Abstract
Rationale: Obstructive sleep apnea, which is characterized by intermittent hypoxia (IH) during sleep, has emerged as an independent risk factor for cardiovascular disease, including atherosclerosis. Leukotriene B4 (LTB4) production is increased in patients with obstructive sleep apnea and negatively correlates to hypoxic levels during sleep, with continuous positive airway pressure therapy decreasing LTB4 production.
Objectives: Determine the potential role of LTB4 in IH-induced atherosclerosis in a monocyte cellular model and a murine model.
Methods: THP-1 cells were exposed to IH for 3, 6, 24, and 48 hours. Macrophage transformation and foam cell formation were assessed after IH exposures. Apolipopotein E (ApoE)−/− or BLT1−/−/ApoE−/− mice were fed an atherogenic diet and exposed to IH (alternating 21% and 5.7% O2 from 7 am to 7 PM each day) for 10 weeks. Atherosclerotic lesion formation in en face aorta was examined by oil red O staining.
Measurements and Main Results: IH increased production of LTB4 and the expression of 5-lipoxygenase and leukotriene A4 hydrolase, the key enzymes for producing LTB4. IH was associated with transformation of monocytes to activated macrophages, as evidenced by increased expression of CD14 and CD68. In addition, IH exposures promoted increased cellular cholesterol accumulation and foam cell formation. The LTB4 receptor 1 (BLT1) antagonist U-75302 markedly attenuated IH-induced changes. Furthermore, IH promoted atherosclerotic lesion formation in ApoE−/− mice. IH-induced lesion formation was markedly attenuated in BLT1−/−/ApoE−/− mice.
Conclusions: BLT1-dependent pathways underlie IH-induced atherogenesis, and may become a potential novel therapeutic target for obstructive sleep apnea–associated cardiovascular disease.
Keywords: obstructive sleep apnea, inflammation, monocyte, atherosclerosis
AT A GLANCE COMMENTARY
Scientific Knowledge on the Subject
Obstructive sleep apnea, which is characterized by intermittent hypoxia (IH) during sleep, has emerged as an independent risk factor for cardiovascular diseases. In recent years, animal models have revealed that IH promotes atherosclerosis. However, the molecular mechanisms underlying IH-induced atherogenesis remain largely undefined.
What This Study Adds to the Field
Leukotriene B4 and its receptor BLT1 play a prominent role in mediating atherogenesis associated with episodic hypoxia during sleep. These findings are consistent with the overarching concept whereby IH triggers inflammatory processes that play a critical role in atherogenesis. Therefore, the leukotriene B4–BLT1 pathway may provide a potential therapeutic target for obstructive sleep apnea–associated atherosclerosis.
Obstructive sleep apnea (OSA) is a highly prevalent disorder throughout the lifespan, which may affect up to 2–5% of the population. OSA has emerged as an independent risk factor for cardiovascular diseases, including atherosclerosis (1, 2). Indeed, independent associations between OSA and the evolution of increased carotid artery intima–media thickness and progressive narrowing of the coronary artery lumen have been reported (3–7). The characteristic disturbances of OSA include intermittent hypoxia (IH), episodic hypercapnia, sleep fragmentation, and increased intrathoracic pressure changes during sleep (8). In recent years, animal models have revealed that IH promotes atherosclerosis (9, 10), systemic hypertension (11), glycemic control disturbances (12, 13), and endothelial dysfunction (14), thereby confirming the contribution of OSA to the metabolic syndrome (15). However, the molecular mechanisms underlying IH-induced atherogenesis remain largely undefined.
It has now become established that atherosclerosis should be viewed as an inflammatory disease (16, 17), such that the mechanistic links between IH and atherosclerosis would likely be mediated by inflammatory processes elicited in the context of IH (18). In the last decade, we established rodent models of IH to enable the exploration of IH-induced pathologic consequences and their underlying mechanisms (19–24). In that context, it became apparent that IH induced prominent oxidative stress and promoted chronic inflammatory processes that could ultimately contribute to IH-associated atherogenesis.
Leukotriene B4 (LTB4) is an eicosanoid lipid derivative of arachadonic acid, the latter being generated by 5-lipoxygenase (5-LO) (25, 26) and leukotriene A4 hydrolase (LTA4H) (27). LTB4 is a proinflammatory mediator that activates multiple leukocyte subsets, leading to cell recruitment, production of reactive oxygen species, and induction of gene expression (28). LTB4 primarily binds its high-affinity G protein–coupled receptor BLT1, which is highly expressed in circulating peripheral blood leukocytes. BLT2 is another functional receptor subtype for LTB4 that exhibits much lower affinity for its ligand, and is ubiquitously expressed at low levels in many human tissues (29, 30). Mounting evidence has suggested that LTB4 is associated with several chronic inflammatory conditions, including atherosclerosis (28, 31). Indeed, LTB4 is a key mediator of inflammation, and genetic ablation of its high-affinity receptor, BLT1, delays the onset of atherosclerosis, suggesting that the LTB4–BLT1 pathway plays a critical role in the development of atherosclerosis (32). Based on these findings, we hypothesized that the LTB4–BLT1 pathway plays a key role in IH-induced atherogenesis. To address our hypothesis, we used both in vitro and in vivo IH models, and explored the LTB4–BLT1 pathway contribution to atherogenesis using both pharmacologic and genetic approaches.
METHODS
Cell Culture and IH Exposures
THP-1 cells were originally obtained from American Tissue Type Cell Collection (ATCC, Manassas, VA) and were cultured in Gibco RPMI 1640 medium. IH exposures were conducted using a custom-designed computer-controlled incubator chamber attached to an external O2–CO2 computer-driven servocontroller (Biospherix, Lacona, NY). This system is able continuously to record the dissolved O2 concentrations in the culture medium and allows for implementation of the desired oxygen concentration profile (see Figure E1 in the online supplement). Cells were exposed to IH or room air (RA) for 3, 6, 24, and 48 hours (see online supplement).
Animals and IH Exposures
Animal care and experimental procedures were performed with approval from animal care committees of the University of Chicago, Chicago, Illinois, and the University of Louisville, Louisville, Kentucky. Apolipopotein E (ApoE)−/−, BLT1−/−ApoE−/− mice were fed with a high-cholesterol atherogenic western diet (TD88137; Harlan Laboratories, Indianapolis, IN). Animals were housed in four identical commercially designed chambers under 12-hour light–dark cycles (Biospherix). After a 10-week IH exposure, mice were anesthetized. The heart and aorta were dissected. Ascending aortas were embedded in optimal cutting temperature (OCT) compound and cryosections were prepared for evaluating atherosclerotic lesions by oil red O staining (see online supplement).
Assessment of Atherosclerotic Lesions
Perfusion fixation, preparation of aortas, and quantification of atherosclerotic lesions were performed as previously described (32) (see online supplement).
Intracellular Lipid Analysis in Macrophages
Free cholesterol and total cholesterol were determined by commercial assay systems (Amplex Red Cholesterol Assay kit; Invitrogen, Carlsbad, CA). Cholesterol ester was estimated by subtracting free cholesterol from total cholesterol. Data were normalized to cellular protein content (see online supplement).
Electrophoretic Mobility Shift Assay
Electrophoretic mobility shift assay was performed using a kit from Panomics (Santa Clara, CA) (see online supplement).
Quantitative Real-Time Polymerase Chain Reaction
The mRNA expression (5-LO, LTA4H, MCP-1, CCR2, CD14, CD68, CD36 SRA, and ABCA1 and ApoA-I) was determined by quantitative real-time polymerase chain reaction.
Oil Red O Staining
Cells were differentiated into macrophages in the presence of phorbol myristate acetate (100 ng/ml) for 24 hours. After being washed three times with phosphate-buffered saline, the differentiated macrophages were cultured in RPMI 1640 plus 5% lipoprotein-deficient serum (LPDS) medium containing 50 μg/ml acetylated low-density lipoprotein (acLDL) and exposed to IH or RA. Cells were fixed in 4% paraformaldehyde and stained with oil red O and hematoxylin.
Assessment of LTB4 Production in Culture Medium and Plasma
LTB4 production was measured by LTB4 ELISA kit (Cayman, Ann Arbor, MI).
Data Analysis
All results are reported as mean ± SEM unless otherwise indicated. Comparisons between the IH and RA groups in the ApoE−/− mice and BLT1−/−ApoE−/− mice were made by using analysis of variance procedures followed by post hoc tests. Statistical significance was assumed at P less than 0.05.
RESULTS
IH Induces a Significant Increased Production of LTB4 and Expression of 5-LO and LTA4H
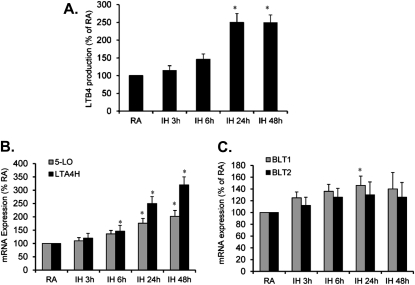
To establish whether LTB4 production and expression of its key enzymes (5-LO and LTA4H) are altered after exposure to IH, THP-1 cells were exposed to IH for 3, 6, 24, and 48 hours. LTB4 concentrations in culture media were significantly increased after 24- and 48-hour IH exposures (*P < 0.05; Figure 1A). mRNA expression of both 5-LO and LTA4H was increased at 24 and 48 hours of IH. However, the magnitude for IH-induced LTA4H expression was markedly greater than 5-LO, suggesting that increased LTA4H expression may play an important role in IH-induced LTB4 production (*P < 0.01; Figure 1B). In contrast, mRNA expression of BLT1 was slightly increased only at 1 day of IH exposures (P < 0.05), but not thereafter. However, no significant changes were detected in BLT2 mRNA expression after IH exposure (P > 0.05; Figure 1C).
Figure 1.
(A) Time course of leukotriene B4 (LTB4) production in culture media after exposure of THP-1 cells to intermittent hypoxia (IH) for 3, 6, 24, and 48 hours. (B) Time course of 5-lipoxygenase (5-LO) and leukotriene A4 hydrolase (LTA4H) mRNA expression in THP-1 cells after exposure to IH for 3, 6, 24, and 48 hours. (C) Time course of BLT1 and BLT2 mRNA expression in THP-1 cells after exposure to IH for 3, 6, 24, and 48 hours. Data are expressed as a percentage of control room air (RA) (mean ± SE; n = 8; * P < 0.01 vs. RA).
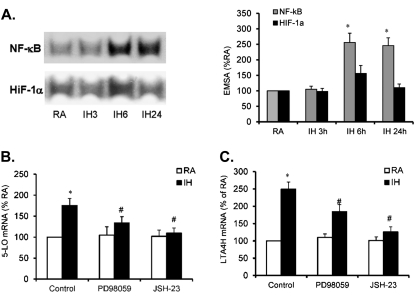
Nuclear Factor-kappa B and Mitogen-activated Protein Kinase Are Involved in Regulating IH-induced Expression of 5-LO and LTA4H
To determine whether the IH-induced mRNA expression of 5-LO and LTA4H is regulated by nuclear factor-kappa B (NF-κB) and hypoxia-inducible factor (HIF)-1α, DNA binding activity for NF-κB and HIF-1α were measured by electrophoretic mobility shift assay after IH exposures for 3, 6, and 24 hours. Increased HIF-1α binding activity occurred only at 6 hours, and was not sustained after longer IH exposures. In contrast, NF-κB DNA binding activity was markedly increased after exposures to IH starting at 6 hours, and remained elevated even at 24 hours (Figure 2A; *P < 0.01), suggesting that IH activates NF-κB, but not HIF-1α in THP-1 cells. To further assess whether NF-κB and mitogen-activated protein kinase (MAPK) are involved in regulating IH-induced mRNA expression of 5-LO and LTA4H, THP-1 cells were treated with JSH-23 (10 μM, a NF-κB inhibitor) and PD98059 (20 μM, a mitogen-activated kinase/ERK kinase 1 [MEK1] inhibitor) or solvent control. After 30 minutes of treatment, the cells were exposed to IH for 24 hours and mRNA expression of 5-LO and LTA4H was examined. IH-induced 5-LO and LTA4H expression were significantly attenuated by either PD98059 or JSH-23 (Figures 2B and 2C; *P < 0.05), indicating that NF-κB and MAPK may play an important role in regulating IH-induced increases in LTB4 production.
Figure 2.
(A) Time course of DNA binding activity of nuclear factor-kappa B (NF-κB) and hypoxia-inducible factor (HIF)-1α after exposure to intermittent hypoxia (IH) for 3, 6, and 24 hours. (B) Effect of PD98059 and JSH-23 on 5-lipoxygenase (5-LO) mRNA expression in THP-1 cells after exposure to IH for 24 hours. (C) Effect of PD98059 and JSH-23 on leukotriene A4 hydrolase (LTA4H) mRNA expression in THP-1 cells after exposure to IH for 24 hours. Data are expressed as a percentage of control RA (mean ± SE; n = 8; *P < 0.01 vs. RA; #P < 0.05 vs. IH).
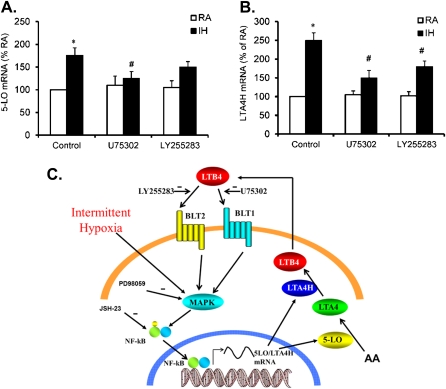
BLT1 and BLT2 Potentiate IH-induced 5-LO and LTA4H Expression
To ascertain whether inhibition of BLT1 and BLT2 effectively suppresses IH-induced increases in 5-LO and LTA4H, THP-1 cells were treated with either U75302 (1 μM, a BLT1 antagonist) or LY255283 (1 μM, a BLT2 antagonist) (solvent served as control) followed by 24-hour IH exposures, after which mRNA expression of 5-LO and LTA4H expression were assessed. Treatment with U75302 effectively suppressed both 5-LO and LTA4H expression (Figures 3A and 3B; #P < 0.01). However, LY255283 only slightly suppressed LTA4H expression but not 5-LO expression (Figures 3A and 3B), suggesting that BLT1 may operate as a component of an amplification loop. Once LTB4 production is triggered by IH, LTB4 may bind to its receptors, BLT1 and BLT2, and further promote the production of LTB4 (see diagram in Figure 3C for more detail).
Figure 3.
(A) Effect of U75302 and LY255283 on 5-lipoxygenase (5-LO) mRNA expression in THP-1 cells after exposure to intermittent hypoxia (IH) for 24 hours. (B) Effect of U75302 and LY255283 on leukotriene A4 hydrolase (LTA4H) mRNA expression in THP-1 cells after exposure to IH for 24 hours. (C) Diagram representing putative signalings of IH-induced leukotriene B4 (LTB4) production and its regulation. Data are expressed as a percentage of control RA (mean ± SE; n = 8; *P < 0.01 vs. RA; #P < 0.05 vs. IH). MAPK = mitogen-activated protein kinase; NF-κB = nuclear factor-kappa B.
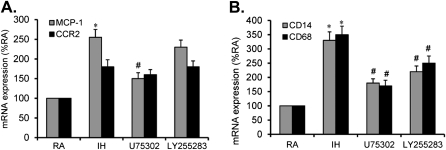
Inhibition of BLT1 or BLT2 Reduces IH-associated Monocyte Recruitment and Macrophage Differentiation
To examine whether IH enhances monocyte recruitment and macrophage differentiation, and whether administration of a BLT1 or a BLT2 blocker reduces these effects, THP-1 cells were exposed to IH with or without U75302 (1 μM) or LY255283 (1 μM) treatment. Monocyte recruitment was determined by measuring the expression of MCP-1 and CCR2, whereas macrophage differentiation was determined by assessing the expression of CD14 and CD68. MCP-1, but not CCR2, was significantly increased after IH exposures (Figure 4A; *P < 0.05). In addition, IH-increased MCP-1 expression was markedly attenuated by U75302, but not by LY255283 (Figure 4A; #P < 0.05), indicating that BLT1 may play a role in mediating IH-induced monocyte recruitment. Furthermore, both CD14 and CD68 were significantly increased after IH exposures (Figure 4B; *P < 0.05), suggesting that IH exposures promote monocyte differentiation. In addition, IH-increased CD14 and CD68 expression was markedly attenuated by either U75302 or LY255283 (Figure 4B; #P < 0.05), indicating that both BLT1 and BLT2 may mediate IH-induced monocyte differentiation.
Figure 4.
(A) Effect of U75302 and LY255283 on MCP-1 and CCR-2 mRNA expression in THP-1 cells after exposure to intermittent hypoxia (IH) for 24 hours. (B) Effect of U75302 and LY255283 on CD14 and CD68 mRNA expression in THP-1 cells after exposure to IH for 24 hours. Data are expressed as a percentage of control RA (mean ± SE; n = 8; *P < 0.01 vs. RA; #P < 0.05 vs. IH).
Inhibition of BLT1 or BLT2 Attenuates IH-induced Cholesterol Accumulation and Foam Cell Formation
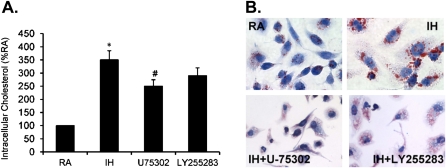
To determine further whether IH increases cellular cholesterol accumulation and foam cell formation and the role of BLT1 or BLT2 in these effects, differentiated THP-1 cells were exposed to IH with or without U75302 (1 μM) or LY255283 (1 μM) treatment. Cellular cholesterol accumulation was determined using a commercial kit, whereas foam cell formation was determined by staining with oil red O. Intracellular cholesterol accumulation was significantly increased by IH exposures in differentiated THP-1 cells (Figure 5A; *P < 0.05). In addition, IH-associated cholesterol accumulation was markedly reduced by U75302, but not by LY255283 (Figure 5A; #P < 0.05), indicating that BLT1 is likely involved. Furthermore, foam cell formation was significantly increased after IH exposures in differentiated THP-1 cells (Figure 5B), and this effect was markedly attenuated by either U75302 or LY255283 (Figure 5B), indicating that both BLT1 and BLT2 may be involved.
Figure 5.
(A) Effect of U75302 and LY255283 on intracellular cholesterol in THP-1 cells after exposure to intermittent hypoxia (IH) for 24 hours. (B) Microscopy images representing the effect of U75302 and LY255283 on foam cell formation in THP-1 cells after exposure to IH for 24 hours. Data are expressed as a percentage of control RA (mean ± SE; n = 8; *P < 0.01 vs. RA; #P < 0.05 vs. IH).
Inhibition of BLT1 or BLT2 Attenuates IH-induced Dysregulation of Cellular Cholesterol Transport Proteins
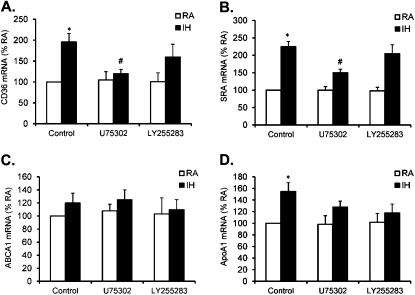
To elicit further the cellular mechanism by which IH promotes cellular cholesterol accumulation in differentiated cells, cholesterol influx was determined by measuring the expression of CD36 and SRA, whereas cholesterol efflux was determined by assessing the expression of ABCA1 and ApoA-I. CD36 and SRA were significantly increased by IH exposure in differentiated THP-1 cells (Figure 6A; *P < 0.05), suggesting that IH exposure enhances influx cholesterol trafficking. In addition, IH-associated CD36 and SRA expression was markedly reduced by both U75302 (1 μM) and LY255283 (1 μM) (Figure 6B; #P < 0.05). Furthermore, expression of ApoA-I was slightly increased after IH exposure in differentiated THP-1 cells (Figure 6C; *P > 0.05), but no significant changes emerged in the expression of ABCA1 (Figure 6D), suggesting that IH exposures do not seem to alter cholesterol efflux. Therefore, IH exposure promotes cholesterol influx and does not affect efflux, thereby contributing to intracellular cholesterol accumulation.
Figure 6.
(A) Effect of U75302 and LY255283 on CD-36 mRNA expression in THP-1 cells after exposure to intermittent hypoxia (IH) for 24 hours. (B) Effect of U75302 and LY255283 on SRA mRNA expression in THP-1 cells after exposure to IH for 24 hours. (C) Effect of U75302 and LY255283 on ABCA1 mRNA expression in THP-1 cells after exposure to IH for 24 hours. (D) Effect U75302 and LY255283 on apolipoprotein A-I mRNA expression in THP-1 cells after exposure to IH for 24 hours. Data are expressed as a percentage of control RA (mean ± SE; n = 8; *P < 0.01 vs. RA; #P < 0.05 vs. IH).
IH Promotes Atherosclerotic Lesion Formation in ApoE−/− Mice and Deletion of BLT1 Attenuates Atherosclerotic Lesion Formation in BLT1−/−/ApoE−/− Mice
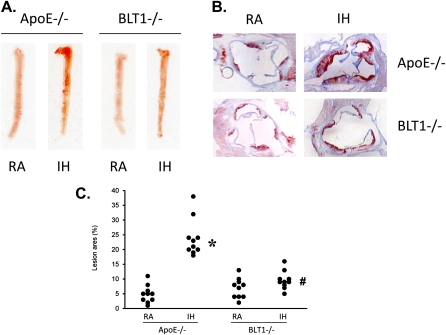
To examine whether the selected IH profile would increase the production of LTB4 and promote atherosclerotic lesion formation in vivo, as recently shown by Jun and colleagues (10), ApoE−/− mice were fed with a high-cholesterol atherogenic western diet, and exposed to IH for 10 weeks. The plasma level of LTB4 production was assessed by an ELISA kit. En face analysis of exposed and control aorta for lesion formation was assessed with Sudan IV staining and by oil red O staining of sections by an investigator who was masked to the treatment group identity. The production of LTB4 in plasma was increased by IH exposure compared with the RA group (data not shown). In the RA-exposed group, there was limited evidence for the presence of lesion formation in either the aorta or the aortic root. In contrast, prominent lesion formations were apparent in both aorta and aortic roots in mice exposed to IH (Figures 7A and 7B), thereby confirming that IH enhances atherogenesis in vivo. To quantitatively assess atherosclerotic lesion formation further, the regions corresponding to atherosclerotic lesions in the aorta and the aortic root were captured and analyzed using image software. In the group exposed to IH, the areas of atherosclerotic lesion in aorta were significantly increased compared with RA controls (n = 10 per group) (Figure 7C). To determine further the effect of BLT1 on IH-associated atherosclerotic lesion formation, BLT1−/− and ApoE−/− double knock-out mice (BLT1−/−ApoE−/− mice) were fed the same western diet and exposed to IH as described previously. In BLT1−/−ApoE−/− mice, the atherosclerotic lesions were prominently reduced compared with ApoE−/− mice (Figures 7A and 7B). In addition, the area of atherosclerotic lesion on aorta was significantly attenuated compared with the lesion formation in ApoE−/− mice (n = 10 per group) (Figure 7C), thereby demonstrating that genetic deletion of BLT1 attenuates IH-associated atherosclerotic lesion formation in vivo, and that BLT1 plays a critical role in IH-associated atherogenesis.
Figure 7.
(A) En face analysis of aortas from apolipoprotein E (ApoE)−/− mice after RA or intermittent hypoxia (IH) exposure for 10 weeks. (B) Oil red O staining on aorta root from ApoE−/− mice after RA or IH exposure for 10 weeks. (C) Quantitative analysis of aortic lesion formation (mean ± SE; n = 10; *P < 0.05 vs. ApoE−/− RA; #P < 0.05 vs. ApoE−/− IH).
DISCUSSION
In this study, we found that LTB4 production and the expression of 5-LO and LTA4H, the key enzymes involved in LTB4 biosynthesis, were induced by IH in a time-dependent fashion. In contrast, BLT1 and BLT2 were globally not affected. However, IH-induced 5-LO and LTA4H expression was reduced by a BLT1 antagonist and by a NF-κB blocker. IH was associated with increased transformation of monocytes to activated macrophages, and promoted increased cellular cholesterol accumulation and foam cell formation, all of which were markedly attenuated by BLT1 blockade. Furthermore, IH promoted in vivo atherosclerotic lesion formation in ApoE−/− mice, whereas genetic deletion of BLT1 abrogated this effect. Therefore, LTB4-BLT1 pathways play an important causative role in IH-induced atherogenesis and may provide a potential novel therapeutic target for OSA-associated atherosclerosis.
Current findings shed some light on the complex interplay between IH-induced production of LTB4 and its regulation. Our original assumption posited that IH may increase the production of LTB4 or lead to up-regulation of LTB4 receptors. Indeed, although robust LTB4 production and up-regulation of the two LTB4 biosynthetic enzymes emerged, the expression of LTB4 receptors, namely BLT1 and BLT2, remained unchanged. A possible explanation for the discrepant effects of IH on LTB4 and its receptors could be a down-regulatory effect on the receptors caused by increased LTB4 bioavailability or alternatively could represent post-translational modifications of LTB4 receptors linked to adaptation processes. Elevated circulating LTB4 concentrations have been reported in untreated patients with OSA, and continuous positive airway pressure treatment effectively reduced LTB4 plasma levels. Interestingly, LTB4 concentrations were correlated with luminal diameter, but not with intima–media thickness (33). However, our study is the first to provide direct evidence on the role of IH in inducing LTB4 production. These observations were somewhat anticipated because expression of 5-LO, 5-lipoxygenase-activating protein (FLAP), and LTA4H can be modified by a variety of stressors, including ischemia and hypoxia (34–37). Thus, IH emerges as an important modulator of LTB4 biosynthesis, and can therefore induce local or systemic inflammatory processes, ultimately contributing to IH-associated atherogenesis. To elucidate potential signaling pathways involved in IH-induced regulation of 5-LO and LTA4H further, we explored DNA binding activity changes in HIF-1α and NF-κB, and pharmacologic manipulation of MAP kinases. Interestingly, IH induced DNA binding activity of NF-κB at 6 and 24 hours. In contrast, IH only induced a slight and transient increase in HIF-1α DNA binding activity at 6 hours. In addition, both MAPK and NF-κB blockers partially inhibited IH-induced 5-LO and LTA4H expression. Because activation of BLT1 and BLT2 may accelerate LTB4 synthesis (38), we explored whether either BLT1 or BLT2 may contribute to IH-induced 5-LO and LTA4H expression and increased LTB4 production. Treatment with a BLT1 antagonist effectively blocked both IH-induced 5-LO and LTA4H expression, whereas a BLT2 inhibitor was almost void of any effect, thereby suggesting that BLT1 is the candidate receptor involved in IH-regulated LTB4 production, at least in part by activating a LTB4–BLT1 amplification loop.
The critical role of LTB4 in the early stages of atherogenesis is now well established (31, 32, 39–43). LTB4 is a potent chemoattractant that facilitates recruitment of monocytes to the inflammatory sites (28). Recruitment of monocytes and leukocyte invasion of the arterial wall are critical steps in the development of atherogenesis (43–45). We have previously shown that LTB4 promotes atherosclerosis by chemoattracting monocytes, providing an amplification loop for monocyte chemotaxis via CCL2 production, and by converting monocytes to foam cells via enhanced expression of CD36 and fatty acid accumulation (32). Current findings strongly support several critical roles for involvement of the LTB4–BLT1 pathway in IH-associated atherogenesis: (1) IH initiates monocyte recruitment as evidenced by increased MCP-1 and CCR2; (2) IH promotes macrophage differentiation, as shown by increased specific macrophage biomarkers in IH-exposed THP-1 cells; (3) IH up-regulated several key elements (e.g., CD36, SRA) that have well-established roles in cholesterol accumulation and foam cell formation, and may further play a critical role in the development of atherosclerosis; and (4) the atherogenic events were attenuated by a BLT1 antagonist (U75302). Although the multiple regulatory mechanisms underlying the expression of these individual genes are unknown, the coordinated regulation of all of these molecules through the activation of BLT1 by IH-induced LTB4 production clearly revealed a putative initiating and integrated step in atherogenesis.
In vivo studies have also consistently supported the view that the LTB4–BLT1 pathway plays a major role in the pathogenesis and progression of atherosclerosis (32, 40–42, 46, 47). ApoE knockout (ApoE−/−) and low density lipoproteins (LDL) receptors knockout mice treated with BLT1 antagonists exhibited reduced lipid accumulation, monocyte infiltration, and smaller atheromata (48). Moreover, levels of adhesion molecules were reduced in mice treated with BLT1 antagonists (48). In rat basophilic cells, LTB4 promotes conversion of monocytes to foam cells through enhanced expression of scavenger receptors (CD36) and subsequent fatty acid accumulation (32). The cumulative evidence from aforementioned studies suggests that the effects of LTB4 in chemotaxis and foam cell formation are mediated by BLT1 and BLT2 receptors (32, 41–43, 48–50). In addition, expression of the 5-LO pathway (FLAP) is increased in atherosclerotic lesions at various stages of development in human aorta and coronary and carotid arteries (47, 51, 52). Furthermore, human genetic studies have shown that a promoter variant of 5-LO is associated with an increase in carotid intima–media thickness in healthy subjects, and certain FLAP haplotypes have been linked to an almost twofold increase in the risk of myocardial infarction or stroke (53). Although studies have explored the role of IH in OSA-associated atherosclerosis (10), its underlying mechanisms were not specifically sought. Here, we not only confirm that IH indeed promotes atherosclerosis, but further demonstrate that genetic deletion of BLT1 greatly attenuates IH-associated atherogenesis. It is possible that IH induces atherosclerosis via mechanisms other than BLT1, but the double knockout is partially protected because of the proatherogenic properties of BLT1, which are independent of IH. Therefore, we exposed ApoE−/−BLT1−/− mice to either RA or IH and compared the lesion formation under those conditions. As illustrated in Figure 7C, the lesion formation in ApoE−/−BLT1−/− mice exposed to IH was not significantly increased compared with the RA group. These results further support the notion that IH-induced atherogenesis is mediated through a LTB4–BLT1 dependent pathway. It is also well established that dyslipidemia plays an important role in the development of atherosclerosis. Therefore, IH-induced hyperlipidemia could also contribute to IH-associated atherogenesis (10). Of note, the biosynthesis of LTB4 is regulated by cholesterol (54) and inhibition of leukotriene may reduce plasma triglyceride (TG) levels (55). However, it remains unclear whether IH-induced hyperlipidemia affects the biosynthesis of LTB4 and downstream atherogenesis. As a methodologic comment, we should point out that IH exposure only provides one aspect of OSA, because sleep fragmentation, episodic hypercapnia, and upper airway collapse are absent in this murine model. Also, the IH profile used here represents a standardization of exposures aiming to reflect severe disease, and as such may not completely represent the variable hypoxic profile characteristics seen in patients with OSA. Because new animal models are being developed to incorporate other elements of OSA (56, 52), assessment of the role played by the LTB4–BLT1 pathway in some or all of the constitutive components of OSA needs to be confirmed.
In summary, we have shown that LTB4 and its receptor BLT1 play a prominent role in the pathophysiologic mechanisms mediating atherogenesis associated with the typical pattern of episodic hypoxia associated with sleep-disordered breathing. These findings are consistent with the overarching concept whereby IH triggers the initiation of an inflammatory process, which is involved in atherogenesis. Thus, the LTB4–BLT1 pathway may provide a potential novel therapeutic target for OSA-associated atherosclerosis.
Supplementary Material
Footnotes
Supported by American Heart Association grant SDG 0930129N and National Institutes of Health grants HL-086662 and HL-65270.
This article has an online supplement, which is accessible from this issue's table of contents at www.atsjournals.org
Originally Published in Press as DOI: 10.1164/rccm.201012-2039OC on April 14, 2011
Authors contributions: Conception and design: R.C.L., B.H., D.G. Data acquisition: R.C.L., S.P.M., J.K. Analysis and interpretation: R.C.L., B.H., S.P.M., J.K., D.G. Manuscript preparation: R.C.L., B.H., D.G.
Authors Disclosure: R.C.L. does not have a financial relationship with a commercial entity that has an interest in the subject of this manuscript. B.H. does not have a financial relationship with a commercial entity that has an interest in the subject of this manuscript. S.P.M. does not have a financial relationship with a commercial entity that has an interest in the subject of this manuscript. J.K. does not have a financial relationship with a commercial entity that has an interest in the subject of this manuscript. D.G. was a consultant for Galleon Pharmaceuticals.
References
- 1.Pack AI, Gislason T. Obstructive sleep apnea and cardiovascular disease: a perspective and future directions. Prog Cardiovasc Dis 2009;51:434–451 [DOI] [PubMed] [Google Scholar]
- 2.Somers VK, White DP, Amin R, Abraham WT, Costa F, Culebras A, Daniels S, Floras JS, Hunt CE, Olson LJ, et al. Sleep apnea and cardiovascular disease: an American Heart Association/American College Of Cardiology Foundation Scientific Statement from the American Heart Association Council for High Blood Pressure Research Professional Education Committee, Council on Clinical Cardiology, Stroke Council, and Council On Cardiovascular Nursing. In collaboration with the National Heart, Lung, and Blood Institute National Center on Sleep Disorders Research (National Institutes of Health). Circulation 2008;118:1080–1111 [DOI] [PubMed] [Google Scholar]
- 3.Drager LF, Bortolotto LA, Lorenzi MC, Figueiredo AC, Krieger EM, Lorenzi-Filho G. Early signs of atherosclerosis in obstructive sleep apnea. Am J Respir Crit Care Med 2005;172:613–618 [DOI] [PubMed] [Google Scholar]
- 4.Leineweber C, Kecklund G, Janszky I, Akerstedt T, Orth-Gomer K. Snoring and progression of coronary artery disease: The Stockholm Female Coronary Angiography Study. Sleep 2004;27:1344–1349 [DOI] [PubMed] [Google Scholar]
- 5.Suzuki T, Nakano H, Maekawa J, Okamoto Y, Ohnishi Y, Yamauchi M, Kimura H. Obstructive sleep apnea and carotid-artery intima-media thickness. Sleep 2004;27:129–133 [DOI] [PubMed] [Google Scholar]
- 6.Drager LF, Bortolotto LA, Krieger EM, Lorenzi-Filho G. Additive effects of obstructive sleep apnea and hypertension on early markers of carotid atherosclerosis. Hypertension 2009;53:64–69 [DOI] [PubMed] [Google Scholar]
- 7.Drager LF, Bortolotto LA, Figueiredo AC, Krieger EM, Lorenzi GF. Effects of continuous positive airway pressure on early signs of atherosclerosis in obstructive sleep apnea. Am J Respir Crit Care Med 2007;176:706–712 [DOI] [PubMed] [Google Scholar]
- 8.Dempsey JA, Veasey SC, Morgan BJ, O'Donnell CP. Pathophysiology of sleep apnea. Physiol Rev 2010;90:47–112 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Savransky V, Nanayakkara A, Li J, Bevans S, Smith PL, Rodriguez A, Polotsky VY. Chronic intermittent hypoxia induces atherosclerosis. Am J Respir Crit Care Med 2007;175:1290–1297 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Jun J, Reinke C, Bedja D, Berkowitz D, Bevans-Fonti S, Li J, Barouch LA, Gabrielson K, Polotsky VY. Effect of intermittent hypoxia on atherosclerosis in apolipoprotein E-deficient mice. Atherosclerosis 2010;209:381–386 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Soukhova-O'Hare GK, Ortines RV, Gu Y, Nozdrachev AD, Prabhu SD, Gozal D. Postnatal intermittent hypoxia and developmental programming of hypertension in spontaneously hypertensive rats: the role of reactive oxygen species and L–Ca2+ channels. Hypertension 2008;52:156–162 [DOI] [PubMed] [Google Scholar]
- 12.Iiyori N, Alonso LC, Li J, Sanders MH, Garcia-Ocana A, O'Doherty RM, Polotsky VY, O'Donnell CP. Intermittent hypoxia causes insulin resistance in lean mice independent of autonomic activity. Am J Respir Crit Care Med 2007;175:851–857 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Polotsky VY, Li J, Punjabi NM, Rubin AE, Smith PL, Schwartz AR, O'Donnell CP. Intermittent hypoxia increases insulin resistance in genetically obese mice. J Physiol 2003;552:253–264 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Philippi NR, Bird CE, Marcus NJ, Olson EB, Chesler NC, Morgan BJ. Time course of intermittent hypoxia-induced impairments in resistance artery structure and function. Respir Physiol Neurobiol 2010;170:157–163 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.O'Donnell CP. Metabolic consequences of intermittent hypoxia. Adv Exp Med Biol 2007;618:41–49 [DOI] [PubMed] [Google Scholar]
- 16.Levy P, Pepin JL, Arnaud C, Baguet JP, Dematteis M, Mach F. Obstructive sleep apnea and atherosclerosis. Prog Cardiovasc Dis 2009;51:400–410 [DOI] [PubMed] [Google Scholar]
- 17.Libby P. Inflammatory mechanisms: the molecular basis of inflammation and disease. Nutr Rev 2007;65:S140–S146 [DOI] [PubMed] [Google Scholar]
- 18.Gozal D, Kheirandish-Gozal L. Cardiovascular morbidity in obstructive sleep apnea: oxidative stress, inflammation, and much more. Am J Respir Crit Care Med 2008;177:369–375 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Burckhardt IC, Gozal D, Dayyat E, Cheng Y, Li RC, Goldbart AD, Row BW. Green tea catechin polyphenols attenuate behavioral and oxidative responses to intermittent hypoxia. Am J Respir Crit Care Med 2008;177:1135–1141 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Goldbart AD, Row BW, Kheirandish-Gozal L, Cheng Y, Brittian KR, Gozal D. High fat/refined carbohydrate diet enhances the susceptibility to spatial learning deficits in rats exposed to intermittent hypoxia. Brain Res 2006;1090:190–196 [DOI] [PubMed] [Google Scholar]
- 21.Gozal D, Nair D, Goldbart AD. Physical activity attenuates intermittent hypoxia-induced spatial learning deficits and oxidative stress. Am J Respir Crit Care Med 2010;182:104–112 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Li RC, Row BW, Kheirandish L, Brittian KR, Gozal E, Guo SZ, Sachleben LR, Jr., Gozal D. Nitric oxide synthase and intermittent hypoxia-induced spatial learning deficits in the rat. Neurobiol Dis 2004;17:44–53 [DOI] [PubMed] [Google Scholar]
- 23.Li RC, Row BW, Gozal E, Kheirandish L, Fan Q, Brittian KR, Guo SZ, Sachleben LR, Jr., Gozal D. Cyclooxygenase 2 and intermittent hypoxia-induced spatial deficits in the rat. Am J Respir Crit Care Med 2003;168:469–475 [DOI] [PubMed] [Google Scholar]
- 24.Row BW, Liu R, Xu W, Kheirandish L, Gozal D. Intermittent hypoxia is associated with oxidative stress and spatial learning deficits in the rat. Am J Respir Crit Care Med 2003;167:1548–1553 [DOI] [PubMed] [Google Scholar]
- 25.Radmark O, Werz O, Steinhilber D, Samuelsson B. 5-Lipoxygenase: regulation of expression and enzyme activity. Trends Biochem Sci 2007;32:332–341 [DOI] [PubMed] [Google Scholar]
- 26.Rakonjac M, Fischer L, Provost P, Werz O, Steinhilber D, Samuelsson B, Radmark O. Coactosin-like protein supports 5-lipoxygenase enzyme activity and up-regulates leukotriene A4 production. Proc Natl Acad Sci USA 2006;103:13150–13155 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Murphy RC, Gijon MA. Biosynthesis and metabolism of leukotrienes. Biochem J 2007;405:379–395 [DOI] [PubMed] [Google Scholar]
- 28.Busse WW. Leukotrienes and inflammation. Am J Respir Crit Care Med 1998;157:S210–S213 [PubMed] [Google Scholar]
- 29.Tager AM, Luster AD. BLT1 and BLT2: the leukotriene B(4) receptors. Prostaglandins Leukot Essent Fatty Acids 2003;69:123–134 [DOI] [PubMed] [Google Scholar]
- 30.Okuno T, Yokomizo T, Hori T, Miyano M, Shimizu T. Leukotriene B4 receptor and the function of its helix 8. J Biol Chem 2005;280:32049–32052 [DOI] [PubMed] [Google Scholar]
- 31.Back M, Hansson GK. Leukotriene receptors in atherosclerosis. Ann Med 2006;38:493–502 [DOI] [PubMed] [Google Scholar]
- 32.Subbarao K, Jala VR, Mathis S, Suttles J, Zacharias W, Ahamed J, Ali H, Tseng MT, Haribabu B. Role of leukotriene B4 receptors in the development of atherosclerosis: potential mechanisms. Arterioscler Thromb Vasc Biol 2004;24:369–375 [DOI] [PubMed] [Google Scholar]
- 33.Lefebvre B, Pepin JL, Baguet JP, Tamisier R, Roustit M, Riedweg K, Bessard G, Levy P, Stanke-Labesque F. Leukotriene B4: early mediator of atherosclerosis in obstructive sleep apnoea? Eur Respir J 2008;32:113–120 [DOI] [PubMed] [Google Scholar]
- 34.Ge QF, Wei EQ, Zhang WP, Hu X, Huang XJ, Zhang L, Song Y, Ma ZQ, Chen Z, Luo JH. Activation of 5-lipoxygenase after oxygen-glucose deprivation is partly mediated via NMDA receptor in rat cortical neurons. J Neurochem 2006;97:992–1004 [DOI] [PubMed] [Google Scholar]
- 35.Kiang JG, Tsen KT. Biology of hypoxia. Chin J Physiol 2006;49:223–233 [PubMed] [Google Scholar]
- 36.Casillan AJ, Gonzalez NC, Johnson JS, Steiner DR, Wood JG. Mesenteric microvascular inflammatory responses to systemic hypoxia are mediated by PAF and LTB4. J Appl Physiol 2003;94:2313–2322 [DOI] [PubMed] [Google Scholar]
- 37.Akisu M, Huseyinov A, Baka M, Yalaz M, Kultursay N. The effect of dietary supplementation with n-3 polyunsaturated fatty acids on the generation of platelet-activating factor and leukotriene B4 in hypoxic-ischemic brain in young mice. Prostaglandins Leukot Essent Fatty Acids 2002;67:429–433 [DOI] [PubMed] [Google Scholar]
- 38.Back M, Bu DX, Branstrom R, Sheikine Y, Yan ZQ, Hansson GK. Leukotriene B4 signaling through NF-kappaB-dependent BLT1 receptors on vascular smooth muscle cells in atherosclerosis and intimal hyperplasia. Proc Natl Acad Sci USA 2005;102:17501–17506 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Riccioni G, Back M, Capra V. Leukotrienes and atherosclerosis. Curr Drug Targets 2010;11:882–887 [DOI] [PubMed] [Google Scholar]
- 40.Kristo F, Hardy GJ, Anderson TJ, Sinha S, Ahluwalia N, Lin AY, Passeri J, Scherrer-Crosbie M, Gerszten RE. Pharmacological inhibition of BLT1 diminishes early abdominal aneurysm formation. Atherosclerosis 2010;210:107–113 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Ahluwalia N, Lin AY, Tager AM, Pruitt IE, Anderson TJ, Kristo F, Shen D, Cruz AR, Aikawa M, Luster AD, et al. Inhibited aortic aneurysm formation in BLT1-deficient mice. J Immunol 2007;179:691–697 [DOI] [PubMed] [Google Scholar]
- 42.Heller EA, Liu E, Tager AM, Sinha S, Roberts JD, Koehn SL, Libby P, Aikawa ER, Chen JQ, Huang P, et al. Inhibition of atherogenesis in BLT1-deficient mice reveals a role for LTB4 and BLT1 in smooth muscle cell recruitment. Circulation 2005;112:578–586 [DOI] [PubMed] [Google Scholar]
- 43.Huang L, Zhao A, Wong F, Ayala JM, Struthers M, Ujjainwalla F, Wright SD, Springer MS, Evans J, Cui J. Leukotriene B4 strongly increases monocyte chemoattractant protein-1 in human monocytes. Arterioscler Thromb Vasc Biol 2004;24:1783–1788 [DOI] [PubMed] [Google Scholar]
- 44.Eriksson EE. Mechanisms of leukocyte recruitment to atherosclerotic lesions: future prospects. Curr Opin Lipidol 2004;15:553–558 [DOI] [PubMed] [Google Scholar]
- 45.Friedrich EB, Tager AM, Liu E, Pettersson A, Owman C, Munn L, Luster AD, Gerszten RE. Mechanisms of leukotriene B4–triggered monocyte adhesion. Arterioscler Thromb Vasc Biol 2003;23:1761–1767 [DOI] [PubMed] [Google Scholar]
- 46.Hlawaty H, Jacob MP, Louedec L, Letourneur D, Brink C, Michel JB, Feldman L, Back M. Leukotriene receptor antagonism and the prevention of extracellular matrix degradation during atherosclerosis and in-stent stenosis. Arterioscler Thromb Vasc Biol 2009;29:518–524 [DOI] [PubMed] [Google Scholar]
- 47.Cipollone F, Mezzetti A, Fazia ML, Cuccurullo C, Iezzi A, Ucchino S, Spigonardo F, Bucci M, Cuccurullo F, Prescott SM, et al. Association between 5-lipoxygenase expression and plaque instability in humans. Arterioscler Thromb Vasc Biol 2005;25:1665–1670 [DOI] [PubMed] [Google Scholar]
- 48.Aiello RJ, Bourassa PA, Lindsey S, Weng W, Freeman A, Showell HJ. Leukotriene B4 receptor antagonism reduces monocytic foam cells in mice. Arterioscler Thromb Vasc Biol 2002;22:443–449 [DOI] [PubMed] [Google Scholar]
- 49.Back M. Inhibitors of the 5-lipoxygenase pathway in atherosclerosis. Curr Pharm Des 2009;15:3116–3132 [DOI] [PubMed] [Google Scholar]
- 50.Sanchez-Galan E, Gomez-Hernandez A, Vidal C, Martin-Ventura JL, Blanco-Colio LM, Munoz-Garcia B, Ortega L, Egido J, Tunon J. Leukotriene B4 enhances the activity of nuclear factor-kappaB pathway through BLT1 and BLT2 receptors in atherosclerosis. Cardiovasc Res 2009;81:216–225 [DOI] [PubMed] [Google Scholar]
- 51.Qiu H, Gabrielsen A, Agardh HE, Wan M, Wetterholm A, Wong CH, Hedin U, Swedenborg J, Hansson GK, Samuelsson B, et al. Expression of 5-lipoxygenase and leukotriene A4 hydrolase in human atherosclerotic lesions correlates with symptoms of plaque instability. Proc Natl Acad Sci USA 2006;103:8161–8166 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Radmark O, Samuelsson B. 5-lipoxygenase: regulation and possible involvement in atherosclerosis. Prostaglandins Other Lipid Mediat 2007;83:162–174 [DOI] [PubMed] [Google Scholar]
- 53.Helgadottir A, Manolescu A, Thorleifsson G, Gretarsdottir S, Jonsdottir H, Thorsteinsdottir U, Samani NJ, Gudmundsson G, Grant SF, Thorgeirsson G, et al. The gene encoding 5-lipoxygenase activating protein confers risk of myocardial infarction and stroke. Nat Genet 2004;36:233–239 [DOI] [PubMed] [Google Scholar]
- 54.Zagryagskaya AN, Aleksandrov DA, Pushkareva MA, Galkina SI, Grishina ZV, Sud'ina GF. Biosynthesis of leukotriene B4 in human polymorphonuclear leukocytes: regulation by cholesterol and other lipids. J Immunotoxicol 2008;5:347–352 [DOI] [PubMed] [Google Scholar]
- 55.Choi JH, Jeon HJ, Park JG, Sonn SK, Lee MR, Lee MN, You HJ, Kim GY, Kim JH, Lee MH, et al. Anti-atherogenic effect of BHB-TZD having inhibitory activities on cyclooxygenase and 5-lipoxygenase in hyperlipidemic mice. Atherosclerosis 2010;212:146–152 [DOI] [PubMed] [Google Scholar]
- 56.Farre R, Nacher M, Serrano-Mollar A, Galdiz JB, Alvarez FJ, Navajas D, Montserrat JM. Rat model of chronic recurrent airway obstructions to study the sleep apnea syndrome. Sleep 2007;30:930–933 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.