 8 |
| Ancestry, B/W/O | 9/14/9 | 31/4/6 | 15/2/2 |
| Smoking history, pack-years | 0 | 19 ± 13 | 34 ± 19 |
| Urine nicotine, ng/ml | Negative | 1,041 ± 1,136 | 1,500 ± 1,459 |
| Urine cotinine, ng/ml | Negative | 1,565 ± 664 | 1,715 ± 1,132 |
| Pulmonary function† | |||
| FEV1 | 106 ± 14 | 106 ± 12 | 104 ± 15 |
| FVC | 108 ± 14 | 111 ± 12 | 108 ± 14 |
| FEV1/FVC | 82 ± 5 | 79 ± 6 | 78 ± 5 |
| TLC | 100 ± 15 | 95 ± 10 | 98 ± 17 |
| DlCO | 95 ± 15 | 91 ± 9 | 70 ± 7 |
| C-reactive protein (mg/dl) | 0.44 ± 0.24 | 0.51 ± 0.51 | 0.41 ± 0.26 |
Definition of abbreviations: B/W/O = black/white/other; DlCO = diffusion capacity of the lung for carbon monoxide; TLC = total lung capacity.
Data are presented as mean ± SD. Normal DlCO value ≥ 80% predicted. There were no differences between the three groups (P > 0.05, all comparisons) except for the low DlCO in group C (P < 0.05, compared to groups A and B), and pack-years, smoking metabolites, sex, and ancestry in group A (P < 0.05, compared to groups B and C).
Combined asymptomatic and symptomatic (cough and/or sputum production) smokers, all with normal lung function. There was no significant difference between asymptomatic and symptomatic smokers in any parameter (P > 0.4, all comparisons, except urine cotinine P < 0.04).
Pulmonary function testing parameters are given as % of predicted value with the exception of FEV1/FVC, which is reported as % observed. For healthy nonsmokers and healthy and symptomatic smokers with DlCO ≥ 80%, FVC, FEV1 and FEV1/FVC are prebronchodilator values. For healthy smokers with DlCO < 80%, FVC, FEV1, and FEV1/FVC are post-bronchodilator values.
In addition, a prospective study population of 60 individuals was assessed using the definitions as described above (Table 2). Prospective cohort 1 included a total of 45 individuals, including healthy nonsmokers (n = 10), healthy smokers with normal spirometry and normal DlCO (n = 20; including asymptomatic active smokers [n = 12] and symptomatic active smokers [n = 8]), and healthy smokers with normal spirometry but low DlCO (n = 15). Prospective cohort 2 assessed a total of 15 individuals classified by serological testing as HIV1+ individuals, including healthy smokers with normal spirometry and normal DlCO (n = 7; including asymptomatic active smokers [n = 5] and symptomatic active smokers [n = 2]) and healthy smokers with normal spirometry but low DlCO (n = 8).
TABLE 2.
PROSPECTIVE STUDY POPULATIONS
| Prospective Cohort 1 |
Prospective Cohort 2 |
||||
| Parameter | Group D: Healthy Nonsmokers with Normal Spirometry and Normal DlCO | Group E: Healthy Smokers with Normal Spirometry and Normal DlCO* | Group F: Healthy Smokers with Normal Spirometry but Low DlCO | Group G: HIV1+ Smokers with Normal Spirometry and Normal DlCO | Group H: HIV1+ Smokers with Normal Spirometry and Low DlCO |
| n | 10 | 20 | 15 | 7 | 8 |
| Sex, male/female | 5/5 | 15/5 | 9/6 | 4/3 | 3/5 |
| Age, yr | 42 ± 12 | 44 ± 9 | 45 ± 10 | 42 ± 7 | 47 ± 3 |
| Ancestry, B/W/O | 4/3/3 | 11/3/6 | 9/3/3 | 5/0/2 | 6/1/1 |
| Smoking history, pack-years | 0 | 21 ± 15 | 23 ± 14 | 33 ± 29 | 30 ± 22 |
| Urine nicotine, ng/ml | Negative | 1,508 ± 1,710 | 1,320 ± 1,453 | 297 ± 301 | 1,557 ± 1,478 |
| Urine cotinine, ng/ml | Negative | 1,593 ± 1,193 | 1,361 ± 1,041 | 1,329 ± 881 | 1,334 ± 704 |
| Pulmonary function† | |||||
| FEV1 | 105 ± 12 | 108 ± 13 | 106 ± 22 | 99 ± 16 | 103 ± 16 |
| FVC | 108 ± 13 | 112 ± 12 | 109 ± 25 | 103 ± 9 | 105 ± 15 |
| FEV1/FVC | 81 ± 5 | 80 ± 6 | 80 ± 7 | 79 ± 8 | 80 ± 7 |
| TLC | 101 ± 19 | 98 ± 15 | 99 ± 16 | 85 ± 7 | 90 ± 10 |
| DlCO | 87 ± 10 | 88 ± 10 | 66 ± 9 | 90 ± 14 | 66 ± 5 |
| C-reactive protein (mg/dl) | 0.6 ± 0.2 | 0.5 ± 0.02 | 0.6 ± 0.2 | 0.6 ± 0.3 | 0.8 ± 1.0 |
Definition of abbreviations: B/W/O = black/white/other; DlCO = diffusion capacity of the lung for carbon monoxide; TLC = total lung capacity.
Data are presented as mean ± SD. Normal DlCO value ≥ 80% predicted. There were no differences between groups D, E, and F (P > 0.05, all comparisons) except for the low DlCO in group F (P < 0.05, compared to groups D and E), and pack-years, smoking metabolites, sex, and ancestry in group D (P < 0.05, compared to groups E, F, G, and H). Except for the low DlCO in group H (P < 0.01, compared to group G) and the urine nicotine level (P < 0.02, comparing group G and H), there were no differences between groups G and H (P > 0.5, all comparisons).
Combined asymptomatic and symptomatic (cough and/or spleen production) smokers, all with normal lung function. There was no significant difference between asymptomatic and symptomatic smokers in any parameter (P > 0.5, all comparisons) except urine cotinine (P < 0.05).
Pulmonary function testing parameters are given as % of predicted value with the exception of FEV1/FVC, which is reported as % observed. For healthy nonsmokers and healthy and symptomatic smokers with DlCO ≥ 80%, FVC, FEV1, and FEV1/FVC are prebronchodilator values. For healthy smokers with DlCO < 80%, FVC, FEV1, and FEV1/FVC are post-bronchodilator values.
Characterization of Plasma EMPs
To quantify EMPs, a standard operating procedure was established (see Figure E1 and Table E1 in the online supplement) based on quality control experiments. Blood was collected in 5-ml sodium citrate tubes (Becton Dickinson, Franklin Lakes, NJ) using a 21-gauge needle and, within 1 hour, centrifuged 10 minutes (160 × g, 23°C) to prepare platelet-rich plasma. Within 5 minutes, the supernatant was further centrifuged 8 minutes (1,000 × g, 23°C) to obtain platelet-poor plasma. Within 5 minutes, 50-μl aliquots of platelet-poor plasma were incubated (45 min, 4°C) with 4 μl of fluorescein-conjugated anti-human PECAM (CD31-FITC, clone WM59, optimized condition) and 5 μl phycoerythrin-conjugated anti-human E-selectin (CD62E-PE, clone 68-5H11; BD PharMingen, San Diego, CA; optimized condition). Four microliters of phycoallocyanine-conjugated anti-human CD42b (CD42b-APC, clone HIP1; optimized condition) was added (45 min, 4°C) to each sample to exclude platelet-derived microparticles. Single and double positive CD42b−CD31+ CD62+ microparticles were determined by simultaneously incubating the plasma with all three specific antibodies. EMP measurements were performed twice to ensure that the measurements were repeatable. CD42b−CD31+ and CD42b−CD62+ microparticle levels were corrected for correlating isotype control antibodies. Five microliters of anti-human CD45-PECy5 (leukocyte marker, clone HI30; optimized condition) was also used to monitor leukocyte MP contamination.
To assess the presence of relative contribution of pulmonary capillary endothelium to the elevated EMPs, CD42b−CD31+ microparticles were costained with 5 μl phycoerythrin-conjugated anti-human ACE (CD143, clone 171417; R&D, Minneapolis, MN; optimized condition) based on the knowledge that ACE is abundantly expressed on pulmonary capillary endothelium (39).
To further evaluate whether the elevated CD42b−CD31+ EMPs were derived from apoptotic endothelial cells, the EMPs were also assessed by annexin V staining for the presence of phosphatidylserine, a marker linked to apoptosis (32, 33, 37). To accomplish this, the EMPs were labeled using phycoerythrin-conjugated annexin V (BD Pharmingen) in the presence of CaCl2 (5 mM) according to manufacturer's recommendation.
EMP phenotype analysis was performed within 15 minutes based on size and fluorescence. Events less than 1.5 μm were identified in forward (size) and side (density) light scatter plots using polystyrene size calibration microspheres (0.2 to 10 μm; Molecular Probes, Invitrogen, Eugene, OR), and analyzed by two- or three-color fluorescence histograms as CD42b−CD31+, CD42b−CD62+, CD42b−CD31+ACE+, or CD42b−CD31+annexin V+ microparticles. EMP levels were assessed by comparison with calibrator Flowcount beads (10-μm diameter; Beckman Coulter, Miami, FL) with a known concentration, using 30-second stop time, with log gain on forward and sideward light scatter and fluorescence. Single antibody conjugates and compensation fluorochrome beads were used for compensation assessment. Samples were acquired at band pass filters: 530 nm (FITC), 585 nm (PE/PI), and 661 nm (APC) with FL4 option. EMPs were quantified by flow cytometry using Cell Quest-Pro software (FACSCalibur; BD Bioscience, San Jose, CA), by investigators blinded to subject status. The data were analyzed using FlowJo software (Tree Star, OR). A high ratio of CD42b−CD62+ to CD42b−CD31+ were defined as “activated” and those with a ratio less than the lowest healthy nonsmoker (< 0.7, see Results) as “apoptotic” (19–21, 34). The percentage of annexin V+ EMPs 2 SDs above that for healthy nonsmokers was considered “apoptotic” (Figure E4C).
Statistical Analysis
We used several linear modeling approaches to test for effects on CD42b−CD31+ EMP level due to phenotype (healthy nonsmoker, healthy smoker with normal spirometry and normal DlCO, and healthy smoker with normal spirometry but low DlCO) and to each of the measured clinical characteristics (DlCO, FEV1, FVC, FEV1/FVC, TLC, and blood pressure); for the former we considered an analysis of variance coding and for latter a regression coding. We performed these tests without any covariates and when including covariates for age, sex, and pack-years; for each we used a regression coding. Inclusion of these covariates did not alter the significance of tests with phenotype or any of the measured clinical characteristics, so only the analyses without covariates are presented. We also performed these same analyses after removing the individuals with diabetes, hypertension, or both. Again, removing these individuals produced no qualitative effect on the test results or significance of any of the tests, so only the analyses including the entire sample are presented. To guard against deviations from parametric assumptions, a nonparametric permutation test was performed for these models; for each permutation we randomized the CD42b−CD31+ EMP values with respect to the samples. The linear model analysis was then applied to each permuted data set and a nonparametric P value was obtained using the ordering of P values obtained from 1,000 permutations. The P values obtained using the parametric and permutation approach were very close and produced no qualitative difference in the outcomes. We therefore present only the parametric analyses.
Results
EMP Levels
Healthy smokers with normal spirometry and normal DlCO had a mild increase in EMP levels compared with healthy nonsmokers, as did symptomatic smokers compared with healthy nonsmokers (P < 10−4 compared with both groups, Figure 1). There was no difference between healthy and symptomatic smokers (P > 0.4). In striking contrast, healthy smokers with normal spirometry (i.e., do not have GOLD criteria COPD) but low DlCO had a significant increase in EMP levels (P < 10−4 compared with healthy nonsmokers; P < 10−3 compared with healthy smokers). A few healthy smokers with normal DlCO and healthy smokers with low DlCO had comorbidities known to be associated with elevated EMPs (systemic hypertension and/or type 2 diabetes); removal of these subjects from the data did not change the results. No individuals had other comorbidities associated with increased circulating EMPs.
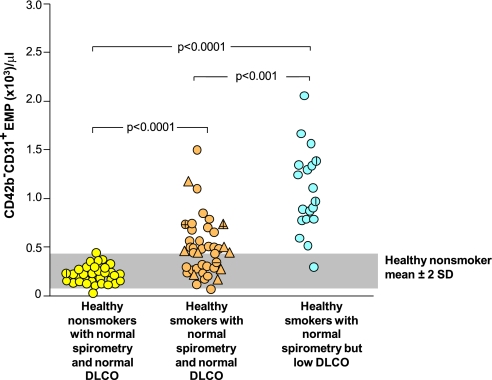
Figure 1.
Levels of CD42b−CD31+ endothelial microparticles (EMPs) per μl in platelet-poor plasma of the study groups. Shown are data for healthy nonsmokers with normal spirometry and normal diffusing capacity of the lung for carbon monoxide (DlCO) (n = 32, yellow circles), healthy smokers with normal spirometry and normal DlCO (combining asymptomatic smokers, n = 32, tan circles, and symptomatic smokers, n = 9, tan triangles), and healthy smokers with normal spirometry and low DlCO (n = 19, blue circles). P values are indicated. For all groups, a vertical line indicates a subject with systemic hypertension, a horizontal line indicates a subject with type 2 diabetes mellitus. The gray shaded area indicates the mean ± 2 SD of CD42b−CD31+ EMP/ml platelet of healthy nonsmokers.
When assessed as percent cumulative frequency of subjects in each group with elevated EMPs, the healthy nonsmoker population was distributed between 0 to 500 EMP/μl, whereas 50% of healthy smokers had EMP levels above the normal range of healthy nonsmokers ± 2 SD (Figure E2). In contrast, 95% of healthy smokers with normal spirometry and low DlCO had EMP levels above the range of healthy smokers, with 52% distributed between 500 and 1,250 EMP/μl and 43% greater than 1,250 EMP/μl. Assessed with all groups together, the best correlations of EMP levels with individual clinical parameters were with pack-years, DlCO, FEV1/FVC, and urine cotinine, with less correlation with urine nicotine, age, blood pressure, or other lung function parameters (Figure E3). Assessed within individual subject groups, there were limited correlations of EMP levels with individual clinical parameters (Table E2). Automated quantification of emphysema levels by transverse chest CT scans also showed a low correlation pattern of emphysema with urine nicotine level, EMPs, or DlCO between all groups (Figure E5) and no differences in emphysema levels between all groups (Figure E6).
None of the covariates were considered significant (P > 0.1) except for pack-years. Therefore, P values for the analysis of variance test are reported without including additional covariates except those involving comparisons of all smoking groups, in which pack-years as covariate was included. There were no qualitative differences in P values obtained from the parametric versus the nonparametric analyses; therefore, the presented results are based on parametric analyses. There was no correlation of EMP levels and age, sex, or ethnicity (P > 0.1, all comparisons).
Source of the EMPs
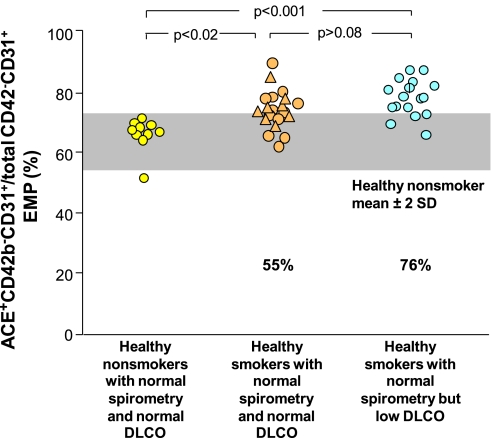
In the context that smoking likely affects multiple vascular beds, the EMPs were assessed for the proportion that were positive for ACE, a surface protein more highly expressed on pulmonary capillary endothelium compared with other endothelial beds (39) (Figure 2). This analysis showed that 55% of the CD42b−CD31+ EMPs in healthy smokers with normal spirometry and normal DlCO were ACE+ beyond that observed for healthy nonsmokers (P < 0.02 compared with healthy nonsmokers), whereas 76% of the CD42b−CD31+ EMPs in healthy smokers with normal spirometry but low DlCO were ACE+ (P < 0.001 compared with healthy nonsmokers) (i.e., the majority of the elevated EMPs in the low DlCO group were derived from pulmonary capillary endothelium).
Figure 2.
Proportion of CD42b−CD31+ endothelial microparticles (EMPs) that express angiotensin-converting enzyme (ACE+). Shown are data for healthy nonsmokers with normal spirometry and normal diffusing capacity of the lung for carbon monoxide (DlCO) (n = 10, yellow circles), healthy smokers with normal spirometry and normal DlCO (combining asymptomatic smokers, n = 12, tan circles, and symptomatic smokers, n = 8, tan triangles), and healthy smokers with normal spirometry and low DlCO (n = 17, blue circles). P values are indicated. For all groups, a vertical line indicates the subject has systemic hypertension. Gray shaded area represents range ± 2 SD of healthy nonsmokers. The % values represent the proportion of individuals in that group who had higher levels of CD42b−CD31+ACE+ EMPs beyond the level observed for healthy nonsmokers.
Apoptotic Versus Activated EMPs
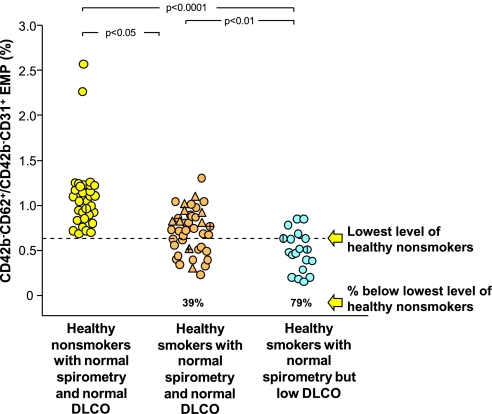
Aside from a few outliers, the CD42b−CD62+/CD42b−CD31+ ratio of the healthy nonsmokers was distributed around a mean of 1.09, with the lowest value 0.7 (Figure 3). On the average, all groups of smokers had some CD42b−CD62+/CD42b−CD31+ EMPs less than the lowest level observed in the healthy nonsmokers (39%, mean level 1.09 ± 0.38, P < 0.05). By far, however, the highest proportion of EMPs with the lowest CD42b−CD62+/CD42b−CD31+ ratio was observed in the healthy smokers with low DlCO (79%, mean level 0.51 ± 0.22 vs. 1.09 ± 0.38 for healthy nonsmokers, P < 10−4).
Figure 3.
Ratio of circulating CD42b−CD62+ to CD42b−CD31+ endothelial microparticles (EMPs) in plasma of healthy nonsmokers with normal spirometry and normal diffusing capacity of the lung for carbon monoxide (DlCO) (n = 32, yellow circles), healthy smokers with normal spirometry and normal DlCO (combining healthy smokers, n = 32, tan circles, and symptomatic smokers, n = 9, tan triangles), and healthy smokers with normal spirometry and low DlCO (n = 19, blue circles). P values are indicated. For all groups, a vertical line indicates the subject has systemic hypertension, a horizontal line indicates the subject has type 2 diabetes mellitus. The dashed line represents the value below any subject in the healthy nonsmoker group. The % values below represent the proportion of individuals in that group below the lowest level of healthy nonsmokers.
Replication in Prospective Cohorts
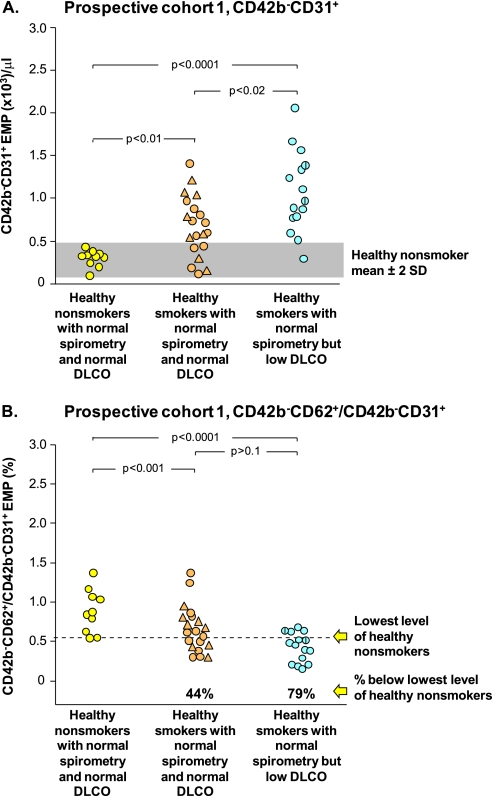
To verify the observations in the initial cohort of elevated EMPs in healthy smokers with normal spirometry but low DlCO, a prospective cohort of 45 individuals was assessed, including healthy nonsmokers, healthy smokers with normal DlCO, and healthy smokers with low DlCO (cohort 1, Table 2, Figure 4). The data in the prospective cohort 1 replicated that in the initial cohort, with significantly increased CD42b−CD31+ EMPs in healthy smokers with normal DlCO compared with healthy nonsmokers (P < 10−4), healthy smokers with low DlCO compared with healthy nonsmokers (P < 10−4), and healthy smokers with low DlCO compared with healthy smokers (P < 0.01; Figure 4A). Likewise, the prospective cohort also had more apoptotic-derived EMPs in healthy smokers with normal DlCO compared with healthy nonsmokers (P < 10−3) and healthy smokers with low DlCO compared with healthy nonsmokers (P < 10−4; Figure 4B). By these criteria, 79% of the EMPs of the healthy smokers with low DlCO were apoptotic-like, as were 44% of the EMPs of the healthy smokers with normal DlCO. The apoptotic nature of the EMPs was confirmed by annexin V staining, with 50% more annexinV+ EMPs in healthy smokers with normal DlCO and 66% more EMPs in healthy smokers with low DlCO compared with healthy nonsmokers (P < 0.002 and P < 10−4, respectively; Figure E4).
Figure 4.
Prospective study cohort 1: plasma endothelial microparticles (EMPs) in a prospective group of healthy nonsmokers with normal spirometry and normal diffusing capacity of the lung for carbon monoxide (DlCO) (n = 10, yellow circles), healthy smokers with normal spirometry and normal DlCO (combining healthy smokers, n = 12, tan circles, and symptomatic smokers, n = 8, tan triangles), and healthy smokers with normal spirometry and low DlCO (n = 15, blue circles). P values are indicated. For all groups, a vertical line indicates the subject has systemic hypertension. (A) Levels of CD42b−CD31+ EMPs in platelet-poor plasma of the study groups. (B) Ratio of circulating CD42b−CD62+ to CD42b−CD31+ EMPs in plasma of study groups. The dashed line represents the value below any subject in the healthy nonsmoker group; the % values below represent the proportion of that group below the lowest level of healthy nonsmokers.
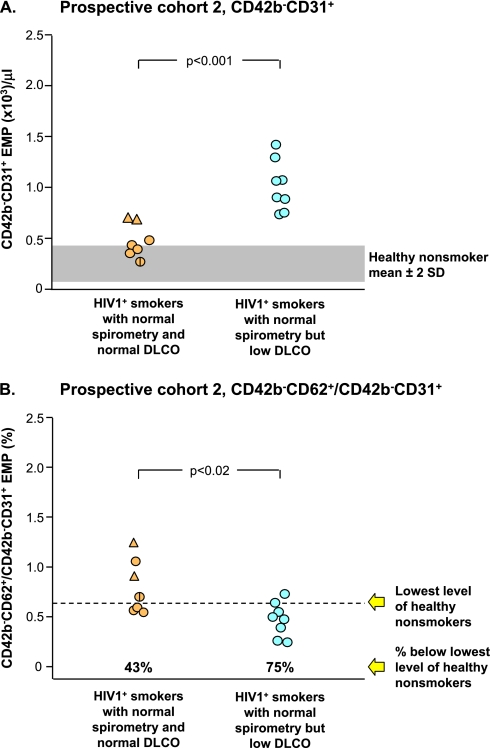
As a further verification that EMPs are elevated in association with early lung destruction in smokers with normal spirometry and low DlCO and based on the knowledge that smokers who are HIV1+ have an accelerated form of emphysema (44), we assessed a second prospective cohort, smokers who were HIV1+, both those with normal spirometry and normal DlCO and those with normal spirometry and low DlCO (cohort 2; Table 2, Figure 5). Parallel to the initial cohort and the first prospective cohort, the HIV1+ low DlCO group had significantly more CD42b−CD31+ EMPs than the HIV1+ with normal DlCO group (P < 10−3; Figure 5A), with 75% of apoptotic-like EMPs in the HIV1+ low DlCO group beyond that of the HIV1− nonsmokers (Figure 5B).
Figure 5.
Prospective study cohort 2: endothelial microparticles (EMPs) in a prospective group of HIV1+ healthy smokers with normal spirometry and normal diffusing capacity of the lung for carbon monoxide (DlCO) (combining healthy smokers, n = 5, tan circles, and symptomatic smokers, n = 2, tan triangles) and HIV1+ healthy smokers with normal spirometry and low DlCO (n = 8, blue circles). P values are indicated. For all groups, a vertical line indicates the subject has systemic hypertension. (A) Levels of CD42b−CD31+ EMPs in platelet-poor plasma of the study groups. (B) Ratio of circulating CD42b−CD62+ to CD42b−CD31+ EMPs in plasma of study groups. The dashed line represents the value below any subject in the healthy nonsmoker group; the % values below represent the proportion of that group below the lowest level of healthy nonsmokers.
Discussion
Based on the knowledge that smoking is the major cause of COPD, that destruction of alveoli is a common component of COPD, and increasing evidence that alveolar destruction may be initiated, in part, by apoptosis of pulmonary capillaries (2–6, 8–14, 38), we hypothesized that smokers with evidence of lung destruction may have elevated plasma levels of EMPs, plasma membrane fragments released when endothelial cells are activated or undergo apoptosis (19–21, 31, 34, 83). As a measure of lung destruction, we used the DlCO, a lung function measure of the functional intactness of the alveolar-capillary bed (15, 16). Healthy smokers and symptomatic smokers with normal spirometry and DlCO had mildly elevated levels of circulating EMPs compared with healthy nonsmokers. Strikingly, however, healthy smokers with normal spirometry but an isolated reduction in DlCO had high levels of circulating EMPs compared with all other groups, with the EMPs likely derived from endothelial cells undergoing apoptosis, and likely mostly from pulmonary endothelium. This observation was replicated in a prospective parallel group of smokers, as well as in HIV1+ smokers with low DlCO.
Endothelial Microparticles
Microparticles are submicron membrane vesicles shed from the plasma membranes of different cell types in response to cell activation, injury, and/or apoptosis (19–21, 31, 34, 83). Microparticles in the plasma of healthy subjects are derived from platelets, leukocytes, and endothelial cells (45–47). EMPs are distinguished from microparticles of other cell types by size, constitutive expression of the platelet–endothelial cell adhesion marker CD31, and the absence of the platelet-specific glycoprotein Ib marker CD42b (19, 21, 45). Apoptosis-induced EMPs are more likely to express only CD31 and show the presence of phosphatidylserine (annexin V) as an apoptotic parameter (32, 33, 37), whereas activation-induced EMPs have increased expression of the inducible endothelial marker CD62 (19–21). Elevated levels of CD42b−CD31+ EMPs have been associated with vascular disease and endothelial dysfunction in patients with acute coronary syndromes, severe hypertension, metabolic syndrome, type 2 diabetes, end-stage renal disease, pulmonary arterial hypertension, subclinical atherosclerosis, heart failure, stroke, thrombotic thrombocytopenic purpura, lupus anticoagulant syndrome and other vasculitides, multiple sclerosis, and sickle cell disease (19, 21, 23–37, 46, 48–64).
One of the burdens of smoking is injury to the lung endothelium (10, 65–67). Consistent with this, we observed that, to some extent, all smoking groups (healthy smokers, symptomatic smokers), had elevation of EMPs compared with healthy nonsmokers. Consistent with this, Heiss and colleagues (68) showed that healthy nonsmokers exposed for 30 minutes to low levels of cigarette smoke had increased EMP levels. Together, the data suggest that smoking per se causes sufficient endothelial changes to mildly raise plasma EMP levels. Moreover, our comparison of the EMP levels of healthy smokers, symptomatic smokers, and smokers with normal spirometry and low DlCO demonstrates significant variation in EMP levels among these smokers, with the highest, by far, in healthy smokers with normal spirometry and low DlCO. Although there is increasing evidence of alveolar destruction initiated, in part, by apoptosis of pulmonary capillaries (2–6, 8–14, 38), more complementary measures of lung vascular damage in addition to DlCO have to be undertaken to underline the association between EMPs and lung destruction. The data in the present study suggest that elevated levels of EMP correlate with an early onset of lung destruction (i.e., normal spirometry/low DlCO group) and that the EMPs may confer to a more apoptotic nature of their parental endothelial origin.
Endothelial Apoptosis and Emphysema
The concept of pulmonary endothelial apoptosis as a primary mechanism in the development of emphysema is supported by the observation of endothelial apoptosis in the lungs of humans with emphysema (8–14). Segura-Valdez and colleagues (69) showed increased DNA fragmentation in the pulmonary capillaries and arteriolar endothelium of individuals with COPD, and Kasahara and colleagues (8–10) reported increased septal cell death (endothelial and epithelial cells) in human emphysematous lungs compared with lungs of nonsmokers or smokers without emphysema. Although the mechanisms associated with this endothelial loss are likely complex, there is evidence that reduced levels of alveolar epithelial-derived vascular endothelial growth factor may play a role (9, 10, 65).
Our study provides a plasma-based assessment of this endothelial destruction by measuring the level of plasma EMPs in smokers without and with alveolar loss as measured by decreased DlCO. The presence of increased levels of CD42b−CD31+ EMPs with a low CD42b−CD62+ to CD42b−CD31+ ratio in individuals with normal spirometry and low DlCO further supports the vascular theory of emphysema by suggesting that apoptosis plays a central role in the early destruction of alveolar endothelium.
Early Detection of Lung Destruction
As defined by the GOLD standards, the diagnosis of COPD is based on lung function criteria as a persistent limitation to forced expiratory airflow after treatment with bronchodilators (38). Although this is a useful unified definition, airflow limitation is a relatively crude measure of lung health, as the lung is redundant, and the GOLD COPD minimum criteria of FEV1/FVC less than 0.7 after bronchodilators occurs only after considerable abnormalities are present (38, 42, 70–73). It has long been recognized that the limitation of forced expiratory airflow observed in COPD can result from intrinsic disease of the airways (chronic bronchitis) and/or destruction of the alveoli (emphysema), with most affected individuals having some contribution of both airway and alveolar disease (2–4, 6, 17, 18). The observation of limitation to forced expiratory airflow after bronchodilators does not indicate whether the cause is intrinsic airway disease and/or alveolar destruction (2–4, 6, 17, 18).
The traditional diagnosis of COPD with emphysema relies on pulmonary function tests demonstrating airflow obstruction and a low DlCO (1, 2, 4, 6, 17, 18, 38, 42). HRCT imaging detects early emphysema by identifying pulmonary tissue with radiologic attenuation below a predetermined threshold, findings that roughly correlate with a low DlCO and pathologic evidence of emphysema (74–80). Although several studies have shown that a significant proportion of asymptomatic smokers have HRCT evidence of emphysema (78, 81–83), early HRCT findings of “emphysema” are not proven to be correlated directly with lung destruction (84–90). Hyperpolarized gas diffusion-weighted magnetic resonance imaging has also been used to identify emphysema, with a correlation of elevated levels of the apparent diffusion coefficient with decreased DlCO (91). We have observed that smokers with normal spirometry and low DlCO are at higher risk for the development of COPD as defined by the GOLD criteria than are smokers with normal spirometry and normal DlCO (92), but there was no direct correlation of emphysema with EMP levels or DlCO. This was not surprising, as healthy smokers with normal spirometry and normal DlCO without any clinical evidence of emphysema showed increased EMP levels as well, indicating that the complexity of the correlation between EMP and smoking-induced early vascular lung endothelium damage may not exclusively rely on the presence of emphysema as detailed by conventional clinical parameters such as DlCO and/or chest HRCT. For future studies it will be of interest to assess measures of endothelial dysfunction to determine if EMP levels are related to early emphysema independent of endothelial dysfunction.
Assessment of EMP levels may provide an early and inexpensive approach to identifying early evidence of emphysema, without the radiation exposure associated with chest HRCT. Interestingly, the smokers with the highest plasma EMP levels are healthy smokers with normal spirometry and isolated low DlCO. This suggests that the vascular-based contributions to the pathogenesis of emphysema may contribute to the early development of emphysema and may identify a point in time where intervention with smoking cessation therapy may prevent the irreversible lung destruction associated with the development of COPD as defined by the GOLD criteria (38). Elevated EMP levels may be a useful biomarker to identify smokers with early emphysema at a stage at which intervention may prevent further permanent lung destruction.
Supplementary Material
Acknowledgments
The authors thank Ann E. Tilley and Timothy P. O'Connor for helpful discussions; Fadi Zakko for help with data acquisition; and Nahla Mohamed for help in preparing this manuscript.
Footnotes
Supported in part by National Institutes of Health grants R01 HL074326, P50 HL084936, and UL1-RR024996.
This article has an online supplement, which is accessible from this issue's table of contents at www.atsjournals.org
Originally Published in Press as DOI: 10.1164/rccm.201012-2061OC on March 11, 2011
Author Disclosure: None of the authors has a financial relationship with a commercial entity that has an interest in the subject of this manuscript.
References
- 1.Weibel ER. Morphological basis of alveolar-capillary gas exchange. Physiol Rev 1973;53:419–495 [DOI] [PubMed] [Google Scholar]
- 2.Hogg JC, Senior RM. Chronic obstructive pulmonary disease - part 2: pathology and biochemistry of emphysema. Thorax 2002;57:830–834 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Barnes PJ. Mediators of chronic obstructive pulmonary disease. Pharmacol Rev 2004;56:515–548 [DOI] [PubMed] [Google Scholar]
- 4.Spurzem JR, Rennard SI. Pathogenesis of COPD. Semin Respir Crit Care Med 2005;26:142–153 [DOI] [PubMed] [Google Scholar]
- 5.Abboud RT, Vimalanathan S. Pathogenesis of COPD. Part I. The role of protease-antiprotease imbalance in emphysema. Int J Tuberc Lung Dis 2008;12:361–367 [PubMed] [Google Scholar]
- 6.Roth M. Pathogenesis of COPD. Part III. Inflammation in COPD. Int J Tuberc Lung Dis 2008;12:375–380 [PubMed] [Google Scholar]
- 7.McElvaney NG, Crystal RG. Proteases and lung injury. : Crystal RG, West JB, Weibel ER, Barnes PJ, The lung: scientific foundations, 2nd ed Philadelphia: Lippencott-Raven Publishers; 1997. pp. 2205–2218 [Google Scholar]
- 8.Kasahara Y, Tuder RM, Cool CD, Voelkel NF. Expression of 15-lipoxygenase and evidence for apoptosis in the lungs from patients with COPD. Chest 2000;117:260S. [DOI] [PubMed] [Google Scholar]
- 9.Kasahara Y, Tuder RM, Taraseviciene-Stewart L, Le Cras TD, Abman S, Hirth PK, Waltenberger J, Voelkel NF. Inhibition of VEGF receptors causes lung cell apoptosis and emphysema. J Clin Invest 2000;106:1311–1319 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Kasahara Y, Tuder RM, Cool CD, Lynch DA, Flores SC, Voelkel NF. Endothelial cell death and decreased expression of vascular endothelial growth factor and vascular endothelial growth factor receptor 2 in emphysema. Am J Respir Crit Care Med 2001;163:737–744 [DOI] [PubMed] [Google Scholar]
- 11.Majo J, Ghezzo H, Cosio MG. Lymphocyte population and apoptosis in the lungs of smokers and their relation to emphysema. Eur Respir J 2001;17:946–953 [DOI] [PubMed] [Google Scholar]
- 12.Aoshiba K, Yokohori N, Nagai A. Alveolar wall apoptosis causes lung destruction and emphysematous changes. Am J Respir Cell Mol Biol 2003;28:555–562 [DOI] [PubMed] [Google Scholar]
- 13.Plataki M, Tzortzaki E, Rytila P, Demosthenes M, Koutsopoulos A, Siafakas NM. Apoptotic mechanisms in the pathogenesis of COPD. Int J Chron Obstruct Pulmon Dis 2006;1:161–171 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Morissette MC, Parent J, Milot J. Alveolar epithelial and endothelial cell apoptosis in emphysema: what we know and what we need to know. Int J Chron Obstruct Pulmon Dis 2009;4:19–31 [PMC free article] [PubMed] [Google Scholar]
- 15.Hughes JM, Bates DV. Historical review: the carbon monoxide diffusing capacity (DLCO) and its membrane (DM) and red cell (Theta. Vc) components. Respir Physiol Neurobiol 2003;138:115–142 [DOI] [PubMed] [Google Scholar]
- 16.Scheid P, Piiper J. Diffusion. : Crystal RG, West JB, Weibel ER, Barnes PJ, The lung: scientific foundations, 2nd ed Philadelphia: Lippincott-Raven Publishers; 1997. pp. 1681–1691 [Google Scholar]
- 17.Cosio Piqueras MG, Cosio MG. Disease of the airways in chronic obstructive pulmonary disease. Eur Respir J Suppl 2001;34:41s–49s [DOI] [PubMed] [Google Scholar]
- 18.Hogg JC. Pathophysiology of airflow limitation in chronic obstructive pulmonary disease. Lancet 2004;364:709–721 [DOI] [PubMed] [Google Scholar]
- 19.Horstman LL, Jy W, Jimenez JJ, Ahn YS. Endothelial microparticles as markers of endothelial dysfunction. Front Biosci 2004;9:1118–1135 [DOI] [PubMed] [Google Scholar]
- 20.Jimenez JJ, Jy W, Mauro LM, Soderland C, Horstman LL, Ahn YS. Endothelial cells release phenotypically and quantitatively distinct microparticles in activation and apoptosis. Thromb Res 2003;109:175–180 [DOI] [PubMed] [Google Scholar]
- 21.Chironi GN, Boulanger CM, Simon A, Dignat-George F, Freyssinet JM, Tedgui A. Endothelial microparticles in diseases. Cell Tissue Res 2009;335:143–151 [DOI] [PubMed] [Google Scholar]
- 22.Berckmans RJ, Neiuwland R, Boing AN, Romijn FP, Hack CE, Sturk A. Cell-derived microparticles circulate in healthy humans and support low grade thrombin generation. Thromb Haemost 2001;85:639–646 [PubMed] [Google Scholar]
- 23.Combes V, Simon AC, Grau GE, Arnoux D, Camoin L, Sabatier F, Mutin M, Sanmarco M, Sampol J, Dignat-George F. In vitro generation of endothelial microparticles and possible prothrombotic activity in patients with lupus anticoagulant. J Clin Invest 1999;104:93–102 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Mallat Z, Benamer H, Hugel B, Benessiano J, Steg PG, Freyssinet JM, Tedgui A. Elevated levels of shed membrane microparticles with procoagulant potential in the peripheral circulating blood of patients with acute coronary syndromes. Circulation 2000;101:841–843 [DOI] [PubMed] [Google Scholar]
- 25.Boulanger CM, Scoazec A, Ebrahimian T, Henry P, Mathieu E, Tedgui A, Mallat Z. Circulating microparticles from patients with myocardial infarction cause endothelial dysfunction. Circulation 2001;104:2649–2652 [DOI] [PubMed] [Google Scholar]
- 26.Bernal-Mizrachi L, Jy W, Jimenez JJ, Pastor J, Mauro LM, Horstman LL, de Marchena E, Ahn YS. High levels of circulating endothelial microparticles in patients with acute coronary syndromes. Am Heart J 2003;145:962–970 [DOI] [PubMed] [Google Scholar]
- 27.Preston RA, Jy W, Jimenez JJ, Mauro LM, Horstman LL, Valle M, Aime G, Ahn YS. Effects of severe hypertension on endothelial and platelet microparticles. Hypertension 2003;41:211–217 [DOI] [PubMed] [Google Scholar]
- 28.Brogan PA, Shah V, Brachet C, Harnden A, Mant D, Klein N, Dillon MJ. Endothelial and platelet microparticles in vasculitis of the young. Arthritis Rheum 2004;50:927–936 [DOI] [PubMed] [Google Scholar]
- 29.Gonzalez-Quintero VH, Smarkusky LP, Jimenez JJ, Mauro LM, Jy W, Hortsman LL, O'Sullivan MJ, Ahn YS. Elevated plasma endothelial microparticles: preeclampsia versus gestational hypertension. Am J Obstet Gynecol 2004;191:1418–1424 [DOI] [PubMed] [Google Scholar]
- 30.Chirinos JA, Heresi GA, Velasquez H, Jy W, Jimenez JJ, Ahn E, Horstman LL, Soriano AO, Zambrano JP, Ahn YS. Elevation of endothelial microparticles, platelets, and leukocyte activation in patients with venous thromboembolism. J Am Coll Cardiol 2005;45:1467–1471 [DOI] [PubMed] [Google Scholar]
- 31.Garcia S, Chirinos J, Jimenez J, Del Carpio MF, Canoniero M, Jy W, Jimenez J, Horstman L, Ahn Y. Phenotypic assessment of endothelial microparticles in patients with heart failure and after heart transplantation: switch from cell activation to apoptosis. J Heart Lung Transplant 2005;24:2184–2189 [DOI] [PubMed] [Google Scholar]
- 32.Amabile N, Guerin AP, Leroyer A, Mallat Z, Nguyen C, Boddaert J, London GM, Tedgui A, Boulanger CM. Circulating endothelial microparticles are associated with vascular dysfunction in patients with end-stage renal failure. J Am Soc Nephrol 2005;16:3381–3388 [DOI] [PubMed] [Google Scholar]
- 33.Werner N, Wassmann S, Ahlers P, Kosiol S. Nickenig G. Circulating CD31+/Annexin V+ apoptotic microparticles correlate with coronary endothelial function in patients with coronary artery disease. Arterioscler Thromb Vasc Biol 2006;26:112–116 [DOI] [PubMed] [Google Scholar]
- 34.Williams JB, Jauch EC, Lindsell CJ, Campos B. Endothelial microparticle levels are similar in acute ischemic stroke and stroke mimics due to activation and not apoptosis/necrosis. Acad Emerg Med 2007;14:685–690 [DOI] [PubMed] [Google Scholar]
- 35.Amabile N, Heiss C, Real WM, Minasi P, McGlothlin D, Rame EJ, Grossman W, De MT, Yeghiazarians Y. Circulating endothelial microparticle levels predict hemodynamic severity of pulmonary hypertension. Am J Respir Crit Care Med 2008;177:1268–1275 [DOI] [PubMed] [Google Scholar]
- 36.Erdbruegger U, Grossheim M, Hertel B, Wyss K, Kirsch T, Woywodt A, Haller H, Haubitz M. Diagnostic role of endothelial microparticles in vasculitis. Rheumatology (Oxford) 2008;47:1820–1825 [DOI] [PubMed] [Google Scholar]
- 37.Bulut D, Tuns H, Mugge A. CD31+/Annexin V+ microparticles in healthy offsprings of patients with coronary artery disease. Eur J Clin Invest 2009;39:17–22 [DOI] [PubMed] [Google Scholar]
- 38.Rabe KF, Hurd S, Anzueto A, Barnes PJ, Buist SA, Calverley P, Fukuchi Y, Jenkins C, Rodriguez-Roisin R, van Weel C, et al. Global strategy for the diagnosis, management, and prevention of chronic obstructive pulmonary disease: GOLD executive summary. Am J Respir Crit Care Med 2007;176:532–555 [DOI] [PubMed] [Google Scholar]
- 39.Danilov SM, Gavrilyuk VD, Franke FE, Pauls K, Harshaw DW, McDonald TD, Miletich DJ, Muzykantov VR. Lung uptake of antibodies to endothelial antigens: key determinants of vascular immunotargeting. Am J Physiol Lung Cell Mol Physiol 2001;280:L1335–L1347 [DOI] [PubMed] [Google Scholar]
- 40.Gordon C, Gudi K, Krause A, Sackrowitz R, Zakko F, Strulovici-Barel Y, Harvey B-G, Crystal RG. Circulating endothelial microparticles as a measure of lung destruction in smokers [abstract]. Am J Respir Crit Care Med 2010;181:A3444. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Macintyre N, Crapo RO, Viegi G, Johnson DC, van der Grinten CP, Brusasco V, Burgos F, Casaburi R, Coates A, Enright P, et al. Standardisation of the single-breath determination of carbon monoxide uptake in the lung. Eur Respir J 2005;26:720–735 [DOI] [PubMed] [Google Scholar]
- 42.Miller MR, Hankinson J, Brusasco V, Burgos F, Casaburi R, Coates A, Crapo R, Enright P, van der Grinten CP, Gustafsson P, et al. Standardisation of spirometry. Eur Respir J 2005;26:319–338 [DOI] [PubMed] [Google Scholar]
- 43.Heijdra YF, Pinto-Plata VM, Kenney LA, Rassulo J, Celli BR. Cough and phlegm are important predictors of health status in smokers without COPD. Chest 2002;121:1427–1433 [DOI] [PubMed] [Google Scholar]
- 44.Diaz PT, King MA, Pacht ER, Wewers MD, Gadek JE, Nagaraja HN, Drake J, Clanton TL. Increased susceptibility to pulmonary emphysema among HIV-seropositive smokers. Ann Intern Med 2000;132:369–372 [DOI] [PubMed] [Google Scholar]
- 45.Piccin A, Murphy WG, Smith OP. Circulating microparticles: pathophysiology and clinical implications. Blood Rev 2007;21:157–171 [DOI] [PubMed] [Google Scholar]
- 46.Jy W, Horstman L, Jimenez JJ, Minagar A, Ahn YS. Circulating cell-derived microparticles in thrombotic and inflammatory disorders. : Minagar A, Alexander JS, Current clinical nerurology: inflammatory disorders of the nervous system. Totowa, NJ: Humana Press Inc; 2007. pp. 91–102 [Google Scholar]
- 47.George FD. Microparticles in vascular diseases. Thromb Res 2008;122:S55–S59 [DOI] [PubMed] [Google Scholar]
- 48.Jimenez JJ, Jy W, Mauro LM, Horstman LL, Ahn YS. Elevated endothelial microparticles in thrombotic thrombocytopenic purpura: findings from brain and renal microvascular cell culture and patients with active disease. Br J Haematol 2001;112:81–90 [DOI] [PubMed] [Google Scholar]
- 49.Minagar A, Jy W, Jimenez JJ, Sheremata WA, Mauro LM, Mao WW, Horstman LL, Ahn YS. Elevated plasma endothelial microparticles in multiple sclerosis. Neurology 2001;56:1319–1324 [DOI] [PubMed] [Google Scholar]
- 50.Shet AS, Aras O, Gupta K, Hass MJ, Rausch DJ, Saba N, Koopmeiners L, Key NS, Hebbel RP. Sickle blood contains tissue factor-positive microparticles derived from endothelial cells and monocytes. Blood 2003;102:2678–2683 [DOI] [PubMed] [Google Scholar]
- 51.Ferreira AC, Peter AA, Mendez AJ, Jimenez JJ, Mauro LM, Chirinos JA, Ghany R, Virani S, Garcia S, Horstman LL, et al. Postprandial hypertriglyceridemia increases circulating levels of endothelial cell microparticles. Circulation 2004;110:3599–3603 [DOI] [PubMed] [Google Scholar]
- 52.Koga H, Sugiyama S, Kugiyama K, Watanabe K, Fukushima H, Tanaka T, Sakamoto T, Yoshimura M, Jinnouchi H, Ogawa H. Elevated levels of VE-cadherin-positive endothelial microparticles in patients with type 2 diabetes mellitus and coronary artery disease. J Am Coll Cardiol 2005;45:1622–1630 [DOI] [PubMed] [Google Scholar]
- 53.Arteaga RB, Chirinos JA, Soriano AO, Jy W, Horstman L, Jimenez JJ, Mendez A, Ferreira A, de Marchena E, Ahn YS. Endothelial microparticles and platelet and leukocyte activation in patients with the metabolic syndrome. Am J Cardiol 2006;98:70–74 [DOI] [PubMed] [Google Scholar]
- 54.Esposito K, Ciotola M, Schisano B, Gualdiero R, Sardelli L, Misso L, Giannetti G, Giugliano D. Endothelial microparticles correlate with endothelial dysfunction in obese women. J Clin Endocrinol Metab 2006;91:3676–3679 [DOI] [PubMed] [Google Scholar]
- 55.Pirro M, Schillaci G, Paltriccia R, Bagaglia F, Menecali C, Mannarino MR, Capanni M, Velardi A, Mannarino E. Increased ratio of CD31+/CD42- microparticles to endothelial progenitors as a aovel marker of atherosclerosis in hypercholesterolemia. Arterioscler Thromb Vasc Biol 2006;26:2530–2535 [DOI] [PubMed] [Google Scholar]
- 56.Sheremata WA, Jy W, Delgado S, Minagar A, McLarty J, Ahn Y. Interferon-beta1a reduces plasma CD31+ endothelial microparticles (CD31+EMP) in multiple sclerosis. J Neuroinflammation 2006;3:23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Simak J, Gelderman MP, Yu H, Wright V, Baird AE. Circulating endothelial microparticles in acute ischemic stroke: a link to severity, lesion volume and outcome. J Thromb Haemost 2006;4:1296–1302 [DOI] [PubMed] [Google Scholar]
- 58.Esposito K, Ciotola M, Giugliano F, Schisano B, Improta L, Improta MR, Beneduce F, Rispoli M, De SM, Giugliano D. Endothelial microparticles correlate with erectile dysfunction in diabetic men. Int J Impot Res 2007;19:161–166 [DOI] [PubMed] [Google Scholar]
- 59.Tushuizen ME, Nieuwland R, Rustemeijer C, Hensgens BE, Sturk A, Heine RJ, Diamant M. Elevated endothelial microparticles following consecutive meals are associated with vascular endothelial dysfunction in type 2 diabetes. Diabetes Care 2007;30:728–730 [DOI] [PubMed] [Google Scholar]
- 60.Wang JM, Huang YJ, Wang Y, Xu MG, Wang LC, Wang SM, Tao J. Increased circulating CD31+/CD42 microparticles are associated with impaired systemic artery elasticity in healthy subjects. Am J Hypertens 2007;20:957–964 [DOI] [PubMed] [Google Scholar]
- 61.Amabile N, Heiss C, Chang V, Angeli FS, Damon L, Rame EJ, McGlothlin D, Grossman W, De MT. Yeghiazarians Y. Increased CD62e(+) endothelial microparticle levels predict poor outcome in pulmonary hypertension patients. J Heart Lung Transplant 2009;28:1081–1086 [DOI] [PubMed] [Google Scholar]
- 62.Ayers L, Ferry B, Craig S, Nicoll D, Stradling JR, Kohler M. Circulating cell-derived microparticles in patients with minimally symptomatic obstructive sleep apnoea. Eur Respir J 2009;33:574–580 [DOI] [PubMed] [Google Scholar]
- 63.Harrison M, Murphy RP, O'Connor PL, O'Gorman DJ, McCaffrey N, Cummins PM, Moyna NM. The endothelial microparticle response to a high fat meal is not attenuated by prior exercise. Eur J Appl Physiol 2009;106:555–562 [DOI] [PubMed] [Google Scholar]
- 64.Jourde-Chiche N, Dou L, Sabatier F, Calaf R, Cerini C, Robert S, Camoin-Jau L, Charpiot P, Argiles A, Dignat-George F, et al. Levels of circulating endothelial progenitor cells are related to uremic toxins and vascular injury in hemodialysis patients. J Thromb Haemost 2009;7:1576–1584 [DOI] [PubMed] [Google Scholar]
- 65.Michaud SE, Dussault S, Groleau J, Haddad P, Rivard A. Cigarette smoke exposure impairs VEGF-induced endothelial cell migration: role of NO and reactive oxygen species. J Mol Cell Cardiol 2006;41:275–284 [DOI] [PubMed] [Google Scholar]
- 66.Yang Q, Underwood MJ, Hsin MK, Liu XC, He GW. Dysfunction of pulmonary vascular endothelium in chronic obstructive pulmonary disease: basic considerations for future drug development. Curr Drug Metab 2008;9:661–667 [DOI] [PubMed] [Google Scholar]
- 67.Edmiston JS, Flora JW, Scian MJ, Li G, Rana GS, Langston TB, Sengupta TK, McKinney WJ. Cigarette smoke extract induced protein phosphorylation changes in human microvascular endothelial cells in vitro. Anal Bioanal Chem 2009;394:1609–1620 [DOI] [PubMed] [Google Scholar]
- 68.Heiss C, Amabile N, Lee AC, Real WM, Schick SF, Lao D, Wong ML, Jahn S, Angeli FS, Minasi P, et al. Brief secondhand smoke exposure depresses endothelial progenitor cells activity and endothelial function: sustained vascular injury and blunted nitric oxide production. J Am Coll Cardiol 2008;51:1760–1771 [DOI] [PubMed] [Google Scholar]
- 69.Segura-Valdez L, Pardo A, Gaxiola M, Uhal BD, Becerril C, Selman M. Upregulation of gelatinases A and B, collagenases 1 and 2, and increased parenchymal cell death in COPD. Chest 2000;117:684–694 [DOI] [PubMed] [Google Scholar]
- 70.Fletcher C, Peto R. The natural history of chronic airflow obstruction. BMJ 1977;1:1645–1648 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Lundback B, Lindberg A, Lindstrom M, Ronmark E, Jonsson AC, Jonsson E, Larsson LG, Andersson S, Sandstrom T, Larsson K. Not 15 but 50% of smokers develop COPD? Report from the Obstructive Lung Disease in Northern Sweden Studies. Respir Med 2003;97:115–122 [DOI] [PubMed] [Google Scholar]
- 72.Bednarek M, Maciejewski J, Wozniak M, Kuca P, Zielinski J. Prevalence, severity and underdiagnosis of COPD in the primary care setting. Thorax 2008;63:402–407 [DOI] [PubMed] [Google Scholar]
- 73.Rennard SI, Vestbo J. Natural histories of chronic obstructive pulmonary disease. Proc Am Thorac Soc 2008;5:878–883 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Coxson HO, Rogers RM, Whittall KP, D'yachkova Y, Pare PD, Sciurba FC, Hogg JC. A quantification of the lung surface area in emphysema using computed tomography. Am J Respir Crit Care Med 1999;159:851–856 [DOI] [PubMed] [Google Scholar]
- 75.Newell JD, Jr, Hogg JC, Snider GL. Report of a workshop: quantitative computed tomography scanning in longitudinal studies of emphysema. Eur Respir J 2004;23:769–775 [DOI] [PubMed] [Google Scholar]
- 76.Madani A, Zanen J, de Maertelaer V, Gevenois PA. Pulmonary emphysema: objective quantification at multi-detector row CT-comparison with macroscopic and microscopic morphometry. Radiology 2006;238:1036–1043 [DOI] [PubMed] [Google Scholar]
- 77.van Beek EJ, Hoffman EA. Functional imaging: CT and MRI. Clin Chest Med 2008;29:195–216 (vii.) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Bastarrika G, Wisnivesky JP, Pueyo JC, Diaz L, Arraiza M, Villanueva A, Alcaide AB, Campo A, Seijo L, de Torres JP, et al. Low-dose volumetric computed tomography for quantification of emphysema in asymptomatic smokers participating in an early lung cancer detection trial. J Thorac Imaging 2009;24:206–211 [DOI] [PubMed] [Google Scholar]
- 79.Cavigli E, Camiciottoli G, Diciotti S, Orlandi I, Spinelli C, Meoni E, Grassi L, Farfalla C, Pistolesi M, Falaschi F, et al. Whole-lung densitometry versus visual assessment of emphysema. Eur Radiol 2009;19:1686–1692 [DOI] [PubMed] [Google Scholar]
- 80.Lynch DA, Newell JD. Quantitative imaging of COPD. J Thorac Imaging 2009;24:189–194 [DOI] [PubMed] [Google Scholar]
- 81.Tylen U, Boijsen M, Ekberg-Jansson A, Bake B, Lofdahl CG. Emphysematous lesions and lung function in healthy smokers 60 years of age. Respir Med 2000;94:38–43 [DOI] [PubMed] [Google Scholar]
- 82.Spaggiari E, Zompatori M, Verduri A, Chetta A, Bna C, Ormitti F, Sverzellati N, Rabaiotti E. Early smoking-induced lung lesions in asymptomatic subjects. correlations between high resolution dynamic ct and pulmonary function testing. Radiol Med (Torino) 2005;109:27–39 [PubMed] [Google Scholar]
- 83.Wilson DO, Weissfeld JL, Balkan A, Schragin JG, Fuhrman CR, Fisher SN, Wilson J, Leader JK, Siegfried JM, Shapiro SD, et al. Association of radiographic emphysema and airflow obstruction with lung cancer. Am J Respir Crit Care Med 2008;178:738–744 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Kuwano K, Matsuba K, Ikeda T, Murakami J, Araki A, Nishitani H, Ishida T, Yasumoto K, Shigematsu N. The diagnosis of mild emphysema. correlation of computed tomography and pathology scores. Am Rev Respir Dis 1990;141:169–178 [DOI] [PubMed] [Google Scholar]
- 85.Remy-Jardin M, Remy J, Gosselin B, Copin MC, Wurtz A, Duhamel A. Sliding thin slab, minimum intensity projection technique in the diagnosis of emphysema: histopathologic-CT correlation. Radiology 1996;200:665–671 [DOI] [PubMed] [Google Scholar]
- 86.Gevenois PA, De Vuyst P, de Maertelaer V, Zanen J, Jacobovitz D, Cosio MG, Yernault JC. Comparison of computed density and microscopic morphometry in pulmonary emphysema. Am J Respir Crit Care Med 1996;154:187–192 [DOI] [PubMed] [Google Scholar]
- 87.Mathews JJ, Maurer AH, Steiner RM, Marchetti N, Criner G, Gaughan JP, Coxson HO. New 133Xe gas trapping index for quantifying severe emphysema before partial lung volume reduction. J Nucl Med 2008;49:771–775 [DOI] [PubMed] [Google Scholar]
- 88.Litmanovich D, Boiselle PM, Bankier AA. CT of pulmonary emphysema–current status, challenges, and future directions. Eur Radiol 2009;19:537–551 [DOI] [PubMed] [Google Scholar]
- 89.Madani A, Van MA, Gevenois PA. Pulmonary emphysema: effect of lung volume on objective quantification at thin-section CT. Radiology 2010;257:260–268 [DOI] [PubMed] [Google Scholar]
- 90.Suga K, Kawakami Y, Koike H, Iwanaga H, Tokuda O, Okada M, Matsunaga N. Lung ventilation-perfusion imbalance in pulmonary emphysema: assessment with automated V/Q quotient SPECT. Ann Nucl Med 2010;24:269–277 [DOI] [PubMed] [Google Scholar]
- 91.Fain SB, Panth SR, Evans MD, Wentland AL, Holmes JH, Korosec FR, O'Brien MJ, Fountaine H, Grist TM. Early emphysematous changes in asymptomatic smokers: detection with 3He MR imaging. Radiology 2006;239:875–883 [DOI] [PubMed] [Google Scholar]
- 92.Harvey B-G, Gordon C, Dvorak A, Crystal RG. Natural history of asymptomatic smokers with normal spirometry and reduced diffusion capacity: do they develop COPD? Am J Respir Crit Care Med 2010;181:A1531 [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.