Abstract
Rationale: Th17 cells comprise a distinct lineage of proinflammatory T helper cells that are major contributors to allergic responses. It is unknown whether cyclooxygenase (COX)-derived eicosanoids regulate Th17 cells during allergic lung inflammation.
Objectives: To determine the role of COX metabolites in regulating Th17 cell differentiation and function during allergic lung inflammation.
Methods: COX-1−/−, COX-2−/−, and wild-type mice were studied in an in vivo model of ovalbumin-induced allergic inflammation and an in vitro model of Th17 differentiation using flow cytometry, cytokine assays, confocal microscopy, real-time polymerase chain reaction, and immunoblotting. In addition, the role of specific eicosanoids and their receptors was examined using synthetic prostaglandins (PGs), selective inhibitors, and siRNA knockdown.
Measurements and Main Results: Th17 cell differentiation in lung, lymph nodes, and bronchoalveolar lavage fluid was significantly lower in COX-2−/− mice after ovalbumin sensitization and exposure in vivo. In vitro studies revealed significantly impaired Th17 cell differentiation of COX-2−/− naive CD4+ T cells with decreased Stat3 phosphorylation and RORγt expression. Synthetic PGF2α and PGI2 enhanced Th17 cell differentiation of COX-2−/− CD4+ T cells in vitro. The selective COX-2 inhibitor, NS-398, and PGF2α receptor and PGI2 receptor siRNA knockdown significantly decreased Th17 cell differentiation in vitro. Administration of synthetic PGs restored accumulation of Th17 cells in lungs of allergic COX-2−/− mice in vivo.
Conclusions: COX-2 is a critical regulator of Th17 cell differentiation during allergic lung inflammation via autocrine signaling of PGI2 and PGF2α through their respective cell surface receptors.
Keywords: Th17 cell, COX-2, asthma, prostaglandins, IL-17
AT A GLANCE COMMENTARY
Scientific Knowledge on the Subject
Cyclooxygenase (COX) enzymes are known to be important regulators of Th1–Th2 balance in allergic lung disease; however, it is not known whether COX-1– or COX-2–derived eicosanoids regulate Th17 cell function, or the mechanisms involved.
What This Study Adds to the Field
This study identifies COX-2 as a key regulator of Th17 cell differentiation and function in allergic lung inflammation via an autocrine loop that involves prostaglandin I2, prostaglandin F2α, and their respective cell surface receptors.
CD4+ T cells play an important role in the initiation of the immune response by providing help to other cells and by taking on a variety of effector functions during the immune response. On antigenic stimulation, naive CD4+ T cells activate, expand, and differentiate into various effector subsets that are characterized by distinct functions and cytokine profiles (1). Th17 cells are a recently discovered CD4+ helper T-cell subset characterized by the production of IL-17A, IL-17F, and IL-22 (2). Although Th1 and Th2 cell differentiation depend on single effector cytokines (IL-12 and IL-4, respectively), Th17 differentiation is induced by the combined activity of transforming growth factor (TGF)-β and IL-6 in mice, or TGF-β and IL-21 (naive T cells) or IL-1β (memory T cells) in humans (3). In both species, these cytokines affect IL-17 production by activating and inducing the expression of key lineage-specific transcription factors, such as Stat3 and the orphan nuclear receptor RORγt (4). IL-17A plays important roles in immune responses, such as delayed-type hypersensitivity, contact hypersensitivity, and allergic airway inflammation (5). IL-17A promotes inflammation by inducing various proinflammatory cytokines and chemokines, recruiting neutrophils, enhancing antibody production, and activating T cells (6). It was previously reported that IL-17A is up-regulated in asthma and nasal polyposis, and in the latter condition its expression is resistant to topical steroids (7, 8).
Cyclooxygenases (COXs) are responsible for the formation of prostaglandins (PGs), which are involved in regulating inflammatory responses (9, 10). The two COX isoforms, COX-1 and COX-2, are expressed at varying levels in different tissues (11). COX-1 is constitutively expressed in most mammalian tissues and cells, whereas COX-2 is inducible in macrophages and other cell types at sites of inflammation (12, 13). PGs exert their actions by binding to a family of G-protein–coupled receptors. These include the DP1 and DP2 subtypes of the PGD2 receptor; the EP1, EP2, EP3, and EP4 subtypes of the PGE2 receptor; the PGF2α receptor (FP); the PGI2 receptor (IP); and the TxA2 receptor (TP) (14). It is known that some of these receptors are expressed during T-cell differentiation, a process that is important in regulating inflammation and immune responses (15).
Recent studies suggest that COX-1 and COX-2 may play important roles in regulating the Th1–Th2 balance in allergic and nonallergic lung diseases (16). These findings demonstrate COX-2–dependent regulation of TGF-β and IL-6, key cytokines that are produced by macrophages and involved in the differentiation of naive CD4+ T cells to Th17 cells. The proinflammatory effects of PGE2 in experimental inflammatory bowel disease (17) and collagen-induced arthritis in mice (18) are mediated through IL-17. In addition, PGE2 favors Th17 expansion and IL-17 production through activation of dendritic cells via a paracrine mechanism that also involves up-regulation of the IL-23 and IL-1 receptors on T cells (19, 20). However, it remains unknown whether (1) COX-1 or COX-2 are critically involved in the Th17 cell differentiation process, (2) COX-derived PGs other than PGE2 are involved in Th17 cell differentiation, and (3) PGs directly influence Th17 cells during differentiation independent of effects on dendritic cells. Therefore, in the present study we used COX-1−/− and COX-2−/− mice to examine whether PGs regulate Th17 cell differentiation in a model of allergic lung inflammation. Our results show that the number of Th17 cells in lung and lymph nodes was dramatically decreased in COX-2−/− mice, but not COX-1−/− mice, compared with wild-type (WT) mice after ovalbumin sensitization and exposure in vivo. There was also significantly impaired Th17 cell differentiation and IL-17A production in COX-2−/− T cells in vitro. COX-2 was expressed in Th17 cells and regulated dose-dependently by IL-6. PG production was increased during Th17 differentiation, a phenomenon that was largely COX-2 dependent. Importantly, synthetic PGF2α and PGI2 partially restored the Th17 cell differentiation defect in COX-2−/− cells in vitro, and systemic administration of PGs restored accumulation of Th17 cells in lungs of allergic COX-2−/− mice in vivo. Finally, Th17 cells expressed prostanoid receptors, and Th17 cell differentiation was significantly reduced by FP and IP receptor antagonists and siRNA knockdown. Together, these results indicate that Th17 cell differentiation during allergic lung inflammation is critically regulated by COX-2–derived PGF2α and PGI2 via an autocrine pathway that involves binding to FP and IP receptors on T cells.
METHODS
Additional details are provided in the online supplement.
Animals
Six- to 10-week-old male COX-1−/− mice, COX-2−/− mice, and WT control mice on a hybrid C57BL6J × 129/Ola genetic background were purchased from Taconic (Germantown, NY) (16). All animal experiments were performed according to the NIH Guide for the Care and Use of Laboratory Animals and approved by the National Institute of Environmental Health Sciences Animal Care and Use Committee.
Ovalbumin-induced Allergic Airway Inflammation Model
Mice were immunized with ovalbumin (20 μg emulsified in 0.2 ml of aluminum hydroxide adjuvant) or vehicle (adjuvant only) by intraperitoneal injection on Days 0 and 1. Starting on Day 14, mice were exposed to 1% ovalbumin (or saline) via nebulizer for 30 minutes per day for 4 consecutive days. Forty-eight hours after the last exposure, mice were killed for tissue collection.
Bronchoalveolar Lavage Fluid and Isolation of Lung and Spleen CD4+ T Cells
Bronchoalveolar lavage fluid (BALF) was collected in 2 ml of phosphate-buffered saline. Naive or allergic CD4+ T cells were isolated using kits from Miltenyi Biotec (Auburn, CA). Additional experiments used cell sorting to obtain naive CD4+ CD25− CD44+low CD62L+ T cells at greater than 99% purity. Cell cultures were maintained in RPMI 1640 with 10% fetal bovine serum. Th17 differentiation was induced as described (4).
Flow Cytometric Analysis and Intracellular Cytokine Staining
Naive CD4+ T cells were differentiated with TGF-β and IL-6 for 5 days and restimulated for 4 hours with 12–0-tetradecanoyl-phorbol-13-acetate, ionomycin, and brefeldin A. Intracellular cytokine staining of fixed cells was analyzed using an LSRII flow cytometer (Becton Dickinson, San Jose, CA).
siRNA Knockdown of IP and FP Receptors
Freshly isolated naive CD4+ T cells from spleens of WT mice were transfected with 20 nmol of siRNAs to the IP receptor, the FP receptor, or negative control using mouse T cell Nucleofector solution (Amaxa, Koln, Germany). Transfected cells were induced to differentiate into Th17 cells and analyzed by fluorescence-activated cell sorter on Day 5.
Analysis of Cytokine Levels
Blood, BALF, or supernatants from T-cell cultures were analyzed for cytokines using commercial kits.
Histopathology and Immunostaining of Lung Tissue
Lungs were perfusion-fixed and stained with hematoxylin and eosin. For immunstaining of lung tissue, the frozen-lung sections were fixed in methanol with 0.3% H2O2 at 4°C, permeabilized with Triton X-100 (0.8%), and stained with fluorescent antibodies. Adjacent sections were stained with hematoxylin and eosin.
Implantation of Osmotic Minipumps for Delivery of Synthetic PGs
PGE2, PGF2α, and the PGI2 analog iloprost (Cayman, Ann Arbor, MI) or vehicle (15% ethanol and sterile saline) were delivered by osmotic minipumps (Alzet, Cupertino, CA) 1 week before ovalbumin exposure.
Eicosanoid Analysis in Blood and BAL Fluid
Eicosanoid levels in BALF were analyzed by liquid chromatography–tandem mass spectrometry as previously described (21).
Statistical Analyses
Data are presented as means ± SEM. Statistical comparisons were performed by randomized-design two-way analysis of variance followed by the Newman-Keuls post hoc test for more than two groups, or by unpaired Student t test for two groups. Statistical significance was defined as P less than 0.05.
RESULTS
COX-2−/− Mice Have Reduced Th17 Cells during Allergic Lung Inflammation In Vivo
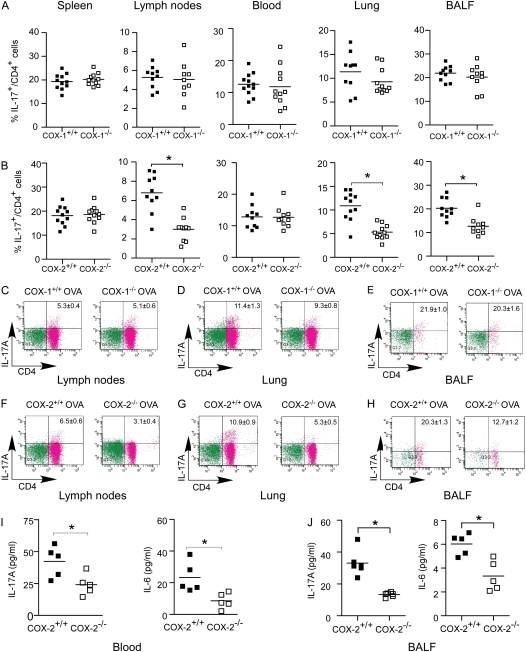
Allergic lung diseases, such as asthma, are hypothesized to result from dysregulated immune responses. A previous study has demonstrated that COX products play an important role in regulating Th1–Th2 balance in both allergic and nonallergic lung diseases (16). Recent progress in characterizing the proinflammatory IL-17 cytokine family has added an additional layer of complexity to the understanding of the regulation of allergic lung inflammation (22). Therefore, to investigate the role of COX-1 and COX-2 in regulating Th17 cells in vivo, we used an established model of ovalbumin sensitization and exposure to induce allergic lung inflammation in COX-1−/−, COX-1+/+, COX-2−/−, and COX-2+/+ mice. After ovalbumin sensitization and exposure, the percentages of IL-17+ CD4+ (Th17) cells in spleen, lymph nodes, blood, lung, and BALF of COX-1−/− and COX-1+/+ mice were comparable (Figures 1A and 1C–1E). In contrast, COX-2−/− mice showed significantly reduced percentages of Th17 cells in lung (5.3% vs. 10.9%), BALF (12.7% vs. 20.3%), and lymph nodes (3.1% vs. 6.5%), but not in spleen and blood, compared with COX-2+/+ mice (Figures 1B and 1F–1H). The absolute number of IL-17+ CD4+ T cells in lung and BALF was also significantly decreased in COX-2−/− mice (see Figure E1 in the online supplement). Consistent with this finding, we found that IL-17A levels in blood and BALF were significantly decreased in COX-2−/− mice compared with COX-2+/+ mice after ovalbumin exposure (Figures 1I and 1J). Interestingly, levels of IL-6 were also significantly decreased in blood and BALF from COX-2−/− mice compared with COX-2+/+ mice after ovalbumin sensitization and exposure (Figures 1I and 1J). Together, these data suggest that Th17 cell numbers, and IL-17A and IL-6 cytokine levels, are reduced in COX-2−/− mice relative to WT during allergic lung inflammation in vivo.
Figure 1.
Reduced Th17 cells in lung, bronchoalveolar lavage fluid (BALF), and lymph nodes of cyclooxygenase (COX)-2−/− mice after ovalbumin (OVA) sensitization and exposure in vivo. COX-1+/+, COX-1−/−, COX-2+/+, and COX-2−/− mice (n = 9–12 each) were sensitized with OVA in adjuvant. Fourteen to 21 days later, mice were exposed to inhaled OVA for 4 consecutive days. The percentages of IL-17A+ CD4+ T cells in spleen, lymph nodes, blood, lung, and BALF from COX-1+/+ versus COX-1−/− mice (A) and COX-2+/+ versus COX-2−/− mice (B) were analyzed by flow cytometry 48 hours after the last OVA exposure. Flow cytometry scattergrams show that the percentage of IL-17A+ CD4+ cells were similar in COX-1+/+ versus COX-1−/− lymph nodes (C), lung (D), and BALF (E). In contrast, COX-2−/− mice had significantly fewer IL-17A+ CD4+ cells in lymph nodes (F), lung (G), and BALF (H). IL-17A and IL-6 concentrations in blood (I) and BALF (J) were measured by ELISA and BioPlex assay 48 hours after the last OVA exposure. For panels A, B, and I, lines indicate the mean, and each symbol (solid squares, COX-1+/+ or COX-2+/+; open squares, COX-1−/− or COX-2−/−) represents an individual mouse. *P < 0.05 versus wild-type.
To further implicate a role for COX-2 in regulating Th17 cells in vivo, and to exclude the contribution of compensatory pathways, such as altered levels of TGF-β, IL-6, or leukocytes in the observed phenotype, we performed an acute COX-2 inhibitor study in WT mice exposed to ovalbumin. Th17 cell percentages in lung (5.18 ± 1.13 vs. 7.25 ± 1.47; n = 6; P < 0.01) and blood (4.05 ± 0.59 vs. 6.07 ± 0.85; n = 6; P < 0.01) were significantly decreased in the COX-2 inhibitor group compared with control after ovalbumin exposure (Figure E2). Th17 cell percentages in BALF (19.12 ± 3.66 vs. 22.42 ± 2.14; n = 6; P > 0.05), lymph nodes (12.03 ± 1.47 vs. 14.21 ± 0.94; n = 6; P > 0.05), and spleen (6.50 ± 1.17 vs. 8.40 ± 0.68; n = 6; P > 0.05) also tended to be lower in the COX-2 inhibitor group, but these differences did not reach statistical significance.
Localization of Th17 Cells to Sites of Allergic Lung Inflammation
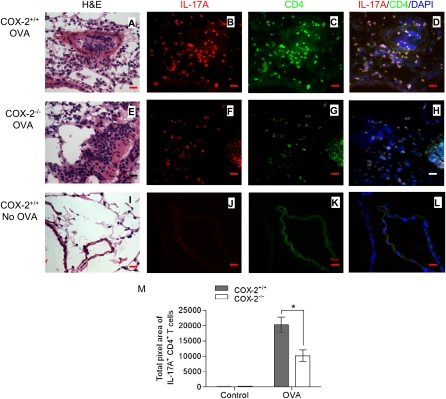
Analysis of lung tissue sections stained with hematoxylin and eosin revealed that allergic COX-2+/+ and COX-2−/− had increased inflammation compared with nonallergic mice that were not sensitized or exposed to ovalbumin (Figures 2A, 2E, and 2I). Consistent with our previous work demonstrating an inhibitory effect of PGs on Th2 immune responses (16, 23), lungs from allergic COX-2−/− mice showed increased airway inflammation compared with lungs from allergic COX-2+/+ mice (Figures 2A and 2E). To examine the localization of Th17 cells in this model, we stained adjacent lung tissue sections with anti-CD4 and anti–IL-17A. In COX-2+/+ mice, there were abundant Th17 cells localized to sites of allergic inflammation (Figures 2B–2D). In contrast, allergic COX-2−/− mice showed markedly reduced numbers of Th17 cells at sites of allergic inflammation (Figures 2F–2H). Th17 cells were not observed in nonallergic mice that were not exposed to ovalbumin (Figures 2J–2L). To quantify the number of Th17 cells in vivo, multiple randomly selected regions (n = 22–29) from each lung section were counted and quantified using MetaMorph software (Sunnyvale, CA). The results demonstrate the presence of significantly more lung IL-17+/CD4+ T cells in COX-2+/+ mice than in COX-2−/− mice after ovalbumin exposure (Figure 2M; P < 0.01). Lung IL-17+/CD4+ T cells of both COX-2+/+ and COX-2−/− mice were significantly increased after ovalbumin exposure compared with control (no ovalbumin exposure). Together, these data indicate that Th17 cells localize to sites of allergic lung inflammation and confirm reduced numbers of Th17 cells in inflammatory loci of COX-2−/− mice exposed to ovalbumin in vivo.
Figure 2.
Reduced Th17 cells in lung of cyclooxygenase (COX)-2−/− mice after ovalbumin (OVA) exposure. COX-2+/+ and COX-2−/− mice were sensitized with OVA in adjuvant (or given adjuvant alone). Fourteen to 21 days later, mice were exposed to inhaled OVA (or inhaled phosphate-buffered saline) for 4 consecutive days. Forty-eight hours after final OVA (or phosphate-buffered saline) exposure, lung tissue sections were stained with hematoxylin and eosin (H&E) and visualized by light microscopy (A, E, and I) (original magnification ×100). Visualization of Th17 cells in COX-2+/+ and COX-2−/− lung tissue sections was accomplished by immunofluorescence staining with phycoerythrin (PE)-labeled anti–IL-17A (B, F, and J) and fluorescein isothiocyanate (FITC)–labeled anti-CD4 (C, G, and K) antibodies. D, H, and L are merged images of anti–IL-17A and PE, anti–CD4 and FITC, and DAPI. All immunofluorescent images are shown at original magnification ×60; numerical aperture, 1.4; scale bar, 25 μm. Results are representative of three independent experiments. Multiple (n = 22–29) randomly selected regions (1,300 × 1,030 pixel2 = 350 × 277 μm2) from each lung section were counted and quantified by a masked observer using MetaMorph software (M); *P < 0.05 versus COX-2+/+.
Regulation of COX-1 and COX-2 Expression during Th17 Cell Differentiation In Vitro and In Vivo
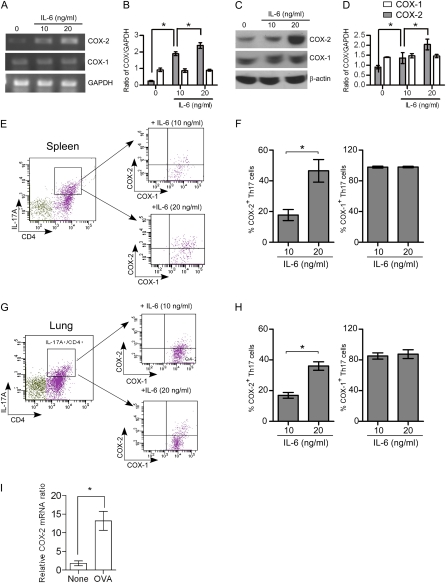
COX-2 is primarily expressed in activated monocytes and macrophages (24); however, T cells are reported to express COX-2 and produce PGE2 after retroviral infection (25). COX-2 expression and PGE2 production are also known to be involved in the inhibitory mechanism of Treg cells (26). To further elucidate the role of COX enzymes in Th17 cell differentiation, the expression of COX-1 and COX-2 in CD4+ T cells during Th17 cell differentiation in vitro was investigated. Naive CD4+ T cells purified from WT mouse spleens were stimulated for 4–5 days to induce Th17 cell differentiation, and COX-1 and COX-2 mRNA and protein levels were examined by real-time polymerase chain reaction (RT-PCR) and immunoblotting, respectively. Untreated, naive CD4+ T cells expressed low levels of COX-2 mRNA and protein (Figures 3A–3D). Treatment of naive CD4+ T cells with anti-CD3, anti-CD28, anti–IFN-γ, TGF-β, and IL-6 increased COX-2 mRNA and protein levels in an IL-6 dose-dependent fashion. In contrast, COX-1 was constitutively expressed in naive CD4+ T cells and mRNA and protein levels were unchanged during Th17 cell differentiation (Figures 3A–3D). We also used flow cytometry to measure intracellular expression of COX-1 and COX-2 during Th17 differentiation. IL-6 significantly and dose-dependently increased the expression of COX-2 protein, but not COX-1 protein, in Th17 cells differentiated from naive CD4+ T cells isolated from spleen (Figures 3E and 3F) and lung (Figures 3G and 3H). To provide further evidence of COX-2 expression in CD4+ T cells that is linked to regulation of Th17 cells in vivo, we isolated CD4+ T cells from lung after ovalbumin exposure and examined COX-2 expression by RT-PCR. The results showed that COX-2 mRNA levels were significantly increased in lung CD4+ cells after ovalbumin exposure (Figure 3I). Together, these results demonstrate that COX-2 is up-regulated during Th17 cell differentiation in vitro and in vivo.
Figure 3.
Regulation of cyclooxygenase (COX)-1 and COX-2 during Th17 cell differentiation from naive CD4+ T cells. Naive CD4+ T cells isolated from spleen were stimulated with or without anti-CD3 (3 μg/ml), anti-CD28 (3 μg/ml), anti–INF-γ (3 μg/ml), transforming growth factor-β (10 ng/ml), and IL-6 (10 or 20 ng/ml) for 4 days to induced Th17 differentiation and then the cells were analyzed for COX-1 and COX-2 expression by real-time polymerase chain reaction (RT-PCR), protein immunoblotting, and flow cytometry. (A) PCR products showing mRNA levels of COX-1, COX-2, and glyceralhehyde phosphate dehydrogenase (GAPDH). (B) COX-1 and COX-2 mRNAs were quantified by densitometry and expressed as the density ratio of COX-1 or COX-2 relative to GAPDH. Results are representative of three independent experiments. (C) Immunoblot analysis of cell lysates for COX-1, COX-2, and β-actin. (D) Protein levels of COX-1 and COX-2 were quantified by densitometry and expressed relative to β-actin. Results are representative of three independent experiments. (E and G) Naive CD4+ T cells were stimulated with anti-CD3, anti-CD28, anti–INF-γ, transforming growth factor-β, and IL-6 (10 and 20 ng/ml) for 4 days, and then stimulated for 4 hours with 12–0-tetradecanoyl-phorbol-13-acetate and ionomycin (500 ng/ml each) in the presence of brefeldin A (1 μg/ml) before intracellular staining with anti-CD4, IL-17, COX-1, and COX-2. Gating on the CD4+ IL-17A+ cell population, COX-1 and COX-2 expression was analyzed by flow cytometry. (F and H) The percent of the COX-1– and COX-2–positive Th17 cells after treatment with different concentrations of IL-6 is shown. Results are representative of three independent experiments. *P < 0.05. (I) Lung CD4+ T cells were isolated after ovalbumin (OVA) exposure in vivo and COX-2 mRNA levels were quantified by RT-PCR; n = 6; *P < 0.01 versus control (no OVA).
Impaired Th17 Cell Differentiation of COX-2−/− Naive CD4+ T Cells In Vitro
To examine the role of COX-2 in Th17 cell differentiation in vitro, we treated equal numbers of naive CD4+ T cells (>95% pure) isolated from WT and COX-2−/− mouse spleens with anti-CD3, anti-CD28, anti–IFN-γ, TGF-β, and IL-6, and quantified IL-17–producing CD4+ T cells by flow cytometry on Day 4. Interestingly, we observed that 6.6% of WT naive CD4+ T cells differentiated into Th17 cells, compared with only 0.7% of COX-2−/− naive CD4+ T cells (Figures 4A and 4B). Thus, there was significantly impaired Th17 cell differentiation of COX-2−/− CD4+ T cells in vitro. Consistent with the role of COX-2 in Th17 cell differentiation, the selective COX-2 inhibitor NS-398 also significantly reduced Th17 differentiation in vitro (Figure 4C).
Figure 4.
Impaired Th17 differentiation in cyclooxygenase (COX)-2−/− naive CD4+ T cells in vitro. (A) Naive CD4+ T cells isolated from spleens of COX-2+/+ and COX-2−/− mice were treated with or without anti-CD3 (3 μg/ml), anti-CD28 (3 μg/ml), anti–INF-γ (3 μg/ml), transforming growth factor (TGF)-β (10 ng/ml), and IL-6 (10 ng/ml) for 4 days. The cells were then stimulated for 4 hours with 12–0-tetradecanoyl-phorbol-13-acetate and inomycin (500 ng/ml each) in the presence of brefeldin A (1 μg/ml) before flow cytometry analysis. Data are representative of three independent experiments. (B) The percentage of naive CD4+ T cells from COX-2+/+ and COX-2−/− mice that differentiated to CD4+ IL-17A+ (Th17) cells is shown. (C) Inhibition of COX-2 with NS-398 during Th17 cell differentiation from naive CD4+ T cells was examined. Naive CD4+ T cells were cultured with anti-CD3 (3 μg/ml), anti-CD28 (3 μg/ml), anti–INF-γ (3 μg/ml), TGF-β (10 ng/ml), and IL-6 (10 ng/ml) in the presence or absence of NS-398 (20 μM) for 4 days. The cells were then stimulated for 4 hours with 12–0-tetradecanoyl-phorbol-13-acetate and inomycin (500 ng/ml each) in the presence of brefeldin A (1 μg/ml) before flow cytometry analysis. (D) The percentage of IL-17A+ CD4+ cells was examined by flow cytometry at different time points (Days 1–7) after treatment with anti-CD3, anti-CD28, anti–INF-γ, TGF-β, and IL-6. (E) Cell culture supernatants from D were collected at different time points and IL-17A levels were assayed by ELISA. (F) Naive CD4+ T cells from COX-2+/+ and COX-2−/− mice were differentiated to Th17 cells in the presence of different amounts of IL-6 (0, 5, 10, and 20 ng/ml) for 4 days and the percent of CD4+ IL-17A+ cells was analyzed by flow cytometry. (G) IL-17A levels in cell culture supernatants from F were assayed by ELISA. For panels C–F, data are representative of three independent experiments; closed circles = COX-2+/+; open circles = COX-2−/−; * P < 0.05 versus COX-2+/+. (H) Total number of CD4+ T cells were enumerated in spleen, lymph nodes, and lung from COX-2+/+ and COX-2−/− mice (n = 10 per group). (I) Splenocytes from COX-2+/+ and COX-2−/− mice were analyzed for CD62L+ and CD4+ expression. Representative scattergrams are illustrated and the percentages of CD62L+ CD4+ cells (mean ± SE; n = 10 per group) are shown. (J) Naive CD4+ CD62L+ T cells from spleens of COX-2+/+ and COX-2−/− mice were first sorted by flow cytometry. These highly purified naive CD4+ CD62L+ T cells were then differentiated into Th17 cells as described in A. On Day 4, the cells were collected and the percentage of IL-17A+ CD4+ cells was analyzed by flow cytometry. (K) Quantification of Th17 cell differentiation of COX-2+/+ and COX-2−/− naive CD4+ CD62L+ T cells is shown.
We also examined the kinetics of Th17 cell differentiation of WT and COX-2−/− naive CD4+ T cells. Th17 cell differentiation of WT naive CD4+ T cells increased daily and attained a maximum on Day 4 of treatment (Figure 4D). In contrast, there was no significant peak of Th17 cell differentiation in COX-2−/− naive CD4+ T cells. Consistent with these data, IL-17A production also peaked on Day 4 in WT naive CD4+ T cells (Figure 4E). In contrast, IL-17A production remained low in COX-2−/− naive CD4+ T cells treated under identical conditions.
IL-6 critically regulates the differentiation potential of naive T cells into Th17 cells. Moreover, allergic COX-2−/− mice have reduced levels of IL-6 (Figures 1I and 1J). Therefore, we tested whether alterations in IL-6 levels were responsible for defective Th17 cell differentiation of COX-2−/− naive CD4+ T cells. We found that Th17 cell differentiation of COX-2+/+ naive CD4+ T cells (Figure 4F) and the ability of these cells to produce IL-17A (Figure 4G) were IL-6 dosage-dependent; however, IL-6 treatment failed to increase Th17 cell differentiation or IL-17A production of COX-2−/− naive CD4+ T cells to levels observed in COX-2+/+ cells (Figures 4F and 4G). Thus, the reduced Th17 cell differentiation in COX-2−/− naive CD4+ T cells does not seem to be caused by reduced IL-6 production.
To exclude the possibility that the Th17 differentiation defect in COX-2−/− mice was caused by altered IL-6 or TGF-β receptor expression in naive CD4+ T cells, we examined the expression of IL-6 and TGF-β receptors by RT-PCR in COX-2−/− naive CD4+ T cells and in Th17 cells in vitro. The results show similar expression of IL-6 and TGF-β receptors in both naive CD4+ T cells and Th17 cells from COX-2−/− and WT mice (Figure E3).
To exclude the possibility that the defect of Th17 cell differentiation in COX-2−/− mice was caused by diminished numbers of naive CD4+ T cells, we examined CD4+ T cell populations in various tissues from COX-2+/+ and COX-2−/− mice by flow cytometry. The number of CD4+ T cells in spleens, lymph nodes, and lungs were comparable between COX-2−/− and COX-2+/+ mice (Figure 4H). Furthermore, the percentage of splenic CD62L+ CD4+ T cells was similar between COX-2−/− and COX-2+/+ mice (Figure 4I).
B cells can produce a variety of cytokines that influence Th17 differentiation (27). To exclude the possibility that the defect of Th17 cell differentiation in COX-2−/− mice was caused by impaired B-cell development, we examined B220+ populations in various tissues from COX-2+/+ and COX-2−/− mice. The number of B220+ cells in blood, spleens, lymph nodes, BALF, and lungs were also comparable between COX-2−/− and COX-2+/+ mice (Figure E4).
Defective Th17 cell differentiation in COX-2−/− mice could also be caused by alterations in other cell types, such as dendritic cells. These cells produce a variety of cytokines that can activate Stat3 and initiate RORγt transcription and Th17 cell differentiation (28). Moreover, PGs secreted from dendritic cells might stimulate Th17 cell differentiation via a paracrine mechanism. Alternatively, PGs secreted by T cells might stimulate dendritic cells to produce IL-23, which can promote differentiation of Th17 cells (20, 29). To eliminate the possibility that contamination of naive CD4+ T cells by other cell types contributed to the Th17 cell differentiation defect in COX-2−/− mice, we sorted naive CD4+ CD62L+ T cells from spleens of COX-2+/+ and COX-2−/− mice by flow cytometry. These highly (>99%) purified cells were then differentiated into Th17 cells in vitro. Consistent with the results described in Figure 4, the percentage of highly purified naive CD4+ CD62L+ T cells from COX-2−/− mice that differentiated into Th17 cells was significantly lower than from COX-2+/+ mice (Figures 4J and 4K). These data suggest that COX-2 products act as T-cell autocrine factors to induce Th17 differentiation.
COX-2−/− Th17 Cells Have Altered Stat3 Phosphorylation and RORγt Expression
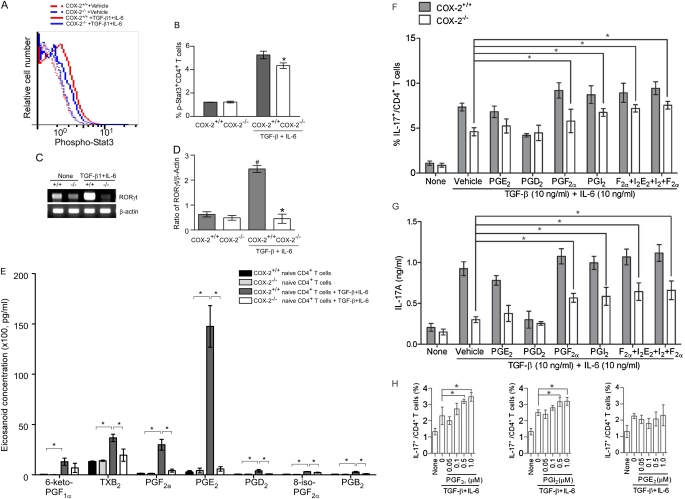
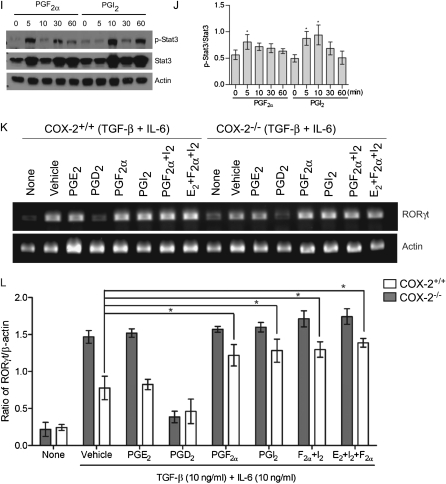
Th17 cell differentiation is known to be accompanied by Stat3 activation and induction of the lineage-specific transcription factor RORγt (30). To determine if the defect in Th17 cell differentiation in COX-2−/− mice was accompanied by altered Stat3 activation and RORγt expression, we examined Stat3 phosphorylation and RORγt mRNA levels during Th17 differentiation in vitro. Stat3 phosphorylation was significantly reduced in Th17 cells differentiated from CD4+ T cells isolated from COX-2−/− mice relative to those isolated from COX-2+/+ mice (Figures 5A and 5B). Likewise, the expression of RORγt was markedly lower in Th17 cells differentiated from naive CD4+ T cells isolated from COX-2−/− spleens compared with those isolated from COX-2+/+ spleens (Figures 5C and 5D).
Figure 5.
Cyclooxygenase (COX)-2–derived prostaglandins (PGs) regulate Th17 cell differentiation in vitro. (A) Stat3 phosphorylation during Th17 differentiation of CD4+ T cells from COX-2+/+ and COX-2−/− mice was assayed using anti–phospho-stat3 (pY705)-PE by flow cytometry. (B) Ratio of phospho-Stat3/total Stat3 during Th17 cell differentiation of COX-2\\plus/\plus+/+ and COX-2−/− naive CD4+ T cells. (C) RORγt mRNA expression in differentiated CD4+ T cells from COX-2+/+ and COX-2−/− mice was analyzed by real-time polymerase chain reaction. (D) Ratio of RORγt to β-actin mRNA during Th17 cell differentiation of COX-2+/+ and COX-2−/− naive CD4+ CD62L+T cells. All data are representative of three independent experiments; #P < 0.05 versus COX-2+/+ naive CD4+ T cells; *P < 0.05 versus COX-2+/+. (E) Eicosanoid levels in supernatants of naive CD4+ T cells and in vitro differentiated Th17 cells from COX-2+/+ and COX-2−/− mice were measured by liquid chromatography/tandem mass spectroscopy. Results are representative of five independent experiments. Effect of synthetic PGs on Th17 cell differentiation of (F) and IL-17A production by (G) naive CD4+ T cells isolated from COX-2+/+ and COX-2−/− mice in vitro. PGs were added at 1 μM on Days 2 and 3 of culture, and culture supernatants were collected on Day 4. IL-17A was measured by ELISA. Results are representative of three independent experiments; *P < 0.05 versus vehicle. (H) Dose dependency of PGI2, PGF2α, and PGE2 (50 nM to 1 μM) on Th17 cell differentiation was examined in vitro; n = 4; *P < 0.05 versus no PG. (I and J) Phospho-Stat3 and total Stat3 levels were determined by immunoblotting before and 5–60 minutes after treatment of naive CD4+ T cells with synthetic PGs (1 μM each). Results are representative of three independent experiments; * P < 0.05 versus time 0. (K and L) RORγt mRNA levels measured by real-time polymerase chain reaction in in vitro differentiated Th17 cells treated with or without synthetic PGs (1 μM each). Results are representative of three independent experiments; * P < 0.05 versus vehicle.
COX-2 Regulates Th17 Cell Differentiation through PGs
To further examine the role of COX-2–derived PGs in regulating T-cell differentiation, eicosanoid levels in supernatants of naive CD4+ T cells and in vitro differentiated Th17 cells from COX-2+/+ and COX-2−/− mice were measured by liquid chromatography–tandem mass spectroscopy. PG levels were low-undetectable in naive CD4+ T cells and not significantly different between the two genotypes (Figure 5E). Levels of each of the PGs increased significantly with Th17 differentiation in cells from COX-2+/+ mice; the largest increases were in PGE2, PGF2α, and 6-keto-PGF1α (the stable prostacyclin metabolite). Importantly, levels of each of the PGs were reduced in Th17 cells differentiated from CD4+ T cells isolated from COX-2−/− mice relative to COX-2+/+ mice (Figure 5E). In addition, immunofluorescent staining showed that Th17 cells from COX-2+/+ mouse lungs produced PGF2α and PGI2 after ovalbumin sensitization and exposure in vivo (Figure E5). Together, these data suggest that PG production is increased during Th17 differentiation and that COX-2 is critical for optimal PG production in Th17 cells.
To determine if the decreased PG production in COX-2−/− Th17 cells resulted in altered systemic levels of these eicosanoids or altered levels in the allergic lung, we measured PG levels in blood and BAL fluid of WT and COX-2−/− mice after ovalbumin sensitization and exposure in vivo. Consistent with our prior published data (16, 23), we observed that PG levels in blood and BAL fluid were not significantly altered in allergic COX-2−/− mice relative to WT (Figure E6). Thus, although COX-2 is critical for optimal production of PGs in Th17 cells, it does not seem to contribute significantly to circulating levels of PGs or to PG production at the whole organ level.
Next, we determined whether PGs could restore the Th17 cell differentiation and IL-17A production defects in COX-2−/− mice. Synthetic PGs (1 μM each) were added to naive CD4+ T cells during Th17 cell differentiation in vitro and the percent of Th17 cells and IL-17A production were measured by flow cytometry and ELISA, respectively. Administration of synthetic PGF2α and PGI2, but not PGD2 or PGE2, partially restored Th17 cell differentiation (Figure 5F) and IL-17A production (Figure 5G) in COX-2−/− cells. PGF2α and PGI2 also increased Th17 differentiation in COX-2+/+ cells, although these changes did not reach statistical significance. In contrast, PGD2 decreased Th17 differentiation and IL-17A production in COX-2+/+ cells (Figures 5F and 5G). To examine the specificity of cellular responses to the prostanoids, we performed a dose-response study of PGF2α, PGI2, and PGE2 on Th17 cell differentiation in vitro over a range of prostanoid concentrations (50 nM to 1 μM). Both PGI2 and PGF2α significantly enhanced Th17 cell differentiation at concentrations as low as 500 nM and showed a trend for enhanced Th17 cell differentiation at lower concentrations (Figure 5H). In contrast, we did not observe a significant effect of PGE2 on Th17 cell differentiation at concentrations as high as 1 μM. Importantly, synthetic PGF2α and PGI2 treatment increased Stat3 phosphorylation in naive CD4+ T cells from WT mice in vitro, whereas Stat3 activation by PGD2 and PGE2 was not observed (Figures 5I and 5J; Figure E7). Likewise, PGF2α and PGI2, but not PGD2 or PGE2, significantly induced RORγt expression in in vitro differentiated COX-2−/− Th17 cells (Figures 5K and 5L). These results suggest that COX-2 directly regulates Th17 cell differentiation in vitro through production of specific PGs.
We then implanted osmotic minipumps containing synthetic PGs to determine if we could restore the COX-2−/− Th17 cell defect in vivo in allergic mice after ovalbumin sensitization and exposure. Th17 cell percentages were increased in lungs, BALF, and lymph nodes of allergic COX-2−/− mice treated with iloprost (a stable PGI2 analog), PGF2α, PGE2, or a combination of iloprost, PGF2α, and PGE2 compared with mice treated with vehicle (Figures 6A–6C). Consistent with these data, IL-17A levels in BALF were significantly increased in COX-2−/− mice treated with iloprost, PGF2α, PGE2, or the combination (Figure 6D). Taken together, these data suggest that COX-2–derived PGs regulate Th17 cell differentiation and IL-17A production in vivo in allergic lung disease.
Figure 6.
Administration of synthetic prostaglandins (PGs) restores Th17 cell percentages in allergic cyclooxygenase (COX)-2−/− mice in vivo. Alzet minipumps filled with PGE2, PGF2α, iloprost, or the combination of PGs were implanted into sensitized COX-2−/− mice. After 1 week, mice were exposed to ovalbumin daily for 4 days. Forty-eight hours after the last ovalbumin exposure, the mice were killed and the percentages of IL-17A+ CD4+ T cells in lung (A), bronchoalveolar lavage fluid (BALF) (B), and lymph nodes (C) were determined by flow cytometry. (D) IL-17 levels in BALF samples from the above mice were assayed for IL-17A by ELISA. Results are representative of three independent experiments. n = 4; *P < 0.05 versus saline vehicle. In A, B, and C, lines indicate the mean, and each symbol represents an individual mouse.
PGs Regulate Th17 Cell Differentiation through Their Cognate Receptors
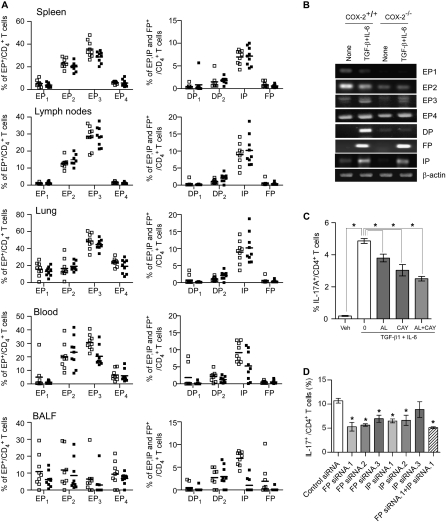
To determine whether COX-2–derived PGs regulate Th17 cell differentiation through their cognate receptors, we first determined the expression of the prostanoid receptors on naive CD4+ T cells isolated from allergic COX-2+/+ and COX-2−/− mice after in vivo ovalbumin sensitization and exposure. The EP1, EP4, DP1, DP2, and FP receptors were present on a low percentage of naive CD4+ T cells isolated from spleen, lymph nodes, lung, blood, and BALF of allergic COX-2+/+ and COX-2−/− mice; there were no significant differences in the percentage of naive CD4+ T cells that expressed these receptors between the two genotypes (Figure 7A). The EP2, EP3, and IP receptors were present on a significantly greater percentage of naive CD4+ T cells isolated from allergic COX-2+/+ and COX-2−/− mouse tissues, but again there were no consistent differences between the two genotypes (Figure 7A).
Figure 7.
Expression of prostaglandin (PG) receptors on CD4+ T cells in vivo and in vitro differentiated Th17 cells from cyclooxygenase (COX)-2+/+ and COX-2−/− mice. (A) The percentage of CD4+ T cells isolated from spleen, lymph nodes, lung, blood, and bronchoalveolar lavage fluid (BALF) of allergic COX-2+/+ and COX-2−/− mice was determined by flow cytometry. Antibodies to each of the PG receptors were conjugated with AlexaFlur-488 or AlexaFlur-594 using a Therm Scientific DyLightTM 488/594 microscale antibody labeling kit. Cell suspensions were prepared and stained with the conjugated receptor antibodies. EP1-EP4, DP1, DP2, FP, and IP receptor-positive CD4+ T cells were quantified by flow cytometry by gating on the IL-17+ CD4+ T cell population. Lines indicate the mean, and each symbol (open squares, COX-2+/+; solid squares, COX-2−/−) represents an individual mouse. (B) Levels of EP1-EP4, DP, FP, and IP receptor mRNAs in naive CD4+ T cells and in in vitro differentiated Th17 cells from COX-2+/+ and COX-2−/− mice. Naive CD4+ T cells isolated from COX-2+/+ and COX-2−/− mice were stimulated with or without anti-CD3, anti-CD28, anti–INF-γ, IL-6, and transforming growth factor (TGF)-β for 4 days. Total RNA was then extracted and reverse transcribed to cDNA for detection of EP1-EP4, DP, FP, and IP receptor expression by real-time polymerase chain reaction. (C) The effects of FP and IP receptor antagonists on Th17 cell differentiation of naive CD4+ T cells were investigated. Naive CD4+ T cells from wild-type mice were differentiated to Th17 cells in the presence or absence of the FP receptor antagonist, AL8810, the IP receptor antagonist, CAY10441, or a combination of AL8810 and CAY10441. The percentage of Th17 cells was analyzed by flow cytometry after 5 days. n = 3; * P < 0.05. (D) The effects of FP and IP receptor knockdown on Th17 differentiation of naive CD4+ T cells were investigated. Naive CD4+ T cells from wild-type mice were transfected with IP receptor siRNAs, FP receptor siRNAs, a combination of IP receptor and FP receptor siRNAs, or control siRNA. Transfected cells were then differentiated in the presence of anti-CD3 (3 μg/ml), anti-CD28 (3 μg/ml), anti–INF-γ (3 μg/ml), TGF-β (10 ng/ml), and IL-6 (10 ng/ml) for 5 days and the percentage of Th17 cells was analyzed by flow cytometry. n = 3; * P < 0.05 versus control siRNA.
We next examined PG receptor expression at the mRNA level by RT-PCR on both naive CD4+ T cells and on in vitro differentiated Th17 cells from COX-2+/+ and COX-2−/− mice. Naive CD4+ splenic T cells expressed EP1, EP2, EP3, and EP4 receptor mRNAs, and to a lesser extent DP, FP, and IP receptor mRNAs (Figure 7B). There were no substantive differences in mRNA levels between COX-2+/+ and COX-2−/− mice. All of the PG receptor mRNAs were detected in in vitro differentiated Th17 cells (Figure 7B). Levels of EP1, EP2, and DP receptor mRNAs were lower in COX-2−/− Th17 cells compared with COX-2+/+ Th17 cells. Thus, the FP and IP receptors were induced in both COX-2+/+ and COX-2−/− cells during Th17 cell differentiation, whereas the DP receptor was not induced in COX-2−/− cells.
To elucidate further the role of PG receptors in Th17 cell differentiation, we examined the effects of selective FP and IP receptor antagonists and siRNA knockdown in vitro. Th17 cell differentiation of naive CD4+ T cells was significantly reduced if either the selective FP receptor antagonist AL8810 or the selective IP receptor antagonist CAY10441 was added to the culture medium (Figure 7C). Likewise, siRNA knockdown of either the FP receptor or the IP receptor significantly reduced Th17 cell differentiation in vitro (Figure 7D; Figure E8). These results indicate that COX-2–derived PGs directly regulate Th17 cell differentiation through both the FP and IP receptors.
DISCUSSION
Th17 cells have been recognized as a unique subset of effector T cells that are distinct from Th1 and Th2 subsets (31–33). They have been implicated as potent effectors of autoimmune disorders, such as multiple sclerosis, psoriasis, arthritis, and inflammatory bowel disease, and allergic disorders, such as asthma (2, 34, 35). COX-derived PGs are known to play important roles in the regulation of inflammatory responses. However, the role of PGs in regulating Th17 cell differentiation and function has remained enigmatic. In this report, we investigated the functions of COX-1 and COX-2 in regulating Th17 cell differentiation and function in an allergic lung inflammation model. Our main findings are as follows: (1) the number of Th17 cells in lung, BALF, and lymph nodes, and the levels of IL-17A in blood and BALF, are decreased in allergic COX-2−/− mice in vivo; (2) COX-2 expression is induced during Th17 cell differentiation in an IL-6 dose-dependent manner; (3) Th17 cell differentiation of naive CD4+ T cells from COX-2−/− mice is impaired in vitro; (4) selective COX-2 inhibition also inhibits Th17 cell differentiation; (5) activation of Stat3 and induction of RORγt are decreased during Th17 cell differentiation in COX-2−/− mice; (6) the production of PGs is increased during Th17 differentiation; (7) PG production during Th17 cell differentiation is lower in COX-2−/− mice; (8) synthetic PGs partially restore the Th17 cell differentiation defect in COX-2−/− mice in vitro and in vivo; and (9) PGs act as autacoids to promote Th17 cell differentiation by acting through their cognate receptors. Together, these data indicate that COX-2, but not COX-1, is a critical regulator of local cytokine production and Th17 cell differentiation during allergic lung inflammation.
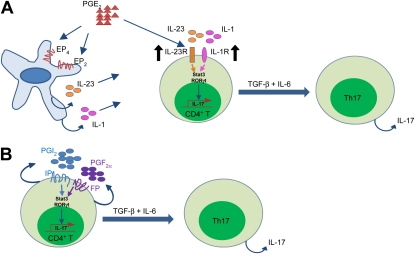
Immune cells produce a variety of PGs that have both proinflammatory and antiinflammatory effects (36). PGE2 is the most abundantly produced prostanoid in the body and has been shown to play an important role in regulating inflammatory processes (37, 38). Indeed, several recent studies suggest a possible role for PGE2 in promoting T-cell differentiation. Activation of the EP4 receptor in T cells and dendritic cells not only facilitates Th1 cell differentiation but also amplifies IL-23–mediated Th17 cell expansion in vitro (19, 29). Administration of an EP4-selective antagonist in vivo decreases accumulation of both Th1 and Th17 cells in regional lymph nodes and suppresses disease progression in mice subjected to experimental autoimmune encephalomyelitis or contact hypersensitivity (39). In vitro differentiation of dendritic cells in the presence of PGE2 alters the IL-12–IL-23 balance and promotes differentiation of Th17 cells via a paracrine mechanism (20). In purified human naive T cells, PGE2 acts via EP2 and EP4 receptors to up-regulate IL-23 receptor and IL-1 receptor expression (19). Furthermore, PGE2 acts in concert with IL-1β and IL-23 to drive RORγt, IL-17, IL-17F, CCL20, and CCR6 expression (29). Thus, previous studies suggest that PGE2 promotes Th17 cell differentiation through an IL-23–IL-1 dependent paracrine pathway that involves dendritic cells (Figure 8A). Despite these studies, it is not known if COX-derived PGs other than PGE2 are involved in Th17 cell differentiation, or if PGs directly influence Th17 cells during the differentiation process, independent of their effects on dendritic cells.
Figure 8.
Proposed mechanisms for the regulation of Th17 cell differentiation by cyclooxygenase (COX)-2–derived prostaglandins (PGs). (A) Paracrine pathway of PGE2 effects on Th17 cell differentiation. Binding of PGE2 to EP2 and EP4 on dendritic cells leads to increased IL-23 and IL-1 expression. In addition, PGE2 induces IL-23R and IL-1R expression on CD4+ T cells. The IL-23 and IL-1 signals lead to activation of Stat3 and up-regulation of RORγt, which in conjunction with transforming growth factor (TGF)-β and IL-6, leads to IL-17 expression. (B) Autocrine pathway of PGI2 and PGF2α effects on Th17 cell differentiation. Binding of PGI2 and PGF2α to IP and FP receptors on CD4+ T cells leads to activation of Stat3 and up-regulation of RORγt, which in conjunction with TGF-β and IL-6 leads to IL-17 expression.
Th17 cell differentiation depends, at least in part, on the local cytokine microenvironment at the differentiation site (40). Key cytokines include TGF-β1, IL-1β, IL-2, IL-6, and IL-23, which are mainly produced by local macrophages, dendritic cells, and neutrophils. Consistent with the importance of locally produced cytokines in directing Th17 differentiation, we observed tissue-specific reductions in Th17 cells in lung and lymph nodes, but not in blood and spleen, of COX-2−/− mice after ovalbumin exposure. It is well known that COX-2 metabolites are involved in allergic lung inflammation (23, 41). PGE2 affects antigen-presenting cell cytokine production, reducing the production of IL-12p35, IL-6, and tumor necrosis factor-α, and increasing the production of IL-10, IL-12p40, and IL-23 (42, 43). Thus, PGE2 may impact the functional characteristics of T cells during priming. Proinflammatory cytokines, such as IL-1β and tumor necrosis factor-α, are dramatically up-regulated in the early phase of allergic lung inflammation and induce COX-2 in many cell types, including dendritic cells, macrophages, and T cells (43). COX-2 metabolites further enhance the secretion of cytokines in dendritic cells, macrophages, and T cells, including TGF-β1, IL-1β, IL-2, IL-6, IL-23, and also increase secretion of chemokines, which are critical for trafficking of other cell types to the site of allergic inflammation (43). Therefore, COX-2 products regulate the cytokine and chemokine microenvironment during Th17 cell differentiation in a tissue-specific fashion.
COX-2 may directly regulate Th17 cell differentiation of naive CD4+ T cells or may indirectly regulate Th17 cell differentiation by acting on other cell types. Several lines of evidence presented herein suggest the presence of a direct effect. First, we used highly purified naive CD4+ CD62L+ T cells from COX-2+/+ and COX-2−/− mice for in vitro studies making confounding effects of other cell types unlikely. Second, we observed similar numbers of CD4+ T cells and B cells in COX-2+/+ and COX-2−/− mice suggesting that impaired T-cell development in COX-2−/− mice was not likely because of altered numbers of these cell types. Third, we observed up-regulation of COX-2 and enhanced PG biosynthesis in isolated CD4+ T cells during Th17 cell differentiation in vitro. Fourth, we observed reduced levels of PGs in Th17 cell cultures from COX-2−/− mice. Finally, PGI2 and PGF2α were expressed in lung Th17 cells, and addition of PGF2α and PGI2 partially restored the Th17 differentiation defect of COX-2−/− cells in vitro. Thus, PGs that are produced by COX-2 in T cells act in an autocrine fashion during the differentiation process (Figure 8B). Previous studies may have overlooked the potent autocrine effects of COX-2–derived metabolites during T-cell differentiation. It should be noted that our data do not exclude the possibility that the reduced Th17 cell numbers in allergic COX-2−/− mice in vivo might be caused, at least in part, by effects of other cell types. In fact, our data showing that PGE2 restores the Th17 differentiation defect in vivo, but not in isolated CD4+ T cells in vitro, suggest an indirect effect of PGE2 on cell types other than T cells, which in turn influences the Th17 cell differentiation process.
Others have observed PGE2 promotion of Th17 cell differentiation in vivo through enhanced IL-23 secretion from dendritic cells (19, 29). In our in vitro experiments, PGE2 did not restore the Th17 cell differentiation defect, whereas PGF2α and PGI2 enhanced Th17 cell differentiation of COX-2−/− naive CD4+ T cells. To our knowledge, this is the first demonstration of an effect of PGF2α and PGI2 on Th17 cells during differentiation. The lack of an effect of PGE2 in our in vitro experiments is likely caused by the exclusion of other cell types from this system. In the absence of dendritic cells, and the paracrine effects of PGE2 on these cells, PGE2 failed to restore the Th17 cell differentiation defect. Interestingly, the combination of PGI2 and PGF2α enhanced, but did not completely restore, Th17 differentiation and IL-17 production in COX-2−/− naive CD4+ cells. Hence, it remains possible that other COX-2 metabolites, or a direct interaction of COX-2 with Stat3 or RORγt, may promote IL-17 production and Th17 cell differentiation (44, 45). In addition, differences in the percent of Th17 cells and IL-17 formation between COX-2−/− and WT mice persist despite PG treatment. This suggests that PG amplification may be secondary to the differential response of WT and COX-2−/− CD4+ cells to the cytokine–antibody cocktail.
Consistent with our previous work demonstrating an inhibitory effect of PGs on Th2 immune responses (23), we found that lungs from allergic COX-2−/− mice showed increased airway inflammation compared with lungs from allergic COX-2+/+ mice. Moreover, Th17 cells were primarily localized to sites of inflammation, which is consistent with the concept that Th17 cell differentiation is dependent on the local inflammatory cytokine milieu. Although there was increased airway inflammation in COX-2−/− mice after ovalbumin sensitization and exposure, fewer Th17 cells were found in lung inflammatory sites in COX-2−/− mice. One possible explanation for this apparent paradox is that allergic COX-2−/− mice also have increased numbers of Th2 cells (Figure E9) that are likely responsible, at least in part, for the enhanced allergic inflammatory response in the lungs of these mice.
Stat3 has been proposed to be a master regulator of Th17 cells, regulating Th17 cell differentiation and cytokine production, and induction of RORγt and IL-23R (46). IL-6 and TGF-β1 induce Th17 differentiation, whereas IL-23 is thought to be more important for in vivo maintenance of Th17 cells (31). Both IL-6 and IL-23 activate Stat3, a process that was found to be necessary for optimal Th17 differentiation. RORγt is a critical transcription factor in Th17 differentiation and is induced by the previously mentioned cytokines in a Stat3-dependent manner (47). PGE2 activates the Stat3 signaling pathway to regulate tumor growth in a variety of human cancers through the EP1 receptor (48). PGI2 induced Stat3 phosphorylation in human erythroleukemia cells (49). Our results demonstrate that Stat3 phosphorylation was lower in Th17 cells differentiated from naive CD4+ T cells isolated from COX-2−/− mice. Stat3 activation by PGE2 and PGD2 was not observed in our studies, perhaps because of low DP, EP1, and EP4 receptor expression on CD4+ T cells. However, PGI2 and PGF2α directly activated Stat3 phosphorylation during Th17 cell differentiation from naive CD4+ T cells in vitro. Importantly, IP and FP receptor antagonists and siRNA knockdown of these receptors reduced Th17 cell differentiation of naive CD4+ T cells in vitro. Together, these results provide compelling evidence that PGs bind to their cognate receptors to facilitate Th17 differentiation.
In summary, our findings demonstrate that COX-2 is a critical regulator of Th17 cell differentiation and function in vitro and in vivo. Moreover, our results provide new mechanistic insights into how COX-2–derived PGs act to enhance Th17 cell differentiation of naive CD4+ T cells. Future studies should examine the role of COX-derived eicosanoids in regulating the differentiation and function of other T-cell subsets.
Supplementary Material
Acknowledgments
The authors thank Dr. Qingqing Wang (Institute of Immunology, Zhejiang University School of Medicine, Hangzhou) and Dr. Hirotake Kasai (Department of Immunology, Duke University Medical Center) for helpful discussion and critical reading of the manuscript. They acknowledge the assistance of Maria Sifre in the flow cytometry center. They also acknowledge the support of Drs. Steven Kleeberger, Michael B. Fessler, and Farhad Imani for providing some experimental reagents.
Footnotes
Supported by the Division of Intramural Research, National Institutes of Health/National Institute of Environmental Health Sciences (Z01 ES025043 and Z01 ES050167).
Contributions of Authors: The work presented here was performed in collaboration with all authors. H.L.: conception, design, performance and interpretation of experiments, and writing the manuscript. J.A.B.: Acquisition and analysis of in vivo, western blot, and ELISA experiments. R.T.D.: Acquisition and analysis of prostanoid mini-pump data. Revision of manuscript. M.L.E.: Acquisition and analysis of LC-MS data, and revision of manuscript. J.P.G.: Acquisition and analysis of RT-PCR data. L.M.D.: Acquisition and analysis of prostanoid mini-pump data. P.M.W.: Acquisition and analysis of lung immunostaining data. C.D.B.: Acquisition and analysis of FACS data. S.M.: Acquisition and analysis of in vivo experimental data. F.B.L.: Acquisition and analysis of LC-MS data. K.B.T.: Design, acquisition and analysis of LC-MS data. D.N.C.: Hypothesis, conception, and design of the study. A.M.J.: Hypothesis, conception, and design of the study. D.C.Z.: Hypothesis, conception, and design of the study, interpretation of data, and revision of the manuscript.
This article has an online supplement, which is accessible from this issue's table of contents at www.atsjournals.org
Originally Published in Press as DOI: 10.1164/rccm.201010-1637OC on April 7, 2011
Author Disclosure: None of the authors has a financial relationship with a commercial entity that has an interest in the subject of this manuscript.
References
- 1.Adamson AS, Collins K, Laurence A, O'Shea JJ. The current status of lymphocyte signaling: new roles for old players. Curr Opin Immunol 2009;21:161–166 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Annunziato F, Cosmi L, Santarlasci V, Maggi L, Liotta F, Mazzinghi B, Parente E, Fili L, Ferri S, Frosali F, et al. Phenotypic and functional features of human Th17 cells. J Exp Med 2007;204:1849–1861 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Annunziato F, Cosmi L, Liotta F, Maggi E, Romagnani S. Human Th17 cells: are they different from murine Th17 cells? Eur J Immunol 2009;39:637–640 [DOI] [PubMed] [Google Scholar]
- 4.Manel N, Unutmaz D, Littman DR. The differentiation of human Th17 cells requires transforming growth factor-beta and induction of the nuclear receptor RORγt. Nat Immunol 2008;9:641–649 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Iwakura Y, Nakae S, Saijo S, Ishigame H. The roles of IL-17A in inflammatory immune responses and host defense against pathogens. Immunol Rev 2008;226:57–79 [DOI] [PubMed] [Google Scholar]
- 6.Cheung PF, Wong CK, Lam CW. Molecular mechanisms of cytokine and chemokine release from eosinophils activated by IL-17A, IL-17F, and IL-23: implication for Th17 lymphocytes-mediated allergic inflammation. J Immunol 2008;180:5625–5635 [DOI] [PubMed] [Google Scholar]
- 7.Molet SM, Hamid QA, Hamilos DL. IL-11 and IL-17 expression in nasal polyps: relationship to collagen deposition and suppression by intranasal fluticasone propionate. Laryngoscope 2003;113:1803–1812 [DOI] [PubMed] [Google Scholar]
- 8.Molet S, Hamid Q, Davoine F, Nutku E, Taha R, Page N, Olivenstein R, Elias J, Chakir J. IL-17 is increased in asthmatic airways and induces human bronchial fibroblasts to produce cytokines. J Allergy Clin Immunol 2001;108:430–438 [DOI] [PubMed] [Google Scholar]
- 9.Chandler DB, Fulmer JD. Prostaglandin synthesis and release by subpopulations of rat alveolar macrophages. J Immunol 1987;139:893–898 [PubMed] [Google Scholar]
- 10.Murata T, Ushikubi F, Matsuoka T, Hirata M, Yamasaki A, Sugimoto Y, Ichikawa A, Aze Y, Tanaka T, Yoshida N, et al. Altered pain perception and inflammatory response in mice lacking prostacyclin receptor. Nature 1997;388:678–682 [DOI] [PubMed] [Google Scholar]
- 11.Hersh EV, Lally ET, Moore PA. Update on cyclooxygenase inhibitors: has a third COX isoform entered the fray? Curr Med Res Opin 2005;21:1217–1226 [DOI] [PubMed] [Google Scholar]
- 12.Patel R, Attur MG, Dave M, Abramson SB, Amin AR. Regulation of cytosolic COX-2 and prostaglandin E2 production by nitric oxide in activated murine macrophages. J Immunol 1999;162:4191–4197 [PubMed] [Google Scholar]
- 13.Gilroy DW, Colville-Nash PR, Willis D, Chivers J, Paul-Clark MJ, Willoughby DA. Inducible cyclooxygenase may have anti-inflammatory properties. Nat Med 1999;5:698–701 [DOI] [PubMed] [Google Scholar]
- 14.Narumiya S. Prostanoids and inflammation: a new concept arising from receptor knockout mice. J Mol Med 2009;87:1015–1022 [DOI] [PubMed] [Google Scholar]
- 15.Rocca B, Spain LM, Pure E, Langenbach R, Patrono C, FitzGerald GA. Distinct roles of prostaglandin H synthases 1 and 2 in T-cell development. J Clin Invest 1999;103:1469–1477 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Carey MA, Germolec DR, Bradbury JA, Gooch RA, Moorman MP, Flake GP, Langenbach R, Zeldin DC. Accentuated T helper type 2 airway response after allergen challenge in cyclooxygenase-1−/− but not cyclooxygenase-2−/− mice. Am J Respir Crit Care Med 2003;167:1509–1515 [DOI] [PubMed] [Google Scholar]
- 17.Sheibanie AF, Yen JH, Khayrullina T, Emig F, Zhang M, Tuma R, Ganea D. The proinflammatory effect of prostaglandin E2 in experimental inflammatory bowel disease is mediated through the IL-23/IL-17 axis. J Immunol 2007;178:8138–8147 [DOI] [PubMed] [Google Scholar]
- 18.Sheibanie AF, Khayrullina T, Safadi FF, Ganea D. Prostaglandin E2 exacerbates collagen-induced arthritis in mice through the inflammatory interleukin-23/interleukin-17 axis. Arthritis Rheum 2007;56:2608–2619 [DOI] [PubMed] [Google Scholar]
- 19.Chizzolini C, Chicheportiche R, Alvarez M, de Rham C, Roux-Lombard P, Ferrari-Lacraz S, Dayer JM. Prostaglandin E2 synergistically with interleukin-23 favors human Th17 expansion. Blood 2008;112:3696–3703 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Khayrullina T, Yen JH, Jing H, Ganea D. In vitro differentiation of dendritic cells in the presence of prostaglandin E2 alters the IL-12/IL-23 balance and promotes differentiation of Th17 cells. J Immunol 2008;181:721–735 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Newman JW, Watanabe T, Hammock BD. The simultaneous quantification of cytochrome P450 dependent linoleate and arachidonate metabolites in urine by HPLC-MS/MS. J Lipid Res 2002;43:1563–1578 [DOI] [PubMed] [Google Scholar]
- 22.Wang YH, Liu YJ. The IL-17 cytokine family and their role in allergic inflammation. Curr Opin Immunol 2008;20:697–702 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Gavett SH, Madison SL, Chulada PC, Scarborough PE, Qu W, Boyle JE, Tiano HF, Lee CA, Langenbach R, Roggli VL, et al. Allergic lung responses are increased in prostaglandin H synthase-deficient mice. J Clin Invest 1999;104:721–732 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Thivierge M, Rola-Pleszczynski M. Up-regulation of inducible cyclooxygenase gene expression by platelet-activating factor in activated rat alveolar macrophages. J Immunol 1995;154:6593–6599 [PubMed] [Google Scholar]
- 25.Rahmouni S, Aandahl EM, Nayjib B, Zeddou M, Giannini S, Verlaet M, Greimers R, Boniver J, Tasken K, Moutschen M. Cyclo-oxygenase type 2-dependent prostaglandin E2 secretion is involved in retrovirus-induced T-cell dysfunction in mice. Biochem J 2004;384:469–476 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Mahic M, Yaqub S, Johansson CC, Tasken K, Aandahl EM. Foxp3+cd4+cd25+ adaptive regulatory T cells express cyclooxygenase-2 and suppress effector T cells by a prostaglandin E2-dependent mechanism. J Immunol 2006;177:246–254 [DOI] [PubMed] [Google Scholar]
- 27.Avalos AM, Latz E, Mousseau B, Christensen SR, Shlomchik MJ, Lund F, Marshak-Rothstein A. Differential cytokine production and bystander activation of autoreactive B cells in response to cpg-a and cpg-b oligonucleotides. J Immunol 2009;183:6262–6268 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Bettelli E, Korn T, Kuchroo VK. Th17: the third member of the effector T cell trilogy. Curr Opin Immunol 2007;19:652–657 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Boniface K, Bak-Jensen KS, Li Y, Blumenschein WM, McGeachy MJ, McClanahan TK, McKenzie BS, Kastelein RA, Cua DJ, de Waal Malefyt R. Prostaglandin E2 regulates Th17 cell differentiation and function through cyclic AMP and EP2/EP4 receptor signaling. J Exp Med 2009;206:535–548 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Ivanov II, Zhou L, Littman DR. Transcriptional regulation of Th17 cell differentiation. Semin Immunol 2007;19:409–417 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Weaver CT, Hatton RD, Mangan PR, Harrington LE. IL-17 family cytokines and the expanding diversity of effector T cell lineages. Annu Rev Immunol 2007;25:821–852 [DOI] [PubMed] [Google Scholar]
- 32.Bettelli E, Oukka M, Kuchroo VK. Th17 cells in the circle of immunity and autoimmunity. Nat Immunol 2007;8:345–350 [DOI] [PubMed] [Google Scholar]
- 33.Dong C. Diversification of T-helper-cell lineages: finding the family root of IL-17-producing cells. Nat Rev Immunol 2006;6:329–333 [DOI] [PubMed] [Google Scholar]
- 34.Kebir H, Kreymborg K, Ifergan I, Dodelet-Devillers A, Cayrol R, Bernard M, Giuliani F, Arbour N, Becher B, Prat A. Human Th17 lymphocytes promote blood-brain barrier disruption and central nervous system inflammation. Nat Med 2007;13:1173–1175 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Hirota K, Yoshitomi H, Hashimoto M, Maeda S, Teradaira S, Sugimoto N, Yamaguchi T, Nomura T, Ito H, Nakamura T, et al. Preferential recruitment of CCR6-expressing Th17 cells to inflamed joints via CCL20 in rheumatoid arthritis and its animal model. J Exp Med 2007;204:2803–2812 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Sousa A, Pfister R, Christie PE, Lane SJ, Nasser SM, Schmitz-Schumann M, Lee TH. Enhanced expression of cyclo-oxygenase isoenzyme 2 (COX-2) in asthmatic airways and its cellular distribution in aspirin-sensitive asthma. Thorax 1997;52:940–945 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Dayer JM, Beutler B, Cerami A. Cachectin/tumor necrosis factor stimulates collagenase and prostaglandin E2 production by human synovial cells and dermal fibroblasts. J Exp Med 1985;162:2163–2168 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Dayer JM, de Rochemonteix B, Burrus B, Demczuk S, Dinarello CA. Human recombinant interleukin 1 stimulates collagenase and prostaglandin E2 production by human synovial cells. J Clin Invest 1986;77:645–648 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Yao C, Sakata D, Esaki Y, Li Y, Matsuoka T, Kuroiwa K, Sugimoto Y, Narumiya S. Prostaglandin E2–EP4 signaling promotes immune inflammation through Th1 cell differentiation and Th17 cell expansion. Nat Med 2009;15:633–640 [DOI] [PubMed] [Google Scholar]
- 40.Kryczek I, Wei S, Zou L, Altuwaijri S, Szeliga W, Kolls J, Chang A, Zou W. Cutting edge: Th17 and regulatory T cell dynamics and the regulation by IL-2 in the tumor microenvironment. J Immunol 2007;178:6730–6733 [DOI] [PubMed] [Google Scholar]
- 41.Nakata J, Kondo M, Tamaoki J, Takemiya T, Nohara M, Yamagata K, Nagai A. Augmentation of allergic inflammation in the airways of cyclooxygenase-2-deficient mice. Respirology 2005;10:149–156 [DOI] [PubMed] [Google Scholar]
- 42.Kalinski P, Vieira PL, Schuitemaker JH, de Jong EC, Kapsenberg ML. Prostaglandin E2 is a selective inducer of interleukin-12 p40 (IL-12p40) production and an inhibitor of bioactive IL-12p70 heterodimer. Blood 2001;97:3466–3469 [DOI] [PubMed] [Google Scholar]
- 43.Sheibanie AF, Tadmori I, Jing H, Vassiliou E, Ganea D. Prostaglandin E2 induces IL-23 production in bone marrow-derived dendritic cells. FASEB J 2004;18:1318–1320 [DOI] [PubMed] [Google Scholar]
- 44.Lo HW, Cao X, Zhu H, Ali-Osman F. Cyclooxygenase-2 is a novel transcriptional target of the nuclear EGFR-Stat3 and EGFRvIII-Stat3 signaling axes. Mol Cancer Res 2010;8:232–245 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Delerive P, Monte D, Dubois G, Trottein F, Fruchart-Najib J, Mariani J, Fruchart JC, Staels B. The orphan nuclear receptor RORalpha is a negative regulator of the inflammatory response. EMBO Rep 2001;2:42–48 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Harris TJ, Grosso JF, Yen HR, Xin H, Kortylewski M, Albesiano E, Hipkiss EL, Getnet D, Goldberg MV, Maris CH, et al. Cutting edge: an in vivo requirement for stat3 signaling in Th17 development and Th17-dependent autoimmunity. J Immunol 2007;179:4313–4317 [DOI] [PubMed] [Google Scholar]
- 47.Ivanov II, McKenzie BS, Zhou L, Tadokoro CE, Lepelley A, Lafaille JJ, Cua DJ, Littman DR. The orphan nuclear receptor RORγt directs the differentiation program of proinflammatory IL-17+ T helper cells. Cell 2006;126:1121–1133 [DOI] [PubMed] [Google Scholar]
- 48.Han C, Demetris AJ, Stolz DB, Xu L, Lim K, Wu T. Modulation of stat3 activation by the cytosolic phospholipase A2α and cyclooxygenase-2-controlled prostaglandin E2 signaling pathway. J Biol Chem 2006;281:24831–24846 [DOI] [PubMed] [Google Scholar]
- 49.Lo RK, Liu AM, Wise H, Wong YH. Prostacyclin receptor-induced stat3 phosphorylation in human erythroleukemia cells is mediated via Galpha(s) and Galpha(16) hybrid signaling. Cell Signal 2008;20:2095–2106 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.