Abstract
Study design
A case series of consecutive patients with chronic low back pain.
Background and purpose
In patients with chronic low back pain (CLBP), the importance of impairments at the hip joints is unclear. However, it has been postulated that impairments at the hip joints may contribute to CLBP. The purpose of this case series was to investigate the short-term outcomes in patients with CLBP managed with impairment-based manual therapy and exercise directed at the hip joints.
Methods
Eight consecutive patients (mean age: 43·9 years) with a primary report of CLBP (>6 months) without radiculopathy were treated with a standardized approach of manual physical therapy and exercise directed at bilateral hip impairments for a total of three sessions over approximately 1 week. At initial examination, all patients completed a numeric rating pain scale (NPRS), Oswestry disability index (ODI), fear-avoidance beliefs questionnaire (FABQ), and patient-specific functional scale (PSFS). At the second and third treatment sessions, each patient completed all outcome measures as well as the Global Rating of Change (GROC).
Results
Five of the eight (62·5%) patients reported ‘moderately better’ or higher (>+4) on the GROC at the third session, indicating a moderate improvement in self-reported symptoms. These five individuals also experienced a 24·4% reduction in ODI scores.
Discussion
This case series suggests that an impairment-based approach directed at the hip joints may lead to improvements in pain, function, and disability in patients with CLBP. A neurophysiologic mechanism may be a plausible explanation regarding the clinical outcomes of this study. A larger, well-controlled trial is needed to determine the potential effectiveness of this approach with patients with CLBP.
Keywords: Chronic low back pain, Hip, Impairment, Lumbar, Manipulation, Manual therapy
Background
From 1992 to 2006, it is estimated that the prevalence of chronic low back pain (CLBP) increased 6·3% and, subsequently, more people with CLBP are seeking treatment.1 Low back pain (LBP) is the most common reason for lost work days,2,3 second most common reason for an individual to see a primary care physician,4 and the third most common reason for hospital admission.5 Approximately one in four individuals have experienced a significant episode of LBP, which prompted them to seek medical attention within the previous 3 months,6 whereas 80% of individuals will experience an episode of LBP during their lifetime.7 The rate of recurrence of LBP is high8,9 and many seek alternative treatment options such as acupuncture.10,11 While 89% of patients with a new episode of LBP have persistent pain and disability at 3 months,10 75–78% of patients continue to have persistent pain and disability at 12 months.10 Additionally, utilization of healthcare resources has increased, with several studies showing significantly increased rates of spinal injections,12–14 surgery,6,15,16 and use of opioid medications.17,18 Individuals with CLBP are more likely to seek care for their symptoms19–21 and utilize more health care resources compared to patients with acute LBP.22–24
Individuals with CLBP are commonly treated by physical therapists, although there is no clear consensus on appropriate management strategy.25–27 Regional interdependence is an emerging paradigm that encourages a more comprehensive management strategy for individuals with musculoskeletal complaints. Wainner and colleagues28 described the term regional interdependence as ‘seemingly unrelated impairments in a remote anatomical region that may contribute to, or be associated with, the patient’s primary complaint’. This approach has been utilized in the management of co-existing pathology in the lumbar spine and lower extremities.8,29 The regional interdependence approach should be considered in individuals with LBP based on the extensive body of literature supporting the anatomical connections and neuromuscular control between the lumbar spine and the hips.29,30
Several studies have reported an association between LBP and hip impairments, including limitations in hip internal rotation,31–35 hip external rotation,31,32,36 total hip rotation,31,37 mobility using the flexion–abduction–external rotation (FABER) test, 34,35 and hip flexion.33 In one study, 48% of older adults with CLBP presented with pain that was reproduced with maximal hip internal rotation and/or the FABER test further implicating the hip in individuals with LBP.38
Furthermore, recent work has demonstrated an association between LBP and impairments in neuromuscular control between the hip and lumbopelvic region. Patients with LBP demonstrated less active hip motion and early compensatory lumbopelvic motion, suggesting altered lumbopelvic control and coordination.30,39
Several studies also suggest a possible clinical relationship between impairments at the hip and LBP. Sembrano and Polly40 estimated that the hips may be a contributing pain generator in approximately 12·5% of patients with LBP. In patients with hip osteoarthritis, the presence of LBP has been shown to be associated with a poor prognosis in terms of pain and disability.41 Finally, radiographic evidence of hip osteoarthritis has been shown to be a risk factor for progression of lumbar disc degeneration42 and 52–58% of older adults with hip or knee osteoarthritis report concurrent LBP.41,43
There is some evidence regarding clinical outcomes for LBP following surgical interventions directed at the hip. The incidence of LBP in patients who have undergone hip fusion has been reported to be 75·8%,44 almost four times higher than the incidence of LBP reported among a similarly aged cohort.45 In one study looking at patients receiving total hip arthroplasty (THA), 49% reported LBP, and more than 20% without pre-operative LBP developed post-operative LBP.46 In 2007, Ben-Galim et al.47 conducted the first clinical study to investigate the direct influence of the hips on the low back. They performed THA procedures on 25 patients with significant concurrent hip and lumbar spine complaints.47 The patients reported a significant reduction in lumbar disability scores and pain at 3-month follow-up as well as 2 years post-THA. The patients in this study clearly had radiographic signs of moderate to severe hip osteoarthritis, which limits the applicability to a general outpatient physical therapy population. However, it does suggest a dynamic interaction between the hips and the lumbar spine.
Brown et al.48 reported that 81% of patients with LBP that were referred to a specialty spine clinic had hip impairments, but there is a dearth of literature related to physical therapy interventions for LBP directed at impairments of the hip. Two case studies have reported successful management of LBP with interventions addressing impairments of hip motion and hip-lumbopelvic control and coordination.49,50 Additionally, Di Lorenzo et al.51 studied patients with first time back pain following open reduction internal fixation for extracapsular hip fractures, and found that interventions targeting the hip resulted in a statistically and clinically significant reduction in LBP, whereas interventions targeting the hip and lumbar spine resulted in a greater reduction in LBP.
Clinical decision making can be challenging when patients present with CLBP with concomitant hip impairments.49 Clinically, we have found that impairment-based manual therapy and exercise for the hips in patients with CLBP can result in noticeable improvements in pain and disability. The aim of the current study was to investigate the response to interventions directed at the hips in a series of patients with CLBP.
Case Description
Patients
Consecutive patients presenting with a chief complaint of chronic LBP (>6 months in duration) were examined for eligibility criteria over a 12-month period (November 2008–2009). Inclusion criteria for this study were a primary report of LBP (between T12 and the gluteal fold) >6 months in duration without radiating pain below the knee, age between 18 and 65 years, a modified Oswestry disability index (ODI) score of ⩾30%, and at least two of the following range of motion (ROM) impairments in one or both hips:
prone internal rotation <30°;
prone external rotation <30°;
supine flexion <110°;
prone extension <10°.
Exclusion criteria included any medical red flags that would contraindicate manual therapy to the hips (i.e. tumor, fracture, metabolic disease, rheumatoid arthritis, osteoporosis, and prolonged history of steroid use), previous surgical or non-surgical management within the last 6 months, signs of nerve root compression (i.e. muscle weakness, hyporeflexia, and decreased sensation), fear-avoidance beliefs questionnaire (FABQ) — work subscale score ⩾34, evidence of central nervous system involvement, pending litigation, insufficient English language skills, recently missed menstrual cycle in women, onset of symptoms from a motor vehicle accident, and inability to comply with treatment protocol. This case series was approved by the Colorado Multiple Institutional Review Board at the University of Colorado, Aurora, CO, USA, and the Institutional Review Board at Sacred Heart University, Fairfield, CT, USA.
Outcome measures
Patients completed several self-report measures at the initial, second and final sessions. Paper versions of the outcome measures, provided by the physical therapist, were completed in the waiting room and returned to a front office staff member. Self-report measures collected at baseline included: modified ODI; FABQ — work and physical activity subscales; the patient-specific functional scale (PSFS); and a numeric pain rating scale (NPRS).
The modified ODI is a 10-question condition-specific measurement of pain and disability for individuals with LBP. Each question is scored from 0 to 5 and summed to determine total score, which is the multiplied by 2 and expressed as a percentage. Higher scores correspond to greater disability. The modified ODI has been shown to be reliable and valid in patients with chronic LBP.52–54 The minimally important clinical difference (MCID) is 6 points or 12% in patients with acute, work-related LBP.52
The FABQ is designed to assess the level of fear-avoidance beliefs in patients with LBP, and consists of two subscales relating to such beliefs about work and physical activity.55,56
The PSFS is a patient-centered measure that requires the patient to list 3–5 activities that are difficult to perform due to their symptoms, injury, or disorder.57,58 The patient rates each of the three activities on a 0–10 point scale where 0 is the inability to perform the activity and 10 is able to perform the activity the same as before the onset of symptoms. The final score of the PSFS is derived by averaging the score for each of the three identified activities. The MCID for the PSFS for individuals with chronic LBP has been determined to be 1·4 points and this scale has been shown to be responsive to clinically important change over time.59
The NPRS is an 11-point Likert scale ranging from 0 (no pain) to 10 (worst imaginable pain) and was used to assess the patient’s level of pain. Each patient was asked to rate the intensity of their current, average, best, and worst pains over the last 24 hours. The average NPRS score was used in reporting results. A two-point change represents the MCID for the NPRS.56,60,61
Examination
A detailed history and physical examination was conducted by the treating physical therapist. Eight patients (six male) with an average age of 43·9 years (range: 18–63 years) were enrolled in the study. The standardized history consisted of: social and occupation background; past medical history; history of the current condition (including a body diagram); previous history of LBP; duration and nature of symptoms; and aggravating/relieving factors.
The physical examination consisted of a postural assessment; a neurological screening examination62 (myotomes, dermatomes, and muscle stretch reflexes); lumbar and hip ROM; accessory motion testing of the thoraco-lumbar spine (T10–L5), pelvis and hips; and assessment of lower extremity, abdominal (including transversus abdominis), and multifidus muscle strength. Details regarding the physical examination can be found elsewhere.49
Protocol
Baseline outcome measures and screening for inclusion criteria were performed by the primary physical therapist as part of the examination. Before the initiation of care, informed consent was obtained from each patient who met the eligibility criteria. Each patient consented to treatment directed at their hips initially, and it was explained to the patient that treatment targeting other impairments, including the lumbar spine, would be continued following the cessation of the study. Following the examination, each patient received manual therapy and exercise directed at one or both hip joints (outlined below). The patient returned to the clinic 2–3 days later and received the same interventions. The final visit was 2–3 days later (total of 7–8 days after enrollment) at which time the procedures were repeated. The initial outcome measures were repeated along with the global rating of change (GROC) at visits 2 and 3.63 The patient was asked to rate their perceived recovery on a scale ranging from −7 (a very great deal worse) to 0 (about the same) to +7 (a very great deal better). Jaeschke and colleagues63 reported that scores on the GROC between ±1 and ±3 represent small changes, ±4–5 represent moderate changes, and ±6–7 represent large changes in the patients’ perceived recovery.
Intervention
Manual therapy
Each patient was examined/re-examined by one of two physical therapists with 4 and 15 years of experience, who worked in outpatient physical therapy settings. All patients received the following interventions: supine long axis distraction thrust manipulation, supine caudal non-thrust manipulation, supine anterior-to-posterior non-thrust manipulation progression, prone posterior-to-anterior non-thrust manipulation in neutral and flexion/abduction/external rotation positions, and mobility exercises targeting the lumbopelvic-hip region. All non-thrust manipulations were performed as grade III or IV oscillations for three bouts of 30 seconds.64 All patients were also instructed to perform mobility and stretching exercises (described below) twice daily as a home exercise program. At each session, interventions were provided in the standardized order outlined above. All interventions are described in Figs. 1–5.
Figure 1.
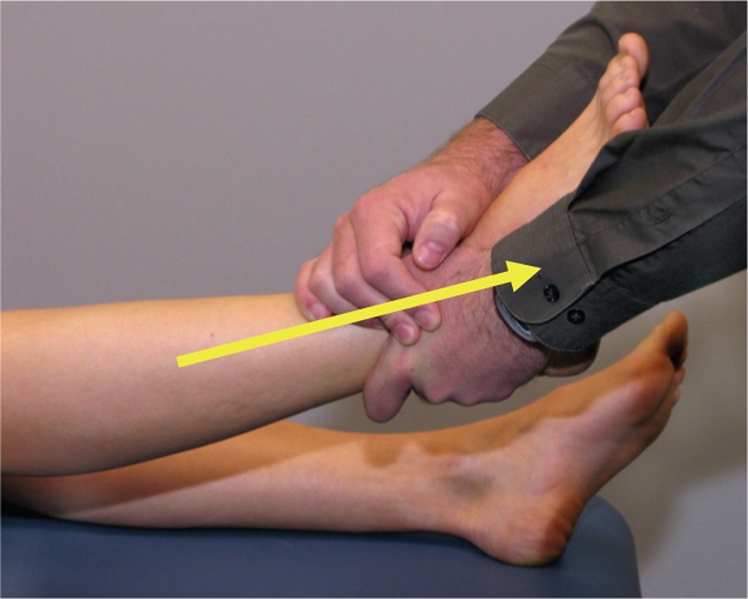
Long-axis distraction manipulation is a high-velocity, end-range, longitudinal traction force to the lower extremity on the acetabulum in supine with the hip in slight flexion, abduction, and varying degrees of internal and external rotation of the lower extremity. Step 1: grasp the patient’s ankle proximal to the malleoli with both hands in a grip comfortable for the patient. Step 2: position the leg in approximately 10–30° of hip flexion and 15–30° of abduction, with slight external rotation. Step 3: gently distract the hip and perform oscillations. Step 4: once the hip is felt to relax, apply a high-velocity, small-amplitude thrust.
Figure 5.
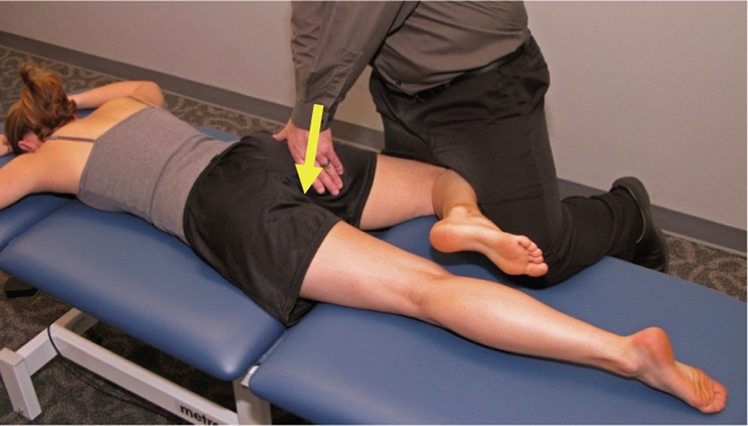
Posterior-anterior mobilization in flexion, abduction, external rotation is a low-velocity, end-range, posterior-to-anterior oscillatory force to the proximal femur in a prone position, with hip flexion, abduction, and external rotation. Step 1: place the patient in prone. Step 2: bring the hip into varying degrees of flexion, abduction, and external rotation. Step 3: contact the proximal hip and use your body to impart an oscillatory, passive mobilizing force in a posterior-to-anterior direction. Step 4: vary the vector of your mobilizing force dependent on the patient’s symptoms and joint stiffness. Step 5: if extremely stiff, start with a pillow under the patient’s left trunk to decrease the amount of hip abduction required: progress to lying flat on the table when it is tolerated by the patient.
Figure 2.
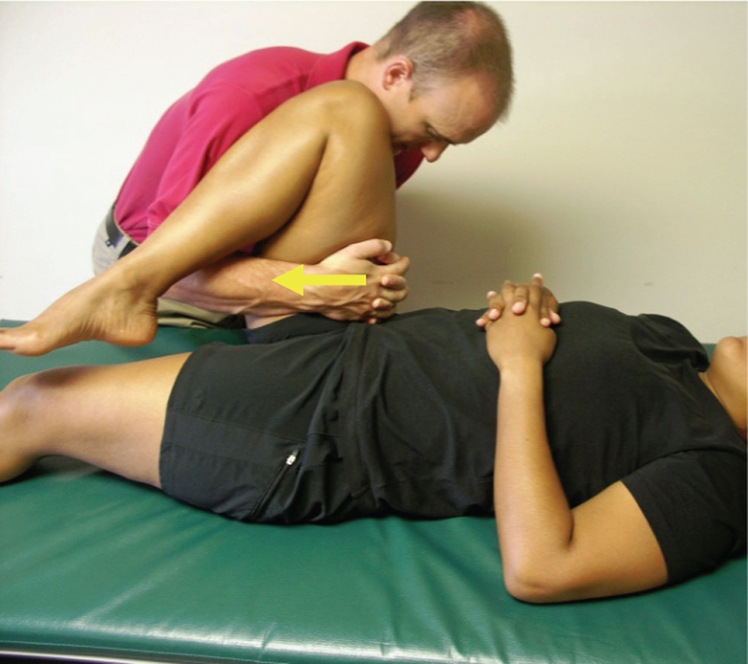
Caudal non-thrust manipulation is a low-velocity, mid-end-range, superior-to-inferior oscillatory force to the femur in a supine position, with hip flexed to 90–100°. Step 1: position the patient in supine and passively flex the hip to 90–100° with neutral rotation. Step 2: place your hands on the anterior aspect of the femur near the joint line of the hip. Step 3: gently distract the femur from the acetabulum in the caudal direction and perform oscillations.
Figure 3.
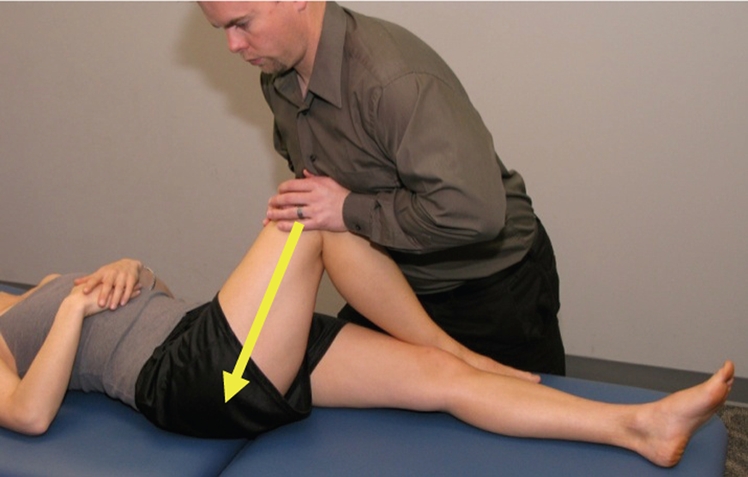
Anterior–posterior hip mobilization progression is a low-velocity, mid-end-range, anteromedial-to-posterolateral oscillatory force to the femur in a supine position, with hip flexion, adduction, and external rotation. Step 1: position the lower extremity with the hip in a position of flexion, adduction, and internal rotation. Step 2: use your body to impart an oscillatory, passive mobilizing force in a posterolateral direction through the long axis of the femur. Step 3: progress the technique by increasing flexion, adduction, and/or internal rotation.
Figure 4.
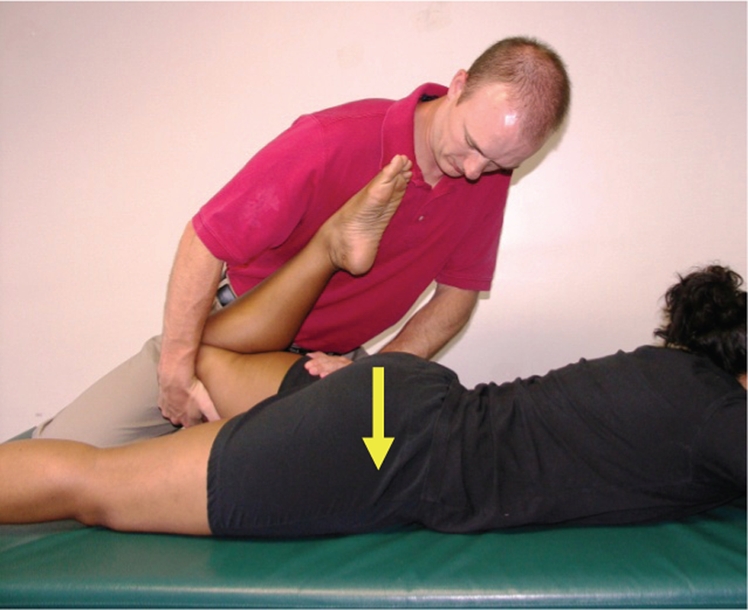
Posterior-to-anterior non-thrust manipulation in neutral is a low-velocity, mid-end-range, posterior-to-anterior oscillatory force to the femur in a prone position. Step 1: position the patient in prone with the knee flexed to 90–100° in neutral abduction and rotation. Step 2: passively extend the hip slightly and apply a posterior to anterior force thru the posterior aspect of the femur slightly distal to the hip.
Home exercise program
The therapist selected two out of four potential exercises based on patient-specific physical examination findings. Four exercises were provided and included a kneeling iliopsoas stretch, kneeling hip internal rotation stretch, supine piriformis stretch, and a prone hip ‘FABER’ stretch. The two exercises were chosen by the therapist based on the primary ROM impairments and/or patient response. Only two exercises were selected to maximize adherence.65 Each subject was instructed to perform two sets of 30-second holds for each exercise, twice daily.
Outcomes
A total of 41 patients with chronic LBP were screened for eligibility criteria. Eight patients satisfied the eligibility requirements and were enrolled in this case series. Of the 33 that did not fulfill the eligibility requirements, four failed to meet the age criteria, 10 did not have at least two hip impairments, six had symptoms distal to the knee, and 13 had a modified ODI score of <30%.
After the three treatment sessions, five (62·5%) of the patients rated their improvement on the GROC as ‘moderately better’ (+4) or higher. Two patients reported ‘no change’ on the GROC at the completion of the study. The patients that reported at least ‘moderate’ improvement for their perceived recovery reported an average reduction of 24·4% on the modified ODI. Three (37·5%) of the patients surpassed the MCID on the modified ODI after the third session. On the NPRS and PSFS, half of the patients reported values that were greater than or equal to the respective MCID. The outcomes for each patient are outlined in Table 1.
Table 1. Outcomes measures among the eight participants.
| Subject | Final global rating of change scores | Initial average pain scores | Follow-up average pain scores | Change in average pain scores | Initial PSFS | Final PSFS | Change in PSFS | Initial ODI (%) | Final ODI (%) | Change ODI (%) |
| 1 | 5 | 7 | 5 | −2* | 2·3 | 7·7 | 5·4* | 48 | 42 | −6 |
| 2 | 0 | 7 | 5 | −2* | 2·3 | 2·7 | 0·4 | 42 | 42 | 0 |
| 3 | 0 | 7 | 8 | +1 | 3·6 | 6 | 2·4* | 62 | 68 | +8 |
| 4 | 4 | 7 | 6 | −1 | 4 | 5 | 1 | 38 | 20 | −18* |
| 5 | 5 | 7 | 7 | 0 | 4 | 2·5 | −1·5 | 42 | 52 | +10 |
| 6 | 5 | 4 | 2 | −2* | 4·7 | 6·7 | 2* | 36 | 16 | −20* |
| 7 | 3 | 5 | 5 | 0 | 5·3 | 6 | 0·7 | 40 | 32 | −8 |
| 8 | 5 | 6 | 4 | −2* | 4 | 6 | 2* | 32 | 18 | −14* |
Note: *Values exceeding minimal clinical important difference.
Discussion
Manual therapy has been shown to be an effective treatment for individuals with LBP60,66 and hip osteoarthritis.67,68 The exact mechanism behind the effectiveness of manual therapy has not been clearly elucidated.69 The current evidence on the mechanisms suggests the mechanical stimulus of manual therapy may elicit a series of neurophysiological responses involving complex interactions between the peripheral and central nervous systems.69
Joint-based manual therapy techniques may exert their effects on both the peripheral and central nervous systems. In the peripheral nervous system, manual therapy has been shown to alter biochemical markers including pro-inflammatory and immunoregulatory cytokines.70–72
There may be effects of manual therapy in the central nervous system including the spinal cord and supraspinal structures. At the level of the spinal cord, it has been postulated that joint-based manual therapy increases signal activity from the muscle proprioceptors.73 Additionally, hypoalgesia and changes in muscle activity following manipulative techniques have been observed.74–76 George et al.75 performed a lumbar spinal thrust manipulation and observed changes in local hypoalgesia. Fernandez-Carneo et al.74 and Vicenzino et al.76 performed cervical spine thrust manipulation and observed hypoalgesia at the lateral elbow. These two studies indicate that hypoalgesia is possible at a site remote to the manual therapy procedure. At the level of the supraspinal structures, it is theorized that there is facilitation or inhibition of varying signals involved in the regulation of pain.69 Additionally, joint-based manual therapy may have an effect on psychological outcomes. Williams and colleagues77 concluded that there were improvements in psychological outcomes following spinal manipulation compared to verbal interventions or other physical interventions.
In the present study, a neurophysiological mechanism may explain, in part, the clinical outcomes in some of our subjects. Over 60% of the patients in this case series reported at least moderate perceived recovery at the end of the study, which may have been modulated by some or all of the components of the previously described neurophysiological mechanism. The relative contribution of peripheral, spinal cord, and supraspinal structures in this study is unknown. The manual therapy package was applied to all patients to potentially ‘activate’ this mechanism. The home exercise program was designed to augment the manual therapy techniques in the hopes that any mechanical or neurophysiological changes would be maintained. It may be that the manual therapy and exercise actually had a therapeutic effect on the lumbar spine.
The clinical decision-making process in patients with CLBP can be challenging.49 Chronic LBP is a complex condition involving both physical and psychosocial variables, and it is often impossible to identify a pathoanatomical source of symptoms.78 Currently, there is no consensus on the most appropriate management strategies for these patients. Several conservative interventions have been discussed in the literature, including exercise programs,25,79,80 cognitive behavioral therapy,81 education,79 and spinal manipulation;79,82 however, no single treatment has been shown to be superior.
While no causal relationship can be drawn from our results, this study may assist clinicians in selecting an initial intervention strategy for some patients with chronic LBP with hip ROM impairments. This may be a potential treatment option for patients that are unable to tolerate interventions directed at the lumbar spine initially. When interpreting these results, caution is recommended. There are several limitations with the present study including lack of blinding, no control group, small sample size, and a short-term follow-up. Future research should include well-designed, randomized clinical trials to evaluate the effectiveness of treating hip impairments in patients with chronic LBP. Additionally, future studies should include a longer-term follow-up following this treatment approach.
Conclusion
There are several studies linking LBP with impairments in hip ROM and neuromuscular control; however, there is little clinical evidence investigating the influence or treatment of these impairments. In this study, an impairment-based regional interdependence approach was implemented including manual therapy and exercise directed at impairments at the hip joint. The results suggest that treatment of identified hip impairments could be a viable initial treatment option for patients with a primary report of CLBP. In this case series, 62·5% (five out of eight) of patients with CLBP treated with manual therapy and exercise targeting the hip joints reported their perceived recovery as ‘moderately better’ and experienced a decrease in disability following these interventions. While the exact mechanism behind this treatment approach is not well understood, a neurophysiological mechanism may be involved. There are several limitations that limit the applicability of the results to larger population and future research should look to clarify the effectiveness and mechanisms behind this approach.
Acknowledgments
The authors would like to thank Edward Foresman, PT, DPT, for assisting with the treatment of patients in this case series. In addition, we would like to graciously thank Robert Mathewson, PT, for his support of our research endeavors. Additionally, we would like to thank Terry Cox, PT, DPT, for his contributions to initial development of the project.
References
- 1.Freburger JK, Holmes GM, Agans RP, Jackman AM, Darter JD, Wallace AS, et al. The rising prevalence of chronic low back pain. Arch Intern Med 2009;169:251–8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Guo HR, Tanaka S, Halperin WE, Cameron LL. Back pain prevalence in US industry and estimates of lost workdays. Am J Public Health 1999;89:1029–35 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Ricci JA, Stewart WF, Chee E, Leotta C, Foley K, Hochberg MC. Back pain exacerbations and lost productive time costs in United States workers. Spine 2006;31:3052–60 [DOI] [PubMed] [Google Scholar]
- 4.Deyo RA, Mirza SK, Martin BI. Back pain prevalence and visit rates: estimates from U. S. national surveys, 2002. Spine 2006;31:2724–7 [DOI] [PubMed] [Google Scholar]
- 5.Volinn E, Turczyn KM, Loeser JD. Theories of back pain and health care utilization. Neurosurg Clin N Am 1991;2:739–48 [PubMed] [Google Scholar]
- 6.Deyo RA, Gray DT, Kreuter W, Mirza S, Martin BI. United States trends in lumbar fusion surgery for degenerative conditions. Spine 2005;30:1441–5 [DOI] [PubMed] [Google Scholar]
- 7.Rydeard R, Leger A, Smith D. Pilates-based therapeutic exercise: effect on subjects with nonspecific chronic low back pain and functional disability: a randomized controlled trial. J Orthop Sports Phys Ther 2006;36:472–84 [DOI] [PubMed] [Google Scholar]
- 8.Fogel GR, Esses SI. Hip spine syndrome: management of coexisting radiculopathy and arthritis of the lower extremity. Spine 2003;3:238–41 [DOI] [PubMed] [Google Scholar]
- 9.Offierski CM, MacNab I. Hip-spine syndrome. Spine 1983;8:316–21 [DOI] [PubMed] [Google Scholar]
- 10.Croft PR, Macfarlane GJ, Papageorgiou AC, Thomas E, Silman AJ. Outcome of low back pain in general practice: a prospective study. BMJ 1998;316:1356–9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Skargren EI, Oberg BE. Predictive factors for 1-year outcome of low-back and neck pain in patients treated in primary care: comparison between the treatment strategies chiropractic and physiotherapy. Pain 1998;77:201–7 [DOI] [PubMed] [Google Scholar]
- 12.Carrino JA, Morrison WB, Parker L, Schweitzer ME, Levin DC, Sunshine JH. Spinal injection procedures: volume, provider distribution, and reimbursement in the U. S. medicare population from 1993 to 1999. Radiology 2002;225:723–9 [DOI] [PubMed] [Google Scholar]
- 13.Friedly J, Chan L, Deyo R. Geographic variation in epidural steroid injection use in medicare patients. J Bone Joint Surg Am 2008;90:1730–7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Weiner BK. Spine update: the biopsychosocial model and spine care. Spine 2008;33:219–23 [DOI] [PubMed] [Google Scholar]
- 15.Deyo RA, Mirza SK. Trends and variations in the use of spine surgery. Clin Orthop Relat Res 2006;443:139–46 [DOI] [PubMed] [Google Scholar]
- 16.Gray DT, Deyo RA, Kreuter W, Mirza SK, Heagerty PJ, Comstock BA, et al. Population-based trends in volumes and rates of ambulatory lumbar spine surgery. Spine 2006;31:1957–63 [DOI] [PubMed] [Google Scholar]
- 17.Luo X, Pietrobon R, Hey L. Patterns and trends in opioid use among individuals with back pain in the United States. Spine 2004;29:884–90 [DOI] [PubMed] [Google Scholar]
- 18.Tacci JA, Webster BS, Hashemi L, Christiani DC. Clinical practices in the management of new-onset, uncomplicated, low back workers’ compensation disability claims. J Occup Environ Med 1999;41:397–404 [DOI] [PubMed] [Google Scholar]
- 19.IJzelenberg W, Burdorf A. Patterns of care for low back pain in a working population. Spine 2004;29:1362–8 [DOI] [PubMed] [Google Scholar]
- 20.Molano SM, Burdorf A, Elders LA. Factors associated with medical care-seeking due to low-back pain in scaffolders. Am J Ind Med 2001;40:275–81 [DOI] [PubMed] [Google Scholar]
- 21.Mortimer M, Ahlberg G, MUSIC-Norrtalje Study Group To seek or not to seek? Care-seeking behaviour among people with low-back pain. Scand J Public Health 2003;31:194–203 [DOI] [PubMed] [Google Scholar]
- 22.Carey TS, Evans A, Hadler N, Kalsbeek W, McLaughlin C, Fryer J. Care-seeking among individuals with chronic low back pain. Spine 1995;20:312–7 [DOI] [PubMed] [Google Scholar]
- 23.Carey TS, Evans AT, Hadler NM, Lieberman G, Kalsbeek WD, Jackman AM, et al. Acute severe low back pain. A population-based study of prevalence and care-seeking. Spine 1996;21:339–44 [DOI] [PubMed] [Google Scholar]
- 24.von Korff M, Lin EH, Fenton JJ, Saunders K. Frequency and priority of pain patients’ health care use. Clin J Pain 2007;23:400–8 [DOI] [PubMed] [Google Scholar]
- 25.Kernan T, Rainville J. Observed outcomes associated with a quota-based exercise approach on measures of kinesiophobia in patients with chronic low back pain. J Orthop Sports Phys Ther 2007;37:679–87 [DOI] [PubMed] [Google Scholar]
- 26.Moseley GL. Evidence for a direct relationship between cognitive and physical change during an education intervention in people with chronic low back pain. Eur J Pain 2004;8:39–45 [DOI] [PubMed] [Google Scholar]
- 27.Guzman J, Esmail R, Karjalainen K, Malmivaara A, Irvin E, Bombardier C. Multidisciplinary bio-psycho-social rehabilitation for chronic low back pain. Cochrane Database Syst Rev 2002;1:CD000963. [DOI] [PubMed] [Google Scholar]
- 28.Wainner RS, Whitman JM, Cleland JA, Flynn TW. Regional interdependence: a musculoskeletal examination model whose time has come. J Orthop Sports Phys Ther 2007;37:658–60 [DOI] [PubMed] [Google Scholar]
- 29.Reiman MP, Weisbach PC, Glynn PE. The hips influence on low back pain: a distal link to a proximal problem. J Sport Rehabil 2009;18:24–32 [DOI] [PubMed] [Google Scholar]
- 30.Scholtes SA, Gombatto SP, van Dillen LR. Differences in lumbopelvic motion between people with and people without low back pain during two lower limb movement tests. Clin Biomech 2009;24:7–12 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Chesworth BM, Padfield BJ, Helewa A, Stitt LW. A comparison of hip mobiility in patients with low back pain and matched healthy subjects. Physiother Can 1994;46:267–74 [Google Scholar]
- 32.Murray E, Birley E, Twycross-Lewis R, Morrissey D. The relationship between hip rotation range of movement and low back pain prevalence in amateur golfers: an observational study. Phys Ther Sport 2009;10:131–5 [DOI] [PubMed] [Google Scholar]
- 33.Sjolie AN. Low-back pain in adolescents is associated with poor hip mobility and high body mass index. Scand J Med Sci Sports 2004;14:168–75 [DOI] [PubMed] [Google Scholar]
- 34.Vad VB, Bhat AL, Basrai D, Gebeh A, Aspergren DD, Andrews JR. Low back pain in professional golfers: the role of associated hip and low back range-of-motion deficits. Am J Sports Med 2004;32:494–7 [DOI] [PubMed] [Google Scholar]
- 35.Vad VB, Gebeh A, Dines D, Altchek D, Norris B. Hip and shoulder internal rotation range of motion deficits in professional tennis players. J Sci Med Sport 2003;6:71–5 [DOI] [PubMed] [Google Scholar]
- 36.Mellin G. Correlations of hip mobility with degree of back pain and lumbar spinal mobility in chronic low-back pain patients. Spine 1988;13:668–70 [PubMed] [Google Scholar]
- 37.van Dillen LR, Bloom NJ, Gombatto SP, Susco TM. Hip rotation range of motion in people with and without low back pain who participate in rotation-related sports. Phys Ther Sport 2008;9:72–81 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Weiner DK, Sakamoto S, Perera S, Breuer P. Chronic low back pain in older adults: prevalence, reliability, and validity of physical examination findings. J Am Geriatr Soc 2006;54:11–20 [DOI] [PubMed] [Google Scholar]
- 39.van Dillen LR, Gombatto SP, Collins DR, Engsberg JR, Sahrmann SA. Symmetry of timing of hip and lumbopelvic rotation motion in 2 different subgroups of people with low back pain. Arch Phys Med Rehabil 2007;88:351–60 [DOI] [PubMed] [Google Scholar]
- 40.Sembrano JN, Polly DW., Jr How often is low back pain not coming from the back? Spine 2009;34:E27–32 [DOI] [PubMed] [Google Scholar]
- 41.Stupar M, Cote P, French MR, Hawker GA. The association between low back pain and osteoarthritis of the hip and knee: a population-based cohort study. J Manipulative Physiol Ther 2010;33:349–54 [DOI] [PubMed] [Google Scholar]
- 42.Hassett G, Hart DJ, Manek NJ, Doyle DV, Spector TD. Risk factors for progression of lumbar spine disc degeneration: the Chingford Study. Arthritis Rheum 2003;48:3112–7 [DOI] [PubMed] [Google Scholar]
- 43.Wolfe F. Determinants of WOMAC function, pain and stiffness scores: evidence for the role of low back pain, symptom counts, fatigue and depression in osteoarthritis, rheumatoid arthritis and fibromyalgia. Rheumatology 1999;38:355–61 [DOI] [PubMed] [Google Scholar]
- 44.Kirkos JM, Papavasiliou KA, Kyrkos MJ, Sayegh FE, Kapetanos GA. The long-term effects of hip fusion on the adjacent joints. Acta Orthop Belg 2008;74:779–87 [PubMed] [Google Scholar]
- 45.Wijnhoven HA, de Vet HC, Picavet HS. Explaining sex differences in chronic musculoskeletal pain in a general population. Pain 2006;124:158–66 [DOI] [PubMed] [Google Scholar]
- 46.Parvizi J, Pour AE, Hillibrand A, Goldberg G, Sharkey PF, Rothman RH. Back pain and total hip arthroplasty: a prospective natural history study. Clin Orthop Relat Res 2010;468:1325–30 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Ben-Galim P, Ben-Galim T, Rand N, Haim A, Hipp J, Dekel S, et al. Hip–spine syndrome: the effect of total hip replacement surgery on low back pain in severe osteoarthritis of the hip. Spine 2007;32:2099–2102 [DOI] [PubMed] [Google Scholar]
- 48.Brown MD, Gomez-Marin O, Brookfield KF, Li PS. Differential diagnosis of hip disease versus spine disease. Clin Orthop Relat Res 2004;419:280–4 [DOI] [PubMed] [Google Scholar]
- 49.Burns SA, Mintken PE, Austin GP. Clinical decision making in a patient with secondary hip-spine syndrome. Physiother Theory Pract 2010; [Epub ahead of print]. [DOI] [PubMed] [Google Scholar]
- 50.Grimshaw PN, Burden AM. Case report: reduction of low back pain in a professional golfer. Med Sci Sports Exerc 2000;32:1667–73 [DOI] [PubMed] [Google Scholar]
- 51.Di Lorenzo L, Forte A, Formisano R, Gimigliano R, Gatto S. Low back pain after unstable extracapsular hip fractures: randomized control trial on a specific training. Eura Medicophys 2007;43:349–57 [PubMed] [Google Scholar]
- 52.Fritz JM, Irrgang JJ. A comparison of a modified Oswestry Low Back Pain Disability Questionnaire and the Quebec Back Pain Disability Scale. Phys Ther 2001;81:776–88 [DOI] [PubMed] [Google Scholar]
- 53.Frost H, Lamb SE, Stewart-Brown S. Responsiveness of a patient specific outcome measure compared with the Oswestry Disability Index v2.1 and Roland and Morris Disability Questionnaire for patients with subacute and chronic low back pain. Spine 2008;33:2450–7 [DOI] [PubMed] [Google Scholar]
- 54.Campbell H, Rivero-Arias O, Johnston K, Gray A, Fairbank J, Frost H, et al. Responsiveness of objective, disease-specific, and generic outcome measures in patients with chronic low back pain: an assessment for improving, stable, and deteriorating patients. Spine 2006;31:815–22 [DOI] [PubMed] [Google Scholar]
- 55.Cleland J, Netter F. Orthopaedic clinical examination: an evidence based approach for physical therapists. Philadelphia, PA: Elsevier Health Sciences; 2005 [Google Scholar]
- 56.Grotle M, Brox JI, Vollestad NK. Reliability, validity and responsiveness of the fear-avoidance beliefs questionnaire: methodological aspects of the Norwegian version. J Rehabil Med 2006;38:346–53 [DOI] [PubMed] [Google Scholar]
- 57.Chatman AB, Hyams SP, Neel JM, Binkley JM, Stratford PW, Schomberg A, et al. The patient-specific functional scale: measurement properties in patients with knee dysfunction. Phys Ther 1997;77:820–9 [DOI] [PubMed] [Google Scholar]
- 58.Westaway MD, Stratford PW, Binkley JM. The patient-specific functional scale: validation of its use in persons with neck dysfunction. J Orthop Sports Phys Ther 1998;27:331–8 [DOI] [PubMed] [Google Scholar]
- 59.Maughan EF, Lewis JS. Outcome measures in chronic low back pain. Eur Spine J 2010;19:1484–94 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Childs JD, Piva SR, Fritz JM. Responsiveness of the numeric pain rating scale in patients with low back pain. Spine 2005;30:1331–4 [DOI] [PubMed] [Google Scholar]
- 61.Cleland JA, Childs JD, Whitman JM. Psychometric properties of the Neck Disability Index and Numeric Pain Rating Scale in patients with mechanical neck pain. Arch Phys Med Rehabil 2008;89:69–74 [DOI] [PubMed] [Google Scholar]
- 62.Kendall F, McCreary E, Provance P. Muscles: testing and function. Baltimore, MD: Williams and Wilkins; 1993 [Google Scholar]
- 63.Jaeschke R, Singer J, Guyatt GH. Measurement of health status. Ascertaining the minimal clinically important difference. Control Clin Trials 1989;10:407–15 [DOI] [PubMed] [Google Scholar]
- 64.Maitland G. Vertebral manipulation. Sydney, NSW: Butterworth; 1986 [Google Scholar]
- 65.Jack K, McLean SM, Moffett JK, Gardiner E. Barriers to treatment adherence in physiotherapy outpatient clinics: a systematic review. Man Ther 2010;15:220–8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Childs JD, Fritz JM, Flynn TW, Irrgang JJ, Johnson KK, Majkowski GR, et al. A clinical prediction rule to identify patients with low back pain most likely to benefit from spinal manipulation: a validation study. Ann Intern Med 2004;141:920–8 [DOI] [PubMed] [Google Scholar]
- 67.MacDonald CW, Whitman JM, Cleland JA, Smith M, Hoeksma HL. Clinical outcomes following manual physical therapy and exercise for hip osteoarthritis: a case series. J Orthop Sports Phys Ther 2006;36:588–99 [DOI] [PubMed] [Google Scholar]
- 68.Hoeksma HL, Dekker J, Ronday HK, Heering A, van der Lubbe N, Vel C, et al. Comparison of manual therapy and exercise therapy in osteoarthritis of the hip: a randomized clinical trial. Arthritis Rheum 2004;51:722–9 [DOI] [PubMed] [Google Scholar]
- 69.Bialosky JE, Bishop MD, Price DD, Robinson ME, George SZ. The mechanisms of manual therapy in the treatment of musculoskeletal pain: a comprehensive model. Man Ther 2009;14:531–8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Teodorczyk-Injeyan JA, McGregor M, Ruegg R, Injeyan HS. Interleukin 2-regulated in vitro antibody production following a single spinal manipulative treatment in normal subjects. Chiropr Osteopat 2010;18:26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Teodorczyk-Injeyan JA, Injeyan HS, Ruegg R. Spinal manipulative therapy reduces inflammatory cytokines but not substance P production in normal subjects. J Manipulative Physiol Ther 2006;29:14–21 [DOI] [PubMed] [Google Scholar]
- 72.Teodorczyk-Injeyan JA, Injeyan HS, McGregor M, Harris GM, Ruegg R. Enhancement of in vitro interleukin-2 production in normal subjects following a single spinal manipulative treatment. Chiropr Osteopat 2008;16:5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Pickar JG, Wheeler JD. Response of muscle proprioceptors to spinal manipulative-like loads in the anesthetized cat. J Manipulative Physiol Ther 2001;24:2–11 [DOI] [PubMed] [Google Scholar]
- 74.Fernandez-Carnero J, Fernandez-de-las-Penas C, Cleland JA. Immediate hypoalgesic and motor effects after a single cervical spine manipulation in subjects with lateral epicondylalgia. J Manipulative Physiol Ther 2008;31:675–81 [DOI] [PubMed] [Google Scholar]
- 75.George SZ, Bishop MD, Bialosky JE, Zeppieri G, Jr, Robinson ME. Immediate effects of spinal manipulation on thermal pain sensitivity: an experimental study. BMC Musculoskelet Disord 2006;7:68. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Vicenzino B, Collins D, Wright A. The initial effects of a cervical spine manipulative physiotherapy treatment on the pain and dysfunction of lateral epicondylalgia. Pain 1996;68:69–74 [DOI] [PubMed] [Google Scholar]
- 77.Williams NH, Hendry M, Lewis R, Russell I, Westmoreland A, Wilkinson C. Psychological response in spinal manipulation (PRISM): a systematic review of psychological outcomes in randomised controlled trials. Complement Ther Med 2007;15:271–83 [DOI] [PubMed] [Google Scholar]
- 78.Laslett M, McDonald B, Tropp H, Aprill CN, Oberg B. Agreement between diagnoses reached by clinical examination and available reference standards: a prospective study of 216 patients with lumbopelvic pain. BMC Musculoskelet Disord 2005;6:28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Dagenais S, Tricco AC, Haldeman S. Synthesis of recommendations for the assessment and management of low back pain from recent clinical practice guidelines. Spine 2010;10:514–29 [DOI] [PubMed] [Google Scholar]
- 80.van Middelkoop M, Rubinstein SM, Kuijpers T, Verhagen AP, Ostelo R, Koes BW, et al. A systematic review on the effectiveness of physical and rehabilitation interventions for chronic non-specific low back pain. Eur Spine J 2010;20:19–39 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Henschke N, Ostelo RW, van Tulder MW, Vlaeyen JW, Morley S, Assendelft WJ, et al. Behavioural treatment for chronic low-back pain. Cochrane Database Syst Rev 2010;7:CD002014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Cecchi F, Molino-Lova R, Chiti M, Pasquini G, Paperini A, Conti AA, et al. Spinal manipulation compared with back school and with individually delivered physiotherapy for the treatment of chronic low back pain: a randomized trial with one-year follow-up. Clin Rehabil 2010;24:26–36 [DOI] [PubMed] [Google Scholar]


