Abstract
The purpose of this Institutional Review Board-approved study was to identify risk factors of caries lesion progression in children enrolled in rural schools in Puerto Rico. A convenience sample of 408 children (5-13 yrs old) was examined at baseline and at 12 and 24 mos with the International Caries Detection and Assessment System (ICDAS). A total of 395 caregivers completed a 25-item questionnaire including socio-demographic, dietary, protective factors, disease experience, and access to care. Caries progression was significant (89% and 91% at 12 and 24 mos, respectively). Multiple-variable models for predicting children with lesion progression and numbers of lesions progressing were calculated for 2 outcome variables (any-progression vs. progression-toward-cavitation). Models developed had areas under the receiver operating characteristic (ROC) curve ranging between 0.70 and 0.79 and were very similar regardless of the outcome (progression criteria), prediction time (12-24 mos), or inclusion (or not) of previous caries experience. Significant predictors of disease progression collected through a parent-completed questionnaire included questions related to caries experience in the child or caregiver, and the caregiver’s rating of the child’s oral health.
Keywords: longitudinal study, Hispanic, dental caries, risk assessment, lesion progression, number of lesions
Introduction
Risk-based prevention and management have been recognized as the cornerstones of modern caries management (Zero et al., 2001; Featherstone, 2003; Fontana and Zero, 2006; Twetman and Fontana, 2009). The fact that the presence of recent restorations is one of the greatest indicators of future caries risk (Zero et al., 2001) only proves that the act of surgically treating the lesion does little to reduce caries risk. Caries risk assessment involves an analysis of the probability that there will be a change in the number (incidence), severity, and/or activity of caries lesions (Fontana and Zero, 2006). Because of the multifactorial nature of the caries process, and the fact that the disease is very dynamic (e.g., lesions can progress and/or regress), studies on risk assessment tend to be complex, with a multitude of variables challenging the prediction at different times during life (Twetman and Fontana, 2009). In addition, risk factors may vary based on race, culture, and ethnicity (Huntington et al., 2002; Shiboski et al., 2003; Eckert et al., 2010; Fontana et al., 2010). For a clinician, the concepts of risk assessment and prognosis are an essential part of clinical decision-making. In fact, the dentist’s overall subjective impression of the patient might have good caries-predictive power (Disney et al., 1992), but it is unclear how this information is incorporated into everyday practice. A recent survey of US practices suggests that a significant proportion of dentists had yet to adopt treatments based on assessment of caries risk (Riley et al., 2010), even when multiple expert-opinion tools are available for children [e.g., Caries Assessment Tool-CAT of the American Academy of Pediatric Dentistry, developed for use in multiple settings (2007); the American Dental Association’s Caries Risk Tool (2008); the Caries Management by Risk Assessment tool (Ramos-Gomez et al., 2007)]. Therefore, a more objective, easy-to-implement, and validated risk tool is highly desirable, particularly for use in non-dental settings (e.g., schools, medical offices), to help target limited human/economic resources toward disease prevention.
The objective of this study was to identify risk factors of caries lesion progression and numbers of lesions progressing in children enrolled in rural schools in the Commonwealth of Puerto Rico. This project is part of a larger prospective longitudinal study to establish the feasibility of using early non-cavitated lesions as a surrogate for cavitated lesions, by studying the natural history of dental caries over 4 yrs (Ferreira-Zandona et al., 2010).
Materials & Methods
This longitudinal study was approved by Indiana University and the University of Puerto Rico. Children (N = 529) in 3 public schools in the area of Aguas Buenas were recruited. For inclusion, parental consent was obtained, and children had to be 5 to 13 yrs of age, provide assent if older than 7 yrs, be available for all examination visits, have at least one permanent molar with at least one unrestored surface, have no medical problem for participation (i.e., need of premedication, epilepsy), and allow examination of the oral cavity.
We examined a convenience sample of 408 children at baseline and 12 and 24 mos to monitor caries development/progression in primary and permanent teeth, using the International Caries Detection and Assessment System (ICDAS) applied by a single calibrated dentist (Ismail et al., 2007). Ten percent of the children were re-examined after each visit to determine intra- and inter-examiner (examiner and back-up examiner) reliability. At each visit, teeth were cleaned with a toothbrush, air-dried, and assessed under light, without magnification. Bitewing radiographs were taken at baseline and annually thereafter. Risk questionnaires were sent home to all caregivers at the 12- and 24-mo examinations. ‘Caregiver’ was defined as the individual consistently responsible for the child’s housing, health, and safety.
A total of 395 caregivers completed and returned the 25-item questionnaire (English version in the Appendix) at both time intervals, which included socio-demographic, dietary, protective factors, disease experience by the child and caregiver, and access to care. The questionnaire was adapted from a previously published one used in preschool children (Fontana et al., 2010). At every examination, caregivers were informed of conditions requiring treatment, and the child was referred for care. Data were analyzed for identification of children with lesion progression and numbers of lesions progressing using 2 types of primary outcomes for predictive modeling:
Any-Progression (caries if ICDAS ≥ 1): Presence of at least one new lesion ICDAS ≥ 1 (i.e., any lesion), one new filling, and/or progression of a lesion from a score of 1-2 (first initial signs of caries lesion) to 3 or higher (established caries), or from a score of 3-4 (established caries) to 5 or higher (severe caries) between the 2 examinations.
Progression-Toward-Cavitation (caries if ICDAS ≥ 3): Presence of at least one new lesion ICDAS ≥ 3, one new filling, and/or progression of a lesion from a score of 1-2 to 3 or higher, or lesions progressing from a score of 3-4 to 5 or higher between the 2 examinations.
Predictors included baseline questionnaire responses and ICDAS examination results. Questionnaire items were categorized as: demographics/access to care, medical history, dental history, dental habits, dietary habits, and protective factors. Repeatability of the ICDAS scores was assessed with 2-way contingency tables and kappa statistics.
Logistic regressions were performed for progression at 12 and 24 mos using each predictor individually. Parsimonious multiple-variable models were developed with a backward-elimination procedure to retain predictors with p < 0.05 in the final model. The area under the receiver operating characteristic (ROC) curve (AUC) was calculated to assess the overall predictive ability of the final models. The use of the AUC is one way of measuring the accuracy of caries risk assessment and is a common way to measure the prognostic ability of risk factors. Baseline ICDAS scores were examined for significance in 2 ways. For models that could be used in non-dental settings, it would be important to assess predictors collected through a questionnaire first, without using the results of a caries examination. Thus, caries experience was not used until the prediction model had been developed, and then was added to see if it improved the prediction. For models that could be used in a dental setting, we started with previous caries experience (dmfs/DMFS at baseline, using ICDAS ≥ 3 as caries), since this would be the easiest variable to obtain, and included additional questionnaire-based predictors as explained previously. A similar process was utilized for the Poisson regression analyses of the numbers of lesions with progression.
Results
Most caregivers were mothers, but also included 19 fathers, 12 grandmothers, and 5 others. The children (49% females, 51% males) were 5 to 13 yrs old at baseline (9.7 ± 2.2 yrs, mean ± standard deviation). The ethnic/racial distribution of the children was self-reported (rounded%): Hispanic (all races), 91%; African-American-Non-Hispanic, < 1%; Other-Non-Hispanic, < 1%; and unknown, 8%. Children were covered by health insurance (86%) through the 1993 Puerto Rico Health Reform, while 48% of caregivers had completed high school, and 51% had technical/college degree. Children had a dmfs/DMFS (ICDAS ≥ 1) = 15.7 ± 12.4 (mean ± standard deviation) and dmfs/DMFS (ICDAS ≥ 3) = 8.2 ± 8.6 at baseline. By the 12-mo examination, 348 (89%) children had at least one surface with Any-Progression and 239 (61%) with a Progression-Toward-Cavitation [dmfs/DMFS (ICDAS ≥ 1) = 17.9 ± 13.8; dmfs/DMFS (ICDAS ≥ 3) = 8.3 ± 8.5]. By the 24-mo examination, 358 (91%) children had at least one surface with Any-Progression and 268 (68%) with a Progression-Toward-Cavitation [dmfs/DMFS (ICDAS ≥ 1) = 16.8 ± 12.4; dmfs/DMFS (ICDAS ≥ 3) = 8.4 ± 8.1]. There were few lesion reversals (for ICDAS ≥ 1 = 1.9 ± 2.4 at 12 mos and 2.1 ± 2.8 at 24 mos; for ICDAS ≥ 3 = 0.2 ± 0.5 at 12 and 24 mos). Repeatability of the ICDAS severity scores at all examinations (mean weighted kappa = 0.72) was acceptable. Radiographic data were not included in the analysis of the present study.
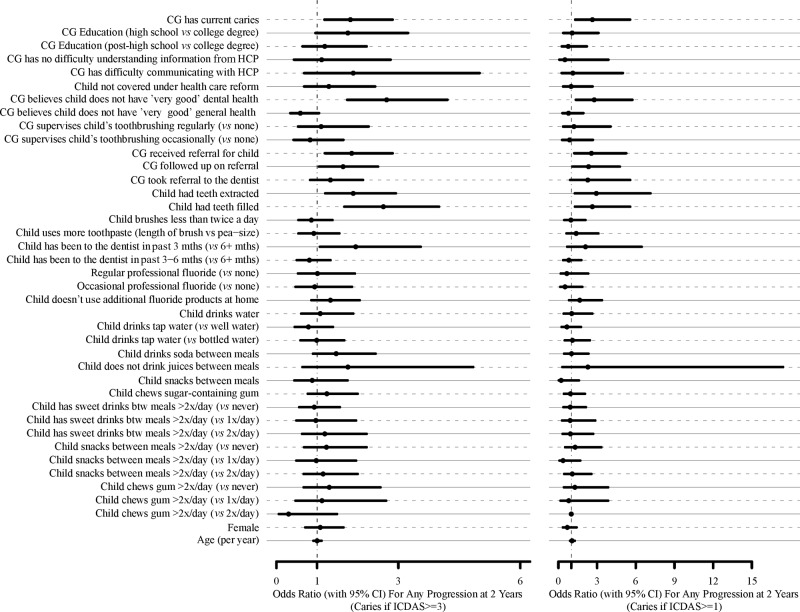
There were very few missing responses for each questionnaire item (2.2 ± 1.8%; range = 0-8.1%). For models developed for use in non-dental settings, predictors of the primary outcome were first examined individually. Significance of individual predictors for identification of a child is shown in the Fig., with lesion progression at 24 mos according to the 2 outcome criteria. The final multiple-variable models for predicting children at risk based on lesion progression at 12 and 24 mos are shown in Table 1. The AUC and identified predictors were in general very similar and ranged from 0.70 to 0.79 regardless of progression criteria and time of follow-up. In general, high sensitivities could be reached, but at the expense of specificity. Although the addition of previous caries experience (dmfs/DMFS at baseline, using ICDAS ≥ 3) does add to the models developed, it does not greatly affect the prediction ability of the model. For models that could be used in a dental setting, predictors and predictive ability of the developed models were in general very similar to the ones developed for non-dental settings, but included fewer questions. The numbers of caries lesion progression counts per questionnaire item for each outcome variable (Table 2) were very similar among questions, and much higher for Any-Progression. The final multiple-variable models for predicting numbers of lesions progressing at 12 and 24 mos are shown in Table 3 (i.e., numbers of lesions are additive across all variables in a model). These models included predictors similar to those developed to identify children with lesions progressing, with the addition of the use of additional fluoride products at home.
Figure.
Odds ratios with 95% confidence intervals for questionnaire items for Progression-1 (caries if ICDAS ≥ 1) and Progression-2 (caries if ICDAS ≥ 3) at 24 mos. CG = Primary caregiver. HCP = Health-care provider.
Table 1.
Multivariate Caries Risk Models for Identification of At-risk Individuals with Lesions Progressing at 12 and 24 Months According to 2 Outcome Criteria
| Model (No dmfs/DMFS) | Model (add dmfs/DMFS at end) | Model (start with dmfs/DMFS then add other variables) | |||||
|---|---|---|---|---|---|---|---|
| Examination Period | Predictors | p-value | Odds Ratio (95% CI) | p-value | Odds Ratio (95% CI) | p-value | Odds Ratio (95% CI) |
| 12 months (ICDAS ≥ 3) | Child had a tooth extracted | 0.0002 | 2.56 ( 1.56, 4.17) | 0.0111 | 1.97 (1.17, 3.32) | 0.0177 | 1.96 (1.12, 3.44) |
| Child had a tooth restored | 0.0029 | 2.08 (1.28, 3.33) | 0.0323 | 1.74 (1.05, 2.89) | 0.0125 | 1.97 (1.16, 3.36) | |
| Time elapsed since last dental visit | 0.0063 | 2.22 (1.19, 4.17) for < 3 months vs. ≥ 6 months |
0.0129 | 2.04 (1.08, 3.85) | 0.0190 | 2.10 (1.06, 4.17) | |
| 0.76 (0.45, 1.30) for 3, 6 months vs. ≥ 6 months | 0.74 (0.43, 1.26) | 0.74 (0.42, 1.32) | |||||
| CG does not consider child’s oral health to be ‘very good’ | 0.0001 | 2.68 (1.67, 4.32) | 0.0005 | 2.37 (1.46, 3.86) | 0.0014 | 2.33 (1.39, 3.90) | |
| CG received a referral for the child | 0.0218 | 1.80 (1.09, 2.98) | |||||
| Child drinks soda between meals | 0.0496 | 1.75 (1.00, 3.08) | |||||
| dmfs/DMFS | 0.0065 | 1.17 (1.05, 1.31) | 0.0260 | 1.14 (1.02, 1.29) | |||
| AUC 0.75, 80% sensitivity, 58% specificity | AUC 0.77, 81% sensitivity, 58% specificity | AUC 0.79, 81% sensitivity, 57% specificity | |||||
| 12 months (ICDAS ≥ 1) | Child had a tooth extracted | 0.0006 | 3.97 (1.45, 10.9) | 0.0067 | 4.05 (1.47, 11.1) | 0.0067 | 4.05 (1.47, 11.1) |
| CG does not consider child’s oral health to be ‘very good’ | 0.0338 | 5.43 ( 2.07, 14.3) | 0.0217 | 2.27 (1.13, 4.59) | 0.0217 | 2.27 (1.13, 4.59) | |
| dmfs/DMFS | 0.0835 | 1.19 (0.98, 1.44) | 0.0835 | 1.19 (0.98, 1.44) | |||
| AUC 0.74, 77% sensitivity, 63% specificity | AUC 0.77, 79% sensitivity, 63% specificity | AUC 0.77, 79% sensitivity, 63% specificity | |||||
| 24 months (ICDAS ≥ 3) | Child had a tooth restored | 0.0004 | 2.38 (1.47, 3.85) | 0.0215 | 1.81 (1.09, 3.00) | 0.0162 | 1.83 (1.12, 2.99) |
| Time elapsed since last dental visit | 0.0270 | 1.93 (1.02, 3.68) for < 3 months vs. ≥ 6 months | 0.0602 | 1.67 (0.85, 3.23) | |||
| 0.76 (0.45, 1.30) for 3, 6 months vs. ≥ 6 months | 0.72 (0.42, 1.23) | ||||||
| CG does not consider child’s oral health to be ‘very good’ | 0.0001 | 2.80 (1.74, 4.48) | 0.0010 | 2.28 (1.40, 3.73) | 0.0029 | 2.05 (1.28, 3.28) | |
| dmfs/DMFS | 0.0012 | 1.21 (1.08, 1.36) | 0.0007 | 1.21 (1.08, 1.35) | |||
| AUC 0.70, 73% sensitivity, 61% specificity | AUC 0.73, 73% sensitivity, 61% specificity | AUC 0.70, 73% sensitivity, 61% specificity | |||||
| 24 months (ICDAS ≥ 1) | CG has current caries | 0.0160 | 2.62 (1.20, 5.71) | 0.0299 | 2.40 (1.09, 5.32) | 0.0097 | 2.77 (1.28, 5.99) |
| Child had a tooth restored | 0.0321 | 2.31 (1.07, 4.98) | 0.1659 | 1.80 (0.78, 4.12) | |||
| CG received a referral for the child | 0.0289 | 2.43 (1.10, 5.38) | 0.0411 | 2.30 (1.03, 5.13) | 0.0157 | 2.61 (1.20, 5.68) | |
| CG does not consider child’s oral health to be ‘very good’ | 0.0363 | 2.33 (1.06, 5.15) | 0.0793 | 2.04 (0.92, 4.55) | |||
| dmfs/DMFS | 0.1318 | 1.18 (0.95, 1.47) | 0.0118 | 1.33 (1.07, 1.66) | |||
| AUC 0.75, 82% sensitivity, 59% specificity | AUC 0.76, 84% sensitivity, 59% specificity | AUC 0.77, 75% sensitivity, 61% specificity | |||||
Table 2.
Number of Caries Lesion Progression Counts per Survey Question Analyzed Individually
| 12-month (ICDAS ≥ 1) | 12-month (ICDAS ≥ 3) | 24-month (ICDAS ≥ 1) | 24-month (ICDAS ≥ 3) | ||||||
|---|---|---|---|---|---|---|---|---|---|
| Question | Count (95% CI) | p-value | Count (95% CI) | p-value | Count (95% CI) | p-value | Count (95% CI) | p-value | |
| CG has current caries | No | 6.80 (5.83, 7.93) | 0.0195 | 2.19 (1.75, 2.75) | 0.0525 | 7.88 (6.81, 9.11) | 0.0478 | 2.96 (2.4, 3.65) | 0.0261 |
| Yes | 8.47 (7.62, 9.41) | 2.85 (2.45, 3.32) | 9.41 (8.5, 10.42) | 3.91 (3.41, 4.5) | |||||
| CG supervises child’s toothbrushing | Regularly | 8.24 (7.15, 9.5) | 0.6827 | 3.11 (2.57, 3.76) | 0.0651 | 9.54 (8.34, 10.92) | 0.2003 | 4.16 (3.48, 4.97) | 0.0501 |
| Occasionally | 7.66 (6.79, 8.65) | 2.42 (2.02, 2.89) | 8.72 (7.77, 9.8) | 3.37 (2.86, 3.97) | |||||
| No | 7.45 (5.75, 9.64) | 2.00 (1.32, 3.02) | 7.36 (5.65, 9.59) | 2.60 (1.76, 3.83) | |||||
| CG education | High school | 8.51 (7.53, 9.62) | 0.0614 | 2.97 (2.5, 3.52) | 0.0149 | 9.92 (8.85, 11.13) | 0.0068 | 3.96 (3.37, 4.65) | 0.0825 |
| Post-high school | 7.29 (6.24, 8.51) | 1.92 (1.49, 2.46) | 7.66 (6.58, 8.93) | 2.93 (2.36, 3.65) | |||||
| College degree | 6.33 (4.95, 8.09) | 2.61 (1.9, 3.57) | 7.30 (5.79, 9.19) | 3.31 (2.44, 4.49) | |||||
| CG received referral for child | No | 7.11 (6.18, 8.18) | 0.0513 | 2.48 (2.04, 3.03) | 0.4232 | 7.82 (6.83, 8.96) | 0.0112 | 3.24 (2.69, 3.91) | 0.1723 |
| Yes | 8.48 (7.60, 9.48) | 2.76 (2.35, 3.24) | 9.75 (8.78, 10.82) | 3.83 (3.3, 4.44) | |||||
| CG followed up on referral | No | 7.19 (6.24, 8.28) | 0.1208 | 2.15 (1.73, 2.66) | 0.0174 | 7.83 (6.82, 8.98) | 0.0231 | 2.93 (2.4, 3.57) | 0.0074 |
| Yes | 8.29 (7.41, 9.27) | 2.95 (2.53, 3.45) | 9.56 (8.6, 10.62) | 4.07 (3.53, 4.69) | |||||
| CG took child to the dentist after referral | No | 7.84 (7.04, 8.75) | 0.8317 | 2.5 (2.13, 2.93) | 0.3174 | 8.51 (7.65, 9.46) | 0.1446 | 3.38 (2.91, 3.92) | 0.1708 |
| Yes | 8.01 (6.85, 9.36) | 2.87 (2.31, 3.56) | 9.73 (8.43, 11.24) | 4.02 (3.3, 4.9) | |||||
| Child had teeth extracted | No | 7.22 (6.4, 8.14) | 0.0461 | 2.20 (1.84, 2.63) | 0.0040 | 7.90 (7.03, 8.87) | 0.0037 | 3.15 (2.68, 3.7) | 0.0191 |
| Yes | 8.63 (7.60, 9.80) | 3.17 (2.66, 3.77) | 10.11 (8.97, 11.39) | 4.15 (3.52, 4.89) | |||||
| Child had teeth filled | No | 6.51 (5.54, 7.64) | 0.0044 | 1.74 (1.35, 2.25) | 0.0001 | 7.42 (6.37, 8.64) | 0.0039 | 2.43 (1.93, 3.06) | 0.0000 |
| Yes | 8.55 (7.70, 9.50) | 3.10 (2.68, 3.57) | 9.66 (8.74, 10.67) | 4.18 (3.66, 4.77) | |||||
| Child brushes twice a day | No | 7.87 (6.69, 9.25) | 0.9406 | 2.53 (2.00, 3.21) | 0.7860 | 8.74 (7.47, 10.23) | 0.8517 | 3.42 (2.75, 4.27) | 0.6415 |
| Yes | 7.81 (7.04, 8.66) | 2.63 (2.27, 3.05) | 8.90 (8.06, 9.83) | 3.64 (3.18, 4.17) | |||||
| Amount of toothpaste child uses | Length of brush | 7.67 (6.94, 8.48) | 0.4745 | 2.66 (2.30, 3.07) | 0.6436 | 8.40 (7.62, 9.26) | 0.0635 | 3.44 (3.01, 3.94) | 0.4532 |
| Bean-size | 8.28 (6.90, 9.93) | 2.47 (1.86, 3.27) | 10.12 (8.56, 11.97) | 3.83 (3.01, 4.88) | |||||
| Child has been to the dentist | Last 3 months | 9.57 (8.13, 11.26) | 0.0256 | 4.22 (3.46, 5.14) | 0.0000 | 10.71 (9.17, 12.52) | 0.0240 | 5.53 (4.59, 6.66) | 0.0000 |
| 3-6 months | 7.18 (6.06, 8.50) | 2.29 (1.80, 2.92) | 7.90 (6.71, 9.30) | 3.11 (2.48, 3.88) | |||||
| 6+ months | 7.38 (6.47, 8.41) | 1.97 (1.60, 2.41) | 8.64 (7.64, 9.77) | 2.90 (2.42, 3.48) | |||||
| Professional fluoride | Regularly | 7.83 (6.95, 8.83) | 0.4847 | 2.61 (2.20, 3.10) | 0.9904 | 9.21 (8.23, 10.30) | 0.4356 | 3.79 (3.25, 4.43) | 0.2340 |
| Occasionally | 7.43 (6.34, 8.69) | 2.64 (2.12, 3.29) | 8.18 (7.02, 9.53) | 3.06 (2.46, 3.82) | |||||
| No | 8.78 (7.05, 10.94) | 2.56 (1.83, 3.60) | 9.24 (7.42, 11.49) | 3.91 (2.91, 5.25) | |||||
| Child uses additional fluoride products at home | No | 8.46 (7.56, 9.47) | 0.0333 | 2.81 (2.38, 3.31) | 0.1762 | 9.42 (8.45, 10.51) | 0.0475 | 3.76 (3.22, 4.38) | 0.2968 |
| Yes | 6.97 (6.06, 8.01) | 2.35 (1.92, 2.87) | 7.92 (6.94, 9.05) | 3.31 (2.75, 3.98) | |||||
| Child drinks water between meals | No | 8.83 (7.27, 10.72) | 0.2552 | 2.46 (1.81, 3.35) | 0.6070 | 8.83 (7.24, 10.76) | 0.9205 | 3.30 (2.48, 4.40) | 0.5006 |
| Yes | 7.77 (7.04, 8.57) | 2.69 (2.34, 3.09) | 8.93 (8.13, 9.80) | 3.68 (3.23, 4.18) | |||||
| Type of water child drinks | Well water | 8.35 (7.01, 9.96) | 0.5726 | 2.41 (1.83, 3.18) | 0.8499 | 9.50 (8.00, 11.27) | 0.5863 | 3.75 (2.95, 4.77) | 0.8144 |
| Bottled water | 7.31 (6.15, 8.70) | 2.61 (2.04, 3.34) | 8.38 (7.08, 9.92) | 3.37 (2.66, 4.26) | |||||
| Tap water | 7.81 (6.85, 8.89) | 2.65 (2.19, 3.20) | 8.78 (7.73, 9.98) | 3.61 (3.03, 4.30) | |||||
| Child drinks soda between meals | No | 7.54 (6.30, 9.02) | 0.6643 | 1.86 (1.38, 2.52) | 0.0073 | 8.48 (7.12, 10.1) | 0.5767 | 3.13 (2.42, 4.04) | 0.2322 |
| Yes | 7.89 (7.13, 8.72) | 2.87 (2.50, 3.30) | 8.98 (8.15, 9.90) | 3.72 (3.25, 4.26) | |||||
| Child drinks juices between meals | No | 9.79 (7.15, 13.4) | 0.1686 | 2.67 (1.61, 4.41) | 0.9503 | 9.17 (6.58, 12.76) | 0.8109 | 3.25 (1.99, 5.31) | 0.6976 |
| Yes | 7.72 (7.06, 8.46) | 2.62 (2.30, 2.99) | 8.79 (8.06, 9.58) | 3.59 (3.18, 4.04) | |||||
| Child has sweet drinks between meals | Never | 7.55 (6.43, 8.85) | 0.7944 | 2.74 (2.20, 3.42) | 0.8632 | 8.64 (7.42, 10.05) | 0.6391 | 3.5 (2.84, 4.33) | 0.3446 |
| 1x/day | 8.69 (6.88, 10.98) | 2.80 (1.98, 3.95) | 10.10 (8.10, 12.60) | 4.57 (3.42, 6.11) | |||||
| 2x/day | 7.62 (6.08, 9.54) | 2.37 (1.69, 3.32) | 8.42 (6.76, 10.47) | 3.22 (2.35, 4.40) | |||||
| >2x/day | 7.9 (6.89, 9.06) | 2.54 (2.08, 3.11) | 8.69 (7.61, 9.92) | 3.39 (2.81, 4.10) | |||||
| Child snacks between meals | Never | 7.97 (6.65, 9.54) | 0.3273 | 2.29 (1.73, 3.04) | 0.2970 | 7.85 (6.52, 9.46) | 0.4352 | 2.99 (2.29, 3.90) | 0.3078 |
| 1x/day | 8.86 (7.19, 10.94) | 3.27 (2.45, 4.37) | 9.86 (8.05, 12.09) | 4.31 (3.28, 5.65) | |||||
| 2x/day | 7.02 (5.98, 8.25) | 2.43 (1.93, 3.05) | 8.91 (7.7, 10.31) | 3.67 (3.00, 4.49) | |||||
| >2x/day | 8.19 (7.00, 9.60) | 2.77 (2.21, 3.48) | 8.96 (7.67, 10.45) | 3.54 (2.85, 4.40) | |||||
| Child snacks between meals | No | 9.18 (7.24, 11.63) | 0.1716 | 2.38 (1.61, 3.51) | 0.5921 | 10.84 (8.67, 13.56) | 0.0830 | 4.6 (3.40, 6.22) | 0.1068 |
| Yes | 7.65 (6.95, 8.42) | 2.66 (2.32, 3.05) | 8.71 (7.94, 9.55) | 3.48 (3.06, 3.96) | |||||
| Child chews gum | Never | 7.61 (6.83, 8.48) | 0.7831 | 2.60 (2.23, 3.03) | 0.9985 | 8.47 (7.63, 9.39) | 0.4559 | 3.5 (3.03, 4.03) | 0.5517 |
| 1x/day | 7.96 (6.17, 10.26) | 2.57 (1.77, 3.72) | 9.39 (7.40, 11.92) | 3.09 (2.14, 4.44) | |||||
| 2x/day | 7.84 (5.26, 11.69) | 2.68 (1.52, 4.73) | 10.79 (7.64, 15.25) | 4.53 (2.83, 7.23) | |||||
| >2x/day | 8.72 (6.91, 11.01) | 2.66 (1.87, 3.78) | 9.60 (7.66, 12.03) | 3.98 (2.93, 5.41) | |||||
| Child chews sugar-containing gum | No | 7.80 (6.77, 8.98) | 0.8463 | 2.76 (2.25, 3.37) | 0.8698 | 8.72 (7.60, 10.02) | 0.7061 | 3.57 (2.96, 4.32) | 0.6346 |
| Yes | 7.94 (7.00, 9.01) | 2.69 (2.24, 3.24) | 9.04 (7.99, 10.22) | 3.8 (3.22, 4.49) | |||||
| CG believes child has ‘very good’ dental health | No | 9.25 (8.37, 10.22) | 0.0000 | 3.05 (2.62, 3.54) | 0.0007 | 10.06 (9.11, 11.11) | 0.0000 | 4.15 (3.62, 4.76) | 0.0003 |
| Yes | 5.47 (4.65, 6.43) | 1.91 (1.51, 2.42) | 6.85 (5.90, 7.97) | 2.63 (2.12, 3.26) | |||||
| CG believes child has ‘very good’ general health | No | 7.46 (6.74, 8.25) | 0.0426 | 2.58 (2.24, 2.99) | 0.6810 | 8.56 (7.77, 9.43) | 0.1219 | 3.39 (2.96, 3.88) | 0.1021 |
| Yes | 9.23 (7.75, 11.00) | 2.76 (2.11, 3.61) | 10.02 (8.44, 11.9) | 4.26 (3.38, 5.36) | |||||
| CG has difficulty understanding information from HCP | No | 7.81 (7.14, 8.55) | 0.6948 | 2.65 (2.33, 3.01) | 0.5743 | 8.77 (8.04, 9.57) | 0.4409 | 3.55 (3.15, 4.01) | 0.5388 |
| Yes | 8.43 (5.86, 12.11) | 2.24 (1.24, 4.03) | 10.1 (7.19, 14.18) | 4.14 (2.6, 6.59) | |||||
| CG has difficulty communicating with HCP | No | 7.65 (6.98, 8.38) | 0.0354 | 2.59 (2.27, 2.96) | 0.5625 | 8.72 (7.99, 9.52) | 0.2195 | 3.55 (3.14, 4.00) | 0.4350 |
| Yes | 10.8 (8.06, 14.48) | 3.00 (1.88, 4.78) | 10.68 (7.9, 14.45) | 4.24 (2.78, 6.46) | |||||
| Child is covered under health care reform | No | 7.32 (5.77, 9.29) | 0.6128 | 2.71 (1.96, 3.75) | 0.7146 | 8.86 (7.10, 11.05) | 0.9290 | 3.36 (2.46, 4.58) | 0.7465 |
| Yes | 7.82 (7.11, 8.60) | 2.54 (2.21, 2.92) | 8.76 (8.00, 9.6) | 3.55 (3.13, 4.02) | |||||
| Race | Black | 8.53 (5.84, 12.44) | 0.2194 | 2.95 (1.73, 5.04) | 0.2992 | 9.47 (6.59, 13.63) | 0.0265 | 5.00 (3.23, 7.73) | 0.0003 |
| Other | 9.50 (7.71, 11.70) | 3.32 (2.48, 4.46) | 11.66 (9.63, 14.11) | 5.55 (4.37, 7.07) | |||||
| Unknown | 8.22 (6.11, 11.06) | 2.13 (1.31, 3.45) | 8.53 (6.35, 11.46) | 2.34 (1.44, 3.83) | |||||
| White | 7.42 (6.69, 8.23) | 2.49 (2.15, 2.90) | 8.29 (7.50, 9.16) | 3.21 (2.79, 3.69) | |||||
| Gender | Female | 7.62 (6.73, 8.62) | 0.5337 | 2.70 (2.27, 3.20) | 0.5779 | 8.34 (7.39, 9.41) | 0.1766 | 3.56 (3.03, 4.19) | 0.9926 |
| Male | 8.05 (7.13, 9.09) | 2.51 (2.09, 3.01) | 9.35 (8.34, 10.49) | 3.56 (3.02, 4.19) | |||||
| Number of counts increases as age increases (per yr) | 1.01 (0.97, 1.05) | 0.6146 | 0.92 (0.87, 0.98) | 0.0068 | 0.99 (0.95, 1.03) | 0.4783 | 0.98 (0.93, 1.04) | 0.4827 | |
Table 3.
Multivariate Caries Risk Models for Identification of Numbers of Lesions Progressing at 12 and 24 Months According to 2 Outcome Criteria
| Model (no dmfs/DMFS) | Model (add dmfs/DMFS at end) | Model (start with dmfs/DMFS then add other variables) | ||||||
|---|---|---|---|---|---|---|---|---|
| Predictors | Examination Period | Count (95% CI) | p-value | Count (95% CI) | p-value | Count (95% CI) | ||
| 12-month (ICDAS ≥ 3) | Child had teeth filled | No | 1.52 (1.15, 2.01) | 0.0003 | 1.58 (1.20, 2.09) | 0.0021 | 1.63 (1.23, 2.14) | 0.0042 |
| Yes | 2.57 (2.12, 3.10) | 2.48 (2.05, 3.00) | 2.46 (2.04, 2.98) | |||||
| Child has been to the dentist: | Last 3 months | 3.10 (2.42, 3.98) | 0.0000 | 2.97 (2.32, 3.82) | 0.0000 | 3.01 (2.35, 3.86) | 0.0000 | |
| 3-6 months | 1.70 (1.29, 2.25) | 1.73 (1.31, 2.28) | 1.75 (1.33, 2.30) | |||||
| 6+ months | 1.46 (1.13, 1.87) | 1.51 (1.18, 1.94) | 1.52 (1.19, 1.95) | |||||
| Child drinks soda between meals | No | 1.60 (1.17, 2.19) | 0.0090 | 1.62 (1.19, 2.20) | 0.0119 | 1.63 (1.20, 2.22) | 0.0104 | |
| Yes | 2.44 (2.06, 2.89) | 2.43 (2.05, 2.87) | 2.46 (2.08, 2.90) | |||||
| CG believes child has ‘very good’ dental health | No | 2.41 (1.96, 2.96) | 0.0036 | 2.31 (1.87, 2.84) | 0.0277 | 2.33 (1.89, 2.87) | 0.0287 | |
| Yes | 1.62 (1.25, 2.09) | 1.70 (1.32, 2.19) | 1.72 (1.34, 2.21) | |||||
| Age | 0.93 (0.87, 0.98) | 0.0103 | 0.95 (0.89, 1.00) | 0.0717 | ||||
| For baseline dmfs/DMFS (ICDAS ≥ 3) | 1.02 (1.01, 1.03) | 0.0026 | 1.02 (1.01, 1.04) | 0.0004 | ||||
| 12-month (ICDAS ≥ 1) | Child had teeth filled | No | 6.16 (5.22, 7.27) | 0.0227 | 6.35 (5.39, 7.48) | 0.0991 | ||
| Yes | 7.70 (6.84, 8.65) | 7.47 (6.64, 8.41) | ||||||
| Child has been to the dentist: | Last 3 months | 8.42 (7.08, 10.00) | 0.0122 | 8.15 (6.86, 9.69) | 0.0422 | 8.51 (7.23, 10.02) | 0.0367 | |
| 3-6 months | 6.11 (5.11, 7.30) | 6.15 (5.15, 7.34) | 6.56 (5.55, 7.75) | |||||
| 6+ months | 6.35 (5.50, 7.34) | 6.52 (5.65, 7.52) | 6.64 (5.80, 7.61) | |||||
| Child uses additional fluoride products at home | No | 7.54 (6.62, 8.58) | 0.0496 | 7.53 (6.63, 8.56) | 0.0486 | |||
| Yes | 6.29 (5.42, 7.30) | 6.30 (5.43, 7.30) | ||||||
| CG believes child has ‘very good’ dental health | No | 8.72 (7.74, 9.83) | 0.0000 | 8.46 (7.50, 9.54) | 0.0000 | 9.15 (8.22, 10.18) | 0.0000 | |
| Yes | 5.43 (4.60, 6.42) | 5.61 (4.75, 6.62) | 5.64 (4.80, 6.63) | |||||
| For baseline dmfs/DMFS (ICDAS ≥ 3) | 1.02 (1.01, 1.03) | 0.0021 | 1.02 (1.01, 1.03) | 0.0009 | ||||
| 24-month (ICDAS ≥ 3) | CG supervises child’s toothbrushing | Regularly | 3.51 (2.78, 4.43) | 0.0437 | 3.47 (2.75, 4.38) | 0.0657 | 3.68 (2.94, 4.61) | 0.0380 |
| Occasionally | 3.06 (2.43, 3.84) | 3.11 (2.47, 3.90) | 3.42 (2.77, 4.23) | |||||
| No | 2.07 (1.33, 3.21) | 2.11 (1.37, 3.27) | 2.19 (1.44, 3.32) | |||||
| CG received referral for child | No | 3.45 (2.56, 4.66) | 0.0473 | 3.56 (2.64, 4.80) | 0.0295 | |||
| Yes | 2.29 (1.70, 3.09) | 2.26 (1.67, 3.05) | ||||||
| CG followed up on referral | No | 2.14 (1.56, 2.92) | 0.0101 | 2.15 (1.58, 2.93) | 0.0096 | |||
| Yes | 3.70 (2.75, 4.97) | 3.74 (2.79, 5.02) | ||||||
| Child had teeth filled | No | 2.32 (1.74, 3.09) | 0.0036 | 2.40 (1.80, 3.19) | 0.0109 | 2.50 (1.91, 3.28) | 0.0031 | |
| Yes | 3.40 (2.70, 4.29) | 3.35 (2.66, 4.23) | 3.65 (2.93, 4.55) | |||||
| Child has been to the dentist: | Last 3 months | 4.23 (3.26, 5.50) | 0.0000 | 4.15 (3.20, 5.38) | 0.0000 | 4.34 (3.38, 5.59) | 0.0000 | |
| 3-6 months | 2.43 (1.82, 3.23) | 2.48 (1.86, 3.29) | 2.67 (2.03, 3.51) | |||||
| 6+ months | 2.16 (1.63, 2.87) | 2.22 (1.67, 2.94) | 2.38 (1.82, 3.10) | |||||
| CG believes child has ‘very good’ dental health | No | 3.51 (2.77, 4.44) | 0.0004 | 3.42 (2.70, 4.32) | 0.0039 | |||
| Yes | 2.25 (1.70, 2.97) | 2.35 (1.78, 3.11) | ||||||
| Race | Black | 3.08 (1.90, 5.00) | 0.0203 | 3.01 (1.86, 4.87) | 0.0163 | 3.16 (1.95, 5.11) | 0.0032 | |
| Other | 3.90 (2.94, 5.18) | 4.01 (3.02, 5.31) | 4.45 (3.42, 5.78) | |||||
| Unknown | 1.99 (1.23, 3.21) | 2.06 (1.28, 3.32) | 2.17 (1.36, 3.47) | |||||
| White | 2.60 (2.14, 3.16) | 2.60 (2.14, 3.16) | 2.73 (2.28, 3.28) | |||||
| For baseline dmfs/DMFS (ICDAS ≥ 3) | 1.02 (1.00, 1.03) | 0.0205 | 1.02 (1.01, 1.03) | 0.0006 | ||||
| 24-month (ICDAS ≥ 1) | CG followed up on referral | No | 7.15 (6.16, 8.31) | 0.0420 | 7.27 (6.27, 8.44) | 0.0818 | ||
| Yes | 8.61 (7.62, 9.72) | 8.52 (7.55, 9.62) | ||||||
| Child had teeth filled | No | 6.98 (5.95, 8.19) | 0.0140 | 7.19 (6.13, 8.43) | 0.0572 | |||
| Yes | 8.82 (7.86, 9.89) | 8.63 (7.69, 9.68) | ||||||
| Child has been to the dentist: | Last 3 months | 9.48 (8.00, 11.24) | 0.0117 | 9.25 (7.80, 10.95) | 0.0325 | |||
| 3-6 months | 6.73 (5.66, 8.01) | 6.82 (5.74, 8.10) | ||||||
| 6+ months | 7.56 (6.60, 8.67) | 7.74 (6.76, 8.86) | ||||||
| Child uses additional fluoride products at home | No | 8.61 (7.59, 9.76) | 0.0376 | 8.63 (7.62, 9.77) | 0.0382 | |||
| Yes | 7.15 (6.20, 8.26) | 7.19 (6.23, 8.28) | ||||||
| CG believes child has ‘very good’ dental health | No | 9.11 (8.07, 10.30) | 0.0015 | 8.90 (7.87, 10.06) | 0.0105 | 9.62 (8.69, 10.65) | 0.0011 | |
| Yes | 6.76 (5.79, 7.88) | 6.97 (5.98, 8.12) | 7.13 (6.15, 8.27) | |||||
| For baseline dmfs/DMFS (ICDAS ≥ 3) | 1.01 (1.00, 1.02) | 0.0061 | 1.02 (1.01, 1.03) | 0.0001 | ||||
Discussion
There are many disparities in dental caries experience in the US, with the disease being particularly prevalent in children from minority (racial and ethnic) and low socio-economic (SES) groups (Beltrán-Aguilar et al., 2005; Dye et al., 2007). Hispanics are the largest racial/ethnic minority group of US children and are in general underserved and high-risk populations because they experience a disproportionate burden of health risk factors, morbidity, suboptimal health status, underuse of health services, and impaired access to care. For example, Puerto Rican children have the highest prevalence of active asthma (14.1%), exceeding by far the prevalence for African-American-Non-Hispanics (10.2%) and Caucasian-Non-Hispanics (7.6%) (CDC, 2010). In our study, disease progression in this rural population was significant over the 24-mo follow-up period (89% and 91% at 12 and 24 mos, respectively), which is in agreement with high caries rates found in other studies of Hispanic population subgroups (Flores et al., 2002).
Objective caries risk assessment is greatly needed and can facilitate the process of early identification of children at high risk and assist in decision-making to tailor appropriate preventive interventions and the periodicity of these services. Unfortunately, “past caries experience” is one of the most powerful predictors of future caries development (Zero et al., 2001). However, from a disease management perspective, this is a less than desirable outcome, since the disease is manifested before it can be accurately predicted, and the ultimate goal of caries management is to prevent disease. It is also an impractical assessment indicator, especially for at-risk populations with difficulties accessing dental care (e.g., those from racial or ethnic minorities and low SES, or rural communities such as the one in this study). In fact, dental care is one of the most difficult health care services for low-income people to obtain, due to a lack of dental insurance, limited dental benefits available through public insurance programs, and a paucity of dentists available to serve these patients (Felland et al., 2008). Strategies to address these problems include promoting risk-based individualized preventive regimens in a variety of settings (e.g., dental and medical offices, schools) (USDHHS, 2000).
Multiple factors have been proposed in caries risk assessment, varying sometimes based on the age group at which they are targeted. Prediction models which include a variety of factors seem to increase the accuracy of the prediction in young children (Fontana et al., 2010b). A recent study in Singapore showed that caries prediction based on a questionnaire reached a sensitivity/specificity of 0.82/0.81 in 3- to 6-year-olds (Gao et al., 2010). However, additional risk factors (e.g., plaque, bacterial tests, salivary factors, exposure to fluoride) do not seem to markedly improve the prediction in older schoolchildren, adolescents, and adults (Disney et al., 1992; Vanobbergen et al., 2001; Stenlund et al., 2002; Twetman and Fontana, 2009). This may be explained in part by the fact that caries experience reflects relatively well both past and current interplay between and among the various etiologic factors. In our study, risk factors related to diet and caries-protective factors and habits (e.g., fluoride exposure, brushing, use of sugarless chewing gum) did not significantly improve the prediction of children at risk in a high-caries-risk population. Interestingly, exposure to additional sources of fluoride was related to the numbers of lesions that progressed. This may be a reflection of treatment recommendations previously given to these children. Our study identified factors associated with caries progression in the child and severity of progression (i.e., numbers of lesions that progressed) that could be measured from a parent-completed questionnaire. Not surprisingly, most of these factors were related to disease experience or rating of oral health, such as whether the child had a tooth extracted or restored, time elapsed since last dental visit, and if the caregiver does not consider the child’s oral health to be ’very good’. These questions could be used in non-dental settings without the need for an oral examination. This has great public health implications for caries management from a resource (financial/personnel) and location (school, medical office, etc.) perspective.
In addition, it has been suggested that the chance to correctly identify non-risk preschoolers and adolescents is greater than a correct identification of individuals with high risk (Twetman and Fontana, 2009). Based on the range of AUC values identified for the developed prediction models in our study (0.70-0.79), the accuracy of the prediction, regardless of whether caries experience is or is not included in the models, was fair.
This study is limited in its conclusions: (1) It is localized in Puerto Rico, with a relatively uniform Hispanic population, and thus results may not be extrapolated to other Hispanic population subgroups. (2) Since we wanted to identify factors that could be collected through a questionnaire, we did not measure other clinical variables that could be associated with caries risk. (3) the lack of radiographic data, it is possible that we may have underestimated some interproximal lesions, if not visible clinically. However, during mixed dentition, interproximal surfaces are more easily visible, and their prevalence in 5- to 13-year-old children is a very minor component of the overall caries experience (Ismail et al., 1988; Macek et al., 2003). (4) Lesion reversals were not included in the analyses, since the focus was on the identification of patients, not surfaces, at risk. Even if some lesions regressed, the patient was considered to be at risk as long as there was some progression within the mouth. (5) We asked about the caregiver’s presence of cavities in the past 2 yrs only. However, several reports have stressed the importance of caregiver’s health on the child’s caries risk (Shearer et al., 2011; Weintraub et al., 2011).
In conclusion, factors related to disease experience or rating of oral health collected through a parent-completed questionnaire were associated with child’s risk of caries lesion development/progression and numbers of lesions progressing, and could be used to screen at-risk children in this rural population. In agreement with the literature, prediction models were fair in their ability to predict caries in this high-caries-risk school-age Hispanic population. Identified factors were similar regardless of progression criteria and time interval. Addition of caries experience as measured from a dental examination did not greatly affect the prediction.
Acknowledgments
We thank Sharon Gwinn, Myrna Hernandez, Melissa Mau, Mildred Riviera, Jennifer Tran, Evaristo Delgado, Hafsteinn Eggertsson, Pedro Hernandez, and OHRI and University of Puerto Rico’s staff for their assistance.
Footnotes
A supplemental appendix to this article is published electronically only at http://jdr.sagepub.com/supplemental.
This study was supported by grant #RO1DE017890-05 from the National Institute of Dental and Craniofacial Research.
Preliminary data from this study were presented at the 2010 IADR General Session in Barcelona, Spain.
The authors declare no potential conflicts of interest with respect to the authorship and/or publication of this article.
References
- American Academy of Pediatric Dentistry (2007). Policy on use of a caries-risk assessment tool (CAT) for infants, children, and adolescents (Reference Manual 2007–2008). Pediatr Dent 29(7 Suppl):1S-271S [PubMed] [Google Scholar]
- American Dental Association (2008). Caries Form (Patients 0-6). URL accessed on 5/25/2011 at: http://www.ada.org/sections/professionalResources/docs/topics_caries_under6.doc
- Beltrán-Aguilar ED, Barker LK, Canto MT, Dye BA, Gooch BF, Griffin SO, et al. (2005). Surveillance for dental caries, dental sealants, tooth retention, edentulism, and enamel fluorosis—United States, 1988-1994 and 1999-2002. MMWR Surveill Summ 54:1-43 [PubMed] [Google Scholar]
- Centers for Disease Control and Prevention (2010). 2007 National Health 11. Interview Survey Data. Table 4-1, Current Asthma Prevalence Percents by Age, United States: National Health Interview Survey Atlanta, GA: US Department of Health and Human Services, CDC; URL accessed on 5/25/2011 at: http://www.cdc.gov/asthma/nhis/07/table4-1.htm [Google Scholar]
- Disney JA, Graves RC, Stamm JW, Bohannan HM, Abernathy JR, Zack DD. (1992). The University of North Carolina Caries Risk Assessment study: further developments in caries risk prediction. Community Dent Oral Epidemiol 20:64-75 [DOI] [PubMed] [Google Scholar]
- Dye BA, Tan S, Smith V, Lewis BG, Barker LK, Thornton-Evans G, et al. (2007). Trends in oral health status: United States, 1988-1994 and 1999-2004. National Center for Health Statistics. Vital Health Stat 11:1-92 [PubMed] [Google Scholar]
- Eckert GE, Jackson R, Fontana M. (2010). Variation of caries risk factors in toddlers and caregivers. Int J Dent pii: 593487. [Epub ahead of print, 2010 Sep 23] (in press) [DOI] [PMC free article] [PubMed]
- Featherstone JD. (2003). The caries balance: contributing factors and early detection. J CA Dent Assoc 31:129-133 [PubMed] [Google Scholar]
- Felland LE, Lauer J, Cunningham PJ. (2008). Community efforts to expand dental services for low-income people. Center for Studying Health System Change, Issue Brief No. 122. URL accessed on 5/25/2011 at: http://www.rwjf.org/publichealth/product.jsp?id=35608 [PubMed]
- Ferreira-Zandona A, Santiago E, Eckert G, Fontana M, Ando M, Zero D. (2010). Use of ICDAS combined with quantitative light-induced fluorescence as a caries detection method. Caries Res 44:317-322 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Flores G, Fuentes-Afflick E, Barbot O, Carter-Pokras O, Claudio L, Lara M, et al. (2002). The health of Latino children. Urgent priorities, unanswered questions, and a research agenda. J Am Med Assoc 288:82-90 [DOI] [PubMed] [Google Scholar]
- Fontana M, Zero D. (2006). Assessing patients’ caries risk. J Am Dent Assoc 137:1231-1239 [DOI] [PubMed] [Google Scholar]
- Fontana M, Jackson R, Eckert G, Swigonski N, Chin J, Ferreira Zandona A, et al. (2010). Longitudinal assessment of caries risk factors in toddlers. J Dent Res 90:209-214 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gao XL, Hsu CY, Xu Y, Hwarng HB, Loh T, Koh D. (2010). Building caries risk assessment models for children. J Dent Res 89:637-643 [DOI] [PubMed] [Google Scholar]
- Huntington N, Kim I, Hughes C. (2002). Caries-risk factors for Hispanic children affected by early childhood caries. Pediatr Dent 24:536-542 [PubMed] [Google Scholar]
- Ismail A, Burt B, Brunelle J, Szpunar S. (1988). Dental caries and periodontal disease among Mexican-American children from five southwestern states, 1982-1983. MMWR CDC Surveill Summ 37:33-45 [PubMed] [Google Scholar]
- Ismail AI, Sohn W, Tellez M, Amaya A, Sen A, Hasson H, et al. (2007). The International Caries Detection and Assessment System (ICDAS): an integrated system for measuring dental caries. Community Dent Oral Epidemiol 35:170-178 [DOI] [PubMed] [Google Scholar]
- Macek MD, Beltrán-Aguilar ED, Lockwood SA, Malvitz DM. (2003). Updated comparison of the caries susceptibility of various morphological types of permanent teeth. J Public Health Dent 63:174-182 [DOI] [PubMed] [Google Scholar]
- Ramos-Gomez FJ, Crall J, Gansky SA, Slayton RL, Featherstone JD. (2007). Caries risk assessment appropriate for the age 1 visit (infants and toddlers). J CA Dent Assoc 35:687-702 [PubMed] [Google Scholar]
- Riley JL, 3rd, Gordan VV, Rindal DB, Fellows JL, Ajmo CT, Amundson C, et al. (2010). Preferences for caries prevention agents in adult patients: findings from the Dental Practice-based Research Network. Community Dent Oral Epidemiol 38:360-370 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shearer DM, Thomson WM, Broadbent JM, Poulton R. (2011). Maternal oral health predicts their children’s caries experience in adulthood. J Dent Res 90:672-677 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shiboski C, Gansky S, Ramos-Gomez F, Ngo L, Isman R, Pollick HF. (2003). The association of early childhood caries and race/ethnicity among California preschool children. J Publ Health Dent 63:38-46 [DOI] [PubMed] [Google Scholar]
- Stenlund H, Mejàre I, Källestål C. (2002). Caries rates related to approximal caries at ages 11-13: a 10-year follow-up study. J Dent Res 81:455-458 [DOI] [PubMed] [Google Scholar]
- Twetman S, Fontana M. (2009). Patient caries risk assessment. Monogr Oral Sci 21:91-101 [DOI] [PubMed] [Google Scholar]
- US Department of Health and Human Services (2000). Oral Health in America: A Report of the Surgeon General—Executive Summary. Rockville, MD: US Department of Health and Human Services, National Institute of Dental and Craniofacial Research, National Institutes of Health; URL accessed on 5/25/2011 at: http://www.surgeongeneral.gov/library/oralhealth/ [Google Scholar]
- Vanobbergen J, Martens L, Lesaffre E, Bogaerts K, Declerck D. (2001). Assessing risk indicators for dental caries in the primary dentition. Community Dent Oral Epidemiol 29:424-434 [DOI] [PubMed] [Google Scholar]
- Weintraub JA, Prakash P, Shain SG, Laccabue M, Gansky SA. (2011). Mothers’ caries increases odds of children’s caries. J Dent Res 89:954-958 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zero D, Fontana M, Lennon ÁM. (2001). Clinical applications and outcomes of using indicators of risk in caries management. J Dent Educ 65:1126-1132 [PubMed] [Google Scholar]