Abstract
Aims
We previously showed decreased cellular coupling and dephosphorylation of the gap junctional protein connexin 43 (Cx43) in left ventricular (LV) myocytes from an arrhythmogenic rabbit model of non-ischaemic heart failure (HF) that was associated with a 2.5-fold increase in the amount of protein phosphatase type 2A (PP2A) co-localized with Cx43. Here, we further explore the molecular mechanisms of enhanced dephosphorylation of Cx43 in HF. p21-activated kinase 1 (PAK1) is a serine–threonine protein kinase that has been shown to activate PP2A.
Methods and results
We found that total PAK1 and activated PAK1 (PAK1-PThr423) were both increased in HF rabbit LV (vs. controls). PAK1 co-immunoprecipitated (co-IP'd) with Cx43 protein and, with HF, co-IP'd PAK1 and PAK1-PThr423 were increased. With failing human LV, PAK1 total protein and PAK1-PThr423 were also increased globally and locally (co-IP'd with Cx43). To further explore the role of PAK1 in modulating Cx43 dephosphorylation and intercellular coupling, we overexpressed active PAK1 in isolated LV myocytes from control rabbits and in HEK293 cells with genetically modified overexpression of Cx43 (HEK293-Cx43). PAK1 overexpression in both rabbit myocytes and HEK293-Cx43 cells significantly increased PP2A activity (globally and at the level of Cx43), increased dephosphorylated Cx43, and markedly reduced intercellular dye coupling. These effects were attenuated with PP2A inhibition using okadaic acid (10 nM).
Conclusions
PAK1 and PP2A are integral components of a macromolecular complex with cardiac Cx43, and increased activation of associated PAK1 can contribute to enhanced Cx43 dephosphorylation and impaired intercellular coupling that may underlie slow conduction in HF.
Keywords: Gap junction, p21-activated kinase, Protein phosphatase, Heart failure
1. Introduction
Sudden death from ventricular tachycardia (VT) degenerating to ventricular fibrillation (VF) accounts for nearly half of the deaths in patients with non-ischaemic (as well as ischaemic) heart failure (HF).1 However, prevention has been ineffective because of lack of understanding of the underlying mechanisms. Although our three-dimensional mapping studies have shown that VT can initiate by a non-reentrant mechanism (such as triggered activity),2 there is slow conduction and non-uniform anisotropy in non-ischaemic HF human hearts3 that could underlie development of reentry, especially during the transition to VF and during VF.4,5 There are substantial data to support that slow conduction and conduction block in failing myocardium involve altered intercellular coupling through cardiac gap junctions from altered expression and distribution of connexin 43 (Cx43).6–10 Down-regulated Cx43 has been found in experimental animal models of HF as well as in the failing human heart.4,8,11,12 Moreover, we12,13 have reported reduced intercellular coupling in left ventricular (LV) myocyte pairs from our arrhythmogenic HF rabbit model as well as in control rabbit LV myocytes in which Cx43 protein was knocked down using siRNA approach. This further supports a potential role of gap junctional proteins, especially Cx43, in altered intercellular coupling.
Cx43 is a phosphoprotein, and phosphorylated Cx43 is the predominant functional form.14 Alterations in phosphorylation state could affect intercellular coupling.15,16 We and others have shown enhanced Cx43 dephosphorylation in LV from animal HF models and from failing human heart.4,7,12 Moreover, we12 reported for the first time that protein phosphatases (PPs) PP1 and PP2A co-localize with Cx43, and with HF there is a 2.5-fold increase in the amount of PP2A co-localized with Cx43 that could underlie the increased dephosphorylation of Cx43 in that setting. We also showed that inhibition of PP2A with okadaic acid improved intercellular dye coupling in HF rabbit LV myocyte pairs. Although emerging evidence indicates that Cx43 dephosphorylation is critical in impaired intercellular coupling in HF,4,12 the mechanisms of regulation of Cx43 dephosphorylation in HF remain poorly understood.
p21-activated kinases (PAKs) are serine–threonine kinases that are expressed in rat hearts.17 Ke et al.17 have reported that PAK1 co-localizes with PP2A and activates PP2A to dephosphorylate cardiac troponin I (cTnI). On the basis of these intriguing findings, we hypothesized that there may be up-regulation and increased activation of PAK1 in HF that could activate PP2A and contribute to Cx43 dephosphorylation. With PAK1 co-localizing with PP2A, we further hypothesized that PAK1 is associated with PP2A at the level of Cx43 and could contribute to Cx43 dephosphorylation by local effects. Thus the aims of this study were to assess PAK1 expression in LV from our rabbit model of HF and from the failing human heart, and to determine the functional role of PAK1 in Cx43 dephosphorylation and intercellular coupling. For the latter aim, we performed in vitro gene transfer with an adenovirus encoding a constitutively active form of PAK1 (AdPAK1) in isolated control rabbit LV myocytes and in a human embryonic kidney 293 cell line with genetically modified stable expression of Cx43 (HEK293-Cx43), and assessed its effects on global and local (at the level of Cx43) PP2A activation, dephosphorylation of Cx43 and intercellular coupling.
2. Methods
See Supplementary material online for additional details.
2.1. Arrhythmogenic rabbit non-ischaemic HF model
HF was induced in adult New Zealand white rabbits (3–4 kg, 5–6 months of age) with volume overload by creation of aortic insufficiency, followed by pressure overload induction 2–6 weeks later by constriction of the thoracic aorta as previously described (Supplementary material online, Table S1).2 The rabbits were sedated with ketamine (35 mg/kg, i.m.) and placed under isoflurane anaesthesia for all surgical procedures. All surgical procedures were performed with monitoring of ECG, temperature, capnography, pulse oximetry, and aortic blood pressure. Surgical anaesthesia depth was confirmed with the absence of pedal and corneal reflexes. Prior to induction of HF (baseline), and at ∼2–4 week intervals, animals underwent echocardiographic examination under sedation with ketamine (30 mg/kg, i.m.). Echocardiographic index of severe LV dysfunction is a LV end-systolic dimension >1.4 cm (>40% increase).2,12 Rabbits were euthanized with ketamine (35 mg/kg, i.m.) followed by Euthasol (saturated pentobarbital solution) injection (50 mg/kg, i.v.), as previously described.2 The LV free wall was flash-frozen in liquid nitrogen as previously described.12 The investigation conforms to the Guide for the Care and Use of Laboratory Animals published by the US National Institutes of Health (NIH Publication No.85-53, revised 1996) and the principles outlined in the Declaration of Helsinki for use of human tissue or subjects. The protocol was also approved by the University of Illinois at Chicago (UIC) and University of Alabama at Birmingham (UAB) Animal Studies Committees.
2.2. Human heart tissue
LV tissue was obtained from seven failing human hearts (ejection fraction 16 ± 2%) after explantation in patients undergoing clinically indicated cardiac transplantation for end-stage idiopathic dilated cardiomyopathy (IDCM) performed at the UIC or Loyola University Chicago Hospital (see Supplementary material online, Table S2). Seven non-failing (NF) human hearts (which could not be used for transplantation) were obtained from Gift of Hope (formerly the Regional Organ Bank of Illinois) as described previously.12 The study was approved by the Human Studies Committees of UIC, Loyola University, UAB, and Gift of Hope.
2.3. Adenoviral infection in isolated rabbit LV myocytes and HEK293-Cx43 cells
Isolated LV myocytes from control rabbits were infected with AdPAK1 (with AdLacZ as a negative control) for 1h in a serum-reduced M199 medium, then cultured in adenovirus-free supplemented M199 medium with electrical pacing for up to 24 h as described previously.13 HEK293-Cx43 cells were cultured in a DMEM medium containing 10% foetal bovine serum and penicillin–streptomycin. The adenoviral infection process was similar to that for isolated rabbit myocytes except that culturing time after adenoviral infection was only 6–8 h.
2.4. Western blot analysis
Western blotting was performed as previously described.12 Primary antibodies were used to detect total Cx43 protein (Cx43-T, Zymed), the non-phosphorylated isoform of Cx43 (Cx43-NP, Zymed),12,18 PAK1 (Zymed), PAK-PThr423 (Santa Cruz), PP2A-PTyr307 (Santa Cruz), PP2A & PP1 (BD Biosciences), and GAPDH (Chemicon). Images were obtained and quantitatively analysed using Quantity-One software (Bio-Rad).
2.5. Co-sedimentation and co-immunoprecipitation
Sedimentation of PP1 and PP2A from rabbit LV homogenates (500 µg total protein) was performed using microcystin-Sepharose beads (UBI).12 Immunoprecipitation (IP) of Cx43 (monoclonal antibody from Chemicon) and PP2Ac (BD Sciences) proteins were performed in LV or HEK-Cx43 lysates as described.12,19,20 Homogenates without microcystin beads or without specific antibody (but with normal mouse IgG) were used as negative controls in all IP experiments. Immunoblotting was then performed with Cx43, PP2Ac, PP2A-PTyr307, PAK1, and PAK-PThr423 antibodies as above.
2.6. PP2A activity assay
PP2A activity in isolated myocytes was determined by the PP2A serine–threonine phosphatase assay according to the manufacturer's protocol (UBI).
2.7. Immunolabelling and confocal microscopy
Suspended isolated rabbit myocytes were attached to laminin-coated glass slides for 30 min, followed by fixing with 4% paraformaldehyde in PBS for 20 min. Cells were blocked in 10% goat serum and 0.2% bovine serum albumin with 0.2% Triton X100. For double staining with Cx43-NP and N-cadherin antibodies, myocytes were first incubated with a Cx43-NP antibody followed by an Alexa Fluor 488-conjugated secondary antibody (Molecular Probes). The cells were then pre-blocked with 10% donkey serum for 1 h, followed by incubating with N-caherin antibody. After washing with PBS, cells were reacted with an Alexa Fluor 568-conjugated secondary antibody (Molecular Probes). Stained cells were visualized by using a laser scanning confocal microscope (Carl Zeiss 510) as previous described.12
2.8. Microinjection of Lucifer Yellow
Four per cent Lucifer Yellow (LY, excitation = 458 nm; emission = 531 nm) and 1% Rhodamine B dextran (excitation = 543 nm; emission = 560 nm) in a buffer containing 150 mmol/L LiCl and 10 mmol/L HEPES were microinjected into one cell of an end-to-end isolated rabbit LV or HEK293-Cx43 cell pair (with an injection pulse pressure of 400 and 200 hPa of 0.1 s duration, respectively). All experiments were performed at 37°C on the stage of a confocal microscope (Carl Zeiss). Cell images were collected every 0.5 s at ×32 magnifications. The extent of intercellular transfer was determined by recording fluorescence intensity in the adjacent (non-injected) cell of the cell pair from confocal images obtained every 12–13 s, as previously described.12,20
2.9. Statistical analysis
All data were presented as means ± SEM. Differences between two groups were evaluated using ANOVA and P < 0.05 was considered to be significant.
3. Results
3.1. Rabbit echocardiographic data
After an average of 9.1 ± 2.0 months, HF rabbit hearts (n = 12) exhibited marked LV dilatation and systolic dysfunction compared with their baseline condition (see Supplementary material online, Table S1) as well as to age-matched controls (n = 23). With HF, LV end-diastolic and end-systolic dimensions increased by 49 and 88%, respectively (both P < 0.001), and mean fractional shortening decreased by 40% (P < 0.001).
3.2. PAK1 expression in rabbit LV and activation of PAK in HF
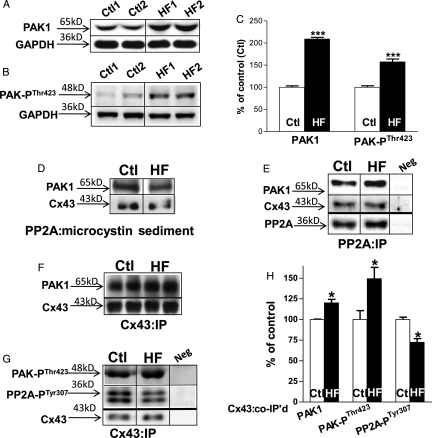
Protein expression of PAK1 was assessed by western blot in age-matched control and HF rabbit LV tissue. PAK1 was found in rabbit LV tissue (Figure 1A) as well as in isolated LV myocytes (data not shown). With HF, PAK1 protein levels increased by 109% vs. controls (n = 12, 12; P < 0.001; Figure 1A and C, left), while the level of activated PAK1 (increased auto-phosphorylation at the threonine 423 site of PAK1, PAK-PThr423) increased by 57% compared with controls (P < 0.001; Figure 1B and C, right).
Figure 1.
Expression and interaction between PAK1, and activated PAK (phosphorylated at Thr423 residue of PAK, PAK-PThr423), PP2A, and Cx43 in control (Ctl) and HF rabbit LV. Immunoblotting images of PAK1 (A), PAK-PThr423 (B), with GAPDH bands (A and B, bottom rows) from control and HF LV tissue with summarized data, normalized to GAPDH (n = 12, 12, ***P < 0.001 vs. Ctl; C). Immunoblotting images of co-sedimented PAK1 and CX43 with microcystin (D) and co-IP'd PAK1 and Cx43 with immunoprecipitated PP2A (with PP2A antibody; n = 3, 3; E). Immunoblotting images of co-IP'd PAK1 (F), PAK-PThr423 (G, top), and activated PP2A (phosphorylation at Tyr307 residue of PP2A, PP2A-PTyr307; G, middle) with immunoprecipitated Cx43 proteins (F and G, bottom) in control and HF rabbit LV with summarized data normalized to pulled-down Cx43 protein (n = 6, 6; *P < 0.05 vs. Ctl, H).
3.3. PAK1 associates with PP2A and Cx43 in rabbit LV
We have previously shown that Cx43 co-localizes with PP2A in rabbit LV,12 and that PAK1 associates with PP2A and modulates PP2A activity in rat heart17 (as well as in the rat brain).21 To explore the interaction of PAK1 with PP2A and Cx43 in rabbit LV, PP2A protein was first isolated from rabbit LV homogenates by binding to microcystin (a specific inhibitor of PP1 and PP2A), followed by immunoblotting analysis. We observed co-sedimented PAK1 reactive bands (Figure 1D, top row) along with Cx43 bands (Figure 1D, bottom row). PP2A protein was then pulled down using a monoclonal PP2A specific-antibody, and the co-immunoprecipitated (co-IP'd) proteins were assessed by immunoblotting with specific antibodies including PAK1 and Cx43. We found PAK1 (Figure 1E, top) was co-IP'd with PP2A protein (Figure 1E, bottom row), while co-IP'd Cx43 was also present (Figure 1E, middle row). To further define the relationship between Cx43 and PAK1 proteins, Cx43 protein was immunoprecipitated with a specific anti-Cx43 antibody. We found that PAK1 protein was associated with Cx43 (co-IP'd PAK1 with Cx43-T antibody, Figure 1F) in rabbit LV.
3.4. Enhanced activation of PAK1 at the level of Cx43 in HF
In our arrhythmogenic rabbit HF model, we have reported an increased associated PP2A with Cx43 that contributes to the enhanced dephosphorylation of Cx43 in HF.12 Here, we found a 20% increase in PAK1 and a 49% increase in PAK-PThr423 that co-IP'd with Cx43 in HF rabbit LV compared with controls (Figure 1F, top; Figure 1G, top; and Figure 1H, left and middle). This enhanced local PAK1 activation in HF was associated with an increased PP2A activation at the level of Cx43 that was evident from a 28% reduction in phosphorylation at the tyrosine 307 site of PP2A (PP2A-PTyr307)22 in Cx43 immunoprecipitates (Cx43:IP; Figure 1G, middle row and Figure 1H, right), as we showed in isolated rat myocytes.17
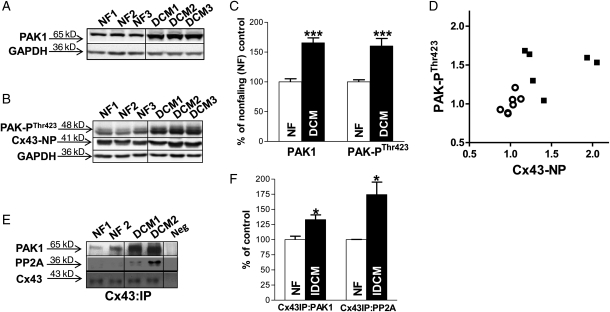
3.5. PAK1 expression in human LV and enhanced PAK1 activation with HF
We also assessed PAK1 protein expression in human NF and failing LV. We found that PAK1 was expressed in human LV, and the amount of PAK1 protein was increased by 66% in LV from patients with IDCM vs. NF controls (n = 7, 6; P < 0.001; Figure 2A and C, left). Moreover, the level of activated PAK1 (PAK-PThr423) in failing human LV was increased by 56% vs. NF controls (P < 0.001; Figure 2B and C, right). Figure 2D shows the level of activated PAK1 (PAK-PThr423) was strongly associated with Cx43 dephosphorylation levels (assessed by levels of Cx43-NP, Figure 2B, middle row).12 In the current study, we found a 33% increase in the amount of PAK1 that co-IP'd with Cx43 in human failing LV compared with NF controls (Figure 2E, top row and Figure 2F, left; n = 4, 4; P < 0.05). Moreover, we confirmed a 74% increase in associated PP2A with Cx43 in the failing human LV (Figure 2E, middle row and Figure 2F, right; n = 4, 4; P < 0.05) that was comparable with findings in LV from our arrhythmogenic HF rabbit model.12
Figure 2.
Expression of PAK1 and PAK1-PThr423 and protein interactions in human IDCM and non-failing (NF) LV. Immunoblotting images of PAK1 (A), PAK-PThr423 and Cx43-NP (B) in human non-failing and IDCM LV tissue with summarized data (normalized to GAPDH) in NF and DCM human LV (n = 7, 6; ***P < 0.001 vs. NF; C). Plot of the amount of PAK-PThr423 and Cx43-NP protein levels (both normalized to NF controls) in NF and DCM LV (n = 7, 6; D). Immunoblotting images show co-IP'd PAK1 and PP2A associated with immunoprecipitated Cx43 (with Cx43 antibody; E). Summarized data of co-IP'ed PAK1 and PP2A with immunoprecipitated Cx43 proteins in NF and DCM human LV (normalized to Cx43; n = 4, 4; *P < 0.05 vs. NF; F).
3.6. PAK1 overexpression dephosphorylates Cx43 and reduces intercellular dye coupling in isolated LV myocytes from control rabbits
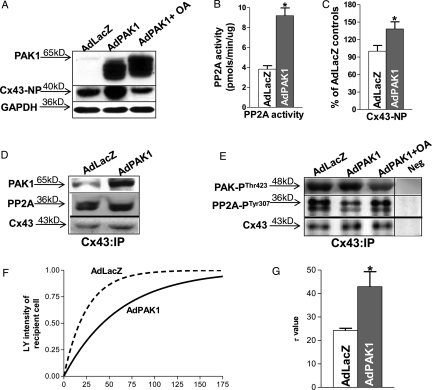
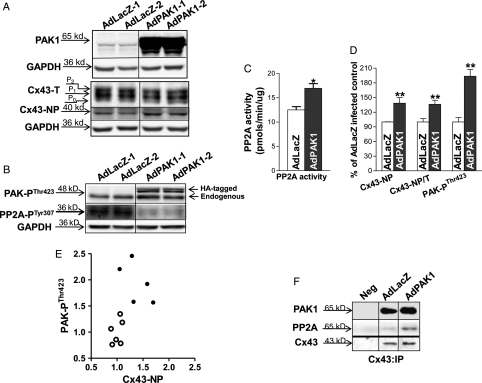
To explore the role of PAK1 in modulating Cx43 dephosphorylation, we performed adenoviral in vitro gene transfer to overexpress constitutively active PAK1 in isolated LV myocytes from control rabbit hearts. Transfection efficiency exceeded 99% as we have previous shown.13 We titrated the multiplicity of infection of AdPAK1 to yield approximately two-fold overexpression of PAK1 at the level of Cx43 (Figure 3D). We found that AdPAK1 overexpression (Figure 3A, top row) led to increased total PP2A activity by 115% (n = 3, 3; P < 0.05 vs. AdLacZ-infected cells; Figure 3B). Moreover, this PAK1 overexpression resulted in a 38% increase in Cx43-NP in cultured rabbit myocytes (n = 3, 3; P < 0.05 vs. AdLacZ controls; Figure 3A, middle row and 3C), while Cx43 total protein was unchanged. There was increased co-IP'd PAK1, PAK-PThr423, and PP2A with Cx43 protein in AdPAK1-overexpressed LV myocytes (Figure 3D and E, top). Activated PP2A at the level of Cx43 (Cx43:IP) was also increased evident from reduced level of PP2A-PTyr307 in active PAK1-overexpressed rabbit LV myocytes (Figure 3E, middle row). In addition, we found that the effects of PAK1 overexpression on Cx43 dephosphorylation and PP2A activation were inhibited by okadaic acid (10 nM, that inhibits PP2A but not PP1;23,24 Figure 3A and E, third lane). These findings suggest that activation of PAK1 contributes to an increased activity of PP2A, which results in an enhanced Cx43 dephosphorylation in active PAK1-overexpressing rabbit myocytes.
Figure 3.
AdPAK1 infected isolated rabbit myocytes. Immunoblots of PAK1 and Cx43-NP bands from AdLacZ- and AdPAK1-infected control rabbit LV myocytes with or without treatment with 10 nmol/L okadaic acid (A). Summarized data show increased levels of PP2A activity (B) and enhanced amount of Cx43-NP; C) in AdPAK1-infected (vs. AdLacZ-infected) control rabbit LV myocytes (normalized to GAPDH). Co-IP'd PAK1 and PP2A (D) and co-IP'd activated PAK-PThr423 and PP2A-PTyr307 (E) with immunoprecipitated Cx43 proteins (Cx43 antibody) in AdLacZ-infected, control myocytes and in AdPAK1-infected control rabbit myocytes with or without okadaic acid treatment. Representative fitted single time courses of LY dye transfer in recipient cell from end-to-end cell pairs of AdLacZ- (black line) and AdPAK1-infected (red line) control rabbit LV myocytes (F). Summarized τ data of LY transfer in recipient cell from AdLacZ- and AdPAK1-infected control rabbit LV myocytes (*P < 0.05; G).
We then assessed the effects of PAK1 overexpression on intercellular dye coupling in LV myocyte cell pairs from control rabbits using LY dye injection into one cell of the pair (with recording of fluorescence in the non-injected cell of the pair) as previous described.12 Figure 3F shows representative fitted single time courses of LY transfer in recipient cells from PAK1-overexpressed and AdLacZ-infected control cell pairs, and Figure 3G shows summarized results demonstrating a significant decrease in dye coupling [evident by the increase in the time constant (tau) of dye transfer from 24 ± 1 to 43 ± 6 s; n = 4, 4; P < 0.05]. These results indicate that increased active PAK1 activates PP2A to dephosphorylate Cx43 and decrease intercellular coupling.
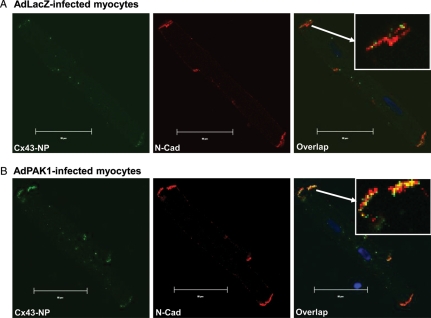
To assess the distribution of Cx43-NP in AdPAK1-infected rabbit myocytes, we performed confocal immunohistochemistry studies. Figure 4A and B shows an increased amount of Cx43-NP signal at the intercalated discs (evident in the N-cadherin colocalization images) in AdPAK1-overexpressed control rabbit LV myocytes vs. AdLacZ-infected controls. This suggests an effect of AdPAK1 on Cx43 dephosphorylation without increasing cytoplasm internalization of Cx43 proteins.
Figure 4.
Confocal fluorescence images of double immunofluorescence staining with Cx43-NP (green) and N-cadherin (N-Cad; red) antibodies in AdLacZ-infected (A) and AdPAK1-infected (B) rabbit LV myocytes. Far right columns of A and B are overlap images of Cx43-NP and N-Cadherin (yellow). Arrows in the overlap images indicate enlarged images of co-localized Cx43-NP and N-Cad at the end of the AdLacZ- and AdPAK1-infected rabbit LV myocytes. Scale bar is 20 μm.
3.7. PAK1 overexpression dephosphorylates Cx43 and reduces intercellular dye coupling in HEK293-Cx43 cells
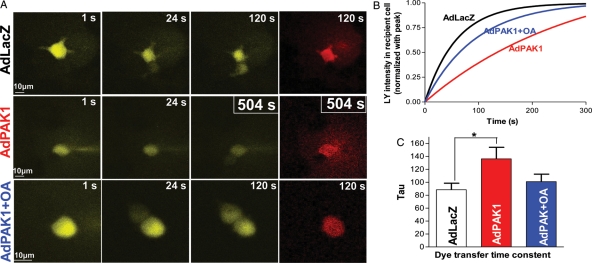
To further confirm the role of PAK1 in Cx43 dephosphorylation and intercellular coupling, we explored the effects of PAK1 overexpression on Cx43 phosphorylation and coupling in an HEK293-Cx43 cell line. AdPAK1 (Figure 5A, top row) increased PP2A activity by 34% (n = 4, 4; P < 0.05; Figure 5C) and Cx43 dephosphorylation (39% increase in Cx43-NP and 36% increase in Cx43-NP/Cx43-total; n = 6, 6; P < 0.05; Figure 5A, fourth row; and Figure 5D left and middle) along with a 93% increase in PAK-PThr423 (n = 6, 6; P < 0.01; Figure 5B, top row and Figure 5D, right). Figure 5E shows a positive relationship between overexpressed active PAK1 and the amount of dephosphorylated Cx43 in HEK293 cells expressing Cx43. We found that PAK1 was associated with Cx43 and PP2A proteins (Figure 5F), indicating interactions between these proteins in HEK293-Cx43 cells similar to our findings in rabbit and human LV myocytes. Intercellular coupling between AdPAK1-infected HEK293-Cx43 cells was markedly reduced [increased dye transfer time constant (τ) of 136.2 ± 17.8 vs. 85.6 ± 10.1 s for AdLacZ controls (n = 4, 4; P < 0.05; Figure 6A–C)]. Moreover, okadaic acid (10 nmol/L, which inhibits PP2A) improved intercellular coupling (τ = 101.0 ± 11.6 s; P = NS vs. AdLacZ controls; Figure 6A–C, blue colour scheme). These results further support our hypothesis that PAK1 contributes to Cx43 dephosphorylation and, ultimately, intercellular coupling through modulation of PP2A activity.
Figure 5.
AdPAK1-infected HEK293-Cx43 cells. Immunoblotting images of PAK1, Cx43-Total (Cx43-T), and Cx43-NP (A), and of PAK-PThr423 and PP2A-PTyr307 (B) bands from AdLacZ- and AdPAK1-infected HEK293-Cx43 cells. Summarized data show increased PP2A activity in AdPAK1-infected HEK293-Cx43 cells (n = 4, 4 *P < 0.05; C). Summarized data show increased Cx43-NP, Cx43-NP/T, and PAK-PThr423 in AdPAK1-infected HEK293-Cx43 cells compared with AdLacZ-infected controls (normalized with GAPDH; n = 6, 6; **P < 0.01; D). Plot of the amount of PAK-PThr423 and Cx43-NP protein levels in AdLacZ (open circles) and AdPAK1-infected HEK293-Cx43 cells (filled circles, n = 6, 6; E). Co-IP'd PAK1 and PP2A with immunoprecipitated Cx43 in AdPAK1- and AdLacZ-infected HEK293-Cx43 cells (F).
Figure 6.
Images of LY dye transfer (yellow) from a single injected cell to an adjacent recipient cell in a cultured HEK293-Cx43 cell pair at 1, 24, and either 120 or 504 s (A). At far right are representative images of co-injected Rhodamine B dextran (Rh, red) that is retained in the injected myocytes. Scale bar at lower left = 10 μm. Representative fitted single time courses of LY transfer in recipient cell from the cell pairs of AdLacZ- (black line) and AdPAK1-infected cells without (red line) or with (blue line) 10 nM okadaic acid treatment (B). Summarized tau data of LY transfer in recipient cell from AdLacZ-infected cell pairs and AdPAK1-infected cell pairs with or without 10 nM OA treatment with the same colour scheme as for Figure 6B (n = 4, 4, 4; *P < 0.05; C).
4. Discussion
In the present study, our major findings are that: (i) global levels of PAK1 and activated PAK1 are increased in both failing rabbit and failing human LV; (ii) PAK1 associates with Cx43 as well as PP2A in both rabbit and human LV; (iii) there is an increase in the amount of activated PAK1 and activated PP2A at the local level of Cx43 in rabbit and human HF; and (iv) adenoviral-induced overexpression of constitutively active PAK1 in isolated rabbit myocytes and in a Cx43-expressing HEK-293 cell line dephosphorylates Cx43 and decreases intercellular coupling. These intriguing findings strongly indicate that increased activated PAK1 plays an important role in PP2A activation at the level of Cx43 proteins and, ultimately, enhanced Cx43 dephosphorylation, and impaired intercellular coupling in HF (that could contribute to VF and sudden death).
4.1. Enhanced Cx43 dephosphorylation with increased associated PP2A in HF
Gap junctional channels, composed of connexins, are specialized membrane structures. The relative amounts and distribution of connexins influence electrical and chemical signal propagation throughout the heart.25,26 Down-regulation as well as altered distribution of Cx43 has been shown to be a common feature in failing heart,9,10 and it is associated with impaired intercellular coupling, as we and others have reported.4,8,11,12 However, Cx43 is a phospho-protein,14 and there is a dynamic process of phosphorylation and dephosphorylation that is critical for forming functional gap junction channels on cell membrane.16,27 Although Cx43 is phosphorylated by a number of kinases,27–29 Cx43 has been shown to be dephosphorylated by PP1 and PP2A.30 Dephosphorylation of Cx43 may play an important regulatory role since PP inhibition enhances gap junctional conductance while stimulation of PPs (e.g. PP1) attenuates it.12,30 Cx43 dephosphorylation during myocardial ischaemia has consistently been associated with uncoupling and slow conduction.18 Thus, in addition to Cx43 down-regulation, alterations in Cx43 phosphorylation state could have dramatic effects on conduction and development of reentry. We reported for the first time that increased dephosphorylation of Cx43 (increased Cx43-NP) is associated with an increased co-localization of PP2A with Cx43 in our arrhythmogenic rabbit HF model.12 This is associated with decreased intercellular coupling (LY dye transfer) with HF, and improved dye coupling (and enhanced Cx43 phosphorylation) after PP2A inhibition with okadaic acid.12,30 However, the mechanism of this enhanced PP2A activation at the level of associated Cx43 in HF remains unknown. Although PP2A is ubiquitously expressed in the heart, studies suggest that PP2A regulates its substrates locally through direct interaction.31 PAK1 has recently been shown to interact with PP2A as a component of functional protein complexes in the rat brain and heart, and it has been shown to be important in the regulation of PP2A activity and its downstream signalling cascade targets. PAK1 auto-phosphorylation activates PP2A by inducing a conformational shift of the PP2A catalytic subunit permitting auto-dephosphorylation at Tyr-307, perhaps through a scaffolding process.21,32,33 Findings from our current study suggest that chronic PAK1 up-regulation and activation in HF, especially locally at the level of Cx43, contribute to this Cx43 dephosphorylation and reduced intercellular coupling.
4.2. PAK1 in rabbit and human heart
PAK1 belongs to a highly conserved family of Ser–Thr protein kinases that are regulated by the Ras-related small G proteins Cdc42 and Rac1.34,35 PAK1 has a number of important physiological effects including roles in cytoskeletal proteins, cardiac contractility, and cell motility.36–38 We recently reported that PAK1 associates with PP2A in rat ventricular myocytes and activates PP2A.17 In the present study, we found that PAK1 is expressed in both rabbit and human LV, and that PAK1 associates with Cx43 and PP2A. These results suggest that PAK1 could be a potential regulator of the phosphorylation state of Cx43 in cardiac myocytes.
4.3. Increased PAK1 expression and activation at the level of Cx43 in failing rabbit and human LV
In our arrhythmogenic HF rabbit model, we have reported increased levels of PP2A co-localized with Cx43.12 In the present study, we found increased global PAK1 expression and activation in HF rabbit LV, and this was associated with enhanced local activation of PAK1 at the level of Cx43. Up-regulated PAK1 expression and local enhancement of PAK1 activation with Cx43 protein were also found in the failing human LV from IDCM patients (compared with that of non-failing human hearts with normal LV function not used for transplantation). PAK1 has been shown to form a functional complex with PP2A in the rat brain, and it modulates various intracellular signalling cascades.21 We recently found that PAK1 overexpression in rat LV myocytes led to an enhanced dephosphorylation of cTnI due to the activation of co-localized PP2A with cTnI.17 Activated PAK1 also regulates cardiac myocytes and pacemaker activity through PP2A activation of the PP2A/PAK1/L-type Ca channel molecular complex.38,39 There is a report of increased PAK1 expression in pressure overload hypertrophy,40 but to the best of our knowledge the present study is the first report of PAK1 regulation in HF. Results from our arrhythmogenic HF rabbit model and human failing heart demonstrate a new role for PAK1 in regulating gap junction function, and suggest that altered PAK1 expression and activation globally and locally (at the level of Cx43) in HF contribute to Cx43 dephosphorylation and decreased intercellular coupling in that setting.
4.4. Active PAK1 overexpression dephosphorylates Cx43 and reduces intercellular dye coupling in isolated rabbit LV myocytes and a culture cell line
To further understand the role of up-regulated PAK1 in enhanced Cx43 dephosphorylation and impaired intercellular coupling in HF, we adenovirally overexpressed active PAK1 in isolated rabbit LV myocytes and in an HEK293 cell line with genetically modified stable expression of Cx43. In both in vitro cellular models, overexpression of constitutively active PAK1 increased PP2A activation (evident by both the reduced level of PP2A-PTyr307 and elevated PP2A activity), but did so without changing total Cx43 or PP2A expression. We show for the first time that PAK1 overexpression can dephosphorylate Cx43 and decrease intercellular dye coupling. Although we find no direct evidence that PAK1 activation phosphorylates Cx43, this marked enhancement of Cx43 dephosphorylation and reduced intercellular dye coupling is attenuated with additional PP2A inhibition. These compelling results in control rabbit myocytes demonstrate that increased PAK1 expression and activation contributes to Cx43 dephosphorylation and reduced intercellular coupling through local PP2A activation.
It is clear that PAK1's effects on activating PP2A could impact on a number of cardiac proteins such as ion channels, Ca handling proteins, and myofilament regulatory proteins.17,38,39 In addition, PAK1 has been shown to regulate L-type calcium channels and delayed rectifier potassium channels activity in sinus node pacemaker cells.36,39 To focus on PAK1's effects on Cx43 and cellular coupling, we performed LY dye transfer studies in electrically quiescent adult myocytes (with no Na-, Ca-, or K-channels active). Our findings of decreased dye coupling in AdPAK1-infected LV myocyte pairs attest to the effects of PAK1 on Cx43 dephosphorylation and intercellular dye coupling rather than on these other channels. Moreover, we confirmed overexpression of PAK1 activates PP2A at the level of Cx43 protein and dephosphorylates Cx43 (to decrease intercellular coupling) in an HEK293 cell line expressing Cx43, thereby excluding the effects of PAK1 on other cardiac ion channels and myofilament proteins. Although alterations of PAK signalling pathway and the effects of PAK1 modulation on conduction velocity in HF remain to be determined, these important proof-of-principle studies provide an important foundation for understanding the role of up-regulated and activated PAK1 in Cx43 dephosphorylation and intercellular coupling.
4.5. Implications
Altered Cx43 has been shown to be associated with impaired intercellular coupling and conduction that could contribute to VF in the failing heart.4,5,7,8,12 To date, the molecular mechanisms by which alterations in Cx43 decrease intercellular coupling and contribute to lethal ventricular arrhythmias in the failing heart remain unclear. Our findings suggest that PAK1 is an important signalling molecule that has impact on the electrophysiological substrate of the failing heart, and that up-regulation and enhanced PAK1 activation in HF contribute to Cx43 dephosphorylation and decreased intercellular coupling in this setting. The finding that PAK1 modulates Cx43 dephosphorylation through PP2A at the level of Cx43 suggests its potential as a novel therapeutic target for the prevention and treatment of ventricular arrhythmias in HF patients.
Supplementary material
Supplementary material is available at Cardiovascular Research online.
Funding
This work was supported by National Institute of Health [grant number HL073966 to (S.M.P.) and HL064035 to (R.J.S.)].
Supplementary Material
Acknowledgements
We would like to thank Weiwei Zhao and Joseph Barchue for their excellent technical support.
Conflict of interest: none declared.
References
- 1.Packer M. Sudden unexpected death in patients with congestive heart failure: a second frontier. Circulation. 1985;72:681–685. doi: 10.1161/01.cir.72.4.681. [DOI] [PubMed] [Google Scholar]
- 2.Pogwizd SM. Nonreentrant mechanisms underlying spontaneous ventricular arrhythmias in a model of nonischemic heart failure in rabbits. Circulation. 1995;92:1034–1048. doi: 10.1161/01.cir.92.4.1034. [DOI] [PubMed] [Google Scholar]
- 3.Anderson KP, Walker R, Urie P, Ershler PR, Lux RL, Karwandee SV. Myocardial electrical propagation in patients with idiopathic dilated cardiomyopathy. J Clin Invest. 1993;92:122–140. doi: 10.1172/JCI116540. doi:10.1172/JCI116540. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Akar FG, Spragg DD, Tunin RS, Kass DA, Tomaselli GF. Mechanisms underlying conduction slowing and arrhythmogenesis in nonischemic dilated cardiomyopathy. Circ Res. 2004;95:717–725. doi: 10.1161/01.RES.0000144125.61927.1c. doi:10.1161/01.RES.0000144125.61927.1c. [DOI] [PubMed] [Google Scholar]
- 5.Poelzing S, Rosenbaum DS. Altered connexin43 expression produces arrhythmia substrate in heart failure. Am J Physiol Heart Circ Physiol. 2004;287:H1762–H1770. doi: 10.1152/ajpheart.00346.2004. doi:10.1152/ajpheart.00346.2004. [DOI] [PubMed] [Google Scholar]
- 6.De Mello WC. Renin-angiotensin system and cell communication in the failing heart. Hypertension. 1996;27:1267–1272. doi: 10.1161/01.hyp.27.6.1267. [DOI] [PubMed] [Google Scholar]
- 7.Akar FG, Nass RD, Hahn S, Cingolani E, Shah M, Hesketh GG, et al. Dynamic changes in conduction velocity and gap junction properties during development of pacing-induced heart failure. Am J Physiol Heart Circ Physiol. 2007;293:H1223–H1230. doi: 10.1152/ajpheart.00079.2007. doi:10.1152/ajpheart.00079.2007. [DOI] [PubMed] [Google Scholar]
- 8.De Mello WC. Cell coupling and impulse propagation in the failing heart. J Cardiovasc Electrophysiol. 1999;10:1409–1420. doi: 10.1111/j.1540-8167.1999.tb00197.x. doi:10.1111/j.1540-8167.1999.tb00197.x. [DOI] [PubMed] [Google Scholar]
- 9.Hesketh GG, Shah MH, Halperin VL, Cooke CA, Akar FG, Yen TE, et al. Ultrastructure and regulation of lateralized connexin43 in the failing heart. Circ Res. 2010;106:1153–1163. doi: 10.1161/CIRCRESAHA.108.182147. doi:10.1161/CIRCRESAHA.108.182147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Severs NJ, Bruce AF, Dupont E, Rothery S. Remodeling of gap junctions and connexin expression in diseased myocardium. Cardiovasc Res. 2008;80:9–19. doi: 10.1093/cvr/cvn133. doi:10.1093/cvr/cvn133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Dupont E, Matsushita T, Kaba RA, Vozzi C, Coppen SR, Khan N, et al. Altered connexin expression in human congestive heart failure. J Mol Cell Cardiol. 2001;33:359–371. doi: 10.1006/jmcc.2000.1308. doi:10.1006/jmcc.2000.1308. [DOI] [PubMed] [Google Scholar]
- 12.Ai X, Pogwizd SM. Connexin 43 downregulation and dephosphorylation in nonischemic heart failure is associated with enhanced colocalized protein phosphatase type 2A. Circ Res. 2005;96:54–63. doi: 10.1161/01.RES.0000152325.07495.5a. doi:10.1161/01.RES.0000152325.07495.5a. [DOI] [PubMed] [Google Scholar]
- 13.Ai X, Zhao W, Pogwizd S. Connexin43 knockdown or overexpression modulates cell coupling in control and failing rabbit left ventricular myocytes. Cardiovasc Res. 2010;85:751–762. doi: 10.1093/cvr/cvp353. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Lau AF, Hatch-Pigott V, Crow DS. Evidence that heart connexin43 is a phosphoprotein. J Mol Cell Cardiol. 1991;23:659–663. doi: 10.1016/0022-2828(91)90975-r. doi:10.1016/0022-2828(91)90975-R. [DOI] [PubMed] [Google Scholar]
- 15.Moreno AP, Saez JC, Fishman GI, Spray DC. Human connexin43 gap junction channels. Regulation of unitary conductances by phosphorylation. Circ Res. 1994;74:1050–1057. doi: 10.1161/01.res.74.6.1050. [DOI] [PubMed] [Google Scholar]
- 16.Kwak BR, Jongsma HJ. Regulation of cardiac gap junction channel permeability and conductance by several phosphorylating conditions. Mol Cell Biochem. 1996;157:93–99. doi: 10.1007/BF00227885. [DOI] [PubMed] [Google Scholar]
- 17.Ke Y, Wang L, Pyle WG, de Tombe PP, Solaro RJ. Intracellular localization and functional effects of P21-activated kinase-1 (Pak1) in cardiac myocytes. Circ Res. 2004;94:194–200. doi: 10.1161/01.RES.0000111522.02730.56. doi:10.1161/01.RES.0000111522.02730.56. [DOI] [PubMed] [Google Scholar]
- 18.Beardslee MA, Lerner DL, Tadros PN, Laing JG, Beyer EC, Yamada KA, et al. Dephosphorylation and intracellular redistribution of ventricular connexin43 during electrical uncoupling induced by ischemia. Circ Res. 2000;87:656–662. doi: 10.1161/01.res.87.8.656. [DOI] [PubMed] [Google Scholar]
- 19.Ai X, Curran JW, Shannon TR, Bers DM, Pogwizd SM. Ca2+/calmodulin-dependent protein kinase modulates cardiac ryanodine receptor phosphorylation and sarcoplasmic reticulum Ca2+ leak in heart failure. Circ Res. 2005;97:1314–1322. doi: 10.1161/01.RES.0000194329.41863.89. doi:10.1161/01.RES.0000194329.41863.89. [DOI] [PubMed] [Google Scholar]
- 20.Bossuyt J, Ai X, Moorman JR, Pogwizd SM, Bers DM. Expression and phosphorylation of the na-pump regulatory subunit phospholemman in heart failure. Circ Res. 2005;97:5580565. doi: 10.1161/01.RES.0000181172.27931.c3. [DOI] [PubMed] [Google Scholar]
- 21.Westphal RS, Coffee RL, Jr, Marotta A, Pelech SL, Wadzinski BE. Identification of kinase-phosphatase signaling modules composed of p70 S6 kinase-protein phosphatase 2A (PP2A) and p21-activated kinase-PP2A. J Biol Chem. 1999;274:687–692. doi: 10.1074/jbc.274.2.687. doi:10.1074/jbc.274.2.687. [DOI] [PubMed] [Google Scholar]
- 22.Chen J, Martin BL, Brautigan DL. Regulation of protein serine-threonine phosphatase type-2A by tyrosine phosphorylation. Science. 1992;257:1261–1264. doi: 10.1126/science.1325671. doi:10.1126/science.1325671. [DOI] [PubMed] [Google Scholar]
- 23.Herzig S, Neumann J. Effects of serine/threonine protein phosphatases on ion channels in excitable membranes. Physiol Rev. 2000;80:173–210. doi: 10.1152/physrev.2000.80.1.173. [DOI] [PubMed] [Google Scholar]
- 24.Bialojan C, Takai A. Inhibitory effect of a marine-sponge toxin, okadaic acid, on protein phosphatases. Specificity and kinetics. Biochem J. 1988;256:283–290. doi: 10.1042/bj2560283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Bruzzone R, White TW, Paul DL. Connections with connexins: the molecular basis of direct intercellular signaling. Eur J Biochem. 1996;238:1–27. doi: 10.1111/j.1432-1033.1996.0001q.x. doi:10.1111/j.1432-1033.1996.0001q.x. [DOI] [PubMed] [Google Scholar]
- 26.Saffitz JE, Davis LM, Darrow BJ, Kanter HL, Laing JG, Beyer EC. The molecular basis of anisotropy: role of gap junctions. J Cardiovasc Electrophysiol. 1995;6:498–510. doi: 10.1111/j.1540-8167.1995.tb00423.x. doi:10.1111/j.1540-8167.1995.tb00423.x. [DOI] [PubMed] [Google Scholar]
- 27.Lampe PD, Lau AF. Regulation of gap junctions by phosphorylation of connexins. Arch Biochem Biophys. 2000;384:205–215. doi: 10.1006/abbi.2000.2131. doi:10.1006/abbi.2000.2131. [DOI] [PubMed] [Google Scholar]
- 28.Ek-Vitorin JF, King TJ, Heyman NS, Lampe PD, Burt JM. Selectivity of connexin 43 channels is regulated through protein kinase C-dependent phosphorylation. Circ Res. 2006;98:1498–1505. doi: 10.1161/01.RES.0000227572.45891.2c. doi:10.1161/01.RES.0000227572.45891.2c. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Godwin AJ, Green LM, Walsh MP, McDonald JR, Walsh DA, Fletcher WH. In situ regulation of cell-cell communication by the cAMP-dependent protein kinase and protein kinase C. Mol Cell Biochem. 1993;127–128:293–307. doi: 10.1007/BF01076779. doi:10.1007/BF01076779. [DOI] [PubMed] [Google Scholar]
- 30.Duthe F, Plaisance I, Sarrouilhe D, Herve JC. Endogenous protein phosphatase 1 runs down gap junctional communication of rat ventricular myocytes. Am J Physiol Cell Physiol. 2001;281:C1648–C1656. doi: 10.1152/ajpcell.2001.281.5.C1648. [DOI] [PubMed] [Google Scholar]
- 31.Janssens V, Goris J. Protein phosphatase 2A: a highly regulated family of serine/threonine phosphatases implicated in cell growth and signalling. Biochem J. 2001;353:417–439. doi: 10.1042/0264-6021:3530417. doi:10.1042/0264-6021:3530417. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Ke Y, Lei M, Solaro RJ. Regulation of cardiac excitation and contraction by p21 activated kinase-1. Prog Biophys Biol. 2008;98:235–250. doi: 10.1016/j.pbiomolbio.2009.01.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Good MC, Zalatan JG, Lim WA. Scaffold proteins: hubs for controlling the flow of cellular information. Science. 2011;332:680–686. doi: 10.1126/science.1198701. doi:10.1126/science.1198701. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Daniels RH, Bokoch GM. p21-activated protein kinase: a crucial component of morphological signaling? Trends Biochem Sci. 1999;24:350–355. doi: 10.1016/s0968-0004(99)01442-5. doi:10.1016/S0968-0004(99)01442-5. [DOI] [PubMed] [Google Scholar]
- 35.Manser E, Leung T, Salihuddin H, Zhao ZS, Lim L. A brain serine/threonine protein kinase activated by Cdc42 and Rac1. Nature. 1994;367:40–46. doi: 10.1038/367040a0. doi:10.1038/367040a0. [DOI] [PubMed] [Google Scholar]
- 36.Bokoch GM. Biology of the p21-activated kinases. Annu Rev Biochem. 2003;72:743–781. doi: 10.1146/annurev.biochem.72.121801.161742. [DOI] [PubMed] [Google Scholar]
- 37.Sells MA, Chernoff J. Emerging from the Pak: the p21-activated protein kinase family. Trends Cell Biol. 1997;7:162–167. doi: 10.1016/S0962-8924(97)01003-9. doi:10.1016/S0962-8924(97)01003-9. [DOI] [PubMed] [Google Scholar]
- 38.Sheehan KA, Ke Y, Wolska BM, Solaro RJ. Expression of active p21-activated kinase-1 induces Ca2+ flux modification with altered regulatory protein phosphorylation in cardiac myocytes. Am J Physiol Cell Physiol. 2009;296:C47–C58. doi: 10.1152/ajpcell.00012.2008. doi:10.1152/ajpcell.00012.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Ke Y, Lei M, Collins TP, Rakovic S, Mattick PA, Yamasaki M, et al. Regulation of L-type calcium channel and delayed rectifier potassium channel activity by p21-activated kinase-1 in guinea pig sinoatrial node pacemaker cells. Circ Res. 2007;100:1317–1327. doi: 10.1161/01.RES.0000266742.51389.a4. doi:10.1161/01.RES.0000266742.51389.a4. [DOI] [PubMed] [Google Scholar]
- 40.Cheng G, Takahashi M, Shunmugavel A, Wallenborn JG, DePaoli-Roach AA, Gergs U, et al. Basis for MAP4 dephosphorylation-related microtubule network densification in pressure overload cardiac hypertrophy. J Biol Chem. 2010;285:38125–38140. doi: 10.1074/jbc.M110.148650. doi:10.1074/jbc.M110.148650. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.