Abstract
Background
The purpose of this study was to evaluate the efficacy and safety of carbohydratederived fulvic acid (CHD-FA) in the treatment of eczema in patients two years and older.
Methods
In this single-center, double-blind, placebo-controlled, parallel-group comparative study, 36 volunteers with predetermined eczema were randomly assigned to receive either the study drug or placebo twice daily for four weeks.
Results
All safety parameters remained within normal limits, with no significant differences in either group. Significant differences were observed for both severity and erythema in the placebo and CHD-FA treated groups, and a significant difference was observed for scaling in the placebo-treated group. With regard to the investigator assessment of global response to treatment, a significant improvement was observed in the CHD-FA group when compared with the placebo group. A statistically significant decrease in visual analog scale score was observed in both groups, when comparing the baseline with the final results.
Conclusion
CHD-FA was well tolerated, with no difference in reported side effects other than a short-lived burning sensation on application. CHD-FA significantly improved some aspects of eczema. Investigator assessment of global response to treatment with CHD-FA was significantly better than that with emollient therapy alone. The results of this small exploratory study suggest that CHD-FA warrants further investigation in the treatment of eczema.
Keywords: fulvic acid, eczema, anti-inflammatory, efficacy, safety
Introduction
Fulvic acid is one of the components of the so-called humic substances, which are formed naturally during the decay of plant and animal residues.1 These substances can be divided into humic acid, fulvic acid, and humin on the basis of solubility in water as a function of pH.2 Fulvic acid is the fraction that is soluble in water under all pH conditions and is generally lower in molecular size, weight, and color intensity when compared with the humic acids.2 Oxifulvic acid has been shown in vitro to have antimicrobial activity3 and to suppress superoxide production by neutrophils. Topical application of oxifulvic acid to laboratory mice has demonstrated clear anti-inflammatory properties.2 Heavy metal-free, carbohydrate-derived fulvic acid (CHD-FA) has been tested in pilot studies, and found to have similar properties when compared with oxifulvic acid.4 Traditionally, CHD-FA has been used to treat a variety of diseases, including skin conditions, although the exact mechanism of action in eczema is not clear due to its complex mixture of acids with different activities and properties, including antimicrobial effects.
The purpose of this study was to evaluate the efficacy and safety of fulvic acid in the treatment of eczema in patients aged two years and older. Eczema is often a recurrent and difficult disease to treat, with topical glucocorticoids used to treat flares and normal emollients used for maintenance therapy. In chronic conditions, topical calcineurin antagonists (eg, pemicrolimus or tacrolimus) are used or may even be supplemented with systemic immunosuppressive therapy in severe cases.
The importance of pH is also recognized in skin diseases. Some diseases or stages are associated with different pH levels. An increase in skin pH may be due to atopic and seborrheic dermatitis, especially acute lesions that are erythematous with exudates and crusts, while chronic atopic dermatitis lesions with lichenification and scaling are only slightly more alkaline than normal skin.5 In many of these diseases, the pathogenesis of alkalinization is not clear, but the higher pH likely predisposes the skin to secondary infections.6
Fulvic acid, with its known anti-inflammatory properties and good safety profile, has the added benefit of being an acid. Reducing the pH of the skin also relieves the itch during eczema. In this study, 3.5% CHD-FA in an emollient (buffered to pH 4.8) was compared with an acidic (pH 4.8) emollient to establish the anti-inflammatory properties of CHD-FA in patients with eczema.
Materials and methods
In this single-center, double-blind, placebo-controlled, parallel-group comparative study, 36 volunteers with known eczema were randomly assigned to receive either the study drug or a placebo emollient applied twice daily for four weeks. Treatment period observations and measurements included improvement in visual analog scale score and investigator global assessment. Blood samples were taken at baseline and again at the end of the study, and included a full blood count, and liver and kidney function tests. Clinical examination, electrocardiogram, documentation of adverse events, and laboratory investigations were also performed to confirm safety.
Healthy males and females over the age of two years were eligible for inclusion in this study. Female patients were required to use reliable contraception if they were of childbearing age. A previous diagnosis of eczema was required. Patients were required to give their written informed consent to participate. Exclusion criteria included renal impairment, liver disease, abnormal liver function tests, hematological abnormalities, any autoimmune disease other than eczema, pregnancy, lactation, participation in any other clinical trial within the previous month, clinical signs of infection, use of concomitant medication, systemic corticosteroids, phototherapy/immune suppression/antihistamines, topical tacrolimus/pemicrolimus within the previous four weeks, or topical corticosteroids within the previous four days.
A CHD-FA 3.5% or placebo (pH 4.8) emollient was applied twice daily to affected areas as well as Epizone A® emollient buffered with acetic acid as needed.
Statistical analysis
Blood parameters, visual analog scale score, and investigator assessment of disease severity were analyzed using the Wilcoxon Rank test at a 95% confidence level. The investigator assessment of global response to treatment was analyzed using the Mann-Whitney U test for nonparametric data. All analysis was done using Graphpad Prism 4.0.
Results
Thirty-six patients completed the trial. Two patients used concomitant medication during the study period and were excluded from the results. No significant differences in kidney function tests were observed between the treatment groups. With regard to liver function tests, a statistically significant difference was observed for aspartate transaminase, although values remained within normal limits, as did the rest of the laboratory parameters for the liver. All safety parameters in the full blood count also remained within normal limits. The only side effect reported was a short-lived burning sensation after application of treatment.
Efficacy
Severity, erythema, vesiculation, fissuring, and scaling were monitored by the investigators during the study. Significant differences were observed for both severity and erythema in the placebo-treated and CHD-FA-treated groups, as well as a significant difference in scaling in the placebo-treated group. The rest of the parameters remained within normal limits (Table 1). Investigator assessment of global response to treatment was performed using the following seven-point scale: 0 = completely clear, 1 = almost clear (about 90%), 2 = marked improvement (75%), 3 = moderate improvement (50%), 4 = slight improvement (25%), 5 = no change (moderate to severe disease) and 6 = worse.7 A significant improvement in global response to treatment was observed in the CHD-FA group when compared with the placebo group (Table 2). A statistically significant decrease in scores on the visual analog scale was observed in both groups, when comparing baseline values with the final results (Table 3).
Table 1.
Investigator assessment of severity of disease
| Placebo (n = 18)
|
CHD-FA (n = 18)
|
|||||||
|---|---|---|---|---|---|---|---|---|
| Base | ±SD | Final | ±SD | Base | ±SD | Final | ±SD | |
| Severity | 2.278 | 0.826 | 1.222* | 0.732 | 1.833 | 0.514 | 0.8331 | 0.707 |
| Erythema | 2.056 | 0.998 | 1.056** | 0.873 | 1.176 | 0.728 | 0.2352 | 0.437 |
| Vesiculation | 0.333 | 0.686 | 0.056 | 0.236 | 0.353 | 0.493 | 0.000 | 0.000 |
| Fissuring | 0.056 | 0.236 | 0.056 | 0.236 | 0.000 | 0.000 | 0.000 | 0.000 |
| Scaling | 0.500 | 0.707 | 0.056*** | 0.236 | 0.176 | 0.529 | 0.059 | 0.243 |
Notes: P = 0.0009;
P = 0.0059;
P = 0.0313;
P < 0.0001;
P = 0.0002 (all by Wilcoxon Signed Rank test). (0 = absent, 1 = mild, 2 = moderate, 3 = moderately severe, 4 = severe.)
Abbreviations: CHD-FA, carbohydrate-derived fulvic acid; SD, standard deviation.
Table 2.
Investigator assessment of global response to treatment
| Scale response (0–6)
|
||
|---|---|---|
| Average | ±SD | |
| Placebo (n = 18) | 2.94 | 1.11 |
| CHD-FA (n = 18) | 1.77* | 1.00 |
Notes: P < 0.05 (by Mann-Whitney U test). 0 = completely clear except for possible residual hyperpigmentation, 1 = almost clear, very significant clearance (about 90%), 2 = marked improvement/significant improvement (about 75%), 3 = moderate improvement (about 50%), 4 = slight improvement (about 25%), but significant disease remaining, 5 = no change (moderate to severe disease), 6 = worse.
Abbreviations: CHD-FA, carbohydrate-derived fulvic acid; SD, standard deviation.
Table 3.
Visual analog scale results.
| Percentage scale response (0–100 mm)
|
||||
|---|---|---|---|---|
| Base | ±SD | Final | ±SD | |
| Placebo (n = 18) | 62.81 | 20.89 | 34.72* | 26.81 |
| CHD-FA (n = 17) | 69.50 | 14.65 | 29.60** | 19.71 |
Notes: P = 0.003;
P = 0.0005 (both Wilcoxon Signed Rank test).
Abbreviations: CHD-FA, carbohydrate-derived fulvic acid; SD, standard deviation.
Discussion
All blood safety parameters remained within acceptable ranges. CHD-FA is a combination of weak acids buffered to pH 4.8, which could account for the burning sensation on application. Extensive clinical safety parameters have already been established for systemically administered CHD-FA (unpublished data). Our trial has further established that changing the route of administration from systemic to topical does not affect the safety profile of the product.
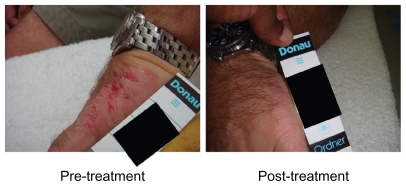
A standard five-point severity grading scale (0 = absent to 4 = severe) has been used to evaluate five characteristics of eczema, ie, severity, erythema, vesiculation, fissuring, and scaling.8 Both treatment groups demonstrated significant improvements in some of the characteristics tested, which is not uncommon in eczema trials, whereby application of an emollient (pH-adjusted) may improve the condition. According to the investigator assessment of global response to treatment, the group treated with CHD-FA showed a significant improvement when compared with the placebo group, thus demonstrating the anti-inflammatory properties of CHD-FA, leading to a significant overall improvement of the condition (Figure 1).
Figure 1.
Photograph of patient before and after treatment.
The severity of eczema was evaluated by the patients using a visual analog scale, defined on a 10 cm line where 0 refers to no eczema and 10 refers to the most severe eczema experienced by the patient.7 A significant decrease was observed for both groups, indicating that both treatments alleviated patient perception of eczema.
Conclusion
In this trial, CHD-FA significantly improved some features of eczema, in particular, overall severity and erythema. Investigator assessment of global response to treatment with CHD-FA was significantly better than that with emollient therapy only. CHD-FA was well tolerated, with no difference in reported side effects, other than a short-lived burning sensation on application. This small exploratory study suggests that further investigation of the clinical use of CHD-FA in eczema is warranted.
Footnotes
Disclosure
The authors report no conflicts of interest in this work.
References
- 1.Snyman JR, Dekker J, Malfeld SCK, van Rensburg CEJ. Pilot study to evaluate the safety and therapeutic efficacy of topical oxifulvic acid in atopic volunteer. Drug Dev Res. 2002;57:40–43. [Google Scholar]
- 2.Van Rensburg CEJ, Malfeld SCK, Dekker J. Topical application of oxifulvic acid suppresses the cutaneous immune response in mice. Drug Dev Res. 2001;53:29–32. [Google Scholar]
- 3.van Rensburg CEJ, Van Straten A, Dekker J. An in vitro investigation of the antimicrobial activity of oxifulvic acid. J Antimicrob Chemother. 2000;46:853. doi: 10.1093/jac/46.5.853. [DOI] [PubMed] [Google Scholar]
- 4.Vrey PJ, Jansen van Rensburg CEJ. Characterization of a novel fulvic acid product derived from a safe, metal-free, naturally-occurring carbohydrate source. Data on file at Department of Pharmacology, University of Pretoria; Pretoria, South Africa: [Google Scholar]
- 5.Levin OL, Silvers SE. The reaction of the skin and its secretions in eczema. Arch Derm Syphilol. 1932;25:825–834. [Google Scholar]
- 6.Chikakane K, Takahashi H. Measurement of skin pH and its significance in cutaneous diseases. Clin Dermatol. 1995;13:299–306. doi: 10.1016/0738-081x(95)00076-r. [DOI] [PubMed] [Google Scholar]
- 7.Barbier N, Paul C, Luger T, Allen R. Validation of the Eczema Area and Severity Index for atopic dermatitis in a cohort of 1550 patients from the pimecrolimus cream 1% randomized controlled clinical trials programme. Br J Dermatol. 2004;150:96–102. doi: 10.1111/j.1365-2133.2004.05696.x. [DOI] [PubMed] [Google Scholar]
- 8.Faghihi G, Iraji F, Shahingohar A, Saidat AH. The efficacy of ‘0.05% Clobetasol + 2.5% zinc sulphate’ cream vs ‘0.05% Clobetasol alone’ cream in the treatment of the chronic hand eczema: Double-blind study. J Eur Acad Dermatol Venereol. 2008;22:531–536. doi: 10.1111/j.1468-3083.2007.02533.x. [DOI] [PubMed] [Google Scholar]