Abstract
Two main proteases cleave enamel extracellular matrix proteins during amelogenesis. Matrix metalloprotease-20 (Mmp20) is the predominant enzyme expressed during the secretory stage, while kallikrein-related peptidase-4 (Klk4) is predominantly expressed during maturation. Mutations to both Mmp20 and Klk4 result in abnormal enamel phenotypes. During a recent whole-genome microarray analysis of rat incisor enamel organ cells derived from the secretory and maturation stages of amelogenesis, the serine protease chymotrypsin C (caldecrin, Ctrc) was identified as significantly up-regulated (> 11-fold) during enamel maturation. Prior reports indicate that Ctrc expression is pancreas-specific, albeit low levels were also noted in brain. We here report on the expression of Ctrc in the enamel organ. Quantitative PCR (qPCR) and Western blot analysis were used to confirm the expression of Ctrc in the developing enamel organ. The expression profile of Ctrc is similar to that of Klk4, increasing markedly during the maturation stage relative to the secretory stage, although levels of Ctrc mRNA are lower than for Klk4. The discovery of a new serine protease possibly involved in enamel development has important implications for our understanding of the factors that regulate enamel biomineralization.
Keywords: amelogenesis, CTRC, enamel, serine proteases, caldecrin, chymotrypsin C, biomineralization
Introduction
Enamel formation is commonly divided into three main stages, including the secretory, transition, and maturation stages, during which ameloblasts secrete and then break down the enamel matrix proteins (EMPs) (Smith, 1998). Proteolytic degradation of EMPs (amelogenin, ameloblastin, enamelin) is required at each stage to allow, ultimately, for full mineralization of the enamel crystals (Bartlett and Simmer, 1999). In the secretory stage, EMPs are enzymatically processed by matrix metalloprotease-20 (Mmp20) (Bartlett and Simmer, 1999). Mmp20 expression decreases during the maturation stage of amelogenesis (Bartlett et al., 1998). Further matrix degradation is required during maturation to allow the enamel crystals to expand in width and thickness, a function that corresponds to serine protease kallikrein-related peptidase-4 (Klk4) (Simmer et al., 1998). Klk4 expression is markedly up-regulated during the maturation stage, being involved with the hardening of the enamel layer across its depth (Hu et al., 2002; Lu et al., 2008; Simmer et al., 2009). Mutations to Mmp20 and Klk4 result in enamel defects known as amelogenesis imperfecta, highlighting the relevance of matrix degradation for the proper development of healthy enamel (Wright et al., 2009).
Studies have suggested the likelihood that additional proteases may also be involved in enamel development (Maycock et al., 2002; Tye et al., 2009). It has also been reported that the whole spectrum of enamel phenotypes associated with amelogenesis imperfecta is not fully accounted for by mutations to Mmp20, Klk4, or the EMPs (Wright, 2006; Wright et al., 2009), opening the possibility for further discoveries of enamel-associated proteases. We recently completed a genome-wide analysis of mRNA obtained from rat enamel organ cells at the secretory and maturation stages (Lacruz et al., in press) and identified that one of the most highly up-regulated gene transcripts in the maturation stage is a serine protease known as chymotrypsin C (caldecrin), encoded by Ctrc (Fig. 1). Ctrc is expressed and secreted primarily in the pancreas, where it functions as a digestive enzyme within the gut (Iio-Akama et al., 1985; Szmola et al., 2011). Human CTRC was also shown to stimulate activation and degradation of human cationic trypsin and promote activation of human procarboxypeptidases A1 and A2 (Nemoda and Sahin-Tóth, 2006; Szmola and Sahin-Tóth, 2007; Szmola et al., 2011). Loss-of-function mutations in CTRC are risk factors for the development of chronic pancreatitis in humans, in all likelihood because of impairment of the protective trypsin-degrading activity (Rosendahl et al., 2008). CTRC was also purified from the pig and rat pancreas and cloned from the rat and human pancreas, as caldecrin, a serum calcium-decreasing factor (Tomomura et al., 1992, 1995, 2002). In vitro, caldecrin inhibited parathormone-stimulated calcium release from cultured fetal long bones and osteoclastogenesis (Tomomura et al., 1992, 1995; Hasegawa et al., 2010). Expression of Ctrc was also demonstrated in the rat brain (Tomomura et al., 2002). Given the marked up-regulation of Ctrc detected by microarray analysis of dental tissues, the aim of this study was to analyze Ctrc expression by quantitative PCR (qPCR) from rat enamel organ epithelium corresponding to various stages of amelogenesis. In addition, we assessed Ctrc levels in the enamel matrix by Western blot.
Figure 1.
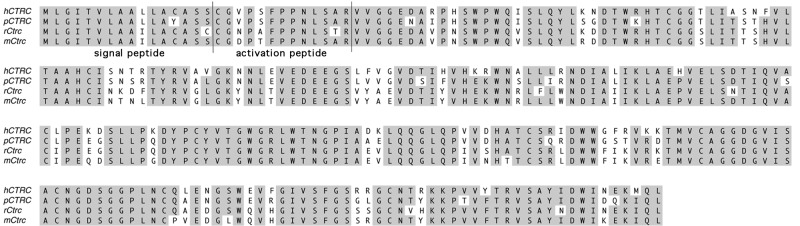
ClustalW alignment of human, porcine, rat, and mouse chymotrypsin C (CTRC) proteins. Chymotrypsin C (encoded by CTRC) contains 8 exons and is located on chromosomes 5q36 (rats) and 1p36.21 (humans). Human and mouse CTRC is 80% homologous, whereas human CTRC and KLK4 protein sequences share ~30% homology. In humans, CTRC is a secreted protein synthesized as a preproenzyme of 268 amino acids, with a signal peptide of 16 amino acids and an activation peptide of 13 amino acids (Tomomura et al., 1995).
Materials & Methods
Animals
All vertebrate animal manipulation complied with Institutional and Federal guidelines.
Real-time PCR
Five adult male Wistar Hannover rats weighing ~100 g were sacrificed, and their mandibles were immediately dissected out. The surrounding tissues were removed, and the mandibles were then frozen in liquid nitrogen and lyophilized as described previously (Smith et al., 2006). The mandibular bone encasing the lower incisors was removed to expose the entire labial enamel surface. Enamel organ cells were collected by gentle scraping from the secretory, early-mid maturation, and mid-late maturation stages with a molar reference line (Smith and Nanci, 1989). Mandibular first molars from 1 rat pup at post-natal days 1, 4, and 8 were also dissected out for real-time PCR analysis.
Total RNA Isolation and Real-time PCR
We extracted total RNA by manually homogenizing the molars and the freeze-dried enamel organ cells obtained from the incisors using a Qiagen RNeasy Minikit (Qiagen, Valencia, CA, USA). Reverse-transcribed PCR was performed with iScript cDNA Synthesis kit (Bio-Rad, Hercules, CA, USA). Real time-PCR (qPCR) reactions were performed with iQTM SYBR® Green Supermix (Bio-Rad) with rat-specific primer pairs as follows: Actb (β-actin; GenBank # NM_031144, forward 5′-AGTGTGAC GTTGACATCCGTA-3′ and reverse 5′-GCCAGGGCAGTAA TCTCCTTCT-3′, product 112 bp); Klk4 (GenBank # NM_00 1004101, forward 5′-GGGATACCTGCCTAGTTTCTGG-3′ and reverse 5′-AGGTGGTACACGGGGTCATAC-3′, product 130 bp); Mmp20 (GenBank # NM_001106800, forward 5′-GGCGAG ATGGTGGCAAGAG-3′ and reverse 5′-CTGGGAAGAGGC GGTAGTT-3′, product 166 bp); and Ctrc (GenBank # NM_ 001077649, forward 5′- AGGACTATCCCTGCTATGTCA-3′ and reverse 5′- ACCACCAGTCCAACCTGGA-3′, product 126 bp). Primers were designed to span intronic regions and are the rat-equivalent to either human or mouse primer pairs identified in “PrimerBank” as tested and ideal for qPCR (http://pga.mgh.harvard.edu/primerbank/index.html). Relative expression of mRNA was calculated by the delta-delta CT method (Livak and Schmittgen, 2001). All values for the mRNA species were normalized to β-actin. We used the Student’s t test to compare expression levels between stages.
Western Blot Analysis
Four rats weighing ~100 g were sacrificed, and their mandibles were immediately dissected out and cleaned of soft tissues. The bone surrounding the labial surfaces of the incisors was carefully removed to isolate enamel organ cells collected by gentle scraping with blunt instruments. Cells were obtained from secretory and maturation stages with the molar reference line (Smith and Nanci, 1989). In addition, the lower first molars from mouse pups at post-natal days 3, 5, and 9 were dissected out. The ameloblast-like cell line LS8, originally derived from newborn mouse molars, was also sampled. Rat and a mouse pancreas were used as Ctrc-positive controls. Enamel matrix protein derivative (Emdogain® or EMD; Straumann USA, LLC, Andover, MA, USA) was used as a source for porcine enamel matrix proteins.
Cell lysates were prepared in ice-cold RIPA (1% NP40, 0.1% SDS, 0.5% DOC, 150 mM NaCl, 50 mM Tris, pH 8.0) and complete mini (Roche Applied Sciences, Indianapolis, IN, USA). Samples were homogenized manually with a pestle prior to sonication. Homogenized samples were cleared at 16,000 rpm x 15 min at 4oC. Proteins were quantified by Micro BCA (Pierce, Rockford, IL, USA) and equally loaded (15 µg/lane) on 10% SDS PAGE resolving gels. A monoclonal antibody against full-length human CTRC, also reactive in the rat, mouse, and pig, was purchased from Abcam (Cambridge, MA, USA; Cat. # ab35694) and used at a dilution of 1:2000.
Results
Relative mRNA Expression of Mmp20, Klk4, and Ctrc mRNA Transcripts in Rat Incisor Enamel Organ Cells
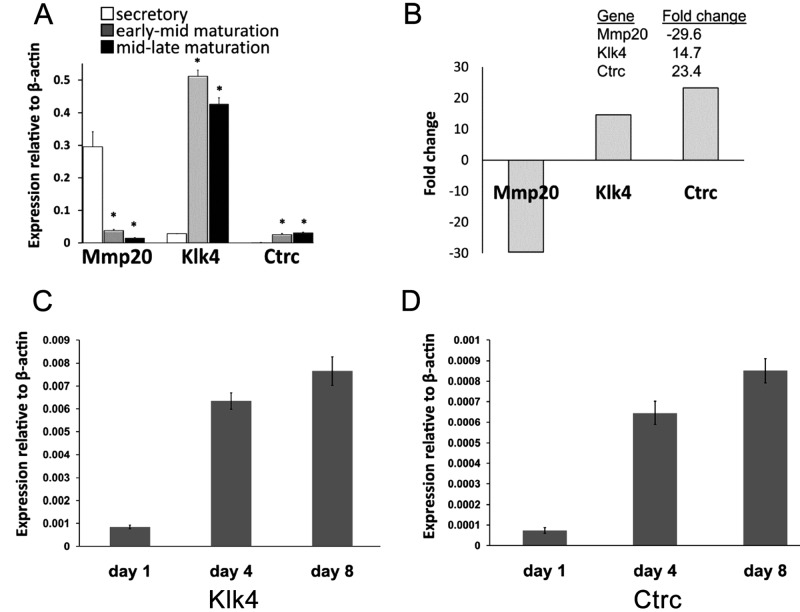
The expression levels of Mmp20, Klk4, and Ctrc, relative to β-actin levels, for secretory, early-mid, and mid-late maturation stage amelogenesis, were assessed by qPCR (Fig. 2A). Mmp20 showed high expression during the secretory stage, but levels decreased in maturation. In contrast, the Klk4 expression was low during the secretory stage, but was then significantly up-regulated in maturation (Student’s t test; p < 0.01), with a greater increase at the onset of maturation relative to mid-late maturation. The overall expression pattern for these 2 genes identified here was in keeping with previous reports describing Mmp20 and Klk4 expression by different methodologies (Bartlett et al., 1998; Bartlett and Simmer, 1999; Hu et al., 2002; Lu et al., 2008). This clearly indicates that the dissected enamel organ epithelium obtained here represents an appropriate source for the characterization of gene expression. Ctrc expression during the secretory stage was low, but showed a ~20-fold increase at the onset of maturation, with high levels maintained during mid-late maturation. In both cases, the differences observed relative to the secretory stage were significant (p < 0.01). Although the absolute expression levels of Ctrc were low compared with those of Klk4, the changes in their levels of expression during the maturation stage were comparable (a ~15-fold change for Klk4 compared with the >20-fold-change for Ctrc) (Fig. 2B).
Figure 2.
Quantitative PCR (qPCR) analysis. (A) Relative expression levels of Mmp20, Klk4, and Ctrc normalized to β-actin during secretory, early-mid maturation, and mid-late maturation stages of amelogenesis. The expression patterns of Mmp20 and Klk4 are in keeping with those reported by other methodologies. Mmp20 was markedly down-regulated in maturation. Expression levels of Klk4 were detected during the secretory stage but significantly increased at the onset of maturation (p < 0.01) (A). The expression level of Ctrc was also significantly up-regulated at the onset of maturation (p < 0.01), but transcript levels appeared to be less abundant than those of Klk4. (*) indicates significant differences (p < 0.01) when secretory and maturation stages were compared by Student’s t test. (B) Fold changes between secretory and mid-late maturation stages for each gene transcript. (C, D) Relative expression of Klk4 (C) and Ctrc (D) in rat mandibular first molars dissected at post-natal days 1, 4, and 8, with marked increases for both Klk4 and Ctrc gene transcripts in days 4 and 8 relative to day 1.
Relative mRNA Expression of Ctrc and Klk4 in Rat Molars
The transition from secretory to maturation stage in rat first molars occurs at about post-natal day 7 (Reith, 1970), but around post-natal day 8 in mice (Simmer et al., 2009). Figs. 2C and 2D show real-time PCR results for Klk4 (Fig. 2C) and Ctrc (Fig. 2D) levels in mandibular first molar teeth extracted from animals on post-natal days 1, 4, and 8. Both Klk4 and Ctrc display similar patterns of expression, with marked increases at post-natal days 4 and 8 relative to day 1. Ctrc increased 9-fold from day 1 to day 4, and 11-fold from day 1 to day 8. This difference was significant in both cases (p < 0.01). Similar fold changes were observed in Klk4 (7- and 9-fold increases, respectively).
Western Blot Analysis of Rat-derived and Mouse-derived Tissues
Fig. 3A shows Western blot analysis of secretory and maturation stage enamel organ cells from rat incisors and from controls. Expression of Ctrc, at the expected band size of ~ 26-30 kDa, was observed in both stages of amelogenesis, albeit the levels observed in the maturation stage were greater than those identified in the secretory stage. Actb (β-actin) served as a control.
Figure 3.
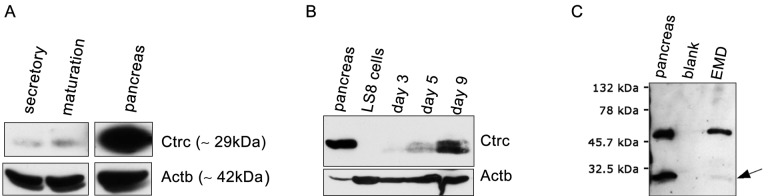
Western blot analysis of Ctrc. (A) Ctrc protein expression level in cells derived from rat incisor enamel organ cells at the secretory and maturation stages. A noticeable increase in Ctrc is observed in the maturation stage relative to the secretory stage. (B) Ctrc protein expression level in cells derived from mouse mandibular first molar teeth at days 3, 5, and 9 and in the ameloblast cell line LS8 derived from newborn mouse molars. There was a significant increase in Ctrc levels as the enamel organ progressed from the secretory stage (day 3) to the maturation stage (day 9). Ctrc expression is absent in LS8 cells. (C) Ctrc expression in the porcine enamel matrix protein derivative (Emdogain® or EMD). A Ctrc-specific band at ˜26-30 kDa (arrow) was identified (C, lane 3; EMD). Ctrc levels in rat and mouse tissues and in EMD are less abundant than the levels of Ctrc seen in the pancreas (C, lane 1). Note that a larger, non-specific band at ˜50 kDa is also apparent for Ctrc (included in C), and these data are consistent with those previously described for Ctrc (Tomomura et al., 1992, 1995).
Fig. 3B shows a Western blot analysis of mouse mandibular first molar teeth extracted at post-natal days 3, 5, and 9. Expression of Ctrc was negligible at day 1, clearly present at day 3, and markedly up-regulated at day 9. Protein extracts from rat and mouse pancreas were included as a positive control for Ctrc. Actb served as a loading control.
Western Blot Analysis of Extract of Porcine Enamel Matrix Proteins Confirms the Presence of Ctrc in the Enamel Matrix
Western blot analysis was performed on Emdogain®, an extract of porcine enamel matrix proteins, and compared with protein extracts from the mouse pancreas (Fig. 3C). CTRC was clearly evident in the pancreas and porcine enamel matrix, although the levels observed in the porcine derivative were marginal compared with those in the rat pancreas. The CTRC-specific band ran ~26-30 kDa, as previously identified (Tomomura et al., 1992, 2002). A larger band (~50 kDa) was present at equal intensity in the pancreas and in Emdogain® (Fig. 3C). Previous studies, with a different antibody, identified a similar band at ~50 kDa in the pancreas and CTRC-transfected cell extracts (Tomomura et al., 1992, 1995), thus in agreement with the earlier work of Tomomura et al. (1992, 1995); this larger band was interpreted as non-specific.
Discussion
Proteolytic processing of enamel matrix proteins is essential for the development of normal enamel. This function is provided by Mmp20 and Klk4. Whereas Mmp20 is the predominant enzyme in the secretory stage and low in maturation, the activity of Klk4 is markedly up-regulated at this latter stage, largely functioning to degrade EMPs within the deeper regions inside the enamel layer, thus contributing to the hardening of enamel (Hu et al., 2002; Simmer et al., 2009). Mutations to KLK4 and MMP20 in humans result in an enamel phenotype characterized by hypomineralization but with seemingly normal thickness (Hart et al., 2004; Kim et al., 2005). However, in the murine model, Mmp20-null mice show both hypoplastic and hypomineralized enamel (Wright et al., 2009). The recently described Klk4-null mice showed normal enamel thickness, with decussating prisms containing loosely packed crystals, and the enamel was rapidly worn after animals were weaned (Simmer et al., 2009). In addition, although these animals showed retention of enamel proteins and overall hypomaturation, some degree of crystal maturation occurred (Simmer et al., 2009). The present study identified that, in addition to Klk4, Ctrc is predominantly expressed during the maturation stage of amelogenesis.
Some parallels can be drawn between Ctrc and Klk4. For instance, both enzymes show limited tissue distributions, both belong to the serine protease family, and both are secreted as inactive proenzymes that need to be proteolytically activated. The distribution of Klk4 has been generally limited to dental tissues and the prostate (Hu et al., 2002), although a recent report identified this enzyme in the kidney and the liver (Seiz et al., 2010). CTRC was first purified from pig pancreas (Folk and Schirmer, 1965), but it was recently identified also in the rat brain (Tomomura et al., 2002). CTRC is secreted as an inactive precursor from the pancreas (chymotrypsinogen C), and it is activated by trypsin in the gut lumen. Trypsin cleaves the Arg29-Val30 peptide bond at the C-terminal end of the CTRC activation peptide. Because Klk4 also cleaves after Arg residues, it is intriguing to speculate that Klk4 may activate CTRC in the enamel. The activation of Klk4 has remained elusive, but recently cathepsin C (also known as dipeptidyl peptidase I, or DPPI) has been suggested to be a possible Klk4 activator (Tye et al., 2009). Thus, a proteolytic activation cascade involving DPPI, Klk4, and CTRC may be operational during enamel development.
The marked change in expression levels of Ctrc between secretory and maturation-stage cells, both at the message and protein levels detected in mice and rats, and its expression in porcine enamel matrix protein extracts suggest that it likely plays an important function in enamel mineralization. A parsimonious role for Ctrc at this stage likely relates to processing of EMPs, as also suggested previously (Tomomura et al., 2002). Furthermore, Ctrc cut sites of cationic trypsin show high specificity for Leu-Glu sites (Szmola and Sahin-Tóth, 2007), a dipeptide sequence present in the sequences of the main EMPs. It is also noteworthy that two recent enamel proteins have been described during maturation-stage amelogenesis, these being the odontogenic, ameloblast-associated protein (Odam) and amelotin (Amtn) (Iwasaki et al., 2005; Moffatt et al., 2006). Processing events of Odam and Amtn are yet to be defined, but Ctrc may be involved in this function, either operating in tandem with Klk4, or alternatively showing a higher affinity for a specific EMP.
This study has identified a novel serine protease known as chymotrypsin C (Ctrc) in rat- and mouse-derived dental tissues. The expression profile of Ctrc in these tissues is similar to that described for Klk4, although the relative expression level of Ctrc is lower than that of Klk4. The marked up-regulation of Ctrc in the maturation stage of amelogenesis, shown by qPCR and Western blot analysis, and its expression in porcine enamel matrix protein derivative, demonstrated here, strongly suggest that Ctrc may play a role in the final stages of enamel mineralization. Despite the fact that Ctrc and Klk4 are expressed at a similar stage of amelogenesis, Ctrc is clearly not able to compensate for Klk4’s digestive functions of the EMP, as evident from the Klk4-null mouse model in which hypomaturation enamel defects were apparent (Simmer et al., 2009). Thus, a possible function for Ctrc may be to hydrolyze peptide fragments generated by Klk4, rather than hydrolyzing larger polypeptide sequences that are known substrates for Klk4 (Hu et al., 2002; Lu et al., 2008). Although Ctrc is accepted as a secreted protease with a well-defined signal peptide, analysis of the data presented here does not exclude the possibility that Ctrc may also function at the cellular level (i.e., near the periphery or within the endosomal/lysosomal system) rather than exclusively at the extracellular level (within the enamel layer). Although the function of Ctrc in enamel is not yet clearly defined, based on the data presented here, it is tempting to suggest that CTRC may become another candidate gene for amelogenesis imperfecta.
Footnotes
This work was supported by grants DE013404 and DE019629 from the National Institutes of Health.
The authors declare no potential conflicts of interest with respect to the authorship and/or publication of this article.
References
- Bartlett JD, Simmer JP. (1999). Proteinases in developing dental enamel. Crit Rev Oral Biol Med 10:425-441 [DOI] [PubMed] [Google Scholar]
- Bartlett JD, Ryu OH, Xue J, Simmer JP, Margolis HC. (1998). Enamelysin mRNA displays a developmentally defined pattern of expression and encodes a protein which degrades amelogenin. Connect Tissue Res 39:101-109 [DOI] [PubMed] [Google Scholar]
- Folk JE, Schirmer EW. (1965). Chymotrypsin C. I. Isolation of the zymogen and the active enzyme: preliminary structure and specificity studies. J Biol Chem 240:181-192 [PubMed] [Google Scholar]
- Hart PS, Hart TC, Michalec MD, Ryu OH, Simmons D, Hong S, et al. (2004). Mutation in kallikrein 4 causes autosomal recessive hypomaturation amelogenesis imperfecta. J Med Genet 41:545-549 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hasegawa H, Kido S, Tomomura M, Fujimoto K, Ohi M, Kiyomura M, et al. (2010). Serum calcium-decreasing factor, caldecrin, inhibits osteoclast differentiation by suppression of NFATc1 activity. J Biol Chem 285:25448-25457 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hu JC, Sun X, Zhang C, Liu S, Bartlett JD, Simmer JP. (2002). Enamelysin and kallikrein-4 mRNA expression in developing mouse molars. Eur J Oral Sci 110:307-315 [DOI] [PubMed] [Google Scholar]
- Iio-Akama K, Sasamoto H, Miyazawa K, Miura S, Tobita T. (1985). Active forms of chymotrypsin C isolated from autolyzed porcine pancreas glands. Biochim Biophys Acta 831:249-256 [DOI] [PubMed] [Google Scholar]
- Iwasaki K, Bajenova E, Somogyi-Ganss E, Miller M, Nguyen V, Nourkeyhani H, et al. (2005). Amelotin—a novel secreted, ameloblast-specific protein. J Dent Res 84:1127-1132 [DOI] [PubMed] [Google Scholar]
- Kim JW, Simmer JP, Hart TC, Hart PS, Ramaswami MD, Bartlett JD, et al. (2005). MMP-20 mutation in autosomal recessive pigmented hypomaturation amelogenesis imperfecta. J Med Genet 42:271-275 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lacruz RS, Smith CE, Bringas P, Jr, Chen Y, Smith SM, Snead ML, et al. (in press). Identification of novel candidate genes involved in mineralization of dental enamel by genome-wide transcript profiling. J Cell Physiol. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Livak KJ, Schmittgen TD. (2001). Analysis of relative gene expression data using real-time quantitative PCR and the 2(-Delta Delta C(T)) method. Methods 25:402-408 [DOI] [PubMed] [Google Scholar]
- Lu Y, Papagerakis P, Yamakoshi Y, Hu JC, Bartlett JD, Simmer JP. (2008). Functions of KLK4 and MMP-20 in dental enamel formation. Biol Chem 389:695-700 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maycock J, Wood SR, Brookes SJ, Shore RC, Robinson C, Kirkham J. (2002). Characterization of a porcine amelogenin preparation, EMDOGAIN, a biological treatment for periodontal disease. Connect Tissue Res 43:472-476 [DOI] [PubMed] [Google Scholar]
- Moffatt P, Smith CE, St-Arnaud R, Simmons D, Wright JT, Nanci A. (2006). Cloning of rat amelotin and localization of the protein to the basal lamina of maturation stage ameloblasts and junctional epithelium. Biochem J 399:37-46 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nemoda Z, Sahin-Tóth M. (2006). Chymotrypsin C (caldecrin) stimulates autoactivation of human cationic trypsinogen. J Biol Chem 281:11879-11886 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reith EJ. (1970). The stages of amelogenesis as observed in molar teeth of young rats. J Ultrastruct Res 30:111-151 [DOI] [PubMed] [Google Scholar]
- Rosendahl J, Witt H, Szmola R, Bhatia E, Ozsvari B, Landt O, et al. (2008). Chymotrypsin C (CTRC) variants that diminish activity or secretion are associated with chronic pancreatitis. Nat Genet 40:78-82 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seiz L, Kotzsch M, Grebenchtchikov NI, Geurts-Moespot AJ, Fuessel S, Goettig P, et al. (2010). Polyclonal antibodies against kallikrein-related peptidase 4 (KLK4): immunohistochemical assessment of KLK4 expression in healthy tissues and prostate cancer. Biol Chem 391:391-401 [DOI] [PubMed] [Google Scholar]
- Simmer JP, Fukae M, Tanabe T, Yamakoshi Y, Uchida T, Xue J, et al. (1998). Purification, characterization and cloning of enamel matrix serine proteinase 1. J Dent Res 77:377-386 [DOI] [PubMed] [Google Scholar]
- Simmer JP, Hu Y, Lertlam R, Yamakoshi Y, Hu JC. (2009). Hypomaturation enamel defects in Klk4 knockout/LacZ knockin mice. J Biol Chem 284:19110-19121 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith CE. (1998). Cellular and chemical events during enamel maturation. Crit Rev Oral Biol Med 9:128-161 [DOI] [PubMed] [Google Scholar]
- Smith CE, Nanci A. (1989). A method for sampling the stages of amelogenesis on mandibular rat incisors using the molars as a reference for dissection. Anat Rec 225:257-266 [DOI] [PubMed] [Google Scholar]
- Smith CE, Nanci A, Moffatt P. (2006). Evidence by signal peptide trap technology for the expression of carbonic anhydrase 6 in rat incisor enamel organs. Eur J Oral Sci 114(Suppl 1):147-153 [DOI] [PubMed] [Google Scholar]
- Szmola R, Sahin-Tóth M. (2007). Chymotrypsin C (caldecrin) promotes degradation of human cationic trypsin: identity with Rinderknecht’s enzyme Y. Proc Natl Acad Sci USA 104:11227-11232 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Szmola R, Bence M, Carpentieri A, Szabo A, Costello CE, Samuelson J, et al. (2011). Chymotrypsin C is a co-activator of human pancreatic procarboxypeptidases A1 and A2. J Biol Chem 286:1819-1827 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tomomura A, Fukushige T, Noda T, Noikura T, Saheki T. (1992). Serum calcium-decreasing factor (caldecrin) from porcine pancreas has proteolytic activity which has no clear connection with the calcium decrease. FEBS Lett 301:277-281 [DOI] [PubMed] [Google Scholar]
- Tomomura A, Tomomura M, Fukushige T, Akiyama M, Kubota N, Kumaki K, et al. (1995). Molecular cloning and expression of serum calcium-decreasing factor (caldecrin). J Biol Chem 270:30315-30321 [DOI] [PubMed] [Google Scholar]
- Tomomura A, Yamada H, Itagaki K, Fujimoto K, Katoh S. (2002). Rat brain expresses serum calcium-decreasing factor (caldecrin). Neurosci Lett 317:17-20 [DOI] [PubMed] [Google Scholar]
- Tye CE, Pham CT, Simmer JP, Bartlett JD. (2009). DPPI may activate KLK4 during enamel formation. J Dent Res 88:323-327 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wright JT. (2006). The molecular etiologies and associated phenotypes of amelogenesis imperfecta. Am J Med Genet A 140:2547-2555 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wright JT, Hart TC, Hart PS, Simmons D, Suggs C, Daley B, et al. (2009). Human and mouse enamel phenotypes resulting from mutation or altered expression of AMEL, ENAM, MMP20 and KLK4. Cells Tissues Organs 189:224-229 [DOI] [PMC free article] [PubMed] [Google Scholar]