Abstract
Several studies have reported that, in Lynch syndrome resulting from mutations of the mismatch repair (MMR) genes, a CA repeat ≤17 within the IGF1 promoter, SNPs within the xenobiotic metabolizing enzyme gene CYP1A1 and SNPs on 8q23.3 and 11q23.1 modify colorectal cancer (CRC) risk in MMR mutation carriers. We analysed the impact of these polymorphisms on CRC risk in 748 French MMR mutation carriers derived from 359 families. We also analysed the effect of the Novel 1 SNP (18q21), which has recently been shown to increase CRC risk in the general population. We observed a significant difference in the CRC-free survival time between males and females, between MSH2 and MSH6 mutation carriers and between MLH1 and MSH6, indicating that this series is representative of Lynch syndrome. In contrast, the univariate log-rank test, as well as multivariate Cox model analysis controlling for familial aggregation and mutated MMR gene, year of birth and gender showed that the polymorphic alleles tested were not associated with a significant CRC risk increase, neither on the entire sample nor among males and females. This discrepancy with previous reports might be explained both by the genetic heterogeneity between the different populations analysed and the allelic heterogeneity of the MMR mutations. We conclude that genotyping of these polymorphisms is not useful to evaluate CRC risk in MMR mutation carriers and to optimize their clinical follow-up.
Keywords: Lynch syndrome, MMR genes, cancer risk, modifier factors
Introduction
Lynch syndrome or hereditary non-polyposis colorectal cancer, the most common form of inherited colorectal cancer (CRC), results from germline mutations within the genes of the mismatch repair (MMR) system, MSH2, MLH1, MSH6 and PMS2 (for a review, see Lynch et al1). In MMR mutation carriers, the main tumour risks are colorectal and endometrial cancers. The cumulative risk at 70 years of CRC has been estimated to be 47–78% in males2, 3, 4 and 30–57% in females,2, 3, 4 and the risk of endometrial cancer to be 25–61%.2, 3 Like in other Mendelian forms of cancer characterized by an incomplete penetrance, one of the main challenges is to identify modifier genetic factors that can modulate the mutation penetrance. Characterization of validated modifier genetic factors should have important clinical consequences in the future since it should then be possible to adapt the follow-up of MMR gene mutation carriers, in terms of nature and timing of investigations, according to modifier alleles.
The modifier genes that have been reported so far in Lynch syndrome have been characterized according to two strategies: several studies have been focused on genes whose implication in CRC development had previously been suggested. One of the first modifier genetic factors identified in Lynch syndrome corresponds to a CA repeat polymorphism present within the IGF1 promoter, 1 kb upstream from the transcriptional initiation site.5, 6 In a study performed in 121 MMR mutation carriers from 59 families, mainly of Caucasian origin, Zecevic et al5 reported that a CA repeat ≤17 was significantly associated with a higher CRC risk and an earlier age of tumour onset. This result was confirmed by an independent study performed in 443 Australian and Polish MMR mutation carriers originated from 269 distinct families.6 Another class of modifier genes reported in Lynch syndrome corresponds to genes encoding xenobiotic metabolizing enzymes involved in environmental carcinogen metabolism. In 129 subjects of South African origin and harbouring the same MLH1 missense mutation, males harbouring the null genotype for the GSTT1 and GSTM1 genes developed cancer earlier than the males harbouring the other genotypes.7 This effect of the GSTT1 and GSTM1 null genotypes was not confirmed in a second study including 257 MMR mutation carriers from 130 families.8 Nevertheless, the authors reported that subjects heterozygous for the CYP1A1 rs1048943 SNP (c.1384A>G; p.Ile462Val) developed CRC earlier than individuals with the homozygous wild-type genotype and that subjects heterozygous for this polymorphism and an additional SNP rs4646903 (Msp1; g.6235T>C) had an increased CRC risk.8
The second strategy, which has recently allowed the detection of modifier genes in Lynch patients, originated from the numerous genome-wide association studies reporting SNPs associated with CRC risk in the general population. Starting from the hypothesis that SNPs acting as risk factors for CRC in the general population might act as risk modifiers in patients harbouring a highly penetrant mutation, Wijnen et al9 recently reported a significant association of CRC risk with rs16892766 (8q23.3) and rs3802842 (11q23.1) in 675 Dutch MMR mutation carriers from 127 families.
In this study, we investigated the impact of these different genetic factors on CRC risk in a large series of French MMR mutation carriers.
Patients and methods
Patients
The study included 748 unselected patients (Table 1) derived from 359 families with Lynch syndrome and recruited from Rouen (n=494) and Lille (n=254) university hospitals in France. All these individuals were confirmed carriers of a deleterious mutation of MSH2, MLH1 or MSH6 and were Caucasian. Among these 748 mutation carriers, 329 (44%) had been diagnosed with CRC prior to inclusion in the study, with a mean age of CRC onset of 43 years (range: 18–82 years). Additionally, 51 mutation carriers (6.8%) had been diagnosed with another tumour belonging to the Lynch syndrome spectrum (endometrial carcinoma, tumour of the urinary tract, ovarian carcinoma, cancer of the stomach and of the small intestine) and 368 (49.2%) had developed no tumour at the time of inclusion.
Table 1. Clinical characteristics of MMR mutation carriers.
| Number of subjects (%) (n=748) | Number of incident CRC cases (n=329) | Log-rank test, P-value | |
|---|---|---|---|
| Gender | |||
| Male | 349 (46.7) | 177 | <0.0001 |
| Female | 399 (53.3) | 152 | |
| Index case | |||
| Yes | 290 (38.8) | 259 | |
| No | 458 (61.2) | 70 | |
| MMR gene mutated | |||
| MSH2 | 414 (55.4) | 178 | 0.0495 (vs MSH6) |
| MLH1 | 267 (35.7) | 115 | 0.0069 (vs MSH6) |
| MSH6 | 67 (8.9) | 36 | |
| Year of birth | |||
| <1940 | 57 (7.6) | 39 (11.8) | |
| 1940–1949 | 106 (14.2) | 73 (22.2) | |
| 1950–1959 | 188 (25.1) | 119 (36.2) | |
| 1960–1969 | 165 (22.1) | 66 (20.1) | |
| 1970–1979 | 129 (17.2) | 26 (7.9) | |
| ≥1980 | 103 (13.8) | 6 (1.8) | |
|
Age |
Mean, min/max |
||
| With CRC | 329 (44) | 43, 18/82 | |
| Without CRC | 419 (56) | 38, 18/78 | |
Genotyping
The IGF1 CA repeat was PCR-amplified using dye-labelled primers and the length of the PCR products was determined after migration on an Applied Biosystems model 3130 Genetic Analyser (PE Applied Biosystems, Foster City, CA, USA) and analysed using the GeneMapper Analysis Software version 4.0 (Applied Biosystems). To calibrate the results, we sequenced, after cloning into a plasmid, the most frequent allele (n=19) and used the corresponding genomic DNA as a reference DNA. The rs16892766 (8q23.3), rs3802842 (11q23.1) SNPs and the rs1048943 (c.1384A>G; p.Ile462Val) and rs4646903 SNPs, both located within the CYP1A1 gene (15q24.1), were genotyped using SNaPshot multiplex assays based on primer extension with dye-labelled dideoxynucleotides (ABI PRISM SNaPshot Multiplex kit, Applied Biosystems). We also analysed, by SNaPshot multiplex analysis, the Novel 1 SNP located on 18q21, which had been shown to be associated with CRC risk in the general population, by altering SMAD7 expression.10 Labelled products were separated using a 25-min run on an ABI Prism 3100 DNA sequencer and data were analysed using the GeneMapper Analysis Software version 4.0 (Applied Biosystems). Primer sequences used for genotyping and conditions of the SNaPshot multiplex assays are available on request.
Statistical analyses
The genotype frequencies were tested for Hardy–Weinberg equilibrium. In all following analyses of time to CRC, first CRC onset was the event of interest, age (in years) was used at the time scale and retrospective follow-up started at birth for all subjects in the study and ended at either first CRC onset for incident CRC cases or age at last follow-up otherwise. These analyses were performed for all subjects and separately for males and females and were repeated for CRC cases only. The association of sex or mutated MMR gene with CRC risk was assessed using the log-rank test. The association of each polymorphism (ie, five SNPs and IGF1 CA repeat length) with CRC risk was assessed using the log-rank test and then the Cox proportional hazard model with stratification on gender (if applicable), mutated MMR gene (MSH2, MLH1, MSH6) and date of birth (<1940, 1940–1949, 1950–1959, 1960–1969, 1970–1979 and ≥1980) and with control for familial clustering. In the Cox model analyses of each of the five SNPs, an overall comparison of the three genotypes was performed and a hazard ratio (HR) and corresponding 95% confidence interval were estimated for homozygous and heterozygous subjects, relative to subjects homozygous for the wild-type allele. Moreover, alternative Cox model analyses of each of the five SNPs considered the number of alleles with the SNP (0, 1, 2) and fitted a trend producing an alternative test of association with CRC risk and an estimate of the CRC HR per mutated allele. Statistical analyses were performed using the SAS software version 9.1 (SAS Institute, Cary, NC, USA).
Results
Among the 748 MMR mutation carriers included in this study, 329 carriers (44%) developed CRC. As shown in Table 1, a significant difference was observed in the age at first CRC occurrence between males and females, with males developing CRC earlier, between MSH2 and MSH6 mutation carriers, between MLH1 and MSH6 mutation carriers, with MSH2 and MLH1 carriers developing CRC earlier, but not between MSH2 and MLH1 mutation carriers (log-rank test, P=0.26). The five SNPs analysed, located on 8q23.3, 11q23.1, 18q21 and within CYP1A1, were in Hardy–Weinberg equilibrium. The distribution of the IGF1 CA repeat alleles was the following: 12 repeats (n=8), 13–15 repeats (n=0), 16 repeats (n=2), 17 repeats (n=28), 18 repeats (n=85), 19 repeats (n=976), 20 repeats (n=262), 21 repeats (n=114) and 22 repeats (n=21) and alleles were dichotomized in ≤17 and ≥18 repeats for the statistical analyses, like in the previous studies.5, 6
In univariate analysis, age at first CRC occurrence did not vary significantly for the five SNPs considered or according to number of IGF1 CA repeats, whether for the whole sample or separately for males and females (Table 2). As indicated in Table 3, the multivariate Cox analysis performed in the total sample of mutation carriers revealed that, for rs16892766 (8q23.3), the homozygosity for the minor C allele present only in three mutation carriers was significantly associated with a decreased risk relative to subjects with AA alleles (HR=0.267, P=0.0271). For the CYPA1 SNPs, we observed a significant difference only in males for one of the SNPs analysed (rs4646903): the CC genotype, detected in four male mutation carriers, was associated with an increased risk relative to subjects with TT alleles (HR=3.496, P=0.0033). Considering that penetrance of MSH6 mutation was found in this study to be lower than that of MSH2 and MLH1 mutations, we restricted the log-rank and Cox analyses to MSH2 and MLH1 carriers and this restriction did not modify the results (data not shown).
Table 2. Allelic frequency of genetic variants and assessment of the associated colorectal risk by the log-rank test.
| Log-rank test P-value | |||
|---|---|---|---|
| Genetic variant | Allelic frequency | All subjects (n=748) | CRC patients (n=329) |
| rs16892766 (8q23.3) | |||
| MAF | 0.07 (C allele) | ||
| Total sample | 0.2188 | 0.0946 | |
| Males | 0.3146 | 0.5209 | |
| Females | 0.7409 | 0.0585 | |
| rs3802842 (11q23.1) | |||
| MAF | 0.28 (C allele) | ||
| Total sample | 0.6206 | 0.2316 | |
| Males | 0.8345 | 0.7805 | |
| Females | 0.7557 | 0.4081 | |
| Novel 1 (18q21) | |||
| MAF | 0.47 (C allele) | ||
| Total sample | 0.9698 | 0.3560 | |
| Males | 0.4676 | 0.3483 | |
| Females | 0.8847 | 0.2349 | |
| rs1048943 (CYP1A) | |||
| MAF | 0.03 (G allele) | ||
| Total sample | 0.7632 | 0.3442 | |
| Males | 0.8957 | 0.5035 | |
| Females | 0.9003 | 0.6082 | |
| rs4646903 (CYP1A) | |||
| MAF | 0.12 (C allele) | ||
| Total sample | 0.9489 | 0.6490 | |
| Males | 0.3701 | 0.7074 | |
| Females | 0.6772 | 0.5724 | |
| IGF1 CA repeat | |||
| MAF | 0.025 (≤17)) | ||
| Total sample | 0.5763 | 0.6002 | |
| Males | 0.2918 | 0.1562 | |
| Females | 0.9457 | 0.4249 | |
Table 3. Evaluation in 748 MMR mutation carriers of the CRC risk associated with 8q23.3, 11q23.1, 18q21, CYP1A1 and IGF1 variants.
| Total sample (N=748) | Males (N=349) | Females (N=399) | ||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Locus | Genotype | N | CRC cases | Hazard ratioa | 95% CI | Pb | N | CRC Cases | HR | 95% CI | Pb | N | CRC cases | HR | 95% CI | Pb |
| 8q23.3 | rs16892766 | |||||||||||||||
| AA | 641 | 277 | 1 | 290 | 142 | 1 | 351 | 135 | 1 | |||||||
| AC | 104 | 50 | 1.184 | 0.866–1.617 | 0.2892 | 57 | 34 | 1.266 | 0.836–1.916 | 0.2656 | 47 | 16 | 1.067 | 0.677–1.680 | 0.7802 | |
| CC | 3 | 2 | 0.267 | 0.083–0.861 | 0.0271 | 2 | 1 | 0.460 | 0.301–0.701 | 0.0003 | 1 | 1 | / | / | <0.0001 | |
| 0.0462 | 0.0002 | <0.0001 | ||||||||||||||
| Per allele | 1.032 | 0.778–1.368 | 0.8283 | 1.117 | 0.770–1.621 | 0.5602 | 0.899 | 0.575–1.405 | 0.6397 | |||||||
| 11q23.1 | rs3802842 | |||||||||||||||
| AA | 380 | 167 | 1 | 178 | 90 | 1 | 202 | 77 | 1 | |||||||
| AC | 321 | 139 | 0.923 | 0.725–1.176 | 0.5165 | 141 | 71 | 0.844 | 0.611–1.167 | 0.3047 | 180 | 68 | 1.019 | 0.723–1.438 | 0.9125 | |
| CC | 47 | 23 | 1.107 | 0.684–1.791 | 0.6793 | 30 | 16 | 1.043 | 0.599–1.815 | 0.8814 | 17 | 7 | 1.222 | 0.543–2.750 | 0.6284 | |
| 0.6491 | 0.5079 | 0.8891 | ||||||||||||||
| Per allele | 0.988 | 0.806–1.210 | 0.9058 | 0.947 | 0.735–1.220 | 0.6740 | 1.052 | 0.778–1.422 | 0.7428 | |||||||
| 18q21 | Novel 1 | |||||||||||||||
| CC | 156 | 72 | 1 | 84 | 45 | 1 | 72 | 27 | 1 | |||||||
| CG | 395 | 165 | 0.956 | 0.704–1.296 | 0.7702 | 185 | 87 | 1.021 | 0.675–1.546 | 0.9215 | 210 | 78 | 0.865 | 0.546–1.370 | 0.5359 | |
| GG | 197 | 92 | 1.006 | 0.727–1.390 | 0.9729 | 80 | 45 | 1.088 | 0.706–1.677 | 0.7007 | 117 | 47 | 0.903 | 0.564–1.447 | 0.6714 | |
| 0.9001 | 0.9087 | 0.8249 | ||||||||||||||
| Per allele | 1.007 | 0.859–1.181 | 0.9326 | 1.044 | 0.841–1.295 | 0.6962 | 0.964 | 0.765–1.215 | 0.7570 | |||||||
| CYP1A1 | rs1048943 | |||||||||||||||
| AA | 708 | 313 | 1 | 331 | 168 | 1 | 377 | 145 | 1 | |||||||
| AG | 40 | 16 | 1.026 | 0.601–1.752 | 0.9249 | 18 | 9 | 0.930 | 0.444–1.948 | 0.8480 | 22 | 7 | 1.168 | 0.583–2.338 | 0.6619 | |
| GG | 0 | 0 | / | / | / | 0 | 0 | / | / | / | 0 | 0 | / | / | / | |
| 0.9249 | 0.8480 | 0.6619 | ||||||||||||||
| Per allele | 1.026 | 0.601–1.752 | 0.9249 | 0.930 | 0.444–1.948 | 0.8480 | ||||||||||
| CYP1A1 | rs4646903 | |||||||||||||||
| TT | 598 | 265 | 1 | 279 | 143 | 1 | 319 | 122 | 1 | |||||||
| TC | 140 | 61 | 1.031 | 0.769–1.382 | 0.8400 | 66 | 32 | 0.909 | 0.601–1.375 | 0.6524 | 74 | 29 | 1.198 | 0.789–1.818 | 0.3956 | |
| CC | 10 | 3 | 1.163 | 0.387–3.495 | 0.7882 | 4 | 2 | 3.496 | 1.517–8.057 | 0.0033 | 6 | 1 | 0.507 | 0.071–3.640 | 0.4997 | |
| 0.9441 | 0.0090 | 0.5434 | ||||||||||||||
| Per allele | 1.039 | 0.801–1.347 | 0.7730 | 1.001 | 0.680–1.474 | 0.9948 | 1.079 | 0.746–1.561 | 0.6874 | |||||||
| IGF1 | CA repeat | |||||||||||||||
| ≥18;≥18 | 710 | 1 | 329 | 1 | 381 | 1 | ||||||||||
| ≥18; ≤17 | 38 | 0.731 | 0.447–1.196 | 0.2127 | 20 | 0.662 | 0.372–1.179 | 0.1611 | 18 | 0.979 | 0.450–2.132 | 0.9579 | ||||
Hazard ratio relative to subjects not carrying the SNP on either allele for each of the five SNPs considered or to subjects with CA repeat length greater than or equal to 18 on both alleles for the IGF1 promoter and obtained from Cox proportional hazard regression stratified on gender (if applicable), mutated MMR gene (MSH2, MLH1, MSH6) and year of birth (<1940, 1940–1949, 1950–1959, 1960–1969, 1970–1979 and ≥1980), and with control for familial aggregation. The per-allele value refers to Cox regression with a trend fitted for number of alleles carrying the SNP (0, 1, 2).
For each of the five SNP variants and each sample (total sample, males, females), the first two P-values refer to separate comparisons of heterozygous and homozygous subjects for the SNPs considered, with subject not presenting the SNP on either allele; the third P-value refers to the overall comparison of heterozygous, homozygous and SNP-free subjects (heterogeneity test); the final P-value refers to the assessment of the trend for the number of alleles carrying the SNP (0, 1, 2). For IGF1 CA repeat length, the P-value refers to the comparison of subjects with CA repeat length less than or equal to 17 on one allele to subjects with CA repeat length greater than or equal to 18 on both alleles. All tests are Wald tests.
As colonoscopy had not been performed in all the unaffected mutation carriers at the time of this study, we then focused the statistical analyses on the 329 MMR mutation carriers with CRC, in order to avoid a bias in the phenotypic evaluation. As indicated in Table 4, the unrestricted multivariate Cox analysis showed that the only remaining significant association was a decreased risk associated with the 8q23.3 CC genotype, but it should be emphasized that, because this genotype was only carried by two CRC cases, no HR estimate could be produced.
Table 4. Evaluation in 329 MMR mutation carriers with CRC of the risk associated with 8q23.3, 11q23.1, 18q21, CYP1A1 and IGF1 variants.
| Total sample (N=329) | Males (N=177) | Females (N=152) | |||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Locus | Genotype | N | Hazard ratioa | 95% CI | Pb | N | HR | 95% CI | Pb | N | HR | 95% CI | Pb |
| 8q23.3 | rs16892766 | ||||||||||||
| AA | 277 | 1 | 142 | 1 | 135 | 1 | |||||||
| AC | 50 | 0.793 | 0.559–1.124 | 0.1920 | 34 | 0.768 | 0.478–1.234 | 0.2749 | 16 | 0.833 | 0.5041.376 | 0.4759 | |
| CC | 2 | / | / | <0.0001 | 1 | / | / | <0.0001 | 1 | / | / | <0.0001 | |
| <0.0001 | <0.0001 | <0.0001 | |||||||||||
| Per allele | 0.666 | 0.481–0.922 | 0.0144 | 0.647 | 0.414–1.009 | 0.0549 | 0.698 | 0.443–0.102 | 0.1227 | ||||
| 11q23.1 | rs3802842 | ||||||||||||
| AA | 167 | 1 | 90 | 1 | 77 | 1 | |||||||
| AC | 139 | 0.941 | 0.738–1.199 | 0.6216 | 71 | 0.840 | 0.593–1.191 | 0.3274 | 68 | 1.067 | 0.751–1.515 | 0.7180 | |
| CC | 23 | 1.234 | 0.694–2.195 | 0.4736 | 16 | 1.034 | 0.548–1.950 | 0.9182 | 7 | 1.815 | 0.664–4.963 | 0.2457 | |
| 0.6151 | 0.5740 | 0.5057 | |||||||||||
| Per allele | 1.028 | 0.831–1.272 | 0.9763 | 0.947 | 0.723–1.239 | 0.947 | 1.170 | 0.854–1.604 | 0.3289 | ||||
| 18q21 | Novel 1 | ||||||||||||
| CC | 72 | 1 | 45 | 1 | 27 | 1 | |||||||
| CG | 165 | 0.828 | 0.588–1.166 | 0.2804 | 87 | 1.081 | 0.701–1.667 | 0.7249 | 78 | 0.571 | 0.315–1.035 | 0.0647 | |
| GG | 95 | 0.741 | 0.516–1.062 | 0.1029 | 45 | 0.867 | 0.554–1.358 | 0.5326 | 47 | 0.570 | 0.305–1.068 | 0.0793 | |
| 0.2637 | 0.5573 | 0.1637 | |||||||||||
| Per allele | 0.865 | 0.726–1.031 | 0.1056 | 0.919 | 0.735–1.149 | 0.4607 | 0.796 | 0.595–1.065 | 0.1238 | ||||
| CYP1A1 | rs1048943 | ||||||||||||
| AA | 313 | 1 | 168 | 1 | 145 | 1 | |||||||
| AG | 16 | 1.107 | 0.609–2.011 | 0.7387 | 9 | 1.105 | 0.502–2.432 | 0.8045 | 7 | 1.110 | 0.447–2.754 | 0.8224 | |
| GG | 0 | / | / | / | 0 | / | / | / | 0 | / | / | / | |
| 0.7387 | 0.8045 | 0.8224 | |||||||||||
| Per allele | 1.107 | 0.609–2.011 | 0.7387 | 1.105 | 0.502–2.432 | 0.8045 | 1.110 | 0.447–2.754 | 0.8224 | ||||
| CYP1A1 | rs4646903 | ||||||||||||
| TT | 265 | 1 | 143 | 1 | 122 | 1 | |||||||
| TC | 61 | 0.988 | 0.725–1.348 | 0.9412 | 32 | 1.070 | 0.696–1.647 | 0.7568 | 29 | 0.902 | 0.572–1.421 | 0.6558 | |
| CC | 3 | 1.741 | 0.925–3.278 | 0.0856 | 2 | 2.276 | 0.890–5.822 | 0.0861 | 1 | 1.241 | 0.759–2.029 | 0.3895 | |
| 0.2094 | 0.2291 | 0.5036 | |||||||||||
| Per allele | 1.038 | 0.786–1.372 | 0.7919 | 1.139 | 0.773–1.678 | 0.5117 | 0.930 | 0.618–1.399 | 0.7277 | ||||
| IGF1 | CA repeat | ||||||||||||
| ≥18;≥18 | 314 | 1 | 166 | 1 | 148 | 1 | |||||||
| ≥18; ≤17 | 15 | 0.710 | 0.397–1.272 | 0.2502 | 11 | 0.562 | 0.297–1.063 | 0.0765 | 4 | 1.709 | 0.470–6.212 | 0.4160 | |
Hazard ratio relative to subjects not carrying the SNP on either allele for each of the five SNPs considered or to subjects with CA repeat length greater than or equal to 18 on both alleles for the IGF1 promoter and obtained from Cox proportional hazard regression stratified on gender (if applicable), mutated MMR gene (MSH2, MLH1, MSH6) and year of birth (<1940, 1940–1949, 1950–1959, 1960–1969, 1970–1979 and ≥1980), and with control for familial aggregation. The per-allele value refers to Cox regression with a trend fitted for number of alleles carrying the SNP (0, 1, 2).
For each of the five SNP variants and each sample (total sample, males, females), the first two P-values refer to separate comparisons of heterozygous and homozygous subjects for the SNPs considered, with subject not presenting the SNP on either allele; the third P-value refers to the overall comparison of heterozygous, homozygous and SNP-free subjects (heterogeneity test); the final P-value refers to the assessment of the trend for the number of alleles carrying the SNP (0, 1, 2). For IGF1 CA repeat length, the P-value refers to the comparison of subjects with CA repeat length less than or equal to 17 on one allele to subjects with CA repeat length greater than or equal to 18 on both alleles. All tests are Wald tests.
Discussion
Like in other cases of Mendelian predisposition to cancer with an incomplete penetrance, the characterization of modifier loci for CRC in Lynch syndrome might allow identification of a subset of mutation carriers who could benefit from a reinforced tumour detection program, such as annual chromocolonoscopy from 20 years of age. This prospect prompted us to evaluate in a large series of 748 MMR carriers the risk conferred by the allelic variants that have recently been reported as risk modifiers for CRC in MMR mutation carriers. Within this series, 44% of the MMR mutation carriers had developed CRC at the time of the analysis; the median age of CRC onset was 43 years among them and we observed a significant difference of CRC penetrance between males and females and between MSH2 and MLH1 mutation versus MSH6 mutation carriers. These results are in agreement with the published studies, which have estimated that the cumulative CRC risk at 70 years is higher in males than in females2, 3, 4 and is lower in MSH6 mutation carriers as compared with MSH2 or MLH1 mutation carriers.2, 3, 11, 12 These findings are in favour of the representativeness of our series with respect to Lynch syndrome.
The distribution of IGF1 CA repeat length was similar to that reported by Zecevic et al4 in 121 MMR mutation carriers, the frequency of the major allele (19 repeats) being 65 and 58% in the two studies, respectively, although we found a lower proportion of alleles with length ≤17 (2.5 versus 5.1%). In the study published by Zecevic et al5 on 121 MMR mutation carriers, the presence of a CA repeat ≤17, detected in 12 subjects, was found to be associated with a higher CRC risk (HR 2.36) and an earlier median age of CRC onset (44 versus 56.5 years), but our study, performed on a larger series, did not allow us to reproduce these results.
For the rs1048943 (c.1384A>G; p.Ile462Val) and rs4646903 SNPs, located within the CYP1A1 gene, we found respective allelic frequencies for the minor allele of 0.03 and 0.12, which correspond to the HapMap values estimated in the European population. Pande et al8 had reported a higher MAF (0.074) for rs1048943 in 257 MMR mutation carriers, but it should be underlined that these carriers were from different ethnicities and included Hispanic and Asian subjects, in whom the allelic frequency of the G allele is known to be higher. In this series of 257 mutation carriers, CRC free survival statistically differed according to rs1048943 genotype, with a 1.78-fold increase of CRC hazard associated with the AG genotype, a result that we did not reproduce in our series of 748 MMR mutation carriers from Caucasian origin. For the second CYPA1 SNP (rs4646903), Pande et al8 had reported that the TC genotype was associated with an increased hazard for earlier CRC and we detected no significant difference in our study for this genotype (Table 3). In contrast, we found that the CC genotype was associated in males with a 3.5-fold increased HR, but this observation should be interpreted with utmost caution, considering the limited number of male mutation carriers presenting this genotype (n=4). For rs16892766 (8q23.3) and rs3802842 (11q23.1) SNPs, we observed respective allele frequencies of 7 and 28%: these frequencies had been, respectively, estimated in the European population at 7%13 and 29%,14 and in 675 Dutch MMR mutation carriers at 10 and 24.9%.9 We did not detect in our series a modification of CRC risk in mutation carriers harbouring the 11q23.1 rs3802842 CC genotype, as previously reported in females. Among the 675 Dutch mutation carriers, the 8q23.3 rs16892766 CC genotype, detected in 9 subjects, was found to be associated with a 2.16-fold CRC risk increase.9 In contrast, we found in our study (Table 3) that this genotype was significantly associated with a decreased CRC risk (HR=0.267), and this significant association was the only one remaining when we restricted the analysis to affected carriers, but the small number of subjects harbouring this genotype (n=3) and presenting a CRC (n=2) must be highlighted.
During the submission of this study, Talseth-Palmer et al15 reported that in MLH1 carriers, but not in MSH2 carriers, the 11q23.1 CC and 8q23.3 AC genotypes were associated with an increased risk, but this significant association detected in 373 Australian mutation carriers was not found in 311 Polish mutation carriers analysed in the same study. We did not replicate either of these associations in the French sample. Indeed, the only association that we detected for these two SNPs in the 267 French MLH1 mutation carriers was a decrease of CRC risk with the 8q23.3 CC genotype (P<0.0001), but as this genotype was carried by only one CRC case, it was not possible to estimate the corresponding HR and due caution should be applied to interpret this finding.
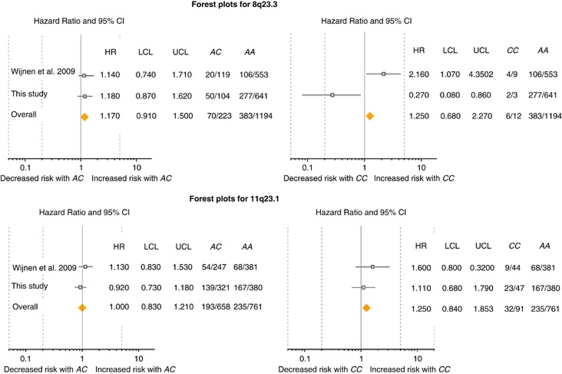
To increase the power of statistical analysis of the 8q23.3 and 11q23.1 SNPs' impact on CRC risk in MMR mutation carriers, we performed a meta-analysis based on the study published by Wijnen et al9 and our results, corresponding to 1423 MMR mutation carriers (Figure 1). In univariate analysis, age at first CRC occurrence varied significantly in male carriers according to the 8q23.3 genotype (AA, AC or CC; P=0.0265 using Fisher's standard method to combine P-values from two log-rank tests), with increased CRC risk associated with the AC genotype and decreased risk associated with the CC genotype. In multivariate Cox analysis, as shown on the Forest plot for the total sample (Figure 1), CRC risk did not vary significantly according to the 8q23.3 genotype. In males, the multivariate Cox analysis showed that the 8q23.3 CC genotype was associated with a decrease in CRC risk (combined fixed-effect weighted sum HR=0.61 relative to AA, P=0.0135). It remains, however, that there were very few males and even fewer male CRC cases carrying the CC genotype in both studies and thus the results of this meta-analysis should be interpreted with caution. Univariate and multivariate Cox analyses did not reveal any significant association between CRC risk and the 11q23.1 genotype (Figure 1).
Figure 1.
Forest plots of the meta-analysis of the CRC risk associated with 8q23.3 and 11q23.1 variants in 1423 MMR mutation carriers. CI, confidence interval; HR, hazard rate; LCL, lower 95% confidence limit; UCL, upper 95% confidence limit. For each panel, the last two columns display the number of subjects with colorectal cancer and the indicated genotype, followed by the overall number of subjects with this genotype.
In conclusion, the evaluation in a series of 748 MMR mutation carriers representative of Lynch syndrome of the IGF1 CA repeat, CYP1A1, 8q23.3 and 11q23.1 SNPs, previously reported as modifier factors for CRC risk in MMR mutation carriers, did not allow us to reproduce the previously published results. The only significant association that we detected both in the whole sample and in the unbiased affected mutation carrier sample was between the 8q23.3 CC genotype and a decreased CRC risk in males, whereas previously published studies had reported an increased CRC risk associated either with this genotype9 or with the heterozygous AC genotype.15 This discrepancy might be explained by the genetic heterogeneity between the different analysed populations. Considering that, in MMR mutation carriers, cancer development requires somatic mutation of several target genes containing repeated sequences, it is likely that numerous polymorphisms affect either the DNA repair or target genes and/or that environmental factors having a role in colorectal carcinogenesis will modify the penetrance of MMR mutations. It is also possible that a key factor modulating the penetrance is the allelic heterogeneity of the MMR mutations. Finally, another explanation of this discrepancy between our results and previous studies is that the previously reported associations were spurious associations due to too small samples. Therefore, we conclude that, from the medical point of view, genotyping of these polymorphisms is not useful to evaluate CRC risk in MMR mutation carriers and to optimize their clinical follow-up.
Acknowledgments
We thank our colleagues who referred to us to Lynch patients. This study was supported by the French Canceropole Nord-Ouest and the French National Cancer Institute (INCa). We are grateful to Mario Tosi for critical review of the manuscript.
The authors declare no conflict of interest.
References
- Lynch HT, Lynch PM, Lanspa SJ, Snyder CL, Lynch JF, Boland CR. Review of the Lynch syndrome: history, molecular genetics, screening, differential diagnosis, and medicolegal ramifications. Clin Genet. 2009;76:1–18. doi: 10.1111/j.1399-0004.2009.01230.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vasen HF, Stormorken A, Menko FH, et al. MSH2 mutation carriers are at higher risk of cancer than MLH1 mutation carriers: a study of hereditary nonpolyposis colorectal cancer families. J Clin Oncol. 2001;19:4074–4080. doi: 10.1200/JCO.2001.19.20.4074. [DOI] [PubMed] [Google Scholar]
- Ramsoekh D, Wagner A, van Leerdam ME, et al. Cancer risk in MLH1, MSH2 and MSH6 mutation carriers; different risk profiles may influence clinical management. Hered Cancer Clin Pract. 2009;7:17. doi: 10.1186/1897-4287-7-17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alarcon F, Lasset C, Carayol J, et al. Estimating cancer risk in HNPCC by the GRL method. Eur J Hum Genet. 2007;15:831–836. doi: 10.1038/sj.ejhg.5201843. [DOI] [PubMed] [Google Scholar]
- Zecevic M, Amos CI, Gu X, et al. IGF1 gene polymorphism and risk for hereditary nonpolyposis colorectal cancer. J Natl Cancer Inst. 2006;98:139–143. doi: 10.1093/jnci/djj016. [DOI] [PubMed] [Google Scholar]
- Reeves SG, Rich D, Meldrum CJ, et al. IGF1 is a modifier of disease risk in hereditary non-polyposis colorectal cancer. Int J Cancer. 2008;123:1339–1443. doi: 10.1002/ijc.23668. [DOI] [PubMed] [Google Scholar]
- Felix R, Bodmer W, Fearnhead NS, van der Merwe L, Goldberg P, Ramesar RS. GSTM1 and GSTT1 polymorphisms as modifiers of age at diagnosis of hereditary nonpolyposis colorectal cancer (HNPCC) in a homogeneous cohort of individuals carrying a single predisposing mutation. Mutat Res. 2006;602:175–181. doi: 10.1016/j.mrfmmm.2006.09.004. [DOI] [PubMed] [Google Scholar]
- Pande M, Amos CI, Osterwisch DR, et al. Genetic variation in genes for the xenobiotic-metabolizing enzymes CYP1A1, EPHX1, GSTM1, GSTT1, and GSTP1 and susceptibility to colorectal cancer in Lynch syndrome. Cancer Epidemiol Biomarkers Prev. 2008;17:2393–2401. doi: 10.1158/1055-9965.EPI-08-0326. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wijnen JT, Brohet RM, van Eijk R, et al. Chromosome 8q23.3 and 11q23.1 variants modify colorectal cancer risk in Lynch syndrome. Gastroenterology. 2009;136:131–137. doi: 10.1053/j.gastro.2008.09.033. [DOI] [PubMed] [Google Scholar]
- Pittman AM, Naranjo S, Webb E, et al. The colorectal cancer risk at 18q21 is caused by a novel variant altering SMAD7 expression. Genome Res. 2009;19:987–993. doi: 10.1101/gr.092668.109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Plaschke J, Engel C, Kruger S, et al. Lower incidence of colorectal cancer and later age of disease onset in 27 families with pathogenic MSH6 germline mutations compared with families with MLH1 or MSH2 mutations: the German Hereditary Nonpolyposis Colorectal Cancer Consortium. J Clin Oncol. 2004;22:4486–4494. doi: 10.1200/JCO.2004.02.033. [DOI] [PubMed] [Google Scholar]
- Baglietto L, Lindor NM, Dowty JG, et al. Risks of Lynch syndrome cancers for MSH6 mutation carriers. J Natl Cancer Inst. 2010;102:193–201. doi: 10.1093/jnci/djp473. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tomlinson IP, Webb E, Carvajal-Carmona L, et al. A genome-wide association study identifies colorectal cancer susceptibility loci on chromosomes 10p14 and 8q23.3. Nat Genet. 2008;40:623–630. doi: 10.1038/ng.111. [DOI] [PubMed] [Google Scholar]
- Tenesa A, Farrington SM, Prendergast JG, et al. Genome-wide association scan identifies a colorectal cancer susceptibility locus on 11q23 and replicates risk loci at 8q24 and 18q21. Nat Genet. 2008;40:631–637. doi: 10.1038/ng.133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Talseth-Palmer BA, Brenne IS, Ashton KA, et al. Colorectal cancer susceptibility loci on chromosome 8q23.3 and 11q23.1 as modifiers for disease expression in lynch syndrome J Med Genet 2010. e-pub ahead of print. [DOI] [PubMed]