Abstract
Rationale: To determine vascular signaling pathways involved in inhaled air pollution (vehicular engine emission) exposure–induced exacerbation of atherosclerosis that are associated with onset of clinical cardiovascular events.
Objectives: To elucidate the role of oxidized low-density lipoprotein (oxLDL) and its primary receptor on endothelial cells, the lectin-like oxLDL receptor (LOX-1), in regulation of endothelin-1 expression and matrix metalloproteinase activity associated with inhalational exposure to vehicular engine emissions.
Methods: Atherosclerotic apolipoprotein E knockout mice were exposed by inhalation to filtered air or mixed whole engine emissions (250 μg particulate matter [PM]/m3 diesel + 50 μg PM/m3 gasoline exhausts) 6 h/d for 7 days. Concurrently, mice were treated with either mouse IgG or neutralizing antibodies to LOX-1 every other day. Vascular and plasma markers of oxidative stress and expression proatherogenic factors were assessed. In a parallel study, healthy human subjects were exposed to either 100 μg PM/m3 diesel whole exhaust or high-efficiency particulate air and charcoal-filtered “clean” air (control subjects) for 2 hours, on separate occasions.
Measurements and Main Results: Mixed emissions exposure increased oxLDL and vascular reactive oxygen species, as well as LOX-1, matrix metalloproteinase-9, and endothelin-1 mRNA expression and also monocyte/macrophage infiltration, each of which was attenuated with LOX-1 antibody treatment. In a parallel study, diesel exhaust exposure in volunteer human subjects induced significant increases in plasma-soluble LOX-1.
Conclusions: These findings demonstrate that acute exposure to vehicular source pollutants results in up-regulation of vascular factors associated with progression of atherosclerosis, endothelin-1, and matrix metalloproteinase-9, mediated through oxLDL–LOX-1 receptor signaling, which may serve as a novel target for future therapy.
Keywords: atherosclerosis, particulate matter, endothelin-1, matrix metalloproteinase, oxidized low-density lipoprotein
AT A GLANCE COMMENTARY
Scientific Knowledge on the Subject
An increasing number of epidemiologic studies show strong correlations between exposure to environmental air pollutants and onset of clinical cardiovascular events; however, the underlying mechanisms between inhalation of pollutants and cardiovascular sequelae are not fully understood.
What This Study Adds to the Field
The research presented in this manuscript identifies the endothelial oxidized low-density lipoprotein receptor, LOX-1, as a possible mediator of air pollution-induced progression of cardiovascular disease. Furthermore, inhalational exposure to vehicular emissions resulted in LOX-1-mediated vascular oxidative stress and expression of matrix metalloproteinase-9 and endothelin-1, both of which are associated with progression of atherosclerosis and atherosclerotic plaque rupture, which can lead to heart attack and stroke.
Epidemiological studies report a significant correlation between exposure to air pollution and increased risk of cardiovascular morbidity and mortality (1–3), including a focused impact of traffic-related sources (2–4). Multiple studies have described detrimental cardiovascular effects of environmental air pollutants, wherein increased expression of factors that are associated with progression of atherosclerosis were observed (5–7); however, the central mechanisms and cell-signaling pathways are still under investigation.
Atherosclerosis is a chronic inflammatory disease of the vasculature characterized by plaque formation, destabilization, and rupture, which can lead to onset of heart attack or stroke. Under the regulation of several stimuli, including genetic, behavioral, and environmental, atherogenesis is a complex sequence of events that includes endothelial cell dysfunction, vascular remodeling, and accumulation of lipids, especially oxidized low-density lipoproteins (oxLDLs) (8). The lectin-like oxLDL receptor-1 (LOX-1) is the major endothelial cell-surface receptor responsible for binding and internalization of oxLDL (9) and is also found expressed on other vascular cell types (reviewed In References 10, 11). Basal LOX-1 expression is reported to be relatively low, but is up-regulated by prooxidative and inflammatory stimuli associated with pathological cardiovascular conditions (11). Furthermore, recent studies suggest that LOX-1 mediates plaque growth, weakening of the fibromuscular cap, and induction of vascular extracellular matrix degradation through matrix metalloproteinase (MMP) activity, all of which lead to plaque destabilization and subsequent thrombus formation (12–15).
We have previously shown that inhalation of gasoline engine emissions (GEE) and diesel engine (DE) emissions lead to elevations in plasma and vascular endothelin-1 (ET-1) (7, 16), a known mediator of early endothelial dysfunction and remodeling in atherosclerosis (17). oxLDL is known to play a role in the regulation of ET-1, as it is reported to increase ET-1 synthesis and ET receptor expression (18). The relationship between oxLDL, ET-1, and vascular MMP activity likely plays an important role in the progression of atherosclerosis as both oxLDL and ET-1 have been shown to increase MMP-9 activity (14, 19).
We have reported that inhalational exposure to both GEE and DE resulted in increased expression of factors associated with the progression of atherosclerosis (7, 16, 20). However, the signaling pathway(s) that mediate expression of these factors are not fully understood. Furthermore, we wanted to examine whether mixed emissions (ME: combined gasoline and diesel engine exhaust) exposures, which more closely model the pollutants humans may be exposed to in a high-traffic setting, might have synergistic effects compared with single emission source exposures. In the present study, we tested (1) whether acute exposure to ME results in increased vascular oxidative stress, and (2) if LOX-1 mediates any of the observed vascular effects in mice and humans. Some of the results of these studies have been previously reported in the form of an abstract (21).
METHODS
An expanded Methods section is available in the online supplement.
Animals and Inhalation Exposure Protocol
Ten-week-old male ApoE−/− mice (Taconic, Hudson, NY) were placed on a high-fat diet (21.2% fat, 1.5 g/kg cholesterol) 30 days before exposure. ApoE−/− mice were inhalationally exposed to either 300 μg particulate matter [PM]/m3 ME (50 μg PM/m3 GEE mixed with 250 μg PM/m3 DE in a preexposure chamber upstream of the animal exposure chamber) (n = 16) or filtered air (FA, n = 16) 6 h/d for 7 days. In separate experiments (data represented in Figure 1), 10-week-old male ApoE−/− mice on a high-fat diet were inhalationally exposed for 6 h/day for 7 days to either FA (n = 8), GEE (60 μg PM/m3, n = 8), or DE (300 μg PM/m3, n = 8), or subchronically for 6 h/d for 50 days to either FA (n = 10), GEE (60 μg PM/m3, n = 8), DE (300 μg PM/m3, n = 8), ME-3 (300 μg PM/m3, n = 10), ME at a lower concentration of PM (ME-1, 100 μg PM/m3, n = 10), ME with PM filtered (MEG, n = 10), sulfate PM only (S PM, 300 μg PM/m3, n = 10), or sulfate PM + ME (300 μg PM/m3, n = 10). All procedures were approved by the Lovelace Respiratory Research Institute Institutional Animal Care and Use Committee and conform to National Institutes of Health Publication No. 85–23. Engine emissions were created as previously described (7, 20, 22). General characteristics of mixed emission exposures (mouse) and diesel emission exposures (humans) in are shown in Table 1.
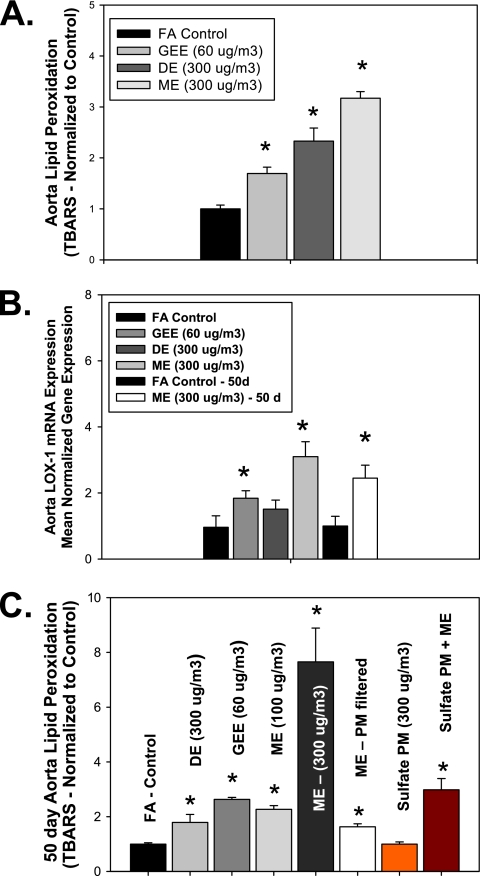
Figure 1.
Lipid peroxidation and aorta lectin-like oxidized low-density lipoprotein receptor (LOX-1) transcriptional changes in ApoE−/− mice exposed to vehicular exhaust. (A) Expression of aortic thiobarbituric acid reactive substances (TBARS) and (B) aortic LOX-1 mRNA levels in ApoE−/− mice exposed for 6 h/d for 7 days to either filtered air (FA, n = 8), gasoline engine emissions (GEE, 60 μg particulate matter [PM]/m3, n = 8), diesel engine emissions (DE, 300 μg PM/m3, n = 8), mixed engine emissions (ME, 50 μg PM/m3 GEE + 250 μg PM/m3 DE emissions, n = 8), filtered air for 50 days (FA – 50d, n = 10), or mixed engine emissions for 50 days (ME, 50 μg PM/m3 GEE + 250 μg PM/m3 DE emissions, n = 8). (C) Aorta lipid peroxidation (TBARS) levels in ApoE−/− mice exposed for 6 h/d for 50 days to either FA (n = 10), GEE (60 μg PM/m3, n = 8), DE (300 μg PM/m3, n = 8), ME-3 (300 μg PM/m3, n = 10), ME-1 (100 μg PM/m3, n = 10), ME with PM filtered (MEG, n = 10), sulfate PM (S PM, 300 μg PM/m3, n = 10), or sulfate PM + ME (300 μg PM/m3, n = 10). Note that data for the DE and GEE exhausts are derived from previous studies (16, 20) normalized to the individual control data for each exposure, presented here to provide reference for the degree of response to the combined emissions exposure. *P ≤ 0.050 compared with FA control mice for each panel.
TABLE 1.
GENERAL CHARACTERISTICS OF EXPOSURE ATMOSPHERES
| PM (μg/m3) | NO2 (ppm) | NO (ppm) | CO (ppm) | |
| Gasoline + Diesel (ME) | 300 | 2 | 16 | 104 |
| Diesel (human exposure) | 106 | 0.4 | 3.5 | 9 |
Definition of abbreviation: PM = particulate matter.
LOX-1 Antibody Treatment Group
ApoE−/− mice in each exposure group were randomly assigned to receive either LOX-1 neutralizing antibody (Ab) (anti-mouse LOX-1/SR-E1 Ab; R&D Systems, Minneapolis, MN) or control (mouse IgG) at 16 μg/ml, 0.1 ml/mouse, intraperitoneally, every other day throughout the exposure. Doses were chosen based on previous studies (23).
Real-Time Reverse Transcriptase Polymerase Chain Reaction
Total RNA was isolated, cDNA was synthesized, and real-time polymerase chain reaction performed and results calculated/normalized, as previously described (7). Primer sequences are available in Table E1 in the online supplement.
Thiobarbituric Acid Reactive Substances Assay
Aortic thiobarbituric acid reactive substances (TBARS) levels were assessed using an assay kit (ZeptoMetrix Corp, Buffalo, NY) per kit instructions.
Western Blot
Aorta LOX-1 and plasma sLOX protein levels were measured and analyzed by Western blot, as previously described (7). Membranes were incubated in anti-mouse LOX-1 (1:3,000; Abcam, Cambridge, MA) and β-actin primary antibodies (1:2,000; Abcam) and rabbit Ab-horseradish peroxidase secondary (1:2,000; Abcam) each for 1 hour at room temperature.
In Situ Zymography
Aortas were sectioned at 6 μm and prepared and analyzed for MMP activity as previously described (7).
Immunohistochemistry
Aorta sections (6 μm) were incubated 1 hour (dark) with either anti–LOX-1 Ab, macrophage/monocyte (MOMA)-2, or von Willebrand factor (each at 1:100; Abcam) and the appropriate secondary Ab (Biotin [1:100; Abcam] or those labeled with Alexa Fluor 488 or 594 for double immunofluorescence [1:100; Invitrogen, Carlsbad, CA]) each for 1 hour at room temperature. Histochemistry sections were visualized with VECTASTAIN ABC-AP kit (Vector Labs, Burlingame, CA), counterstained with hematoxylin, and cover-slipped. Slides were imaged by light or fluorescent microscopy, digitally recorded, and analyzed by image densitometry using Image J software (National Institutes of Health, Bethesda, MD). Quantification was determined by calculating the total fluorescence or percentage or stain-positive area to the total cross-sectional vessel wall area. Double immunofluorescence was quantified by merging Alexa 488 (fluorescein isothiocyanate) and Alexa 594 (Cy3) signals into Red-Green-Blue (RGB) images. Colocalization was determined by quantifying total fluorescence of overlayed signals from minimum of three slides, two sections each, three regions from each section (n = 3–4 per group).
oxLDL Analysis
oxLDL was quantified as previously described (20).
Human Plasma sLOX Assays
Banked samples were analyzed from exposures of healthy subjects (n = 10; 18–40 yr old) exposed to 100 μg/m3 DE whole exhaust or high-efficiency particulate air and charcoal-filtered “clean” air for 2 hours, on separate occasions, as previously described (7). Blood was collected preexposure and 30 minutes and 24 hours postexposure. LOX ELISA (DuoSet#1798 LOX-1/SR-E1; R&D Systems) was performed per manufacturer instructions. Two subjects were eliminated due to lack of sample. Samples were coded and randomized to ensure that the assay was conducted under blinded conditions; data were then decoded and analyzed by another contributor (M.J.C.). All procedures were approved by the Lovelace Respiratory Research Institute Institutional Review Board (IRB) and all subjects provided informed consent. Lipid panel analyses for total cholesterol (C), high-density lipoprotein–C, and triglycerides were performed at LabCorp (Burlington, NC) within 24 hours using standardized automated methods; LDL-C and very low-density lipoprotein (VLDL)-C are reported as calculated values.
Statistical Analysis
Data are expressed as mean ± SEM. One-way analysis of variance with a post hoc Holm-Sidak test was used for analysis of multiple groups; human samples were analyzed with repeated measures analysis of variance. The relationship between specific serum lipids and the magnitude of the sLOX response was analyzed by linear regression. Statistical analyses were conducted in GraphPad Prism v5.02. A P < 0.05 was considered statistically significant.
RESULTS
Vehicular Emissions Exposure Increases Vascular Reactive Oxygen Species, oxLDL, and LOX-1 Expression
Although previous studies report vascular effects of PM, DE, or GEE, alone (5, 7, 16, 20, 24), the ME atmosphere used for the present exposures was notable for the novel combination of high PM levels from DE and high volatile organic carbon levels from GEE (Table 1). We have previously reported that acute exposure to GEE results in elevated vascular oxidative stress and also circulating oxLDL (7). The acute inhalational exposure of ApoE−/− mice to ME in the current set of experiments also resulted in a significant increase in lipid peroxidation, as quantified by TBARs (Figure 1A) compared with FA control mice. Although both GEE (60 μg PM/m3) and DE (300 μg PM/m3) exposures significantly increased vascular TBARS above control levels, mixing these two emissions (ME, 300 μg PM/m3) results in an even further increase in TBARS values (Figure 1A), suggesting that the mixed emissions exposures produce more oxidative stress. We also quantified the role of emission exposures in vascular LOX-1 expression. GEE exposure resulted in a significant increase in vascular LOX-1 expression, whereas DE exposure only modestly increased LOX-1 expression in ApoE−/− mice (Figure 1B). As observed with lipid peroxidation, acute ME exposure results in the most significant increase in vascular LOX-1 mRNA expression, compared with FA controls after both 7-day and 50-day exposures (Figure 1B). These findings suggest that although GEE and DE both increase vascular lipid peroxidation, the components of GEE are likely the primary mediators of increased vascular LOX-1 expression in our ME exposures. However, there is a clear synergistic effect of the ME on vascular LOX-1 expression, as it is more up-regulated in the ME then either GEE or DE alone. Such observations confirm the importance of studying multiple-source pollutant mixtures on pathways involved in cardiovascular disease, as well as overall human health, in future risk-assessment studies.
To determine if alterations in vascular oxidative stress were different in acute versus chronic exposures, we ran TBARS analysis on multiple sets of aortas from chronic inhalational exposures (6 h/d, 7 d/wk for 50 d) to different components and concentrations of vehicular emissions in atherosclerotic ApoE−/− mice. A synergistic increase in vascular lipid peroxidation was observed in ME-exposed versus single-source emissions, after 50 d of exposure (Figure 1C). Exposure to PM-filtered ME led to a negligible change in vascular lipid peroxidation, suggesting that the PM fraction is responsible for mediating the majority of vascular oxidative stress. For comparison, exposure to a nonvehicular PM, secondary sulfate, resulted in no observable increase in aortic TBARS (Figure 1C). However, the PM-filtered ME atmosphere combined with the sulfate PM resulted in a significant increase in vascular TBARS (Figure 1C), indicating a vital interaction between particles and gases in driving these systemic effects. Thus, the toxicity of ambient PM from nonvehicular sources may be exacerbated through coincubation with traffic emissions, potentially explaining the epidemiological associations between cardiovascular outcomes and roadway proximity or related factors (2, 3).
LOX-1 Ab Treatment Attenuates TBARS, oxLDL, and Vascular Expression of ET-1 and MMP-9
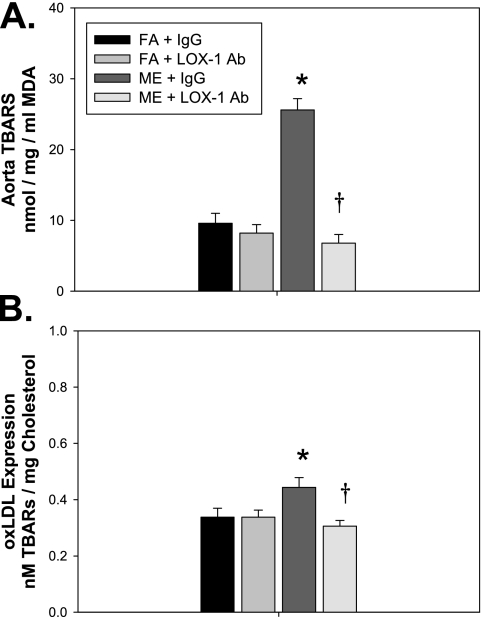
We have previously reported that exposure to GEE results in increased expression of MMP-9 and ET-1 in the vasculature of ApoE−/− mice (7, 20), in addition to elevations in oxidative stress. To determine the role of LOX-1 signaling in mediating vascular lipid peroxidation, as well as vascular expression of ET-1 and MMP-9, associated with atherosclerotic plaque growth and rupture, ApoE−/− mice were treated with either LOX-1 neutralizing Ab (LOX-1 Ab: R&D Systems; anti-mouse LOX-1/SR-E1 Ab: 16 μg/ml, 0.1 ml/mouse) or vehicle (mouse IgG control, 16 μg/ml, 0.1 ml/mouse) intraperitoneally every other day throughout the 7-day exposure to either FA or ME. LOX-1 Ab treatment resulted in reduced vascular TBARS levels in both control and ME-exposed mice (Figure 2A) as well as decreased oxLDL (Figure 2B) compared with IgG-treated ME-exposed mice. As TBARS are a general indicator of oxidative stress, ME exposure/LOX-1–mediated elevations in aortic reactive oxygen species levels were also confirmed with dihydroethidium staining (Figure E1).
Figure 2.
Anti–lectin-like oxidized low-density lipoprotein receptor (LOX-1) neutralizing antibody decreases aortic lipid peroxidation and oxidized low-density lipoprotein (oxLDL) levels in mixed emissions (ME)-exposed ApoE−/− mice. Expression of (A) aorta thiobarbituric acid reactive substances (TBARS) and (B) plasma oxLDL levels in ApoE−/− mice exposed for 6 h/d for 7 days to either filtered air (FA, n = 8), mixed engine emissions (ME, 50 μg PM/m3 gasoline engine emissions + 250 μg PM/m3 diesel engine emissions, n = 8) concurrent with either anti–LOX-1 Ab (LOX-1, n = 8) or IgG (mouse IgG control, n = 8) every other day for 7-day exposure period. *P ≤ 0.050 compared with FA control mice for each panel.
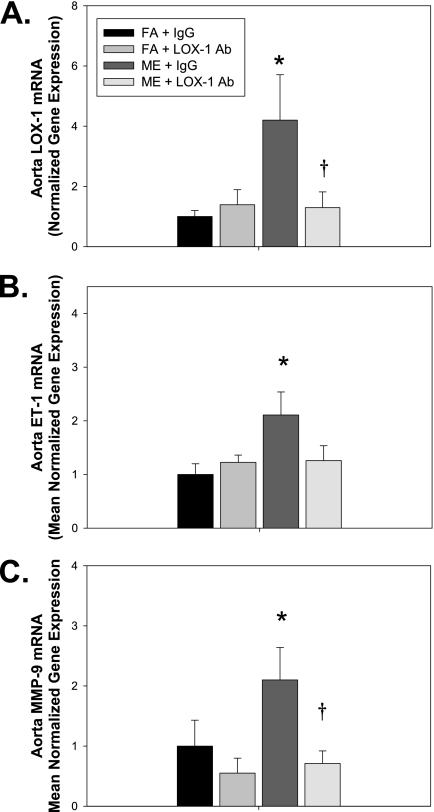
For the LOX-1 Ab treatment experiments, acute ME exposure–mediated expression of vascular LOX-1 was confirmed at the transcriptional (mRNA) and protein (histology) level. Inhalational exposure to ME resulted in significant increase in LOX-1 mRNA expression in ApoE−/− mice compared with FA control mice, which is attenuated through LOX-1 Ab treatment (Figure 3A). ME exposure–mediated increase in aorta LOX-1 was confirmed using immunohistochemistry for LOX-1 (Figure E2B), as was the decrease in LOX-1 expression with anti–LOX-1 Ab treatment (Figures E2C and E2D). Additionally, LOX-1 Ab treatment attenuated ME-mediated vascular expression of ET-1 (Figure 3B) and MMP-9 (Figure 3C) mRNA. Interestingly, both ME exposure and LOX-1 Ab treatment resulted in an increase in tissue inhibitor of matrix metalloproteinases (TIMP)-1 and TIMP-2 mRNA expression (Figure E3); however, further experiments will be needed to determine whether there are alterations in vascular TIMP–MMP binding. These data suggest that ME exposure mediates expression of factors associated with exacerbation of atherosclerosis, namely oxidative stress, ET-1, and MMP-9, via LOX-1 receptor signaling.
Figure 3.
Mixed engine emission (ME) exposure–mediated increases in vascular expression of endothelin (ET)-1 and matrix metalloproteinase (MMP)-9 mRNA are attenuated with anti–lectin-like oxidized low-density lipoprotein receptor (LOX-1) neutralizing antibody. Aorta (A) LOX-1, (B) ET-1, and (C) MMP-9 mRNA expression, as determined by real-time polymerase chain reaction (PCR), in ApoE−/− mice exposed inhalationally for 6 h/d for 7 days to either filtered air (FA, n = 8) or ME (50 μg PM/m3 gasoline engine emissions + 250 μg PM/m3 diesel engine emissions, n = 8) concurrent with either anti–LOX-1 Ab (LOX-1, n = 8) or IgG (mouse IgG control, n = 8) treatment every other day for 7-day exposure period. *P ≤ 0.050 compared with FA control mice and †P ≤ 0.050 compared with ME (+IgG) exposed. Data show mean normalized gene expression (to glyceraldehyde 3-phosphate dehydrogenase) ± SEM, as determined by real-time PCR. Representative aorta LOX-1 protein expression, as determined by Western blot, in either FA (+IgG), FA (+ LOX-1 antibody), ME (+IgG), or ME (+LOX-1 antibody).
Vehicular Emission Exposure Regulation of LOX-1 Expression
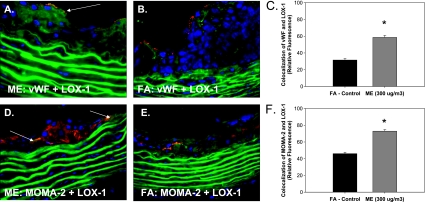
As LOX-1 is located on multiple different vascular and plaque cell types, we used double immunofluorescence to determine LOX-1 localization. We observe LOX-1 expression on vascular smooth muscle cells in the tunica media, as well as on endothelial cells in the intimal layer and colocalized with macrophages in atherosclerotic plaques. We see a significant up-regulation of LOX-1 in the endothelial cells layer (Figure 4A, yellow fluorescence) and within the plaque region (Figure 4D, yellow fluorescence) of ApoE−/− mice exposed to ME, compared with FA controls (Figure 4B and 4E), as represented in graphs showing colocalization of von Willebrand factor and LOX-1 (Figure 4C) and also colocalization of MOMA-2 and LOX-1 (Figure 4F). Such observations suggest that acute vehicular emission exposure up-regulates LOX-1 expression in the vasculature, with a significant increase in expression in the endothelial cells and within atherosclerotic plaques.
Figure 4.
Mixed emission exposure (ME)-mediated vascular lectin-like oxidized low-density lipoprotein receptor (LOX-1) localization. Representative double immunofluorescence localization of alterations in LOX-1 expression in the tunica intima (endothelial cells) using von Willebrand factor antibody (A, B, red fluorescence) or in the atherosclerotic plaque using monocyte/macrophage (MOMA)-2 antibody (D, E, red fluorescence). Green fluorescence indicates LOX-1 expression, which is observed in the tunica intima and tunica media layers of the vessel. LOX-1 expression in the ME-exposed group in (A) the intimal and (D) the plaque region compared with LOX-1 expression in the filtered air (FA) control group in (B) the intimal and (E) the plaque region. Yellow fluorescence indicates colocalization of both LOX-1 and von Willebrand factor in ME and FA aortas (A and B, respectively, and graph C) or both LOX-1 and MOMA-2 in ME and FA aortas (D and E, respectively, and graph F). Fluorescence was quantified in a minimum of three slides, two sections each, three regions from each section (n = 3–4 per group).
LOX-1 Mediates Vehicular Emission Exposure–induced Monocyte/Macrophage Infiltration into the Vascular Wall
To elucidate whether ME exposure–mediated increases in LOX-1 receptor results in enhanced monocyte recruitment and macrophage infiltration into the vascular wall and atherosclerotic plaque region, MOMA-2 immunohistochemistry staining was quantified in aortas from the study mice. MOMA-2 staining revealed an increase in monocyte/macrophage infiltration in the systemic vasculature of ME-exposed ApoE−/− mice (Figure 5A) compared with control mice (Figure 5B). LOX-1 Ab treatment significantly decreased MOMA staining in ME-exposed ApoE−/− mice (Figure 5C); however, a reduction in MOMA staining was also observed in LOX-1 Ab-treated ApoE−/− mice exposed to FA (Figure 5D), graphical quantification of which is displayed in Figure 5E. Such findings confirm that LOX-1 regulates monocyte/macrophage infiltration in the vasculature, independent of exposure, and inhibition of the LOX-1 receptor attenuates ME-mediated increases in monocyte/macrophage infiltration in the vessel wall.
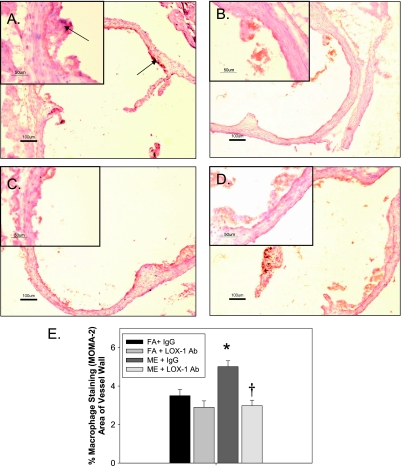
Figure 5.
Monocyte/macrophage (MOMA)-2 staining in aortic valve from ME-exposed ApoE−/− mice treated with lectin-like oxidized low-density lipoprotein receptor (LOX-1) neutralizing antibody for 7 days. (A) Mixed emissions-exposed (ME) + IgG (mouse IgG control); (B) filtered air (FA) control + IgG (mouse IgG control); (C) ME + anti-LOX antibody (Ab) (LOX-1); (D) FA + anti-LOX Ab (LOX-1); (E) analysis of % stained area for sections (n = 6–8 sections per animal, n = 5 animals per exposure/treatment group); MOMA-2–stained areas were divided by total lesion area. *P < 0.050 compared with FA control, †P ≤ 0.050 compared with ME-exposed. Red/brown staining indicates positive MOMA-2 area; arrows indicate increased MOMA-2 regions in tunica intima and atherosclerotic plaque regions. 2× pictures with 10× insets.
Vehicular Emissions Exposure Increases MMP Activity, Which Is Decreased through LOX-1 Ab Treatment
As MMP activity is regulated transcriptionally, translationally, and through inhibition of TIMP binding, we used in situ zymography to quantify acute ME-mediated effects of MMP-9 and -2 (gelatinase) activity in the aorta, and we assessed the role of LOX-1 in mediating vascular MMP activity. Consistent with gasoline emissions (7, 24), aorta gelatinase activity was increased with ME exposure (Figure 6A) compared with FA controls (Figure 6B). LOX-1 Ab treatment attenuated MMP-9 activity, predominantly in the medial layer, compared with FA controls (Figure 6C, arrows, and Figure 6D, respectively). Densitometric quantification of relative fluorescence from all samples analyzed is shown in Figure 6E.
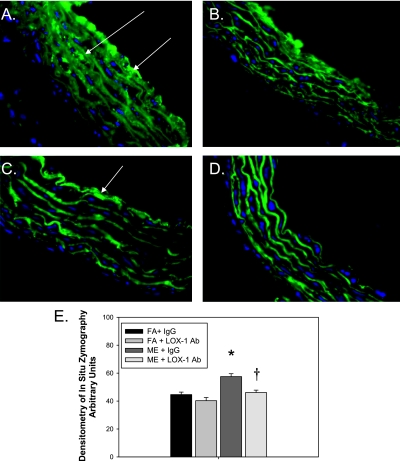
Figure 6.
In situ zymography in aortas from mixed emission (ME)-exposed ApoE−/− mice treated with lectin-like oxidized low-density lipoprotein receptor (LOX-1) neutralizing antibody for 7 days. (A) ME + IgG (mouse IgG control); (B) filtered air (FA) control + IgG (mouse IgG control); (C) ME + anti-LOX-1 antibody (Ab) treatment (LOX-1); (D) FA + anti–LOX-1 Ab treatment (LOX-1); (E) densitometric quantification. *P < 0.050 compared with control, †P ≤ 0.050 compared with ME-exposed. Green fluorescence indicates gelatinase activity; blue fluorescence is 4′,6-diamidino-2-phenylindole nuclei staining. Six sections per sample, n = 5–6 per exposure/treatment group were used for quantification analysis. Arrows indicate increased gelatinase activity; note increased tunica intimal and medial gelatinase activity in ME-exposed ApoE−/− mice versus baseline activity (predominately in the intimal layer) in FA controls and ME+LOX Ab-treated ApoE−/− mice.
Human Exposures to Diesel Emissions Result in Increased Plasma Soluble LOX-1
LOX-1 is known to be cleaved by proteases from the cell surface and released as a circulating factor, soluble LOX-1 (sLOX-1) (25). sLOX is reported to be found increased in association with acute coronary syndrome (26) and thus may serve as a novel biomarker for early diagnosis of a clinical cardiovascular event. Because plasma sLOX-1 expression was found to be significantly increased in ME-exposed ApoE−/− mice (Figures 7A and 7B, representative graph), we tested whether plasma sLOX-1 may serve as a useful translational biomarker for human models of exposure as well. Plasma samples collected from humans exposed to DE for only 2 hours showed a significant elevation in sLOX-1 (Figure 7C). Intrasubject variation was apparent in baseline values of sLOX-1; however, the plasma sLOX-1 concentration and activity were uniformly elevated post DE exposure as compared with changes induced by filtered air exposure. (Tables E2 and E3). We examined the relationship between plasma lipid levels (triglycerides, total cholesterol, VLDL, LDL, oxLDL, and HDL) and found several interesting correlations for the magnitude of response immediately after exposures (Table 2). Specifically, triglycerides (Figure 7D), total cholesterol, and VLDL all displayed significant trends related to sLOX response. At 24 hours, the serum lipids were not as predictive, although this may relate to a lack of power in the available samples.
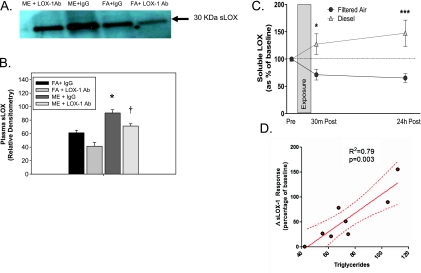
Figure 7.
Human and mouse plasma sLOX levels in response to ME and DE exposure. (A) ApoE−/− mouse plasma soluble lectin-like oxidized low-density lipoprotein receptor (sLOX) levels in response to either filtered air (FA) or mixed exhaust (ME) exposure concurrent with anti-LOX antibody (Ab) (LOX-1, LOX-1/SR-E1 antibody) or IgG (IgG control) as measured by Western blot, and quantified by (B) densitometry from three separate experiments (n = 3 per group). Arrow indicates 30-kD band confirmed as sLOX. (C) Human plasma sLOX levels in response to filtered air (FA) versus diesel exhaust (DE, 100 μg/m3) exposure for 2 hours, as quantified by ELISA. Data presented as the difference from preexposure to each postexposure value (30 min post and 24 h post). (D) Linear regression of the relationship between the magnitude of relative sLOX response (at 30 min post) between FA and DE exposures and the serum triglyceride levels for each human subject. Details of the relationships between exposure and other plasma lipids are presented in Table 2. *P < 0.050 compared with control, †P ≤ 0.050 compared with ME-exposed, ***P < 0.001 compared with control.
TABLE 2.
LINEAR REGRESSION COEFFICIENTS (β) ± 95% CONFIDENCE INTERVALS AND P VALUES FOR THE PREDICTIVE VALUE OF SERUM LIPIDS ON SOLUBLE LECTIN-LIKE OXIDIZED LOW-DENSITY LIPOPROTEIN RECEPTOR RESPONSE IN HUMANS
| 30 min |
24 h |
|||
| β | P Value | β | P Value | |
| Triglycerides | 1.899 ± 0.402 | 0.003 | 1.097 ± 1.241 | 0.410 |
| LDL cholesterol | 1.485 ± 0.873 | 0.140 | 2.634 ± 1.193 | 0.069 |
| HDL cholesterol | 0.402 ± 1.200 | 0.750 | 0.437 ± 1.822 | 0.819 |
| VLDL cholesterol | 9.391 ± 2.007 | 0.003 | 5.540 ± 6.137 | 0.401 |
| oxLDL | 2.654 ± 1.718 | 0.173 | 5.005 ± 2.290 | 0.071 |
| Total cholesterol | 1.541 ± 0.584 | 0.039 | 2.156 ± 0.953 | 0.064 |
Definition of abbreviations: HDL = high-density lipoprotein; LDL = low-density lipoprotein; oxLDL = oxidized low-density lipoprotein; VLDL = very low-density lipoprotein.
DISCUSSION
The present study demonstrates that short-term inhalation of ME results in up-regulation of oxLDL and vascular LOX-1 expression in atherosclerotic ApoE−/− mice. LOX-1, which mediates many of the atherogenic effects of oxLDL in the vascular wall (9, 10, 12), is found up-regulated in both human and animal models of atherosclerosis (12, 27), and its expression is associated with events leading to plaque rupture (13, 28). Although an increasing number of epidemiological studies report a positive correlation between acute exposure to traffic-generated air pollutants and an increased risk of clinical cardiovascular events (2, 3, 29), the underlying mechanisms leading to onset of acute myocardial infarction (AMI) and/or stroke have not yet been fully elucidated, making it difficult to identify vulnerable populations. LOX-1 activation is known to mediate key downstream inflammatory signaling pathways, superoxide generation (O2− ▪), increased monocyte adhesion to endothelial cells (reviewed in Reference 30), and endothelial nitric oxide synthase uncoupling, collectively associated with endothelial dysfunction and progression of atherosclerosis as well as apoptosis and induction of MMPs (14, 27), which are associated with plaque rupture. Based on these findings, it is plausible that LOX-1 plays a role in mitigating systemic vascular effects of traffic-related emissions.
Our previous observations of GEE-induced oxLDL and vascular lipid peroxidation (7) led us to question whether LOX-1 might play a significant role in driving vascular inflammation and oxidative stress, as oxLDL has been shown to directly regulate expression of LOX-1 in the vasculature (31). Although multiple components of vehicular emissions might account for the increase in production of oxLDL with exposure, at least one main component, diesel PM, has been reported to increase oxidative modification of LDL (32) in vitro, although it is readily conceivable that multiple other components may also be responsible for oxidation of LDL. Certainly, our findings of significantly reduced vascular lipid peroxidation (TBARS) when PM was filtered from the ME exposures suggest that the PM is vital to the systemic effect of ME; previous studies found the principal gases (CO, NO, and NO2) were unable to drive the lipid peroxidation induced by gasoline emissions (24). However, when the filtered gases from ME were combined with a seemingly innocuous sulfate particulate, we observed substantial increases in vascular TBARS. Interactions between gases and particles may therefore be crucial to the effects observed in our model, and such interactions may help to explain effects of traffic-related morbidity and mortality in locations where vehicular emission–generated PM may be low, but there is other background PM present (e.g., resuspended road dust).
ET-1 has also been shown to increase LOX-1 expression and augment oxLDL uptake in the vasculature (33). We have previously reported a significant increase in circulating and vascular expression of ET-1 with both acute and subchronic exposure to inhaled single-source vehicular emissions (7, 20); our findings in this study with inhalational exposure to a mixed source of vehicular emissions are in agreement. There are likely complex “positive feedback”–type signaling mechanisms involved as oxLDL has been shown to mediate expression of ET-1 (18), which in turn increases nicotinamide adenine dinucleotide phosphate (NADPH) oxidase activity (as does LOX-1 [30]) and O2− ▪ production that can further oxidize LDL, producing more oxLDL. Thus, it is plausible that increased production of oxLDL and/or ET-1 may serve as mediators driving expression of vascular LOX-1 with ME exposure. However, given that we have only examined a single time point for LOX-1 Ab intervention in the present study, we are not able to discern those events leading to LOX-1 expression. The ligand(s) responsible for linking pulmonary exposures with LOX-1 activity in the systemic vasculature remains uncertain; as LOX-1 is considered a promiscuous receptor, any number of potential modified lipids or proteins may be involved, and thus more in depth in vitro and in vivo experiments will be needed to identify specific LOX-1 ligands(s) and/or signaling pathways that mediate this response.
To gain a further understanding of its role in progression and/or exacerbation of cardiovascular pathologies, it is important to determine downstream signaling pathways activated by ME-induced LOX-1 expression. Progression of atherosclerosis is characterized by endothelial dysfunction and chronic inflammation of the vascular wall, associated with increased adhesion molecules that promote recruitment, attachment, and subsequent infiltration of monocytes that will differentiate into macrophages and mediate lipid accumulation (via scavenger receptors, such as the LOX-1 receptor) in the vascular wall (34). Monocyte/macrophage infiltration was assessed by MOMA-2 staining in this study, and whereas control mice show high baseline levels of MOMA-2 staining in the intimal layer of the aorta and plaque region, MOMA-2 staining is significantly increased in aortas of ME-exposed ApoE−/− mice. LOX-1 inhibition resulted in a notable decrease in MOMA-2 staining in ME-exposed ApoE−/− mice, which was also seen in anti–LOX-1 Ab-treated FA controls. Such findings confirm that LOX-1 mediates, at least in part, monocyte/macrophage infiltration in the vasculature of atherosclerotic vessels, which is further exacerbated through inhalational exposure to vehicular-generated air pollutants.
We also report an increase in LOX-1–mediated expression of MMP-9 mRNA and gelatinase (MMP-9, -2) activity in the vasculature of ME-exposed ApoE−/− mice. As MMP-9 has been identified as a novel predictor of cardiovascular mortality (35), and its activity is known to promote atherosclerotic plaque instability (36), it is plausible to suspect that LOX-1–mediated MMP-9 activity may play an important role in mediating air pollution–induced cardiovascular events such as AMI. Although anti–LOX-1 Ab treatment results in a significant decrease in MMP-9 expression and activity, there is still an increase in gelatinase (MMP-9, -2) activity in the intimal layer of the aorta in the ME-exposed animals. This observation may have multiple explanations. First, there are other receptors that mediate LDL uptake (e.g., CD-36, SR-A, SR-B1, etc.) in the vasculature that may also account for regulation of MMP activity. Additionally, we have not yet determined whether TIMP–MMP binding is altered with exposures. Further studies will be needed to determine whether LOX-1 is directly regulating MMP-9 expression and/or TIMP inhibition of vascular MMP expression, resulting from ME exposures. Interestingly, we did observe an increase in both TIMP-1 and TIMP-2 vascular expression with anti–LOX-1 Ab treatment in ApoE−/− mice, which were also significantly increased with ME exposure. The regulation in expression of TIMPs in this study is likely due to different mechanistic pathways because we observe an up-regulation in expression with both LOX-1 inhibition and ME exposure (where LOX-1 expression is increased), which is not unexpected because TIMPs are known to have multiple MMP-independent functions in both endothelial and vascular smooth muscle cells (37).
Like most cell surface receptors with a single transmembrane domain, LOX-1 can be cleaved and released in a soluble form (sLOX) (25), circulating levels of which may serve as a novel biomarker for clinical cardiovascular events (26). We found sLOX increased significantly in plasma from our ME-exposed ApoE−/− mice compared with FA control mice. In addition, in banked samples from human exposures to DE, sLOX levels were found to be elevated immediately after exposure (2-h DE exposure), with even further increases observed 24 hours after exposure. These effects correlated well with baseline circulating lipid concentrations among the subjects, suggesting a possible link between cardiovascular disease risk and severity of response to DE. It is likely that exposure to vehicular emissions results in a combination of increased LOX-1 expression and increased vascular protease activity, thereby contributing to the observed elevations in sLOX; however, other biological and genetic factors may also contribute to sLOX formation. Exposure to filtered air resulted in reductions in sLOX levels and thus the effects of diesel were determined based on relative sLOX change (i.e., change from preexposure control samples). The reduction of sLOX after the exposure regimen is not completely clear, but we speculate that the exposure protocol, consisting of both a 2- to 3-hour fast along with exercise between blood sampling, acts to down-regulate the vascular expression of this protein. Of importance, several polymorphisms have been identified in the LOX-1 gene, variation of which may account for both LOX-1 activity and plasma sLOX levels (38). Interestingly, at least one known LOX-1 polymorphism (C-to-T substitution in the 3′ untranslated region) is associated with increased risk for coronary artery disease (39). Thus, identification of LOX-1 gene polymorphisms associated with alterations in LOX-1 function may serve as a novel method to identify individuals who are more susceptible to onset of a cardiovascular event when exposed to high levels of environmental air pollutants.
In conclusion, our findings show that acute exposure to ubiquitous environmental air pollutants from vehicular sources results in up-regulation of the oxLDL receptor, LOX-1. Furthermore, our data suggest that LOX-1 mediates the extrapulmonary expression and activity of several proatherosclerotic pathways, including vascular MMP-9, macrophage/monocyte infiltration, and ET-1 production, resulting from inhaled combustion emissions. It is likely that LOX-1 is only one of several immunomodulatory receptors, such as CD36 or Toll-like receptors, that link pulmonary exposures to systemic vascular inflammation and oxidative stress via the innate immune system. Indeed, another recent study has found a similar role for TLR4 receptors in linking pulmonary exposures to ambient particulate matter to systemic vascular insults (40). These studies combined offer new insights into the role of innate immune regulation in mediating cardiovascular health effects of air pollution. We observed elevations in plasma sLOX in both our animal model and humans exposed to vehicular emissions, suggesting that our findings are translatable and that sLOX may serve as a novel biomarker in future air pollution exposure risk-assessment research. As atherosclerosis is the primary underlying pathological determinant of onset of AMI and stroke, mechanisms that drive progression of plaque development and/or rupture, in response to exposure to environmental air pollutants, require further investigation and clarification to determine effective prevention and treatment options.
Supplementary Material
Acknowledgments
The authors thank Guy Herbert and Nadine Mathews for their technical support.
Footnotes
Supported by National Institute of Environmental Health Sciences grants R00ES016586 (A.K.L.) and R01ES014639 (M.J.C.), the Health Effects Institute grant EPA G09C10329 (J.D.M., M.J.C.), and United States Environmental Protection Agency grant EPA STAR R83399001–0 (A.K.L., M.J.C.).
The content of this manuscript is solely the responsibility of the authors and does not necessarily represent the official views of the National Institute of Environmental Health Sciences, the National Institutes of Health, or the Health Effects Institute. The research described in this article has been reviewed by the National Health and Environmental Effects Research Laboratory, U.S. Environmental Protection Agency, and approved for publication. Approval does not signify that the contents necessarily reflect the views and policies of the Agency nor does mention of trade names or commercial products constitute endorsement or recommendation for use.
Author contributions: Conception and design: A.K.L., M.C.M., J.D.M., M.J.C. Conducted experiments: A.K.L., J.L., M.H., M.C.M., J.D.M., M.J.C. Analysis and interpretation: A.K.L., J.L., M.H., M.C.M., J.S., M.J.C. Drafting the manuscript for important intellectual content: A.K.L., J.S., M.J.C.
This article has an online supplement, which is accessible from this issue's table of contents at www.atsjournals.org
Originally Published in Press as DOI: 10.1164/rccm.201012-1967OC on April 14, 2011
Author Disclosure: None of the authors has a financial relationship with a commercial entity that has an interest in the subject of this manuscript.
References
- 1.Pope CA, III, Muhlestein JB, May HT, Renlund DG, Anderson JL, Horne BD. Ischemic heart disease events triggered by short-term exposure to fine particulate air pollution. Circulation 2006;114:2443–2448 [DOI] [PubMed] [Google Scholar]
- 2.Hoffmann B, Moebus S, Möhlenkamp S, Stang A, Lehmann N, Dragano N, Schmermund A, Memmesheimer M, Mann K, Erbel R, et al. , for the Heinz Nixdorf Recall Study Investigative Group Residential exposure to traffic is associated with coronary atherosclerosis. Circulation 2007;116:489–496 [DOI] [PubMed] [Google Scholar]
- 3.Peters A, von Klot S, Heier M, Trentinaglia I, Hörmann A, Wixhmann HE, Löwel H. Cooperative health research in the region of Augsburg study group. Exposure to traffic and the onset of myocardial infarction. N Engl J Med 2004;351:1721–1730 [DOI] [PubMed] [Google Scholar]
- 4.Grahame TJ, Schlesinger RB. Cardiovascular health and particulate vehicular emissions: a critical evaluation of the evidence. Air Qual Atmos Health 2010;3:3–27 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Sun Q, Wang A, Jin X, Natanzon A, Duquaine D, Brook RD, Aguinaldo JG, Fayad ZA, Fuster V, Lippmann M, et al. Long-term air pollution exposure and acceleration of atherosclerosis and vascular inflammation in an animal model. JAMA 2005;21:3003–3010 [DOI] [PubMed] [Google Scholar]
- 6.Brook RD, Rajagopalan S, Pope CA, III, Brook JR, Bhatnagar A, Diez-Roux AV, Holguin F, Hong Y, Luepker RV, Mittleman MA, et al. ; on behalf of the American Heart Association Council on Epidemiology and Prevention, Council on the Kidney in Cardiovascular Disease, and Council on Nutrition, Physical Activity and Metabolism Particulate matter air pollution and cardiovascular disease. An update to the scientific statement from the American Heart Association. Circulation 2010;121:2331–2378 [DOI] [PubMed] [Google Scholar]
- 7.Lund AK, Lucero J, Lucas S, Madden M, McDonald J, Campen MJ. Vehicular emissions induce vascular MMP-9 expression and activity associated with endothelin-1 mediated pathways. Arterioscler Thromb Vasc Biol 2009;29:511–517 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Berliner JA, Heinecke JW. The role of oxidized lipoproteins in atherogenesis. Free Radic Biol Med 1996;20:707–727 [DOI] [PubMed] [Google Scholar]
- 9.Sawamura T, Kume N, Aoyama T, Moriwaki H, Hoshikawa H, Aiba Y, Tanaka T, Miwa S, Katsura Y, Kita T, et al. An endothelial receptor for oxidized low-density lipoprotein. Nature 1997;386:73–77 [DOI] [PubMed] [Google Scholar]
- 10.Reiss AB, Anwar K, Wirkowski P. Lectin-like oxidized low density lipoprotein receptor -1 (LOX-1) in atherogenesis: a brief review. Curr Med Chem 2009;16:2641–2652 [DOI] [PubMed] [Google Scholar]
- 11.Navarra T, Del Turco S, Berti S, Basta GJ. The lectin-like oxidized low-density lipoprotein receptor-1 and its soluble form: cardiovascular implications. J Atheroscler Thromb 2010;17:317–331 [DOI] [PubMed] [Google Scholar]
- 12.Mehta JL, Chen J, Hermonat PL, Romeo F, Novelli G. Lectin-like oxidized low-density lipoprotein receptor (LOX-1): a critical player in the development of atherosclerosis and related disorders. Cardiovasc Res 2006;69:36–45 [DOI] [PubMed] [Google Scholar]
- 13.Ishino S, Mukai T, Kume N, Asano D, Ogawa M, Kuge Y, Minami M, Kita T, Shiomi M, Saji H. Lectin-like oxidized LDL receptor-1 (LOX-1) expression is associated with atherosclerotic plaque instability–analysis in hypercholesterolemic rabbits. Atherosclerosis 2007;95:48–56 [DOI] [PubMed] [Google Scholar]
- 14.Li D, Liu L, Chen H, Sawamura T, Ranganathan S, Mehta JL. LOX-1 mediates oxidized low-density lipoprotein-induced expression of matrix metalloproteinases in human coronary artery endothelial cells. Circulation 2003;107:612–617 [DOI] [PubMed] [Google Scholar]
- 15.White SJ, Sala-Newby GB, Newby AC. Overexpression of scavenger receptor LOX-1 in endothelial cells promotes atherogenesis in the ApoE(−/−) mouse model. Cardiovasc Pathol (In press) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Campen MJ, Lund AK, Knuckles TL, Conklin DF, Bishop B, Young D, Sielkop SK, Seagrave JC, Reed MD, McDonald JD. Inhaled diesel emissions alter atherosclerotic plaque composition in Apo E−/− mice. Toxicol Appl Pharmacol 2010;242:310–317 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Ihling C, Szombathy T, Bohrmann B, Brockhaus M, Schaefer HE, Loeffler BM. Coexpression of endothelin-converting enzyme-1 and endothelin-1 in different stages of human atherosclerosis. Circulation 2001;104:864–869 [DOI] [PubMed] [Google Scholar]
- 18.Haug C, Schmid-Kotsas A, Zorn U, Schuett S, Gross HJ, Gruenert A, Bachem MG. Endothelin-1 synthesis and endothelin B receptor expression in human coronary artery smooth muscle cells and monocyte-derived macrophages is up-regulated by low density lipoproteins. J Mol Cell Cardiol 2001;33:1701–1712 [DOI] [PubMed] [Google Scholar]
- 19.Ergul A, Portik-Dobos V, Giulumian AD, Molero MM, Fuchs LC. Stress up-regulates arterial matrix metalloproteinase expression and activity via endothelin A receptor activation. Am J Physiol Heart Circ Physiol 2003;285:H2225–H2232 [DOI] [PubMed] [Google Scholar]
- 20.Lund AK, Knuckles TL, Obat Akata C, Shohet R, McDonald JD, Seagrave JC, Campen MJ. Exposure to gasoline exhaust results in alterations of pathways involved in atherosclerosis. Toxicol Sci 2007;95:485–494 [DOI] [PubMed] [Google Scholar]
- 21.Lund AK, Lucero J, Mathews N, Harman M, Lucas S, Campen MJ. Vascular lectin-like-OxLDL scavenger receptor (LOX-1) mediates oxidative stress, endothelin-1, and matrix metalloproteinase expression in the vasculature of vehicular engine emissions-exposed mice [abstract]. Circulation 2009;120:S1154 [Google Scholar]
- 22.McDonald JD, Barr EB, White RK, Chow JC, Schauer JJ, Zielinska B, Grosjean E. Generation and characterization of four dilutions of diesel engine exhaust for a subchronic inhalation study. Environ Sci Technol 2004;38:2513–2522 [DOI] [PubMed] [Google Scholar]
- 23.Xu S, Gao X, Potter BJ, Cao J-M, Zhang C. Anti-LOX-1 rescues endothelial function in coronary arterioles in atherosclerotic ApoE knockout mice. Arterioscler Thromb Vasc Biol 2007;27:871–877 [DOI] [PubMed] [Google Scholar]
- 24.Campen MJ, Lund AK, Doyle-Eisele M, McDonald JD, Knuckles JL, Rohr A, Knipping E, Mauderly JL. A comparison of vascular effects from complex and individual air pollutants indicates a toxic role for monoxide gases. Environ Health Perspect 2010;118:921–927 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Murase T, Kume N, Kataoka H, Minami M, Sawamura T, Masaki T, Kita T. Identification of soluble forms of lectin-like oxidized LDL receptor-1. Arterioscler Thromb Vasc Biol 2000;20:715–720 [DOI] [PubMed] [Google Scholar]
- 26.Hayashida K, Kume N, Murase T, Minami M, Nakagawa D, Inada T, Tanaka M, Ueda A, Kominami G, Kambara H, et al. Serum soluble lectin-like oxidized low-density lipoprotein receptor-1 levels are elevated in acute coronary syndrome. A novel marker for early diagnosis. Circulation 2005;112:812–818 [DOI] [PubMed] [Google Scholar]
- 27.Kataoka H, Kume N, Miyamoto S, Minami M, Moriwaki H, Murase T, Sawamura T, Masaki T, Hashimoto N, Kita T. Expression of lectin-like oxidized low-density lipoprotein receptor-1 in human atherosclerotic lesions. Circulation 1999;99:3110–3117 [DOI] [PubMed] [Google Scholar]
- 28.Kuge Y, Kume N, Ishino S, Takai N, Ogawa Y, Mukai T, Minami M, Shiomi M, Saji H. Prominent lectin-like oxidized low density lipoprotein (LDL) receptor-1 (LOX-1) expression in atherosclerosis lesions is associated with tissue factor expression and apoptosis in hypercholesterolemic rabbits. Biol Pharm Bull 2008;31:1475–1482 [DOI] [PubMed] [Google Scholar]
- 29.Bhaskaran K, Hajat S, Haines A, Herrett E, Wilkinson P, Smeeth L. Effects of air pollution on the incidence of myocardial infarction. Heart 2009;95:1746–1759 [DOI] [PubMed] [Google Scholar]
- 30.Ogura S, Kakino A, Sato Y, Fujita Y, Iwamoto S, Otsui K, Ysohimoto R, Sawamura T. LOX-1: the multifunctional receptor underlying cardiovascular dysfunction. Circ J 2009;73:1993–1999 [DOI] [PubMed] [Google Scholar]
- 31.Aoyama T, Sawamura T, Furutani Y, Matsuuoka R, Yoshida MC, Fujiwara H, Masaki T. Structure and chromosomal assignment of the human lectin-like oxidized low density lipoprotein receptor (LOX-1) gene. Biochem J 1999;339:177–184 [PMC free article] [PubMed] [Google Scholar]
- 32.Ikeda M, Shitashige M, Yamasaki H, Sagai M, Tomita T. Oxidative modification of low density lipoprotein by diesel exhaust particles. Biol Pharm Bull 1995;18:866–871 [DOI] [PubMed] [Google Scholar]
- 33.Morawietz H, Duerrschmidt N, Niemann B, Galle J, Sawamura T, Holtz J. Augmented endothelial uptake of oxidized low-density lipoprotein in response to endothelin-1. Clin Sci (Lond) 2002;103:9S–12S [DOI] [PubMed] [Google Scholar]
- 34.Quehenberger O. Thematic review series: the immune system and atherogenesis. Molecular mechanisms regulating monocyte recruitment in atherosclerosis. J Lipid Res 2005;46:1582–1590 [DOI] [PubMed] [Google Scholar]
- 35.Blankenberg S, Rupprecht HJ, Poirier O, Bickel C, Smieja M, Hafner G, Meyer J, Cambein F, Tiret L. Plasma concentrations and genetic variation of matrix metalloproteinase 9 and prognosis of patients with cardiovascular disease. Circulation 2003;107:1579–1585 [DOI] [PubMed] [Google Scholar]
- 36.de Nooijer R, Verkleij CJ, von der Thusen JH, Jukema JW, van der Wall EE, van Berkel TJ, Baker AH, Biessen EA. Lesional overexpression of MMP-9 promotes intraplaque hemorrhage in advanced lesions, but not earlier stages of atherogenesis. Arterioscler Thromb Vasc Biol 2006;26:340–346 [DOI] [PubMed] [Google Scholar]
- 37.Vanhoutte D, Heymans S. TIMPs and cardiac remodeling: embracing the MMP-independent-side of the family. J Mol Cell Cardiol 2010;48:445–453 [DOI] [PubMed] [Google Scholar]
- 38.Brinkley TE, Kume N, Mitsuoka H, Brown MD, Phares DA, Ferrell RE, Kita T, Hagberg JM. Variation in the human lectin-like oxidized low-density lipoprotein receptor (LOX-1) gene is associated with plasma soluble LOX-1 levels. Exp Physiol 2008;93:1085–1090 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Chen Q, Reis SE, Kammerer C, Craig WY, LaPierre SE, Zimmer EL, McNamara DM, Pauly DF, Sharaf B, Holubkow R, et al. Genetic variation in lectin-like oxidized low density lipoprotein receptor 1 (LOX-1) gene and the risk of coronary artery disease. Circulation 2003;107:3146–3151 [DOI] [PubMed] [Google Scholar]
- 40.Kampfrath T, Maiseyeu A, Ying Z, Shah Z, Deiuliis JA, Xu X, Kherada N, Brook RD, Reddy KM, Padture NP, et al. Chronic fine particulate matter exposure induces systemic vascular dysfunction via NADPH oxidase and TLR4 pathways. Circ Res 2011;108:716–726 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.