Abstract
Objectives
Brain metastases (BMs) often advance the course of non-small cell lung cancer (NSCLC). We performed an observational study in order to investigate the possible correlation of selected clinical and epidemiological factors with BM appearance in patients suffering from different histological subtypes of NSCLC stage I–IV.
Methods
The study included 161 consecutive patients with NSCLC. Analyzed data included patient- and tumor-related characteristics.
Results
Thirty-nine patients (24.2%) presented BMs within 12 (0–36) weeks of diagnosis. BMs decreased the mean overall survival significantly (15.6 versus 50.7 weeks, P < 0.001), with hazard ratio (95% confidence interval) 3.60 (2.42–5.35). The age of the patients with BM was significantly lower than that of the patients without BM (60.8 ± 8.9 versus 66.5 ± 8.5, P < 0.001). Patients with BM had significantly higher pack-years consumption (75.9 ± 23.9 versus 58.9 ± 31.9, P = 0.003) and larger tumor size compared with patients without BM (size in mm: 55.1 ± 20.1 versus 45.9 ± 19.3, P = 0.012). The presence of BM was also correlated with the absence of lung (P < 0.001), bone (P = 0.005), and adrenal (P = 0.046) metastases.
Conclusion
Younger NSCLC patients with high tobacco consumption, large tumor size, and absence of metastases in other organs (lung, bones, adrenal metastases) are at high risk of BM appearance during the course of NSCLC and are candidates for prophylactic cranial irradiation early in the course of the disease.
Keywords: NSCLC, brain metastases, clinical and epidemiological factors, PCI
Introduction
Lung cancer was the leading cause of death from cancer in Europe in 2006, with 334,800 deaths (19.7% of total).1 Non-small cell lung cancer (NSCLC) is the most common type of lung cancer, representing more than 80% of lung cancer cases.2
Brain metastases (BMs) are a frequent complication of NSCLC, especially in patients with locally advanced disease.3,4 The addition of chemotherapy to radiation therapy (RT) reduces distant metastases and significantly improves survival.5,6 However, chemoradiotherapy is shown not to reduce the rate of BM,5 but to be associated with increased rates of overall brain failure (21%–54%) and an increased incidence of the brain as the first site of relapse (15%–30%).5–8 These findings emphasize the need for treatment specifically directed at brain micrometastases.
Prophylactic cranial irradiation (PCI) has been demonstrated to reduce the incidence or delay the onset of BM in patients with locally advanced NSCLC, after initial treatment in numerous selected nonrandomized and randomized studies.3,7,9–16 Nevertheless, during the last decade only few studies assessed the clinical and epidemiological factors associated with high risk of BM appearance in NSCLC patients with locally advanced disease at diagnosis.14,17–20 In these studies, several factors such as duration of survival after diagnosis, performance status, chemotherapy regimens, age at diagnosis, sex, and lung cancer histotype and stage have been associated with the risk of BM development.
The authors of this paper hypothesized that among NSCLC patients of stage I–IV may exist a group of patients at high risk of presenting BM that may be protected using PCI. This group should be identified in order to serve as target for future studies of PCI application in NSCLC.
We performed an observational study in order to investigate the possible correlation of selected clinical and epidemiological factors with BM appearance in patients suffering from different histological subtypes of NSCLC stage I–IV.
Methods
The study’s cohort
We recruited 161 consecutive patients with a new diagnosis of NSCLC, between January 2003 and March 2009. Patients’ selection criteria were as follows: confirmed diagnosis of NSCLC and appropriate staging. The sixth edition of the tumor–node–metastasis (TNM) classification was used.21
All patients were treated with surgery and/or chemotherapy and/or radiotherapy according to the current guidelines.22,23 They were evaluated every 3–6 months, depending on the curative or palliative nature of the initial treatment.
For each patient, the following variables were recorded at the time of diagnosis: age, sex, tobacco consumption, comorbidities, TNM status at diagnosis, tumor histotype, computed tomography (CT) scan features (central/peripheral location, side, lung lobe, size, cavitation, pleural effusion), and bronchoscopic findings. During the study period, the variables of patients with BM were registered and compared with those of patients without BM. All patients gave their informed consent, and the study was approved by the Ethics Committee of the “Sotiria” Chest Diseases Hospital, Athens.
Statistical analysis
Mean values (and standard deviation [SD]) or median values (and interquartile range [IR]) were used to describe quantitative variables. For the comparison of quantitative variables without normal distribution between two groups, and between three or more different groups, the Mann– Whitney test and Kruskal–Wallis test were used, respectively. To compare normal distributed quantitative variables between two groups and between three or more different groups, Student’s t-test and analysis of variance test were used, respectively.
To control for type I errors, due to multiple comparisons, Bonferroni correction was used, by which the significance level is defined as 0.05/k (k = number of comparisons). Logistic regression analysis (stepwise method) was used in order to find independent factors associated with BM presentation. Odds ratios (ORs) and 95% confidence intervals (CIs) were computed from the results of logistic analysis. Kaplan–Meyer method was used to estimate survival curves. To compare survival curves, log rank tests were used. Statistical significance was set at 0.05, and all P-values are two tailed. For the statistical analysis, SPSS Statistics 17.0 (IBM Corporation, Somers, NY) and STATA 9.0 (Stata Corp, College Station, TX) programs were used.
Results
Description of the cohort
Patient’s characteristics are summarized in Table 1. Most of the patients were males (88.8%), with mean age (±SD) 65.1 ± 8.9 years and mean tobacco consumption (±SD) of 63.0 ± 31.0 pack-years.
Table 1.
Patient- and disease-related characteristics
| Characteristic | n (%) |
|---|---|
| Patient-related variables | |
| Sex | |
| Male/female | 143 (88.8)/18 (11.2) |
| Age | |
| Mean ± SD | 65.1 ± 8.9 |
| Pack-years | |
| Mean ± SD | 63.0 ± 31.0 |
| COPD | |
| No/yes | 89 (55.3)/72 (44.7) |
| Arterial hypertension | |
| No/yes | 98 (60.9)/63 (39.1) |
| Coronary disease | |
| No/yes | 132 (82.0)/29 (18.0) |
| Diabetes mellitus | |
| No/yes | 136 (84.5)/25 (15.5) |
| Gastritis/ulcer | |
| No/yes | 138 (85.7)/23 (14.3) |
| Hypothyroidism | |
| No/yes | 154 (95.7)/7 (4.3) |
| Other comorbidity | |
| No/yes | 130 (80.7)/31 (19.3) |
| Disease-related variables | |
| Histotype | |
| Non-differentiated NSCLC | 49 (30.4) |
| Squamous | 49 (30.4) |
| Adenocarcinoma | 59 (36.6) |
| Large cell carcinoma | 4 (2.6) |
| Location | |
| Central/peripheral | 138 (85.7)/23 (14.3) |
| Bronchoscopic findings | |
| Mass | 36 (22.4) |
| Infiltration | 109 (67.7) |
| None | 16 (9.9) |
| Lung tumor side | |
| Right/left | 84 (52.2)/77 (47.8) |
| Lung tumor lobe | |
| Upper | 113 (70.2) |
| Middle | 8 (5.0) |
| Inferior | 40 (24.8) |
| Lung tumor size | |
| Mean ± SD | 48.1 ± 19.8 |
| Other tumor characteristics | |
| Pleural effusion | 59 (36.6) |
| Cavitation | 12 (7.5) |
| None | 90 (55.9) |
| T classification (brain metastases)a | |
| T1/T2 | 13 (8.1)/55 (34.2) |
| T3/T4 | 24 (14.9)/69 (42.9) |
| N classification (brain metastases)a | |
| N0/N1 | 38 (23.6)/16 (9.9) |
| N2/N3 | 70 (43.5)/37 (23.0) |
| Lung metastasis | |
| No/yes | 101 (62.7)/60 (37.3) |
| Bones metastasis | |
| No/yes | 102 (63.4)/59 (36.6) |
| Liver metastasis | |
| No/yes | 124 (77.0)/37 (23.0) |
| Adrenal metastasis | |
| No/yes | 126 (78.3)/35 (21.7) |
| Other metastasis | |
| No/yes | 144 (89.4)/17 (10.6) |
| Metastasis brain | |
| No/yes | 122 (75.8)/39 (24.2) |
| Diagnosis to brain metastases time (weeks) | |
| Median (IR) | 12 (0–36) |
| Number of brain metastases | |
| 0/1 | 122 (75.8)/16 (9.9) |
| 2/>2 | 6 (3.7)/17 (10.6) |
| Brain metastasis side | |
| Right | 12 (30.8) |
| Left | 9 (23.0) |
| Bilateral | 18 (46.2) |
| Brain metastasis lobe | |
| Frontal | 9 (23.1) |
| Parietal | 9 (23.1) |
| Occipital | 1 (2.6) |
| Cerebellum | 2 (5.1) |
| ≥2 | 18 (46.2) |
Note: TNM (tumor–node–metastasis) classification.21
Abbreviations: COPD, chronic obstructive pulmonary disease; IR, interquartile range; NSCLC, non-small cell lung cancer; SD, standard deviation.
Most of the tumors were located centrally (85.7%). Therefore, they were located within the range of fiber bronchoscopy, which revealed mainly mucosal or submucosal infiltration (67.7%). Most of the tumors were on the right lung (52.2%) and on the upper lobes (70.2%). The mean size (±SD) of the tumors, measured on CT scanners, was 48.1 ± 19.8 mm. Almost one-third (36.6%) were accompanied by pleural effusion at presentation. During the disease course, 37.3% of the patients presented lung, 36.6% bone, 23% liver, and 21.7% adrenal metastases.
BMs
BMs were presented in 24.2% of the patients. The median time (IR) of BM appearance was 12 (0–36) weeks from diagnosis. At the time of BM presentation, most of the patients were classified as T4 (42.9%) and N2 (43.5%) by the TNM classification. A total of 59% of the BMs were ≥2, mostly unilateral (53.8%).
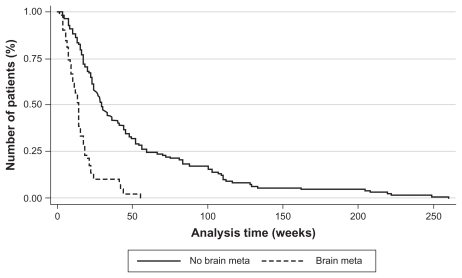
The overall survival of the cohort was influenced by the presence of BM (Figure 1). Survival time of the patients with BM was shorter compared with those without BM: 15.6 weeks (standard error [SE] = 1.9) versus 50.7 weeks (SE = 4.8, P < 0.001). The hazard ratio, upon Cox model, for the presence of BM was 3.60 (95% CI 2.42–5.35, P < 0.001).
Figure 1.
Kaplan–Meier estimation of overall survival (patients with or without brain metastases).
Abbreviation: meta, metastasis.
The age of patients with BM was significantly lower compared with that of the patients without BM (60.8 ± 8.9 versus 66.5 ± 8.5, P < 0.001) (Table 2). Furthermore, patients with BM had significantly higher pack-years consumption (75.9 ± 23.9 versus 58.9 ± 31.9, P = 0.003) and larger tumor size compared with patients without BM (size in mm: 55.1 ± 20.1 versus 45.9 ± 19.3, P = 0.012). The presence of BM was also correlated with the absence of lung (P < 0.001), bone (P = 0.005), and adrenal (P = 0.046) metastases.
Table 2.
Correlation of brain metastases with patient- and disease-related features (univariate analysis)
| Feature | Brain metastases
|
P χ2 test | |
|---|---|---|---|
| No (N) | Yes (N) | ||
| Patient-related variables | |||
| Sex | |||
| Male/female | 108/14 | 35/4 | 0.833 |
| Age | |||
| Mean ± SD | 66.5 ± 8.5 | 60.8 ± 8.9 | <0.001a |
| Pack-years | |||
| Mean ± SD | 58.9 ± 31.9 | 75.9 ± 23.9 | 0.003a |
| COPD | |||
| No/yes | 68/54 | 21/18 | 0.836 |
| Arterial hypertension | |||
| No/yes | 77/45 | 21/18 | 0.302 |
| Diabetes mellitus | |||
| No/yes | 102/20 | 34/5 | 0.592 |
| Coronary disease | |||
| No/yes | 98/24 | 34/5 | 0.332 |
| Hypothyroidism | |||
| No/yes | 118/4 | 36/3 | 0.361b |
| Gastritis/ulcer | |||
| No/yes | 105/17 | 33/6 | 0.822 |
| Other comorbidity | |||
| No/yes | 97/25 | 33/6 | 0.481 |
| Disease-related variables | |||
| Histotype | |||
| Non-differentiated NSCLC | 33 | 16 | 0.586b |
| Squamous | 38 | 11 | |
| Adenocarcinoma | 48 | 11 | |
| Large cell carcinoma | 3 | 1 | |
| Location | |||
| Central/peripheral | 105/17 | 33/6 | 0.822 |
| Bronchoscopic findings | |||
| Mass | 29 | 7 | 0.151 |
| Infiltration | 78 | 31 | |
| None | 15 | 1 | |
| Lung tumor side | |||
| Right/left | 62/60 | 22/17 | 0.543 |
| Lung tumor lobe | |||
| Upper | 86 | 27 | 0.657 |
| Middle | 5 | 3 | |
| Inferior | 31 | 9 | |
| Lung tumor size | |||
| Mean ± SD | 45.9 ± 19.3 | 55.1 ± 20.1 | 0.012a |
| Other tumor characteristics | |||
| Pleural effusion | 48 | 11 | 0.399 |
| Cavitation | 8 | 4 | |
| None | 66 | 24 | |
| T classification (brain metastases)c | |||
| T1/T2 | 10/37 | 3/18 | 0.138 |
| T3/T4 | 22/53 | 2/16 | |
| N classification (brain metastases)c | |||
| N0/N1 | 29/10 | 9/6 | 0.550 |
| N2/N3 | 53/30 | 17/7 | |
| Lung metastasis | |||
| No/yes | 67/55 | 34/5 | <0.001 |
| Bone metastasis | |||
| No/yes | 70/52 | 32/7 | 0.005 |
| Liver metastasis | |||
| No/yes | 91/31 | 33/6 | 0.195 |
| Adrenal | |||
| No/yes | 91/31 | 35/4 | 0.046 |
| Other metastasis | |||
| No/yes | 107/15 | 37/2 | 0.205 |
Notes: Student’s t-test;
Fisher’s exact test;
TNM (tumor–node–metastasis) classification.21
Abbreviations: COPD, chronic obstructive pulmonary disease; NSCLC, non-small cell lung cancer; SD, standard deviation.
Patients with right-sided BM presented a significantly lower rate of arterial hypertension (16.7% versus 83.8%, P = 0.050) (Table 3). None of the patients with unilobar BM suffered from diabetes, compared with patients with multilobar (≥2 lobes) metastases (P = 0.015) (Table 4).
Table 3.
Univariate analysis of brain metastases side
| Metastasis side | P χ2 test | |||
|---|---|---|---|---|
| Right (N) | Left (N) | Bilateral (N) | ||
| Patient-related variables | ||||
| Sex | ||||
| Male/female | 11/1 | 9/0 | 15/3 | 0.546 |
| Age | ||||
| Mean ± SD | 57.7 ± 8.3 | 62.6 ± 9.9 | 61.9 ± 8.7 | 0.349a |
| Pack-years | ||||
| Mean ± SD | 80.8 ± 20.2 | 84.0 ± 19.6 | 68.6 ± 26.8 | 0.202a |
| COPD | ||||
| No/yes | 6/6 | 5/4 | 10/8 | 1.000 |
| Arterial hypertension | ||||
| No/yes | 10/2 | 4/5 | 7/11 | 0.050 |
| Coronary disease | ||||
| No/yes | 12/0 | 9/0 | 13/5 | 0.054 |
| Diabetes mellitus | ||||
| No/yes | 11/1 | 9/0 | 14/4 | 0.406 |
| Gastritis/ulcer | ||||
| No/yes | 12/0 | 7/2 | 17/1 | 0.216 |
| Hypothyroidism | ||||
| No/yes | 12/0 | 6/3 | 15/3 | 0.063 |
| Other comorbidity | ||||
| No/yes | 11/1 | 7/2 | 15/3 | 0.740 |
| Disease-related variables | ||||
| Histotype | ||||
| Non-differentiated NSCLC | 6 | 2 | 7 | 0.194 |
| Squamous | 2 | 4 | 5 | |
| Adenocarcinoma | 3 | 3 | 6 | |
| Large cell carcinoma | 1 | 0 | 0 | |
| Location | ||||
| Central/peripheral | 10/2 | 8/1 | 15/3 | 1.000 |
| Diagnosis to brain metastases time (weeks) | ||||
| Median (IR) | 18 (4–40) | 0 (0–26) | 14 (0–36) | 0.632b |
Notes: Analysis of variance;
Kruskall–Wallis test.
Abbreviations: COPD, chronic obstructive pulmonary disease; IR, interquartile range; NSCLC, non-small cell lung cancer; SD, standard deviation.
Table 4.
Univariate analysis of brain metastases lobes
| Metastasis lobe | P Fisher’s exact test | ||
|---|---|---|---|
| 1 lobe (N) | ≥2 lobes (N) | ||
| Patient-related variables | |||
| Sex | |||
| Male/female | 19/2 | 16/2 | 1.000 |
| Age | |||
| Mean ± SD | 61.0 ± 9.5 | 60.6 ± 8.3 | 0.891a |
| Pack-years | |||
| Mean ± SD | 79.1 ± 19.8 | 72.2 ± 28.0 | 0.377a |
| COPD | |||
| No/yes | 12/9 | 9/9 | 0.656b |
| Arterial hypertension | |||
| No/yes | 13/8 | 8/10 | 0.276b |
| Diabetes mellitus | |||
| No/yes | 21/0 | 13/5 | 0.015 |
| Coronary disease | |||
| No/yes | 20/1 | 14/4 | 0.104 |
| Hypothyroidism | |||
| No/yes | 19/2 | 17/1 | 1.000 |
| Gastritis/ulcer | |||
| No/yes | 18/3 | 15/3 | 1.000 |
| Other comorbidity | |||
| No/yes | 17/4 | 16/2 | 0.667 |
| Disease-related variables | |||
| Histotype | |||
| Non-differentiated NSCLC | 7 | 8 | 0.088 |
| Squamous | 5 | 6 | |
| Adenocarcinoma | 8 | 4 | |
| Large cell carcinoma | 1 | 0 | |
| Location | |||
| Central/peripheral | 17/4 | 16/2 | 0.667 |
| Diagnosis to brain metastases time (weeks) | |||
| Median (IR) | 17 (0–32) | 8 (0–36) | 0.922c |
Notes: Student’s t-test;
Pearson’s χ2 test;
Mann–Whitney test.
Abbreviations: COPD, chronic obstructive pulmonary disease; IR, interquartile range; NSCLC, non-small cell lung cancer; SD, standard deviation.
According to regression analysis, age, tobacco consumption in pack-years, and absence of lung or bone metastases represented independent prognostic factors for the appearance of BM (Table 5). In particular, an increase of age reduced the possibility of BM appearance (OR 0.91; 95% CI 0.87–0.96, P < 0.001). Conversely, increasing cigarette consumption increased the possibility of BM appearance (OR 1.02; 95% CI 1.001–1.030, P = 0.006). Patients without lung and bone metastases had 76% and 70% higher possibility of presenting BM, respectively.
Table 5.
Correlation of brain metastases with patient- and disease-related features (multivariate analysis)
| Variable | Odds ratio | 95% | CI | P |
|---|---|---|---|---|
| Age | 0.91 | 0.87 | 0.96 | <0.001 |
| Pack-years | 1.02 | 1.01 | 1.03 | 0.006 |
| Lung metastasis | ||||
| No | 1.00a | |||
| Yes | 0.24 | 0.08 | 0.69 | 0.008 |
| Bone metastasis | ||||
| No | 1.00a | |||
| Yes | 0.30 | 0.11 | 0.81 | 0.018 |
Note: Represents referral class.
Discussion
The main finding of this observational study was that younger NSCLC patients with high tobacco consumption, large tumor size, and absence of other metastases are at high risk of developing BMs during the course of their disease.
BM appearance and survival
Robnett et al reported that the timing of chest irradiation can influence the risk of brain recurrences: the rate of BM is 27% in patients receiving induction chemotherapy before thoracic RT compared with 15% in patients who are treated with concurrent chemoradiation.17 The 2-year actuarial rate of BM is 39% versus 20%. The authors hypothesize that early aggressive locoregional and systemic treatment could better control regional disease, which in turn affects the development of brain relapses. In accordance with these findings, BMs presented in 39 out of 161 patients (24.2%) in this present study. The rate of BM is quite similar to the rate which has been previously reported by Robnett et al for patients who were not treated with concurrent chemoradiotherapy. The lack of a radiotherapy department in the “Sotiria” Chest Diseases Hospital renders impossible the application of concurrent chemoradiotherapy and therefore leads to the application of the sequential module.
Once diagnosed, BMs are mostly treated with wholeb-rain radiotherapy, having a response rate of 45%–81% in NSCLC.24,25 The overall survival of NSCLC patients with BM is poor, reported to be 3–6 months, despite medical treatment.26 The overall survival of the patients in this present study with BM was also poor, approximately 4 months.
Patients who are at high risk of developing BM
The delay of BM appearance is expected to improve prognosis of NSCLC patients. To achieve this, we need objective means to indicate patients at high risk for developing BM. Some studies have already been oriented towards this direction. Biologic agents like neuron specific enolase, carcinoembryonic antigen, serum sodium levels, or numerous molecular markers have been correlated with the development of BM and a shorter survival.26–28
Nevertheless, specific phenotypic characteristics may also serve as surrogate prognostic factors. Earlier studies correlated the presence of BM with advanced stage, NSCLC histotypes, delay of lung radiotherapy, younger age, and large tumor size.28–32 However, few studies assessed in this regard tobacco consumption, comorbidities, CT scanner tumor characteristics, or the presence of metastases other than BMs.
Age at diagnosis
Age < 60 years was shown to be associated with an increased risk of BM.30,33,34 In this present study, younger age (60.8 ± 8.9 years) was correlated with a higher possibility of BM appearance (Table 2). However, younger patients with BM present a better performance status and longer survival, while they may tolerate aggressive treatment better and are willing to accept a higher risk of toxicity than older patients.26,35
T and N status
T4 initial status was associated with increased risk of BM in a multivariate analysis of 305 patients with localized NSCLC.30 The N2 status was found to be predictive of BM by Jacobs et al and by Tang et al.36,37
In this study, lung tumor size was correlated with the appearance of BM (55.1 ± 20.1 cm) (Table 2). This finding is in agreement with the study of Mujoomdar et al.31 However, no correlation was found with the T status itself. T status, as well as N status, has been correlated with BM outbreak in recent studies.30,31
As is the case in the study of Shi et al, the authors of this present study found most of the primary tumors to be located in the right lung and in the upper lobes.32 These frequent locations of lung tumor did not seem to correlate with the appearance of BM.32 Central or peripheral location of primary lung tumor was not found to be correlated to BM, which is in agreement with the study of Mujoomdar et al.31
M status
Previous studies speculate that the spread of lung cancer to the thoracic lymphatic system and to the brain could also relate to the presence of distant metastatic disease in other organs.31 So far, no study has confirmed this hypothesis. On the contrary, in this present study, appearance of BM was correlated with the absence of metastases in other organs, like lung, bone, and adrenal glands. Except adrenal metastases,27 synchronous metastases in other organs have not been correlated with median survival, probably as a result of already poor prognosis of the BM.26
Tobacco consumption
Smoking status has already been correlated with poor prognosis and shorter overall survival in lung cancer patients,18 but no correlation was found with BM. In this study’s cohort, high tobacco consumption (75.9 ± 23.9 pack-years) was correlated with the outbreak of BM.
NSCLC histological subtype
In previous studies, non-squamous lung cancer, mainly lung adenocarcinoma, showed higher prevalence of BM development.30–32 In this study, no correlation was found between NSCLC histotype and BM appearance. This discordance is probably a result of the small number of allocated groups and the relatively large number of unspecified NSCLC tumors in the present study.
PCI
Prophylactic cranial irradiation (PCI) has been demonstrated to reduce the incidence or delay the onset of BM in patients with locally advanced NSCLC after initial treatment.3,7,9–16 Thus, identification of risk population for BM development is pertinent. Specific phenotypes of patients at higher risk for BM development could serve as candidates of PCI and could allow early intervention, which seems more promising than the palliative approach.
Limitations
The patients in this current study were treated with sequential rather than concurrent chemoradiotherapy despite the current treatment guidelines. This limitation of the study is due to the lack of a radiotherapy department in the “Sotiria” Chest Diseases Hospital.
The pathologic data lack molecular markers, which could be related to the overall survival as is the case in many recent studies. In fact, during the study period, molecular data were not available.
Implications
This study records the deleterious effect of BMs on NSCLC patient survival, enriches the high risk profile with more features, and contributes to the discussion of pathophysiologic mechanisms underlying the brain involvement in NSCLC. More studies are needed in order to elucidate these issues.
Conclusion
Younger NSCLC patients with high tobacco consumption, large tumor size, and absence of other metastases (lung, bones, adrenal metastases) are at high risk of BM appearance during the course of NSCLC and may be candidates for PCI early in the course of their disease. Apart from genome-based studies, phenotype-based studies may contribute to future lung cancer therapy.
Footnotes
Disclosure
The authors report no conflicts of interest in this work.
References
- 1.Ferlay J, Autier P, Boniol M, Heanue M, Colombet M, Boyle P. Estimates of the cancer incidence and mortality in Europe in 2006. Ann Oncol. 2007;18:581–592. doi: 10.1093/annonc/mdl498. [DOI] [PubMed] [Google Scholar]
- 2.Walker S. Updates in non-small cell lung cancer. Clin J Oncol Nurs. 2008;2:587–596. doi: 10.1188/08.CJON.587-596. [DOI] [PubMed] [Google Scholar]
- 3.Albain KS, Rusch VW, Crowley JJ, et al. Concurrent cisplatin/etoposide plus chest radiotherapy followed by surgery for stages IIIA (N2) and IIIB non-small-cell lung cancer: Mature results of Southwest Oncology Group Phase II study 8805. J Clin Oncol. 1995;13:1880–1892. doi: 10.1200/JCO.1995.13.8.1880. [DOI] [PubMed] [Google Scholar]
- 4.Andre F, Grunenwald D, Pujol JL, et al. Patterns of relapse of N2 nonsmall-cell lung carcinoma patients treated with preoperative chemotherapy: Should prophylactic cranial irradiation be reconsidered? Cancer. 2001;91:2394–2400. [PubMed] [Google Scholar]
- 5.Cox JD, Scott CB, Byhardt RW, et al. Addition of chemotherapy to radiation therapy alters failure patterns by cell type within non-small cell carcinoma of lung (NSCCL): Analysis of Radiation Therapy Oncology Group (RTOG) trials. Int J Radiat Oncol Biol Phys. 1999;43:505–509. doi: 10.1016/s0360-3016(98)00429-5. [DOI] [PubMed] [Google Scholar]
- 6.Cooper JD, Silverman S, Clement JA. Prophylactic cranial irradiation for lung cancer patients at high risk for development of cerebral metastasis: Results of a prospective randomized trial conducted by the Radiation Therapy Oncology Group. Int J Radiat Oncol Biol Phys. 1991;21:637–643. doi: 10.1016/0360-3016(91)90681-s. [DOI] [PubMed] [Google Scholar]
- 7.Stuschke M, Eberhardt W, Pottgen C, et al. Prophylactic cranial irradiation in locally advanced nonsmall-cell lung cancer after multimodality treatment: Long-term follow-up and investigations of late neuropsychologic effects. J Clin Oncol. 1999;17:2700–2709. doi: 10.1200/JCO.1999.17.9.2700. [DOI] [PubMed] [Google Scholar]
- 8.Andre F, Grunenwald D, Pujol JL, et al. Patterns of relapse of N2 nonsmall-cell lung carcinoma patients treated with preoperative chemotherapy: Should prophylactic cranial irradiation be reconsidered? Cancer. 2001;91:2394–2400. [PubMed] [Google Scholar]
- 9.Strauss GM, Herndon JE, Sherman DD, et al. Neoadjuvant chemotherapy and radiotherapy followed by surgery in stage IIIA non-small cell carcinoma of the lung: Report of a Cancer and Leukemia Group B phase II study. J Clin Oncol. 1992;10:237–1244. doi: 10.1200/JCO.1992.10.8.1237. [DOI] [PubMed] [Google Scholar]
- 10.Rusch VW, Griffin BR, Livingston RB. The role of prophylactic cranial irradiation in regionally advanced non-small cell lung cancer. A Southwest Oncology Group Study. J Thorac Cardiovasc Surg. 1989;98:535–539. [PubMed] [Google Scholar]
- 11.Skarin A, Jochelson M, Sheldon T, et al. Neoadjuvant chemotherapy in marginally resectable stage III M0 non-small cell lung cancer: Longterm follow-up in 41 patients. J Surg Oncol. 1989;40:266–274. doi: 10.1002/jso.2930400413. [DOI] [PubMed] [Google Scholar]
- 12.Russell AH, Pajak TE, Selim HM, et al. Prophylactic cranial irradiation for lung cancer patients at high risk for development of cerebral metastasis: Results of a prospective randomized trial conducted by the Radiation Therapy Oncology Group. Int J Radiat Oncol Biol Phys. 1991;21:637–643. doi: 10.1016/0360-3016(91)90681-s. [DOI] [PubMed] [Google Scholar]
- 13.Umsawasdi T, Valdivieso M, Chen TT, et al. Role of elective brain irradiation during combined chemoradiotherapy for limited disease non-small cell lung cancer. J Neuro Oncol. 1984;2:253–259. doi: 10.1007/BF00253278. [DOI] [PubMed] [Google Scholar]
- 14.Cox JD, Stanley K, Petrovich Z, Paig C, Yesner R. Cranial irradiation in cancer of the lung of all cell types. JAMA. 1981;245:469–472. [PubMed] [Google Scholar]
- 15.Pöttgen C, Eberhardt W, Grannass A, et al. Prophylactic cranial irradiation in operable stage IIIA non-small-cell lung cancer treated with neoadjuvant chemoradiotherapy: Results from a German multicenter trial. J Clin Oncol. 2007;25:4987–4992. doi: 10.1200/JCO.2007.12.5468. [DOI] [PubMed] [Google Scholar]
- 16.Yavuz AA, Topkan E, Onal C, Yavuz MN. Prophylactic cranial irradiation in locally advanced non-small cell lung cancer: Outcome of recursive partitioning analysis group I patients. J Exp Clin Cancer Res. 2008;27:80. doi: 10.1186/1756-9966-27-80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Robnett TJ, Machtay M, Stevenson JP, Algazy KM, Hahn SM. Factors affecting the risk of brain metastases after definitive chemoradiation for locally advanced non-small-cell lung carcinoma. J Clin Oncol. 2001;19:1344–1349. doi: 10.1200/JCO.2001.19.5.1344. [DOI] [PubMed] [Google Scholar]
- 18.Law A, Karp DD, Dipetrillo T, Daly BT. Emergence of increased cerebral metastasis after high-dose preoperative radiotherapy with chemotherapy in patients with locally advanced non-small cell lung carcinoma. Cancer. 2001;92:160–164. doi: 10.1002/1097-0142(20010701)92:1<160::aid-cncr1304>3.0.co;2-x. [DOI] [PubMed] [Google Scholar]
- 19.Ceresoli GL, Reni M, Chiesa G, et al. Brain metastases in locally advanced nonsmall cell lung carcinoma after multimodality treatment: Risk factors analysis. Cancer. 2002;95:605–612. doi: 10.1002/cncr.10687. [DOI] [PubMed] [Google Scholar]
- 20.Carolan H, Sun AY, Bezjak A, et al. Does the incidence and outcome of brain metastases in locally advanced non-small cell lung cancer justify prophylactic cranial irradiation or early detection? Lung Cancer. 2005;49:109–115. doi: 10.1016/j.lungcan.2004.12.004. [DOI] [PubMed] [Google Scholar]
- 21.Sobin LH, Wittekind C the International Union Against Cancer (UICC), editors. TNM Classification of Malignant Tumors. 6th ed. New York, NY: Wiley-Liss; 2002. pp. 99–103. [Google Scholar]
- 22.Alberts M. Lung Cancer Guidelines. Chest. 2003;123:1–2. [Google Scholar]
- 23.Alberts M. Diagnosis and management of lung cancer. Executive summary. Chest. 2007;132:1–19. doi: 10.1378/chest.07-1860. [DOI] [PubMed] [Google Scholar]
- 24.Addeo R, Caraglia M, Faiola V, et al. Concomitant treatment of brain metastasis with whole brain radiotherapy [WBRT] and temozolomide [TMZ] is active and improves quality of life. BMC. 2007;7:18. doi: 10.1186/1471-2407-7-18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Ma S, Xu Y, Deng Q, Yu X. Treatment of brain metastasis from nonsmall cell lung cancer with whole brain radiotherapy and Gefitinib in a Chinese population. Lung Cancer. 2009;65:198–203. doi: 10.1016/j.lungcan.2008.10.028. [DOI] [PubMed] [Google Scholar]
- 26.Jacot W, Quantin X, Boher JM, et al. Brain metastases at the time of presentation of non-small cell lung cancer: A multi-centric AERIO* analysis of prognostic factors. Br J Cancer. 2001;84:903–909. doi: 10.1054/bjoc.2000.1706. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Penel N, Brichet A, Prevost B, et al. Prognostic factors for synchronous brain metastases from lung cancer. Lung Cancer. 2001;33:143–154. doi: 10.1016/s0169-5002(01)00202-1. [DOI] [PubMed] [Google Scholar]
- 28.Arrieta O, Saavedra-Perez D, Kuri R, et al. Brain metastasis development and poor survival associated with carcinoembryonic antigen (CEA) level in advanced non-small cell cancer: A prospective analysis. BMC Cancer. 2009;9:119. doi: 10.1186/1471-2407-9-119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Robnett T, Machtay M, Stevenson J, et al. Factors affecting the risk of brain metastases after definitive chemoradiation for locally advanced non-small-cell lung carcinoma. J Clin Oncol. 2001;19:1344–1349. doi: 10.1200/JCO.2001.19.5.1344. [DOI] [PubMed] [Google Scholar]
- 30.Bajard A, Westeel V, Dubiez A. Multivariate analysis of factors predictive of brain metastases in localized non-small cell lung carcinoma. Lung Cancer. 2004;45:317–323. doi: 10.1016/j.lungcan.2004.01.025. [DOI] [PubMed] [Google Scholar]
- 31.Mujoomdar A, Austin J, Malhota R, et al. Clinical predictors of metastatic disease to the brain from non-small cell lung carcinoma: Primary tumor size, cell type, and lymph node metastases. Radiology. 2007;242:882–888. doi: 10.1148/radiol.2423051707. [DOI] [PubMed] [Google Scholar]
- 32.Shi A, Digumarthy S, Temel J, Halpern EF, Kuester LB, Aquino SL. Does initial staging or tumor histology better identify asymptomatic brain metastases in patients with non-small cell lung caner? J Thorac Oncol. 2006;1:205–210. doi: 10.1016/s1556-0864(15)31569-0. [DOI] [PubMed] [Google Scholar]
- 33.Ceresoli GL, Reni M, Chiesa G, et al. Brain metastases in locally advanced non-small cell lung carcinoma after multimodality treatment: Risk factors analysis. Cancer. 2002;95:605–612. doi: 10.1002/cncr.10687. [DOI] [PubMed] [Google Scholar]
- 34.Schouten LJ, Rutten J, Huveneers HA, Twinjstra A. Incidence of brain metastases in a cohort of patients with carcinoma of the breast, colon, kidney, and lung and melanoma. Cancer. 2002;94:2698–2705. doi: 10.1002/cncr.10541. [DOI] [PubMed] [Google Scholar]
- 35.Gore E. Brain metastases in very young patients with lung cancer are still brain metastases. Onkologie. 2008;31:297–268. doi: 10.1159/000134005. [DOI] [PubMed] [Google Scholar]
- 36.Jacobs RH, Awan A, Bitran JD, et al. Prophylactic cranial irradiation in adenocarcinoma of the lung: A possible role. Cancer. 1987;59:2016–2019. doi: 10.1002/1097-0142(19870615)59:12<2016::aid-cncr2820591208>3.0.co;2-4. [DOI] [PubMed] [Google Scholar]
- 37.Tang SG, Lin FJ, Leung VM. Impact of cranial irradiation in adenocarcinoma of the lung. J Formos Med Assoc. 1993;92:413–419. [PubMed] [Google Scholar]