Abstract
Objective
To evaluate the effects of neurodynamic mobilization on the fluid dynamics of the tibial nerve in cadavers.
Background
Evidence showing patients benefit from neural mobilization is limited. Mechanisms responsible for changes in patient symptoms are unclear.
Methods
Bilateral lower limbs of six unembalmed cadavers (n = 12) were randomized into matched pairs and dissected to expose the tibial nerve proximal to the ankle. Dye composed of Toulidine blue and plasma was injected into the nerve. The longitudinal dye spread was measured pre- and post-mobilization. The experimental group received the intervention consisting of 30 repetitions of passive ankle range of motion over the course of 1 minute. The matched control limb received no mobilization. Data were analysed using a 2×2 repeated measures ANOVA with subsequent t-tests for pairwise comparisons.
Results
Mean dye spread was 23.8±10.2 mm, a change of 5.4±4.7% in the experimental limb as compared to 20.7±6.0 mm, a change of −1.5±3.9% in the control limb. The ANOVA was significant (P⩽0.02) for interaction between group (experimental/control) and time (pre-mobilization/post-mobilization). t-test results were significant between pre- and post-mobilization of the experimental leg (P = 0.01), and between control and experimental limbs post-mobilization (P⩽0.02).
Conclusion
Passive neural mobilization induces dispersion of intraneural fluid. This may be clinically significant in the presence of intraneural edema found in pathological nerves such as those found in compression syndromes.
Keywords: Cadaver, Compression, Entrapment, Nerve, Neurodynamic, Neuropathy, Tibial
Introduction
Clinicians incorporate neurodynamic techniques for assessment and treatment of neuromuscular disorders. These disorders include compression syndromes or other neuromuscular conditions that may be accompanied by neuropathic pain.1–5 Damaged nerves exhibit predictable pathophysiological responses including impaired nerve mobility,6–9 increased mechanosensitivity,8,10 impaired nerve conduction,11–13 nerve tissue ischemia,14,15 axonal transport inhibition,16–22 and intraneural edema.15,23–28 Impaired nerve mobility and increased mechanosensitivity provide the basis for existing studies of neurodynamic techniques. These studies examined neurodynamic techniques with respect to quantification of strain29 and excursion,30–37 as well as symptom reproduction.8,34
Impaired nerve mobility and mechanosensitivity can be clinically assessed by measuring changes in joint range of motion,38,39 pain reproduction,4,8,10,40–42 or change of symptoms following neurodynamic mobilization.43 Some authors reported an improvement of symptoms43 and increased mobility39 when using neural mobilization as part of the treatment regime, while others found no significant improvement in pain or mobility following neural mobilization.44 In a review of neural mobilization studies, Ellis and Hing found limited evidence to support the use of this modality.45 To our knowledge, there is no published study to date that investigates neurodynamic techniques with respect to nerve function or other pathophysiological responses, such as ischemia, impaired axonal transport, or intraneural edema.
Intraneural edema is a common response to nerve injury,23,25–27 and is intimately involved in the proliferation of damage to nerve structure and function.25,46 Edema is found in peripheral nerves that have been subject to trauma such as compression,13,23,24,47 excessive tension events,14,20,48 or vibration.21,26 Even mild injury may result in epineurial edema,23 but compression that is prolonged or of great magnitude leads to a breach of the diffusion barriers created by both the perineurium and microvasculature, resulting in endoneurial edema.23 The absence of lymphatic vessels in the endoneurium limits drainage of this edema,47 thereby creating a ‘mini-compartment syndrome’ within the nerve.25 This ‘mini-compartment syndrome’, due to the increase in endoneurial pressure, subsequently leads to fibrosis and adhesions, impairing intrafascicular gliding. This loss of intrafascicular gliding creates an internal stretch lesion (Fig. 1).14,46,47,49 Fibrosis may be accompanied by nerve enlargement, resulting in additional compression due to intraneural thickening,50 as well as increased extraneural compression from adjacent structures.51,52 The effects of this enlargement will be more pronounced when the nerve traverses a small space such as an osteofibrous tunnel that is often the site of a compression syndrome.51,52
Figure 1.
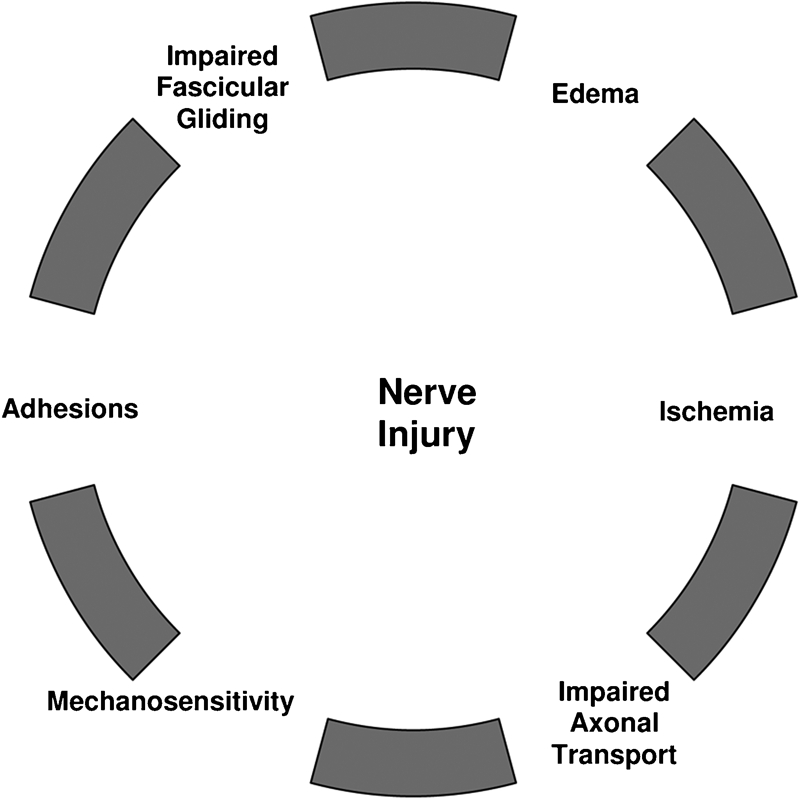
Pathophysiological responses due to nerve injury. Edema propagates the cycle.
Neurodynamic mobilization techniques are often used clinically for restoring nerve mobility39 and decreasing pain.43,53,54 In the lower extremity, neurodynamic techniques incorporate ankle range of motion for neurodynamic testing2,4,29,40,41,55,56 and treatment.57 Moreover, repetitive ankle motion (e.g. ankle dorsiflexion and plantar flexion) is often used clinically to assist in fluid dispersion in the presence of generalized ankle swelling, as well as increase ankle joint mobility regardless of effects on nerve mobility. Despite anecdotal reports of positive treatment outcomes when using neurodynamic techniques,5,43,53,54,57 the mechanisms responsible for these outcomes remain unclear. The reduction of neural edema has been cited as a possible benefit to neurodynamic mobilization techniques43 but has not been demonstrated.
The purpose of this study was to examine the effects of neurodynamic mobilization on simulated intraneural edema in the tibial nerve at the ankle in cadavers. The current investigators hypothesized that neurodynamic mobilization performed by passive ankle dorsiflexion and plantar flexion would induce dispersion in the tibial nerve at the ankle. Demonstrating that intraneural fluid can be dispersed by means of ankle range of motion may help identify one mechanism responsible for the benefits of neurodynamic mobilization.
Materials and Methods
Specimens
Seven unembalmed cadavers (three females and four males) with a mean age of 72±10.4 years, mean height of 166±12.8 cm, and mean weight of 66±15.5 kg (Table 1), were used for this pre-test–post-test matched control study. By random selection within each cadaver, one limb was designated as the experimental limb and the other limb was designated as a control limb. Data were not collected on one control limb due to loss of power in the digital caliper. Therefore, a total of six matched limb pairs (n = 12) were used for analysis.
Table 1. Cadaver demographics.
| Cadaver no. | Age | Sex | Height (cm) | Weight (kg) | Cause of death |
| 1 | 80 | F | 149.8 | 43.2 | Pneumonia |
| 2 | 52 | F | 162.5 | 72.7 | Metastatic carcinoma of anus, systemic HTN |
| 3 | 74 | M | 175.2 | 68.2 | Protein calorie malnutrition, prostate CA, RA, CAD, old CVA |
| 4 | 71 | F | 154.9 | 50 | CAD, HTN, COPD |
| 5 | 74 | M | 182.8 | 81.8 | CVA |
| 6 | 80 | M | 172.7 | 77.3 | COPD |
| Mean | 72.0 | 166.0 | 66.0 | ||
| SD | 10.36 | 12.72 | 15.50 |
Note: CA = cancer; RA = rheumatoid arthritis; CAD = coronary artery syndrome; CVA = cerebral vascular disorder; HTN = hypertension; COPD = chronic obstructive pulmonary disorder.
Position
Before stabilizing limb position, all ankles underwent repetitive ankle dorsiflexion and plantar flexion in order to break the mild but present rigor mortis. Cadaver position was standardized in supine with limbs externally rotated at the hip to 25° and abducted to 10°. The amount of hip rotation was determined by the natural leg position, available range of motion, and ability to access the tibial nerve at the ankle during measurement trials. Wooden blocks and stabilization straps were used to stabilize the position of the limbs. The straps were placed in such a manner as to avoid compression on any part of the nerve and to stabilize without interfering with movement of the ankle.
Dissection
With the exception of the dissection window, the cadavers remained intact so as not to disturb the nerve bed proximal or distal to the ankle. A dissection window (10×4 cm) was incised just proximal to the distal tip of the medial malleolus and the resulting skin flap was retracted posteriorly. The subcutaneous tissue was excised proximal to the flexor retinaculum and retracted posteriorly along with the skin flap. The deep fascia was excised to expose the neurovascular bundle comprised of the tibial nerve, posterior tibial artery, and posterior tibial vein. The neurovascular bundle was separated by blunt dissection in order to free the tibial nerve and allow visualization of the length of the nerve within the dissection window. The vascular components were retracted and with ‘T-pins’ fixated posteriorly unless the anatomical arrangement required an anterior fixation to allow a better view of the tibial nerve. In order to maintain visualization of the initial injection site, the site was marked with a coloured marker and a ‘T-pin’ was inserted in the intact tissue anterior to the injection site as an additional reference point (Fig. 2).
Figure 2.
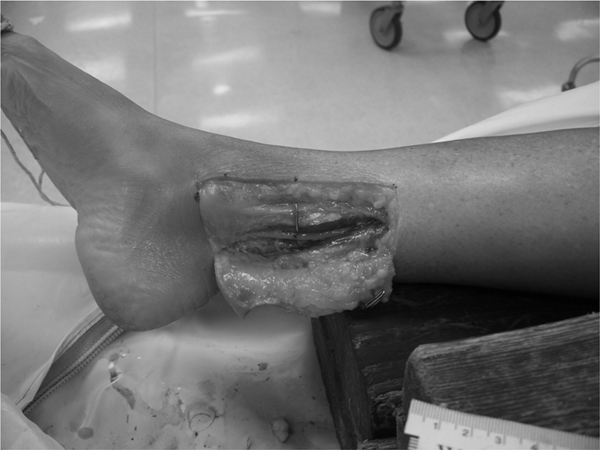
Before dye injection, location of window for dissection was identified and marked starting just proximal to the distal tip of the medial malleolus. The skin flap was incised and the neurovascular bundle was separated in order to free the tibial nerve from all adjacent tissue. This was necessary in order to visualize and measure the nerve. The injection site was marked with a coloured marker as well as a T-pin.
Dye injection procedure
The dye mixture was composed of plasma and Toluidine blue used in a previous investigation.58 The dye injection procedure was piloted using embalmed cadavers and the amount of dye injected was determined by the amount needed to reach the threshold of leakage through the injection site. Using a syringe and a 0.45×23 mm needle, the predetermined amount of dye (0.2 cc), was injected with steady, mild pressure just beneath the epineurium59 of the tibial nerve, 5 cm proximal to the distal edge of the medial malleolus (Fig. 3). This bolus of fluid created an initial dye spread, which was measured proximally and distally by means of a digital caliper (Wiha Digimax 6′ precision digital caliper; Wihatools.com LLC, Monticello, MN, USA). The point of measurement was determined by the most distinct border. Indistinct, barely visible borders were not considered to be reliable sites for measurements (Fig. 4).
Figure 3.
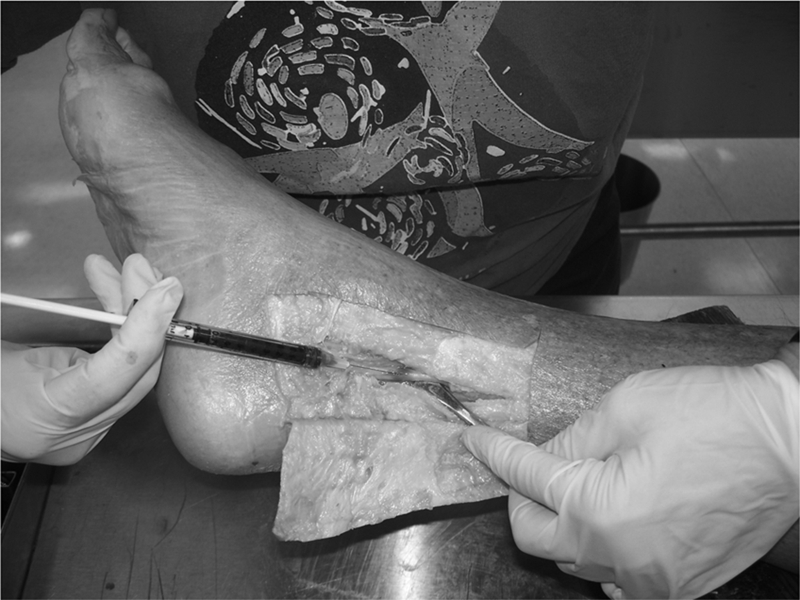
Dye injection (pilot study). The injection amount and method were piloted on embalmed cadavers. The amount of dye needed was determined by the ability to create a bolus without leaking from the nerve. The needle was injected just beneath the external epineurium.
Figure 4.
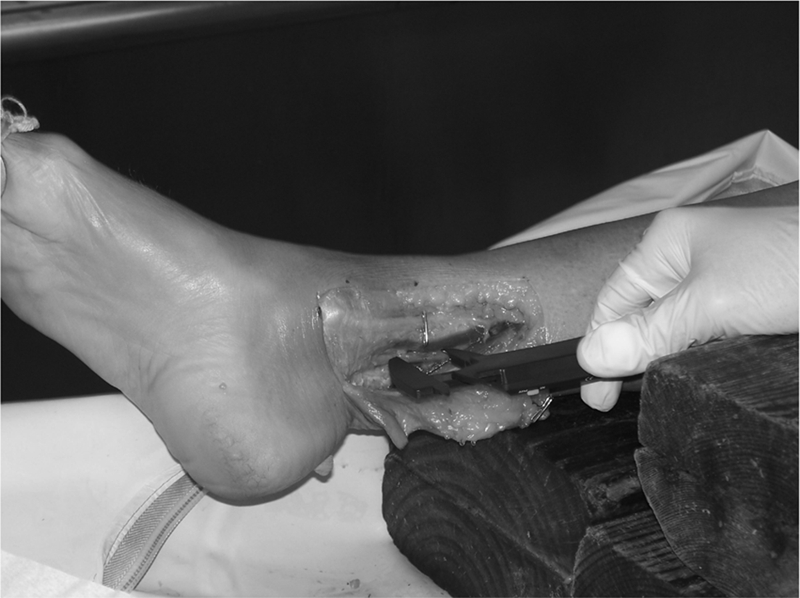
Caliper measurements. The measurements were made by one investigator who was blinded to the digital reading. The average of three successive measurements at each 2-minute interval was recorded.
Measurements
Once the initial dye spread was established, longitudinal dye spread was measured at 2-minute intervals until stabilized. In order to reduce measurement errors, dye spread measurements were taken three times and averaged. Stabilization was determined by successive 2-minute interval dye spread measurements that were within 0.5 mm of each other. Once the dye spread stabilized, the neurodynamic mobilization intervention was performed.
Intervention
Repeated passive ankle dorsiflexion and plantar flexion to end range were used as the neurodynamic mobilization intervention. This motion is a component of treatment for lower extremity nerve pathology.40,53 According to Butler,40 the full range of motion corresponds to a grade three mobilization as described by Maitland. The intervention for this study consisted of 1 minute of passive ankle dorsiflexion/plantar flexion repetitions performed rhythmically at a rate of 30 repetitions per minute. The rhythm was maintained at a constant rate by means of a software metronome (TempoPerfect v1.02). This rate of movement was established to create a clinically applicable intervention parameter and maximize performance uniformity (Fig. 5).
Figure 5.
Neural mobilization was performed through 30 repetitions of ankle dorsiflexion/plantar flexion to end range.
Statistical analysis
Reliability
Intrarater reliability of caliper measurements was assessed with data collected from four repeated measurements of both limbs from two embalmed cadavers taken during two separate trials. These measurements were taken at 2-minute intervals, similar to the same time interval used for the final data collection. As in the final data collection, the investigator taking the measurements was blinded to the results of the caliper measurements. Data were analysed by means of intraclass correlation coefficient (ICC).
Descriptive data
The range, mean, standard deviation, and confidence intervals were determined for the length of the dye spread from the time of injection, at each 2-minute interval and post-mobilization. The same descriptive information was determined for the percent change in dye following intervention, total change in dye length, and total range of motion at the ankle.
Inferential statistics
Statistical analysis was performed using SPSS (v17.0). The independent variables were group (control versus experimental limbs) and time (pre-mobilization versus post-mobilization). The dependent variable was the amount of longitudinal dye dispersion. Because ankle range of motion has clinical application, the total amount of range (from dorsiflexion to plantar flexion) was considered to be a possible influencing factor (covariate). Therefore, inferential analysis was performed with an ANCOVA using range of motion as a covariate in pre- and post-intervention in each group (i.e. control and experimental conditions). In the event that the results were not significant, that is, range of motion was not determined to be a covariate, inferential analysis was pre-planned to proceed using 2×2 repeated measures ANOVA. Any interaction was further analysed using t-tests for post hoc pairwise comparison. Since the pre-test data had been normalized to have a common baseline, there was no need to analyse the pre-control versus pre-experimental grouping. The pairwise comparisons were made between the following groups of limbs: (1) pre-control versus post-control; (2) post-control versus post-experimental; and (3) pre-experimental versus post-experimental.
Results
The results showed significant mobilization effects in that there was increased fluid dispersion within the tibial nerve after the intervention. This significance was found with respect to time/intervention (experimental limb, pre- versus post-mobilization) as well as across groups (experimental versus control limb) post-mobilization (Fig. 6).
Figure 6.
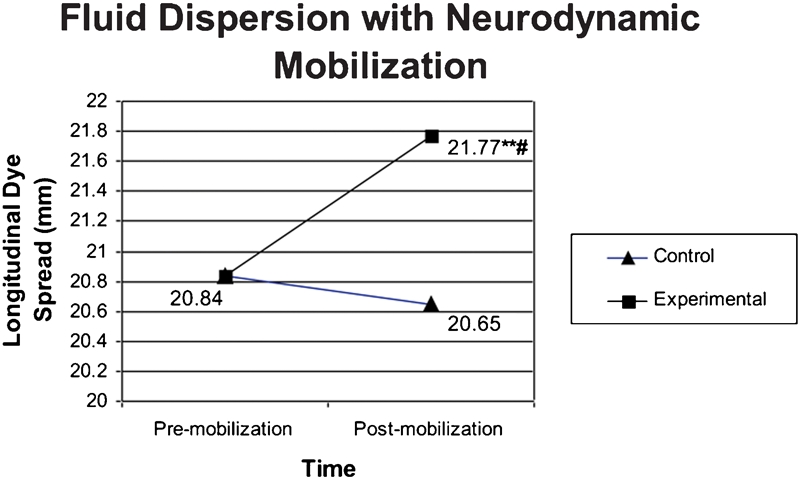
Mean fluid dispersion after mobilization. Statistical significance based on t-test results. At pre-test, the experimental group data were normalized to the control group data. **P⩽0.05, significantly different from pre-mobilization condition (P = 0.009); #P⩽0.05, significantly different from control group (P = 0.022).
Reliability
Intrarater reliability for caliper measurements of dye dispersion using ICC was found to be 0.987 with a 95% confidence interval of 0.949–0.997.
Descriptive statistics
Following the intervention in the experimental leg, the dye spread ranged from 13.8 to 37.0 mm with a mean±SD of 23.8±10.2 mm. In the absence of the intervention, but within the same time period, the measurements of the control leg ranged from 12.6 to 30.3 mm with a mean±SD of 20.7±6.0 mm. The mean total movement of dye was −0.2±0.7 mm in the control leg and 0.9±0.6 mm in the experimental leg (Table 2).
Table 2. Descriptive data.
| Control leg |
Experimental leg |
|||||
| Range | Mean±SD | 95% confidence intervals | Range | Mean±SD | 95% confidence intervals | |
| Initial dye spread (mm) | 6.6–29.5 | 17.1±8.5 | 10.3–23.9 | 10.1–33.6 | 20.9±9.6 | 13.2–28.6 |
| Dye stabilized (mm) | 13.8–29.7 | 20.8±5.6 | 15.0–26.7 | 12.1–36.2 | 22.9±10.5 | 11.9–33.8 |
| Dye post-mobilization (mm) | 12.6–30.3 | 20.7±6.0 | 14.4–26.9 | 13.8–37.0 | 23.8±10.2 | 13.1–34.5 |
| Time to stabilize (min.) | 10–22 | 14.0±4.4 | 10.5–17.5 | 10–16 | 13.0±2.1 | 11.3–14.7 |
| Total length change (mm) | (−)1.2–0.6 | (−)0.2±0.7 | (−)0.7–0.4 | 0.3–1.6 | 0.9±0.6 | 0.5–1.4 |
| Change post-mobilization (%) | (−)8.5–2.0 | (−)1.5±3.9 | (−)4.7–1.6 | 0.9–13.5 | 5.4±4.7 | 1.7±9.1 |
| Total arc of motion | 30–54° | 38.7±6.3 | 32.1–45.2 | 23°–54° | 41.3±10.7 | 30.1–52.6 |
Inferential statistics
Owing to the variability of initial dye spread, the data were normalized in order to establish a standardized baseline (Table 3). According to the ANCOVA results, range of motion was not found to be a covariate. Therefore, as pre-planned, the effects of the intervention (repeated passive ankle dorsiflexion–plantar flexion to end range) were analysed by means of a repeated measures 2×2 ANOVA. The 2×2 ANOVA was found to be significant at P⩽0.022 for interaction between groups (control and experimental) and time (pre- and post-mobilization). In order to clarify this interaction, subsequent post hoc pairwise analyses via t-tests were performed. No significance (P⩽0.539) was found with analysis of the pre- and post-control group. The t-test analysis for the experimental group, pre- and post-test mobilization was significant (P⩽0.009). Additionally, post-mobilization t-test comparing fluid dispersion between the experimental group and control group was found to be significant (P⩽0.022) (Table 4).
Table 3. Normalized data.
| Cadaver | Control pre-mobilization (mm) | Control post-mobilization (mm) | Transformed experimental pre-mobilization (mm) | Transformed experimental post-mobilization (mm) |
| 1 | 17.21 | 17.37 | 17.21 | 18.79 |
| 2 | 18.67 | 19.04 | 18.67 | 19.44 |
| 3 | 21.84 | 21.50 | 21.84 | 23.47 |
| 4 | 13.78 | 12.61 | 13.78 | 14.54 |
| 5 | 29.66 | 30.27 | 29.66 | 30.17 |
| 6 | 23.85 | 23.11 | 23.85 | 24.18 |
| Mean | 20.84 | 20.65 | 20.84 | 21.77 |
| SD | 5.58 | 5.96 | 5.58 | 5.40 |
Note: The pre-mobilization data were normalized by means of a constant determined by the difference between the stabilized (pre-mobilization) dye spread of the control and the experimental limbs. This established a standardized baseline. The constant was applied to the experimental limb in both pre- and post-mobilization measurements of dye spread. SD = standard deviation.
Table 4. Inferential statistics.
| Test | Independent variables | F-Statistic | df | Effect size (partial eta squared) | Power | Statistical results (P⩽0.05) |
| ANCOVA | Pre-control versus post-control | 3.590 | 1 | 0.435 | 0.308 | ⩽0.131 |
| ANCOVA | Pre-experimental versus post-experimental | 0.987 | 1 | 0.198 | 0.121 | ⩽.0.377 |
| 2×2 ANOVA (repeated measures) | Group† by time‡ interaction | 10.724 | 1 | 0.682 | 0.745 | ⩽0.022*** |
| t-test | Pre-control versus post-control | 0.435 | 1 | 0.08 | 0.084 | ⩽0.539 |
| t-test | Post-control versus post-experimental | 10.724 | 1 | 0.682 | 0.745 | ⩽0.022*** |
| t-test | Pre-experimental versus post-experimental | 17.259 | 1 | 0.775 | 0.908 | ⩽0.009*** |
Group = control limb/experimental limb
Time = pre-mobilization/post-mobilization
Significant at P⩽0.05.
Discussion
Previous investigative studies of neurodynamic mobilization have focused on quantitative findings with respect to nerve mechanics during limb movement.29,30,34,60–62 While these studies provide valuable information regarding peripheral nerve movement and strain, they provide no insight into the mechanisms responsible for the benefits of using neurodynamic mobilization. Furthermore, no studies were found that address the effects of neurodynamic mobilization on nerves exhibiting pathophysiological responses such as impaired nerve conduction, ischemia, inhibited axonal transport, or intraneural edema.
The current study examined the effects of neurodynamic mobilization on intraneural edema in the cadaveric tibial nerve. The authors’ hypothesis that neurodynamic mobilization would induce fluid dispersion in the cadaveric tibial nerve at the ankle was supported by statistically significant dye dispersion after the mobilization. The dye in the control limb receded in three limbs and spread minimally in the other three, resulting in a ‘negative’ mean dye dispersion. It was surmised that without mechanical influence, the dye pooled due to the influence of gravity, and/or rebounded due to the change in pressure gradient after the injection. On the other hand, there was significant dye dispersion after the mobilization in the experimental limb indicating that there was a treatment effect due to the intervention. Comparison between the experimental limb and the control limb also produced significant results supporting the use of neurodynamic mobilization to induce fluid dispersion in the tibial nerve at the ankle.
The use of cadavers eliminated confounding physiological effects, such as changes due to blood flow, axonal transport, or temperature, thus pointing to the purely mechanical effects of passive neural mobilization. Because the tibial nerve was dissected free of all adjacent tissue and eliminated any external interfaces, the response to the mobilization appeared to be due to intraneural mechanics.
The mechanisms responsible for the dye spread with mobilization may include fluid movement with intrafascicular gliding, and/or transverse contraction of the nerve as it lengthens.63 Transverse contraction of the nerve results in an increase in intrafascicular pressure due to changes in volume.63 During the mobilization technique, the tibial nerve alternately elongated and shortened which may have provided a temporary increase in intraneural pressure followed by a period of relaxation. Although speculative, it appears that this repetitive or ‘pumping’ action may have created a flushing of the dye and an alteration of the intraneural pressure as the intraneural fluid was dispersed.
The clinical importance of limiting intraneural pressure is supported by the results of studies that demonstrated the detrimental effects of increased levels of intraneural pressure on neural tissue.15,23 Maintaining healthy nerve function using neurodynamic techniques may occur by promoting uninterrupted axonal transport, thereby preventing deposition of mechanosensitivity elements, the presence of which results in pain and limited neural movement.8,22 In the early stages of stretch injury or compression, the ability to prevent or at least reduce edema may prevent or slow the inhibition of blood flow,15,23 thus preventing the sequelae leading to impaired axonal transport,18,20 demyelination,11,24 loss of elasticity due to fibrosis or adhesions,46,63 and ultimately to alteration in nerve structure and function.19,24,27 The ability to promote fluid dispersion using range of motion may provide a means to break the cycle of edema formation leading to fibrosis,50 which may in turn lead again to edema formation. These findings may be even more important when considering interventions for previously damaged nerves64,65,66,67 or nerves compromised by pathology such as diabetes.68
In the current study, the limbs were positioned in hip and knee extension and the ankles were moved from plantar flexion to dorsiflexion in a manner that is consistent with the generally accepted position for performance of passive ankle range of motion in the clinic. It was assumed that the amount of strain reached at dorsiflexion was well within the limits of strain normally tolerated by the nerve.69 Furthermore, the pressure created by normal elongation, which occurs during range of motion, was assumed to be well tolerated by the nerve.
The ability to mechanically induce fluid dispersion by means of passive neurodynamic mobilization is a substantial finding as it sheds light on the physiological benefits of this technique that could ultimately influence neurophysiological function. Clinical conditions resulting in loss of active motion may prohibit more active participation in mobilization. This is especially important when endoneurial edema is present because of the limited physiological flushing or fluid dispersion due to the absence of an endoneurial lymphatic system.
The effects that gliding or compression from extraneural interfaces along the intact neurovascular bundle have on fluid dispersion are still unknown. A combination of the intraneural mechanical effects described in this study, along with extraneural mechanical and physiological effects may provide further collective beneficial effects on fluid dispersion in the nerve. Future studies should give consideration to examining the combination of the intraneural mechanical effects combined with extraneural effects. The effect of passive versus active neurodynamic mobilization also warrants investigation.
In vivo studies would be helpful in assessing the benefits of neurodynamic mobilization in both normal subjects and in those presenting with pathology. In vivo studies using diagnostic ultrasound (DUS) demonstrate that this type of imaging is a valid tool for use in measuring longitudinal and transverse movement of nerve tissue during neural mobilization.7,9,32,62,70 In addition, DUS has the ability to detect pathological changes in nerve morphology52,71,72 and can be useful in visualizing the structures within an osteofibrous tunnel.51,73 The ability of DUS to detect morphological changes as well as nerve movement makes this an excellent tool for further exploring the direct effects of neurodynamic mobilization on nerve tissue mechanics. Finally, limb position is an important consideration in the clinical use of neurodynamic mobilization as there is a mutual relationship between joint position and the amount of strain and excursion.29,34,60,61 Therefore, additional studies investigating various limb positions on fluid dispersion are warranted.
Limitations of this current study include the inability to generalize the findings to live subjects due to active physiological processes, the inability to completely generalize the results to other peripheral nerves or nerve roots, and the inability to generalize the findings to nerves exhibiting a loss of elasticity due to fibrosis or adhesions,46,63 which may alter the manner in which fluid is dispersed.
Conclusion
Passive mobilization in the form of ankle dorsiflexion and plantar flexion to end range induces a significant increase in fluid dispersion in the tibial nerve of human cadaveric specimen. Neurodynamic mobilization may be beneficial in preserving nerve function by limiting intraneural fluid accumulation, thus preventing the adverse effects of intraneural edema.
Acknowledgments
The authors would like to thank Texas Tech University Health Sciences Center for use of the Cadaveric Anatomy Laboratory and the School of Allied Health Sciences for use of the Center for Rehabilitation Research, Clinical Anatomy Research Laboratory and for donation of cadaveric specimens.
References
- 1.Maitland GD. Negative disc exploration: positive canal signs. Aust J Physiother 1979;25:129–34 [DOI] [PubMed] [Google Scholar]
- 2.Maitland GD. The Slump Test: examination and treatment. Aust J Physiother 1985;1:215–9 [DOI] [PubMed] [Google Scholar]
- 3.Shacklock M. Neurodynamics. Physiotherapy 1995;81:9–16 [Google Scholar]
- 4.Butler D. The sensitive nervous system. Adelaide, SA: Noigroup; 2000 [Google Scholar]
- 5.Ekstrom RA, Holden K. Examination of and intervention for a patient with chronic lateral elbow pain with signs of nerve entrapment. Phys Ther 2002;82:1077–86 [PubMed] [Google Scholar]
- 6.Greening J, Smart S, Leary R, Hall-Craggs M, O’Higgins P, Lynn B. Reduced movement of median nerve in carpal tunnel during wrist flexion in patients with non-specific arm pain. Lancet 1999;354:217–8 [DOI] [PubMed] [Google Scholar]
- 7.Greening J, Lynn B, Leary R, Warren L, O’Higgins P, Hall-Craggs M. The use of ultrasound imaging to demonstrate reduced movement of the median nerve during wrist flexion in patients with non-specific arm pain. J Hand Surg Br 2001;26:401–6 [DOI] [PubMed] [Google Scholar]
- 8.Greening J, Dilley A, Lynn B. In vivo study of nerve movement and mechanosensitivity of the median nerve in whiplash and non-specific arm pain patients. Pain 2005;115:248–53 [DOI] [PubMed] [Google Scholar]
- 9.Hough AD, Moore AP, Jones MP. Reduced longitudinal excursion of the median nerve in carpal tunnel syndrome. Arch Phys Med Rehabil 2007;88:569–76 [DOI] [PubMed] [Google Scholar]
- 10.Dilley A, Lynn B, Pang SJ. Pressure and stretch mechanosensitivity of peripheral nerve fibres following local inflammation of the nerve trunk. Pain 2005;117:462–72 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Dahlin LB, Shyu BC, Danielsen N, Anderson SA. Effects of nerve compression or ischaemia on conduction properties of myelinated and non-myelinated nerve fibres. An experimental study in the rabbit common peroneal nerve. Acta Physiol Scand 1989;136:97–105 [DOI] [PubMed] [Google Scholar]
- 12.Wall EJ, Massie JB, Kwan MK, Rydevik BL, Myers RR, Garfin SR. Experimental stretch neuropathy. Changes in nerve conduction under tension. J Bone Joint Surg Br 1992;74:126–9 [DOI] [PubMed] [Google Scholar]
- 13.Brown R, Pedowitz R, Rydevik B, Woo S, Hargens A, Massie J, et al. Effects of acute graded strain on efferent conduction properties in the rabbit tibial nerve. Clin Orthop Rel Res 1993;296:288–94 [PubMed] [Google Scholar]
- 14.Lundborg G, Rydevik B. Effects of stretching the tibial nerve of the rabbit. A preliminary study of the intraneural circulation and the barrier function of the perineurium. J Bone Joint Surg Br 1973;55:390–401 [PubMed] [Google Scholar]
- 15.Lundborg G. Structure and function of the intraneural microvessels as related to trauma, edema formation, and nerve function. J Bone Joint Surg Am 1975;57:938–48 [PubMed] [Google Scholar]
- 16.Rydevik B, McLean WG, Sjostrand J, Lundborg G. Blockage of axonal transport induced by acute, graded compression of the rabbit vagus nerve. J Neurol Neurosurg Psychiatry 1980;43:690–8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Dahlin LB, Sjostrand J, McLean WG. Graded inhibition of retrograde axonal transport by compression of rabbit vagus nerve. J Neurol Sci 1986;76:221–30 [DOI] [PubMed] [Google Scholar]
- 18.Dahlin LB, McLean WG. Effects of graded experimental compression on slow and fast axonal transport in rabbit vagus nerve. J Neurol Sci 1986;72:19–30 [DOI] [PubMed] [Google Scholar]
- 19.Dahlin LB, Lundborg G. The neurone and its response to peripheral nerve compression. J Hand Surg Br 1990;15:5–10 [DOI] [PubMed] [Google Scholar]
- 20.Tanoue M, Yamaga M, Ide J, Takagi K. Acute stretching of peripheral nerves inhibits retrograde axonal transport. J Hand Surg Br 1996;21:358–63 [DOI] [PubMed] [Google Scholar]
- 21.Yan JG, Matloub HS, Sanger JR, Zhang LL, Riley DA. Vibration-induced disruption of retrograde axoplasmic transport in peripheral nerve. Muscle Nerve 2005;32:521–6 [DOI] [PubMed] [Google Scholar]
- 22.Dilley A, Bove GM. Disruption of axoplasmic transport induces mechanical sensitivity in intact rat C-fibre nociceptor axons. J Physiol 2008;586:593–604 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Rydevik B, Lundborg G. Permeability of intraneural microvessels and perineurium following acute, graded experimental nerve compression. Scand J Plast Reconstr Surg 1977;11:179–87 [DOI] [PubMed] [Google Scholar]
- 24.Rydevik B, Nordborg C. Changes in nerve function and nerve fibre structure induced by acute, graded compression. J Neurol Neurosurg Psychiatry 1980;43:1070–82 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Lundborg G, Myers R, Powell H. Nerve compression injury and increased endoneurial fluid pressure: a “miniature compartment syndrome”. J Neurol Neurosurg Psychiatry 1983;46:1119–24 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Lundborg G, Dahlin LB, Danielsen N, Hansson HA, Necking LE, Pyykko I. Intraneural edema following exposure to vibration. Scand J Work Environ Health 1987;13:326–9 [DOI] [PubMed] [Google Scholar]
- 27.Dahlin LB, Archer DR, McLean WG. Axonal transport and morphological changes following nerve compression. An experimental study in the rabbit vagus nerve. J Hand Surg Br 1993;18:106–10 [DOI] [PubMed] [Google Scholar]
- 28.Rempel D, Dahlin L, Lundborg G. Pathophysiology of nerve compression syndromes: response of peripheral nerves to loading. J Bone Joint Surg Am 1999;81:1600–10 [DOI] [PubMed] [Google Scholar]
- 29.Alshami A, Babri AS, Souvlis T, Coppieters MW. Strain in the tibial and plantar nerves with foot and ankle movements and the influence of adjacent joint positions. J Appl Biomech 2008;24:368–76 [DOI] [PubMed] [Google Scholar]
- 30.Wright TW, Glowczewskie F, Wheeler D, Miller G, Cowin D. Excursion and strain of the median nerve. J Bone Joint Surg Am 1996;78:1897–903 [DOI] [PubMed] [Google Scholar]
- 31.Erel E, Dilley A, Greening J, Morris V, Cohen B, Lynn B. Longitudinal sliding of the median nerve in patients with carpal tunnel syndrome. J Hand Surg Br 2003;28:439–43 [DOI] [PubMed] [Google Scholar]
- 32.Dilley A, Lynn B, Greening J, DeLeon N. Quantitative in vivo studies of median nerve sliding in response to wrist, elbow, shoulder and neck movements. Clin Biomech 2003;18:899–907 [DOI] [PubMed] [Google Scholar]
- 33.Dilley A, Summerhayes C, Lynn B. An in vivo investigation of ulnar nerve sliding during upper limb movements. Clin Biomech 2007;22:774–9 [DOI] [PubMed] [Google Scholar]
- 34.Coppieters MW, Alshami AM, Babri AS, Souvlis T, Kippers V, Hodges PW. Strain and excursion of the sciatic, tibial, and plantar nerves during a modified straight leg raising test. J Orthop Res 2006;24:1883–9 [DOI] [PubMed] [Google Scholar]
- 35.Coppieters MW, Alshami AM. Longitudinal excursion and strain in the median nerve during novel nerve gliding exercises for carpal tunnel syndrome. J Orthop Res 2007;25:972–80 [DOI] [PubMed] [Google Scholar]
- 36.Babbage CS, Coppieters MW, McGowan CM. Strain and excursion of the sciatic nerve in the dog: biomechanical considerations in the development of a clinical test for increased neural mechanosensitivity. Vet J 2007;174:330–6 [DOI] [PubMed] [Google Scholar]
- 37.Echigo A, Aoki M, Ishial S, Yamaguchi M, Nakamura M, Sawada Y. The excursion of the median nerve during nerve gliding exercise: an observation with high-resolution ultrasonography. J Hand Ther 2008;21:221–8 [DOI] [PubMed] [Google Scholar]
- 38.Pahor S, Toppenberg R. An investigation of neural tissue involvement in ankle inversion sprains. Man Ther 1996;1:192–7 [DOI] [PubMed] [Google Scholar]
- 39.Herrington L. Effect of different neurodynamic mobilization techniques on knee extension range of motion in the slump position. J Man Manip Ther 2006;14:101–7 [Google Scholar]
- 40.Butler DS. Mobilization of the nervous system. London: Churchill Livingstone; 1991 [Google Scholar]
- 41.Kinoshita M, Okuda R, Morikawa J, Jotoku T, Abe M. The dorsiflexion–eversion test for diagnosis of tarsal tunnel syndrome. J Bone Joint Surg 2001;83:1835–9 [DOI] [PubMed] [Google Scholar]
- 42.Coppieters MW, Kurz K, Mortensen TE, Richards NL, Skaret IA, McLaughlin LM, et al. The impact of neurodynamic testing on the perception of experimentally induced muscle pain. Man Ther 2005;10:52–60 [DOI] [PubMed] [Google Scholar]
- 43.Coppieters MW, Bartholomeeusen KE, Stappaerts KH. Incorporating nerve-gliding techniques in the conservative treatment of cubital tunnel syndrome. J Manipulative Physiol Ther 2004;27:560–8 [DOI] [PubMed] [Google Scholar]
- 44.Scrimshaw S, Mayer C. Randomized controlled trial of neural mobilization after spinal surgery. Spine 2001;26:2647–52 [DOI] [PubMed] [Google Scholar]
- 45.Ellis RF, Hing WA. Neural mobilization: a systematic review of randomized controlled trials with an analysis of therapeutic efficacy. J Man Manip Ther 2008;16:8–22 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Abe Y, Doi K, Kawai S. An experimental model of peripheral nerve adhesion in rabbits. J Plastic Surg Br 2005;58:533–40 [DOI] [PubMed] [Google Scholar]
- 47.Lundborg G, Dahlin LB. Anatomy, function, and pathophysiology of peripheral nerves and nerve compression. Hand Clin 1996;12:185–93 [PubMed] [Google Scholar]
- 48.Driscoll PJ, Glasby MA, Lawson GM, et al. An in vivo study of peripheral nerves in continuity: biomechanical and physiological responses to elongation. J Orthop Res 2002;20:370–5 [DOI] [PubMed] [Google Scholar]
- 49.Rydevik B, Lundborg G, Nordborg C. Intraneural Tissue reactions induced by internal neurolysis. Scand J Plast Reconstru Surg 1976;10:3–8 [DOI] [PubMed] [Google Scholar]
- 50.Sakurai M, Miyasaka Y. Neural fibrosis and the effect of neurolysis. J Bone Joint Surg 1986;68:483–8 [DOI] [PubMed] [Google Scholar]
- 51.Martinoli C, Bianchi S, Gandolfo N, Valle M, Simonetti S, Derchi LE. US of nerve entrapments in osteofibrous tunnels of the upper and lower limbs. Radiographics 2000;20:S199–17 [DOI] [PubMed] [Google Scholar]
- 52.Peer S, Kovacs P, Harpf C, Bodner G. High-resolution sonography of lower extremity peripheral nerves: Anatomic correlation and spectrum of disease. J Ultrasound Med 2002;21:315–22 [DOI] [PubMed] [Google Scholar]
- 53.Cleland JA, Hunt GC, Palmer J. Effectiveness of neural mobilization in the treatment of a patient with lower extremity neurogenic pain: a single-case design. J Man Manip Ther 2004;12:143–152 [Google Scholar]
- 54.Cleland JA, Childs JD, Palmer JA, Eberhart S. Slump stretching in the management of non-radicular low back pain: a pilot clinical trial. Man Ther 2006;11:279–86 [DOI] [PubMed] [Google Scholar]
- 55.Walsh J, Flatley M, Johnston N, Bennett K. Slump test: sensory responses in asymptomatic subjects. J Man Manip Ther 2007;15:231–8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Alshami AM, Babri AS, Souvlis T, Coppieters MW. Biomechanical evaluation of two clinical tests for plantar heel pain: the dorsiflexion–eversion test for tarsal tunnel syndrome and the windlass test for plantar fasciitis. Foot Ankle Int 2007;28:499–505 [DOI] [PubMed] [Google Scholar]
- 57.Murphy DR, Hurwitz EL, Gregory AA, Clary R. A non-surgical approach to the management of lumbar spinal stenosis: a prospective observational cohort study. BMC Musculoskeletal Disorders 2006;7:16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Gilbert KK, Apte G, James CR, Smith M. Neural and fluid dynamics of the lumbosacral nerve roots during lower limb movements in cadavers. 2010; to be published. [Google Scholar]
- 59.van Hoof T, Gomes GT, Audenaert E, Verstraete K, Kerckaert I, D’Herde K. 3D computerized model for measuring strain and displacement of the brachial plexus following placement of reverse shoulder prosthesis. Anat Rec 2008;291:1173–85 [DOI] [PubMed] [Google Scholar]
- 60.Gilbert KK, Brismee JM, Collins DL, James CR, Shah RV, Sawyer SF, et al. 2006 young investigator award winner: lumbosacral nerve root displacement and strain: Part 1. A novel measurement technique during straight leg raise in unembalmed cadavers. Spine 2007;32:1513–20 [DOI] [PubMed] [Google Scholar]
- 61.Gilbert KK, Brismee JM, Collins DL, James CR, Shah RV, Sawyer SF, et al. 2006 young investigator award winner: lumbosacral nerve root displacement and strain: Part 2. A comparison of 2 straight leg raise conditions in unembalmed cadavers. Spine 2007;32:1521–5 [DOI] [PubMed] [Google Scholar]
- 62.Ellis R, Hing W, Dilley A, McNair P. Reliability of measuring sciatic and tibial nerve movement with diagnostic ultrasound during a neural mobilisation technique. Ultrasound Med Biol 2008;34:1209–2008 [DOI] [PubMed] [Google Scholar]
- 63.Millesi H, Zoch G, Reihsner R. Mechanical properties of peripheral nerves. Clin Orthop Rel Res 1995;314:76–83 [PubMed] [Google Scholar]
- 64.Hurst LC, Weissberg D, Carroll RE. The relationship of the double crush to carpal tunnel syndrome (an analysis of 1,000 cases of carpal tunnel syndrome). J Hand Surg Br 1985;10:202–4 [DOI] [PubMed] [Google Scholar]
- 65.Dellon AL, Mackinnon SE. Chronic nerve compression model for the double crush hypothesis. Ann Plast Surg 1991;26:259–64 [DOI] [PubMed] [Google Scholar]
- 66.Augustijn P, Vanneste J. The tarsal tunnel syndrome after a proximal lesion. J Neurol Neurosurg Psychiatry 1992;55:65–7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Mackinnon SE. Double and multiple "crush" syndromes. Double and multiple entrapment neuropathies. Hand Clin 1992;8:369–90 [PubMed] [Google Scholar]
- 68.Dellon AL, Mackinnon SE, Seiler WA. Susceptibility of the diabetic nerve to chronic compression. Ann Plast Surg 1988;20:117–20 [DOI] [PubMed] [Google Scholar]
- 69.Grewal R, Xu J, Sotoreanos DG, Woo SL. Biomechanical properties of peripheral nerves. Hand Clin 1996;12:195–203 [PubMed] [Google Scholar]
- 70.Dilley A, Greening J, Lynn B, Leary R, Morris V. The use of cross-correlation analysis between high-frequency ultrasound images to measure longitudinal median nerve movement. Ultrasound Med Biol 2001;27:1211–8 [DOI] [PubMed] [Google Scholar]
- 71.Kincaid BR, Barrett SL. Use of high-resolution ultrasound in evaluation of the forefoot to differentiate forefoot nerve entrapments. J Am Podiatr Med Assoc 2005;95:429–32 [DOI] [PubMed] [Google Scholar]
- 72.de Kool BS, van Neck JW, Blok JH, Walbeehm ET, Hekking I, Visser GH. Ultrasound imaging of the rabbit peroneal nerve. J Peripher Nerv Syst 2005;10:369–74 [DOI] [PubMed] [Google Scholar]
- 73.Nagaoka M, Matsuzaki H. Ultrasonography in tarsal tunnel syndrome. J Ultrasound Med 2005;24:1035–40 [DOI] [PubMed] [Google Scholar]


