Abstract
The association between fluoride and risk for osteosarcoma is controversial. The purpose of this study was to determine if bone fluoride levels are higher in individuals with osteosarcoma. Incident cases of osteosarcoma (N = 137) and tumor controls (N = 51) were identified by orthopedic physicians, and segments of tumor-adjacent bone and iliac crest bone were analyzed for fluoride content. Logistic regression adjusted for age and sex and potential confounders of osteosarcoma was used to estimate odds ratios (OR) and 95% confidence intervals (CI). There was no significant difference in bone fluoride levels between cases and controls. The OR adjusted for age, gender, and a history of broken bones was 1.33 (95% CI: 0.56-3.15). No significant association between bone fluoride levels and osteosarcoma risk was detected in our case-control study, based on controls with other tumor diagnoses.
Keywords: fluoride, osteosarcoma, case-control study, bone, oncology, epidemiology
Introduction
Osteosarcoma, a rare, painful, primary malignant bone tumor, is more prevalent in males (Homa et al., 1991), in the long bones (Patel and Benjamin, 2005), and in individuals < 20 yrs old (Gurney et al., 1999).
Chemicals and genetic factors have been suggested as risk factors of osteosarcoma (Miller et al., 1996), while ionizing radiation is the only documented environmental risk factor for bone cancer (Steiner, 1965; Tucker et al., 1987). A National Toxicology Program (NTP) study concluded that there was “equivocal evidence” of carcinogenic activity of sodium fluoride in male rats that were given extremely high doses (100 ppm and 175 ppm) for 2 yrs (NTP, 1990). Other animal studies have not provided evidence of an association between fluoride and osteosarcoma (Maurer et al., 1990; NTP, 1992).
Numerous descriptive studies, with self-reported or ecological level data used to determine fluoride exposure from drinking water, failed to demonstrate an association (Hoover et al., 1976; Doll and Kinlen, 1977, 1978; Newbrun, 1977; Hrudey et al., 1990; Mahoney et al., 1991; Freni and Gaylor, 1992). Similarly, case-control studies have not found any significant association between osteosarcoma risk and fluoridated drinking water (McGuire et al., 1991; Moss et al., 1995) or total lifetime fluoride (Gelberg et al., 1995). One exploratory analysis reported an increased risk among a subset of males exposed to fluoride in drinking water during childhood (Bassin et al., 2006).
Fluoride has an affinity for calcified tissues, with 99% of fluoride in the body contained within the skeleton. Thus, bone fluoride levels can serve as a biomarker for chronic fluoride exposure, providing a more objective measure of fluoride exposure. The purpose of this study was to evaluate whether fluoride levels in bone are associated with the occurrence of osteosarcoma.
Methods
Study Population
Patients were identified by physicians in the orthopedic departments from 9 hospitals across the US between 1993 and 2000. The study sample included incident cases of primary osteosarcoma, including osteoblastic, parosteal, and periosteal subtypes, and two control groups: tumor controls, patients with newly diagnosed malignant bone tumors; and orthopedic controls, surgical patients with benign tumors or non-neoplastic conditions. Since tumor controls were the only ones with available bone specimens for assay, they comprised the control series for this report. The study was approved by the Institutional Review Boards of the respective hospitals, Harvard Medical School, and the Medical College of Georgia.
All eligible patients who consented to participate were interviewed in person during hospitalization, pre-admission, or post-admission. Medical information was requested for all living patients born in the US. Patients who completed at least 80% of the questionnaire were considered to be enrolled in the study. Although the study protocol called for matching of cases and controls based on gender, age (± 5 yrs), and distance from their medical center, this approach was abandoned early in the study, since it proved to be a barrier to recruiting controls. Thus, all available tumor patients were recruited, and the statistical analysis was adjusted for age and gender.
Exposure and Outcome Assessment
Cancer diagnoses were confirmed by pathology reports. Specimens of both the tumor and normal bone adjacent to the margins of tumor tissue, herein referred to as tumor-adjacent bone, were collected from cases and tumor controls during surgery. Given that bone at the tumor site was destroyed as a result of the tumor, tumor-adjacent bone was analyzed for fluoride content. In some centers, a segment of bone from the iliac crest was also requested for cases, to assess the correlation between fluoride in iliac crest bone and in tumor-adjacent bone.
Methods used to measure fluoride concentration in the bone specimens have been described in detail elsewhere (Medina et al., 2006). A 4- to 6-mg portion of bone was ashed, pulverized, and analyzed for fluoride concentrations (ash weight, mg F/kg, or ppm) according to a method developed by Taves (1968) and modified by Whitford (1996). With blinding to the case or control status of the bone specimens, each specimen was analyzed in duplicate; if measurements differed by more than 10%, another specimen was analyzed. Deer bone specimens with known fluoride concentrations were included in each batch of specimens for quality control (Medina et al., 2006) and confirmed the validity of the bone fluoride assay procedure.
Statistical Analysis
We used Chi-square and Wilcoxon rank-sum tests to evaluate differences in patient characteristics and median fluoride concentrations between tumor-adjacent bone and iliac crest bone. We also evaluated all specimens of tumor-adjacent bone and iliac crest bone among the cases, taking into account the within-person correlation for those patients who had both types of bone specimens. We used Spearman’s correlation to assess the correlation between fluoride iliac crest bone and fluoride in tumor-adjacent bone.
In the subset of matched cases and controls, we used Wilcoxon’s signed-rank test to evaluate if there were a difference in the median fluoride concentration in tumor-adjacent bone. Since this subset represented less than 25% of cases that provided bone, an unmatched analysis comparing median fluoride concentration in tumor-adjacent bone among all cases with control bone was conducted by a Wilcoxon rank-sum test.
We used both conditional and unconditional logistic regression to estimate the age- and sex-adjusted odds ratios (OR) and 95% confidence intervals (CI) to account for the initial matching. Fluoride measurements were transformed to a natural logarithmic scale to improve normality (Pagano and Gauvreau, 2000). Age- and sex-adjusted analysis was carried out for variables that were considered to be potential confounders of osteosarcoma: race/ethnicity; patient’s, mother’s, and father’s education; combined household income; whether the patient ever lived in an urban area; and patient’s past medical history (history of broken bones, other bone diseases, other cancers, receiving radiation for diagnosis or treatment prior to the present diagnosis); and variables with p value of ≤ 0.25 in the demographic-adjusted analysis were considered as potential confounders. The missing indicator method was used for patients missing information on household income and parents’ level of education (Greenland and Finkle, 1995). Both manual and automated stepwise selection approaches were used to determine potential confounders to be included in the final risk-adjusted model. An exploratory analysis was also conducted among patients < 45 yrs old and < 20 yrs old. However, this study did not have sufficient power for a subgroup analysis among patients < 20 yrs old. Statistical analysis was carried out in SAS Version 9.1 (SAS Institute, Cary, NC, USA).
Results
In total, 314 patients were eligible for enrollment (200 cases; 114 controls), and 296 patients (94%) completed the questionnaire (188 cases; 108 controls). Of these, 194 patients (142 cases; 52 controls) provided either tumor-adjacent or iliac crest bone for assay of fluoride content in bone. Eighteen patients were deceased, did not complete the questionnaire, or were otherwise lost to follow-up (12 cases; six controls). In total, 257 bone specimens were analyzed for fluoride content (200 tumor-adjacent bone; 57 iliac crest bone). Bone from six patients (five cases; one control) had fluoride levels below 100 mg F/kg, and thus were considered to be tissue other than bone (G. Whitford, personal communication).
Among patients who provided bone, there were no differences between cases and controls in enrollment site, race/ethnicity, patient’s and mother’s education level, combined household income, and whether they ever lived in an urban area. The median age of controls was higher than that of cases (p < 0.001); gender-specific age differences were also significant, with controls being older, on average, than cases for both males (p < 0.001) and females (p = 0.02) (Table 1). There was a greater proportion of male cases than female cases (p = 0.03), and fathers of cases were significantly more likely to have higher education levels than those of controls (p = 0.02) (Table 1). Comparisons between all subjects who provided bone specimens versus those who did not are included in the Appendix Table 1.
Table 1.
Patient Characteristics of Osteosarcoma Cases (N = 137) and Tumor Controls (N = 51) Who Provided a Bone Specimena
| Cases (%) (N = 137)b | Tumor Controls (%) (N = 51)b | p Valuec (Chi-square) | ||
|---|---|---|---|---|
| Site of enrollment | MGH, Boston, MA | 24 (17.5) | 7 (13.7) | 0.58d |
| Creighton Univ./St. Joseph’s, Omaha, NE | 3 (2.2) | 4 (7.8) | ||
| University of Nebraska, Omaha | 12 (8.8) | 1 (2.0) | ||
| University of Chicago | 37 (27.0) | 20 (39.2) | ||
| Rush Presbyterian, Chicago, IL | 13 (9.5) | 4 (7.8) | ||
| University of Florida, Gainesville | 21(15.3) | 7 (13.7) | ||
| University of California, Los Angeles | 15 (11.0) | 7 (13.7) | ||
| Cleveland Clinic | 10 (7.3) | 1 (2.0) | ||
| Children’s Nat’l Med. Ctr., Washington, DC | 2 (1.5) | 0 (0) | ||
| Gender | Male | 73 (53.3) | 36 (70.6) | 0.03 |
| Female | 64 (46.7) | 15 (29.4) | ||
| Race/Ethnicity | White, Non-Hispanic | 112 (81.8) | 41 (80.4) | 0.83e |
| Hispanic | 12 (8.8) | 1 (2.0) | ||
| Black, Non-Hispanic | 8 (5.8) | 6 (11.8) | ||
| Asian and Pacific Islander | 3 (2.2) | 0 (0) | ||
| Other | 2 (1.5) | 3 (5.9) | ||
| Patient’s education | Less than high school | 74 (54.0) | 18 (35.3) | 0.07 |
| HS/equivalent/post-HS training/some college | 43 (31.4) | 22 (43.1) | ||
| College or post-grad | 20 (14.6) | 11 (21.6) | ||
| Missing | 0 (0) | 0 (0) | ||
| Mother’s education | Less than high school | 14 (10.2) | 9 (17.7) | 0.28f |
| HS/equivalent/post-HS training/ some college | 85 (62.0) | 30 (58.8) | ||
| College or post-grad | 36 (26.3) | 10 (19.6) | ||
| Missing | 2 (1.5) | 2 (3.9) | ||
| Father’s education | Less than high school | 15 (11.0) | 13 (25.5) | 0.02 |
| HS/equivalent/post-HS training/ some college | 72 (52.6) | 17 (33.3) | ||
| College or post-grad | 44 (32.1) | 16 (31.4) | ||
| Missing | 6 (4.4) | 5 (9.8) | ||
| Combined household income | ≤ $40,000$40,001 - $60,000 | 54 (37.2)27 (19.7) | 18 (35.3)13 (25.5) | 0.84 |
| > $60,000 | 38 (27.7) | 13 (25.5) | ||
| Missing | 18 (13.1) | 7 (13.7) | ||
| Urban | Ever lived in urban area | 119 (86.9) | 47 (92.2) | 0.32 |
| Never lived in urban area | 18 (13.1) | 4 (7.8) | ||
| Age (yrs) | 0 – 14 | 37 (27.0) | 9 (17.7) | < 0.001 |
| 15 – 29 | 72 (52.6) | 12 (23.5) | ||
| 30 – 44 | 13 (9.5) | 9 (17.7) | ||
| 45 and older | 15 (10.9) | 21 (41.2) | ||
| Median age (yrs) | Overall | 17.6 | 41.3 | < 0.001g |
| Males | 17.0 | 42.0 | < 0.001g | |
| Females | 17.0 | 39.0 | 0.02g |
There were 194 patients who provided tumor-adjacent bone specimens; however, fluoride concentrations from specimens (five cases and one tumor control) were below < 100 mg F/kg bone ash and were not included in the analysis. In total, 188 patients were used in the analysis.
Percentages do not add up to 100 because of rounding.
Chi-square testing differences between cases and tumor controls with bone specimens.
For the comparisons, patients from MGH and Children’s Nat’l Med. Ctr. were grouped together, since they are in the same region; patients from Creighton Univ. and the Univ. of Nebraska were grouped together, since they are in the same city; and patients from the Univ. of Chicago and Rush Presbyterian were grouped together, since they are in the same city.
Comparing White, Non-Hispanics with all other racial/ethnic groups.
Fisher’s exact test.
Wilcoxon rank sum.
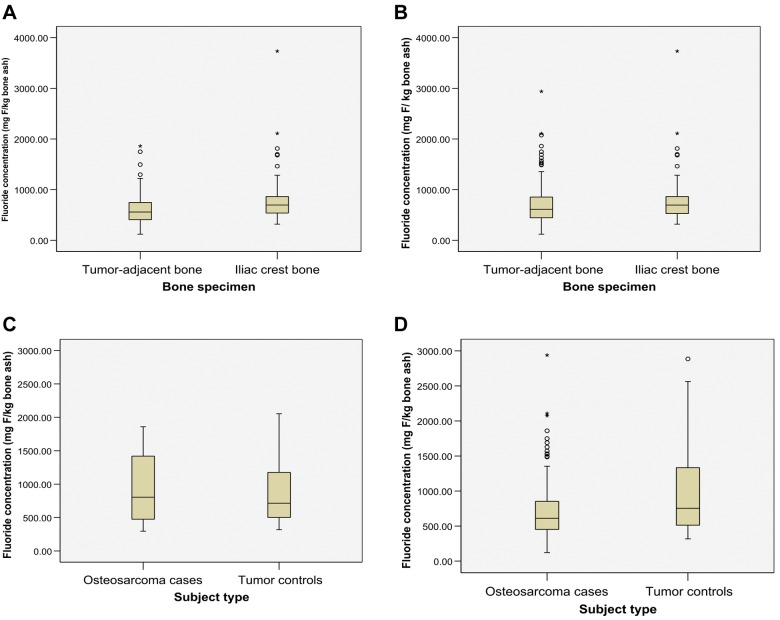
Among the 53 cases who provided both tumor-adjacent and iliac crest bone specimens, there was a significantly higher fluoride concentration in iliac crest bone than in tumor-adjacent bone (median = 697 vs. 558 mg F/kg bone ash, p < 0.001) (Fig., a). However, when all specimens of tumor-adjacent bone (N = 137) and iliac crest bone (N = 54) from cases were included, the median fluoride concentrations in iliac crest bone were not significantly higher than those in tumor-adjacent bone (median = 695 vs. 611 mg F/kg bone ash, p = 0.10) (Fig., b). In a validation study examining the fluoride content between iliac crest bone and tumor-adjacent bone among cases, the Spearman correlation was 0.61 (p < 0.001).
Figure.
Box plots of interquartile range (IQR), range, and median fluoride concentrations (mg/kg bone ash). (A) Tumor-adjacent bone vs. iliac crest bone among cases that contributed both samples: IQR = 407.0-746.5 vs. 538.0-863.0; range = 121.0-1859.5 vs. 318.5-3732.5; median = 558.0 vs. 696.5 (p < 0.001, Wilcoxon signed-rank test). (B) Tumor-adjacent bone vs. iliac crest bone among all cases: IQR = 444.5-853.0 vs. 527.0-863.5; range = 121.0-2939.0 vs. 318.5-3732.5; median = 611.0 vs. 696.5 (p = 0.10, Wilcoxon rank-sum test). (C) Tumor-adjacent bone in matched cases vs. tumor controls: IQR = 474.8-1419.5 vs. 501.0-1176.5; range = 296.0-1859.5 vs. 317.5-2053.5; median = 803.8 vs. 714.3 (p = 0.63, Wilcoxon signed-rank test). (D) Tumor-adjacent bone among all cases vs. tumor controls: IQR = 451.5-853.0 vs. 501.5-1359.0; range = 121.0-2939.0 vs. 317.5-2885.0; median = 611.0 vs. 754.0 (p = 0.0008, Wilcoxon rank-sum test).
There was no significant difference in the median fluoride concentration in bone between the matched osteosarcoma case and tumor control pairs (N = 32) (median = 804 vs. 714 mg F/kg of bone ash, p = 0.63) (Fig., c). When bone specimens from all cases (N = 137) and controls (N = 51) were included in an unmatched analysis, the median bone fluoride concentration in tumor-adjacent bone was significantly higher in controls than in cases (median = 754 vs. 611 mg F/kg of bone ash, p = 0.01) (Fig., d).
There were no differences in the results of the conditional and unconditional analyses; thus, the results of the unconditional analyses are reported given the increased power for detecting associations with bone fluoride. In the age- and sex-adjusted analysis, OR = 1.22 for an increase in bone fluoride from the 25th percentile (463.5 ppm) to the 75th percentile (943.3 ppm), representing an OR = 1.32 (95% CI, 0.58-3.03) for a 1-unit increase in the natural log of fluoride (ppm). After adjustment for age and gender, history of broken bones, other bone diseases, other cancer diagnoses, and history of receiving radiation prior to illness were significant covariates (see Appendix Table 2). The OR for log bone fluoride adjusted for these predictors, age, and gender was 1.23 (95% CI, 0.51-2.97) (Table 2). With a stepwise selection method to determine the final model adjusted for age and gender, a history of broken bones remained as a significant predictor, and the final adjusted OR was 1.33 (95% CI: 0.56-3.15) (Table 2).
Table 2.
Odds Ratios and 95% CI for a 1-unit Increase in Natural Log of Fluoride Concentration (ppm) in Bone and Risk of Osteosarcoma: (A) for All Osteosarcoma Cases and Tumor Controls and (B) among Patients under 45 Years of Age
| OR | 95% CI | p Value | |
|---|---|---|---|
| A. For all osteosarcoma cases and tumor controls | |||
| Age- and sex-adjusted modela | 1.32 | (0.58, 3.03) | 0.51 |
| Fully adjusted modelb | 1.23 | (0.51, 2.97) | 0.65 |
| Risk-adjusted modelc | 1.33 | (0.56, 3.15) | 0.58 |
| B. Among patients younger than 45 yrs old | |||
| Age- and sex-adjusted modela | 1.23 | (0.48, 3.16) | 0.67 |
| Fully adjusted modeld | 1.27 | (0.49, 3.35) | 0.62 |
| Risk-adjusted modele | 1.23 | (0.48, 3.16) | 0.67 |
Includes only age and gender.
Includes all variables that were significant at p ≤ 0.25 in the age- and sex-adjusted analysis (history of broken bones, other cancers, other bone diagnosis, and received radiation prior to illness), plus age and gender.
Includes history of broken bones, plus age and gender.
Includes history of broken bones (significant at p ≤ 0.25 in the age- and sex-adjusted analysis), plus age and gender.
Includes age and gender and no other variables.
In an analysis restricted to patients < 45 yrs old (123 cases; 30 controls), history of broken bones was the only predictor of osteosarcoma risk at the p = 0.25 level, adjusted for age and gender. However, the final risk-adjusted model included only age and gender (OR = 1.23, 95% CI: 0.48-3.16) (Table 2).
Discussion
The results of the present study are similar to results of several other case-control studies that included histories of fluoride exposure based on community water fluoride concentrations or from other fluoride sources, such as toothpaste and supplements (McGuire et al., 1991; Gelberg et al., 1995; Moss et al., 1995).
The higher median fluoride concentration of controls compared with that of cases in this study is likely due to the fact that control patients tended to be older than the cases. In this study, fluoride content in bone in both cases and controls increased with age (moderate positive correlation, data not shown), which is similar to findings in studies that looked at the relationship between bone fluoride content and age (Parkins et al., 1974; Eble et al., 1992; Richards et al., 1994).
Previous ecological and case-control studies that relied on historic residential information were limited in that they did not reflect the true exposure of fluoride at the individual level; thus, such studies are subject to the “ecological fallacy” (Aschengrau and Seage, 2003). They also did not take into account population mobility between fluoridated and non-fluoridated areas, or changes in population size and age (Freni and Gaylor, 1992), or potential confounders.
In this study, cases were all recruited from academic referral centers for bone cancer and thus were not a random sample of osteosarcoma patients. Controls were also bone cancer patients recruited from these same centers, and thus likely reflect the same source population as the cases. Although there was a difference in participation rates in the bone donation component, with 76% of the cases and 48% of the controls participating, it is unlikely that any enthusiasm for participation was related to bone fluoride levels.
Misclassification of exposure and/or outcome is always a concern in observational studies; however, given the laboratory measurement of fluoride exposure and the histologic confirmation of cases, misclassification bias is likely to be minimized in this study. The coefficient of variation for deer bone specimens, included in each batch of specimens as quality control, was 0.03, further decreasing the likelihood of substantial non- random misclassification bias.
There are also some potential drawbacks to the use of bone fluoride measurements. For example, if risk is related to exposures at a specific time in life, rather than total accumulated dose, this metric would not be optimal. Also, it is possible that fluoride concentrations in bone may be influenced by the disease, or that concentrations in tumor tissue are not representative of pre- disease levels. For this reason, we chose normal tumor adjacent bone from the surgical specimens instead of the tumor tissue. It is possible that bone metabolism in the vicinity of the tumor could also be disrupted, however, we chose other bone tumors as controls. If such a circumstance prevailed, it would likely affect the controls in a manner similar to the cases. To address this concern, we compared fluoride levels in bone adjacent to the tumor with those in bone from a distant site (iliac crest) from the same patients. While the absolute levels were different, as anticipated from the different kinds of bone involved, there was a highly significant rank order correlation between the fluoride levels from these 2 locations, lending some confidence to the validity of the adjacent bone measures (data not shown).
If fluoride levels were related to bone cancer in general, the current study design would be unable to detect this. There is no published evidence of such an association.
The major advantage of this study is the use of bone fluoride concentrations as the measure of fluoride exposure, rather than estimating fluoride exposure in drinking water (Bassin et al., 2006). Since 99% of the body burden of fluoride is located in calcified tissues, and fluoride concentration is dependent upon the amount and duration of exposure as well as the rate of bone turnover (Turner et al., 1993), if chronic fluoride intake was a risk factor for osteosarcoma, then it would be reasonable to expect that cases would have significantly higher bone fluoride concentrations than tumor controls. This study did not demonstrate an association between fluoride levels in bone and osteosarcoma.
Acknowledgments
The authors acknowledge the participation of the patients, hospitals, and surgeons in this study.
Footnotes
A supplemental appendix to this article is published electronically only at http://jdr.sagepub.com/supplemental.
This study was funded by the National Institute of Environmental Health Sciences, National Institutes of Health (NIH) grant 5R01ESO6000, National Institute of Dental and Craniofacial Research (NIH) grant T32DE07151, and National Cancer Institute (NIH). Data collection was conducted by Westat, Inc. This manuscript is based on a dissertation submitted to the faculty of the Harvard School of Public Health in partial fulfillment of the requirements for the degree of DrPH in the Department of Epidemiology (FMK). A portion was presented at the American Public Health Association 134th Annual Meeting and Exhibition, Boston, MA, 2006 (FMK).
CWD has written reviews of the literature for several companies that sell, reimburse for, or do research on preventive dentistry products, most notably GlaxoSmithKline, Colgate- Palmolive, Dentsply, Quintile, Delta Dental Plans, and the United States Public Health Service (USPHS). CH has done limited consulting with Procter & Gamble. All other authors have no conflict of interest to disclose.
Contributor Information
Collaborators: M.C. Gebhardt, M.T. Scarborough, S. Gitelis, J.J. Eckardt, J.R. Neff, M.J. Joyce, M. Malawer, M. McGuire, and H.C. Anderson
References
- Aschengrau A, Seage GR. (2003). Essentials of epidemiology in public health. Sudbury, MA: Jones and Bartlett Publishers [Google Scholar]
- Bassin EB, Wypij D, Davis RB, Mittleman MA. (2006). Age-specific fluoride exposure in drinking water and osteosarcoma (United States). Cancer Causes Control 17:421-428 [DOI] [PubMed] [Google Scholar]
- Doll R, Kinlen L. (1977). Fluoridation of water and cancer mortality in the U.S.A. Lancet 1:1300-1302 [DOI] [PubMed] [Google Scholar]
- Doll R, Kinlen L. (1978). Cancer mortality and fluoridation. Lancet 1:150. [PubMed] [Google Scholar]
- Eble DM, Deaton TG, Wilson FC, Bawden JW. (1992). Fluoride concentrations in human and rat bone. J Public Health Dent 52:288-291 [DOI] [PubMed] [Google Scholar]
- Freni SC, Gaylor DW. (1992). International trends in the incidence of bone cancer are not related to drinking water fluoridation. Cancer 70: 611-618 [DOI] [PubMed] [Google Scholar]
- Gelberg KH, Fitzgerald EF, Hwang SA, Dubrow R. (1995). Fluoride exposure and childhood osteosarcoma: a case-control study. Am J Public Health 85:1678-1683 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Greenland S, Finkle WD. (1995). A critical look at methods for handling missing covariates in epidemiologic regression analyses. Am J Epidemiol 142:1255-1264 [DOI] [PubMed] [Google Scholar]
- Gurney JG, Swensen AR, Bulterys M. (1999). Malignant bone tumors. In: Cancer incidence and survival among children and adolescents: United States SEER Program 1975-1995, National Cancer Institute, SEER Program. Smith MAS, Ries LA, Gurney JG, Linet M, Tamra T, Young JL, et al., editors. Bethesda, MD: NIH Pub. No. 99-4649, pp. 99-110 [Google Scholar]
- Homa DM, Sowers MR, Schwartz AG. (1991). Incidence and survival rates of children and young adults with osteogenic sarcoma. Cancer 67:2219-2223 [DOI] [PubMed] [Google Scholar]
- Hoover RN, McKay FW, Fraumeni JF. (1976). Fluoridated drinking water and the occurrence of cancer. J Natl Cancer Inst 57:757-768 [DOI] [PubMed] [Google Scholar]
- Hrudey SE, Soskolne CL, Berkel J, Fincham S. (1990). Drinking water fluoridation and osteosarcoma. Can J Public Health 81:415-416 [PubMed] [Google Scholar]
- Mahoney MC, Nasca PC, Burnett WS, Melius JM. (1991). Bone cancer incidence rates in New York State: time trends and fluoridated drinking water. Am J Public Health 81:475-479 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maurer JK, Cheng MC, Boysen BG, Anderson RL. (1990). Two-year carcinogenicity study of sodium fluoride in rats. J Natl Cancer Inst 82: 1118-1126 [DOI] [PubMed] [Google Scholar]
- McGuire SM, Vanable ED, McGuire MH, Buckwalter JA, Douglass CW. (1991). Is there a link between fluoridated water and osteosarcoma? J Am Dent Assoc 122:38-45 [DOI] [PubMed] [Google Scholar]
- Medina N, Douglass CW, Whitford GM, Hoover RN, Fears TR. (2006). A reproducibility study for a fluoride assay in bone. Cancer Epidemiol Biomarkers Prev 15:1035-1037 [DOI] [PubMed] [Google Scholar]
- Miller RW, Boice JD, Curtis RE. (1996). Bone cancer. In: Cancer epidemiology and prevention. Schottenfeld D, Fraumeni JF, Jr, editors. New York: Oxford University Press; pp. 946-958 [Google Scholar]
- Moss ME, Kanarek MS, Anderson HA, Hanrahan LP, Remington PL. (1995). Osteosarcoma, seasonality, and environmental factors in Wisconsin, 1979-1989. Arch Environ Health 50:235-241 [DOI] [PubMed] [Google Scholar]
- Newbrun R. (1977). The safety of water fluoridation. J Am Dent Assoc 94:301-304 [DOI] [PubMed] [Google Scholar]
- NTP (1990). National Toxicology Program Technical Report on the Toxicology and Carcinogenesis Studies of Sodium Fluoride (CAS No. 7681-49-4) in F344/N Rats and B6C3F1 Mice (Drinking Water Studies), Technical Report No. 393 Research Triangle Park, NC: NIH Publ. No. 91-2848 National Institutes of Health, Public Health Service, U.S. Department of Health and Human Services [Google Scholar]
- NTP (1992). National Toxicology Program Supplemental 2-Year Study of Sodium Fluoride in Male F344 Rats (CAS No. 7681-49-4). Study No. C55221D Research Triangle Park, NC: National Institutes of Environmental Health Sciences [Google Scholar]
- Pagano M, Gauvreau K. (2000). Principles of biostatistics. 2nd ed. Pacific Grove, CA: Duxbury Thomas Learning [Google Scholar]
- Parkins FM, Tinanoff N, Moutinho M, Anstey MB, Waziri MH. (1974). Relationships of human plasma fluoride and bone fluoride to age. Calcif Tissue Res 16:335-338 [DOI] [PubMed] [Google Scholar]
- Patel SR, Benjamin RS. (2005). Soft tissue and bone sarcomas and bone metastases. In: Harrison’s principles of internal medicine. Kasper DL, Braunwald E, Fauci AS, Hauser SL, Longo DL, Jameson JL, Isselbacher KJ, editors. New York: McGraw-Hill Co., Inc., pp. 610-613 [Google Scholar]
- Richards A, Mosekilde L, Sogaard CH. (1994). Normal age-related changes in fluoride content of vertebral trabecular bone—relation to bone quality. Bone 15:21-26 [DOI] [PubMed] [Google Scholar]
- Steiner GC. (1965). Postradiation sarcoma of bone. Cancer 18:603-612 [DOI] [PubMed] [Google Scholar]
- Taves D. (1968). Determination of submicromolar concentrations of fluoride in biological specimens. Talanta 15:1015-1023 [DOI] [PubMed] [Google Scholar]
- Tucker MA, D’Angio GJ, Boice JD, Strong LC, Li FP, Stovall M, et al. (1987). Bone sarcomas linked to radiotherapy and chemotherapy in children. N Engl J Med 317:588-593 [DOI] [PubMed] [Google Scholar]
- Turner CH, Boivin G, Meunier PJ. (1993). A mathematical model for fluoride uptake by the skeleton. Calcif Tissue Int 52:130-138 [DOI] [PubMed] [Google Scholar]
- Whitford GM. (1996). Metabolism and toxicity of fluoride. 2nd ed. San Francisco, CA: S. Karger Publishers [Google Scholar]