Abstract
Background: A higher folate intake is associated with a decreased colorectal cancer risk in observational studies, but recent evidence suggests that excessive folate supplementation may increase colorectal cancer risk in some individuals. Therefore, mandatory folic acid fortification of grain products in the United States may have unintended negative consequences.
Objective: We examined the association between folate intake and colorectal cancer risk, including 8.5 y of postfortification follow-up.
Design: We examined the association between folate intake and colorectal cancer in the NIH-AARP Diet and Health Study—a US cohort study of 525,488 individuals aged 50–71 y initiated in 1995–1996. Dietary, supplemental, and total folate intakes were calculated for the pre- and postfortification periods (before and after 1 July 1997) based on a baseline food-frequency questionnaire. HRs and 95% CIs were calculated by using multivariable Cox proportional hazards regression models.
Results: During follow-up through 31 December 2006 (mean follow-up: 9.1 y), 7212 incident colorectal cancer cases were identified. In the postfortification analysis (6484 cases), a higher total folate intake was associated with a decreased colorectal cancer risk (HR for ≥900 compared with <200 μg/d: 0.70; 95% CI: 0.58, 0.84). The highest intakes specifically from supplements (HR: 0.82; 95% CI: 0.72, 0.92) or from diet (HR: 0.81; 95% CI: 0.67, 0.97) were also protective. The pattern of associations was similar for the prefortification period, and no significant differences between time periods were observed.
Conclusions: In this large prospective cohort study that included 8.5 y of postfortification follow-up, folate intake was associated with a decreased colorectal cancer risk. Given that the adenoma-carcinoma sequence may take ≥10 y, additional follow-up time is needed to fully examine the effect of folic acid fortification.
INTRODUCTION
Folate is a water-soluble B vitamin that is essential to the human diet and can be found naturally in fruit, vegetables, beans, and other foods. Folic acid, a synthetic form of folate that has greater stability and bioavailability, is added to many processed foods and supplements, including multivitamins. Folate is linked to cancer through its role in one-carbon metabolism, in which adequate folate in its various forms is required for the production of nucleotides used in DNA synthesis and repair (1). One-carbon metabolism also supplies the methyl groups for the methylation of DNA, which in turn influences gene expression and has been associated with cancer risk (1–3). Most observational studies have observed a decreased colorectal cancer risk of participants with a higher folate intake (4). A recent summary report from the World Cancer Research Fund/American Institute for Cancer Research concluded that there was a clear inverse dose-response relation between folate from foods and colorectal cancer risk (5), and a large pooled analysis reported a significant inverse association for total folate intake (6). Although inverse associations have been observed for blood concentrations of folate as well, the evidence is less consistent than for folate intake (4, 7, 8).
A recent clinical trial reported an increased risk of multiple and advanced colorectal adenomas for subjects who were supplemented with folic acid (9), and laboratory studies have indicated that high folate status may promote colorectal carcinogenesis, depending on the dosage and timing of the exposure (10). These results inspired the hypothesis that whereas low folate status may predispose normal colorectal mucosa to the development of cancer, excessive supplementation with folic acid or supplementation after the initiation of carcinogenesis may increase risk of colorectal cancer in some individuals (11, 12).
Possible adverse effects caused by high intakes of folic acid are particularly relevant in the United States and Canada, which mandated fortification of grain products with folic acid in 1998 to prevent neural tube defects. Fortification effectively increased folic acid intake (13) and folate status (14, 15) of the US population, and the incidence of neural tube defects decreased substantially after fortification (16). However, concerns have been raised regarding the safety of high exposure to folic acid through the combination of fortified foods and supplements (17, 18). Previous studies showing an inverse association between folate intake and colorectal cancer risk were conducted in populations without folic acid fortification, but evidence in the context of a fortified food supply is needed. We conducted a study of folate intake and colorectal cancer risk in the NIH-AARP Diet and Health Study, which included 8.5 y of follow-up after mandatory fortification.
SUBJECTS AND METHODS
Study population
Details of the NIH-AARP Diet and Health Study were described previously (19). In brief, the study was initiated in 1995–1996 when 3.5 million self-administered questionnaires were mailed to 50–71-y-old AARP members residing in selected states (California, Florida, Louisiana, New Jersey, North Carolina, and Pennsylvania) or metropolitan areas (Atlanta, Georgia and Detroit, Michigan). Responses were received from 617,119 men and women, of who 567,169 successfully completed the baseline questionnaire (exclusions included 27,552 who did not fill out a substantial portion of the questionnaire, 13,442 who did not complete the questionnaire because they were not the intended recipients, 8,028 who had >10 recording errors, 99 who reported consuming <10 foods, 6 who did not report sex, and 823 who asked to be dropped from the study). The study was approved by the National Cancer Institute Special Studies Institutional Review Board, and informed consent was obtained from all participants.
We excluded 179 duplicate questionnaires, 582 individuals who died or moved out of the study area before baseline, 6 withdrawals, 15,760 proxy respondents, 4994 individuals with self-reported colon cancer before baseline, 1180 with end-stage renal disease before baseline, 7829 individuals with any cancer diagnosis (except for nonmelanoma skin cancer) as identified by a cancer registry match before baseline, and 4530 with a cancer cause of death record but no cancer registry record. We further excluded 4715 individuals who reported extreme total energy intakes and 1906 who reported extreme dietary folate intakes. Extreme intakes were considered those beyond twice the interquartile range of sex-specific Box-Cox-transformed intakes. The final analytic cohort consisted of 525,488 individuals. Of these, 322,206 responded to a second questionnaire in late 1996 that included questions on medication use and colorectal cancer screening; 214,483 individuals responded to a follow-up questionnaire in 2004 that included another assessment of colorectal cancer screening.
Cancer ascertainment
Incident colorectal cancer cases were identified during follow-up through 31 December 2006 by using probabilistic linkage with state and metropolitan area cancer registries, which identified >90% of all cancers in a validation study (20). Information on anatomic site and histology was obtained via the cancer registries, and the vital status of all participants was updated annually by linkage to the Social Security Administration Death Master File and the cancer registries. Members of the study were followed periodically for changes of address by linkage to the US Postal Service's National Change of Address database, a commercial address change database, and by processing undeliverable mail, other address change services, or direct communication from the participants. In addition, we expanded the cancer ascertainment area by adding the registries of Arizona and Texas to follow-up participants who moved to those states. Approximately 95% of study participants resided in the covered registry areas. Follow-up was terminated when participants moved out of the registry areas or when participants died regardless of residential history.
Cases were defined as those with a diagnosis of a first primary invasive colorectal cancer (International Classification of Diseases for Oncology, 3rd ed, codes C180-189, C260, C199, and C209) (21). For site-specific analyses, colorectal cancers were further classified by anatomic site as proximal colon (C180–184), distal colon (C185–187), and rectum (C199, C209). The 46 participants who had cancers of the colon and rectum diagnosed on the same day were considered cases for both sites.
Dietary assessment
Dietary intake was assessed at baseline by using a grid-based version of the National Cancer Institute's DHQ.4 This self-administered questionnaire was designed to measure usual intake over the past 12 mo and asked participants to report the frequency of consumption and portion size typically consumed for 124 food items. Nutrient values for the foods listed in the DHQ were determined by the method of Subar et al (22) using the USDA's survey nutrient database, which provides pre- and postfortification folate contents for foods and supplemented by the Nutrition Data System for Research's nutrient database for the amounts of natural and synthetic folate (22, 23). In a validation substudy, the deattenuated energy-adjusted correlation coefficients between intake from the DHQ and 24-h dietary recalls were generally in the range of 0.4 to 0.6, including 0.64 and 0.69 for folate in men and women, respectively (24). The frequency and duration of supplemental folic acid use was derived from the use of 3 types of multivitamins [Stress-tabs (Inverness Medical Inc) type, Therapeutic or Theragran (Bristol-Myers Squibb) type, and One-a-Day (Bayer Corp) type].
Statistical analysis
Cox proportional hazards regression models, with age as the underlying time metric, were used to determine HRs and 95% CIs. Follow-up started at the age at cohort entry, defined as the date of the baseline questionnaire, and ended at the earlier of age at colorectal cancer diagnosis or age at censoring, defined as the earliest of the following: other cancer diagnosis, death, relocation from the registry areas, or end of follow-up (31 December 2006). The proportional hazards assumption was evaluated by assessing the significance of interaction terms between follow-up time and the folate exposure variables by using likelihood ratio tests; no significant violations were observed.
Univariate and multivariable-adjusted models were constructed for folate intake: dietary folate, supplemental folic acid, total folate, intake of folate naturally occurring in foods, and synthetic folic acid. Separate analyses were conducted for the prefortification (baseline to 30 June 1997) and postfortification (1 July 1997 to 31 December 2006) time periods by using pre- and postfortification food folate values, respectively. The postfortification analyses assumed that participants maintained the same diet and multivitamin use reported at baseline. Folic acid fortification of grain products in the United States was mandated to occur by 1 January 1998, but we used 1 July 1997 as the start date because most manufacturers initiated fortification early in anticipation of the deadline (14). We tested for differences in folate effects between the pre- and postfortification periods by including a time-dependent interaction of time period by folate intake and using a Wald test.
All food and nutrient variables except supplemental folic acid were adjusted for total energy intake by using the residual method (regression of intake on calories) (25). Total folate intake was calculated as energy-adjusted dietary intake plus unadjusted supplemental intake. Categorization of folate exposures was based on absolute intake cutoffs, which were chosen a priori to encompass the range of reported intake. Sensitivity analyses were carried out by using quintile categorization of the folate variables, and a continuous 100-μg change in folate intake was also examined. Tests for linear trend used a continuous variable in which each category was assigned the median folate intake value within that category. The possibility of a nonlinear relation between dietary folate and risk of colorectal cancer was examined nonparametrically by using restricted cubic splines (26), with 4 knots placed at the 5th, 35th, 65th, and 95th percentiles of folate intake. Likelihood ratio tests were used to test for nonlinearity and for overall association of the spline curves with colorectal cancer risk.
Parsimonious multivariable models were constructed to adjust for potential confounders of the folate–colorectal cancer association. Risk factors for colorectal cancer were identified a priori, and potential confounders were defined as variables that were correlated (Spearman's r > 0.10) with both the exposure and the outcome. Additional colorectal cancer risk factors were added to the multivariable model, and those that were not associated with colorectal cancer and did not affect the folate estimates (<5% change in the HR estimates) were dropped from the final model. The final model included sex, smoking, physical activity, use of aspirin or NSAIDs, BMI, and quintiles of intake for dietary calcium and red meat. Covariates were incorporated in the model as ordinal trend variables when appropriate, and separate indicator variables were included for missing responses (<5% for all variables). Variables evaluated as potential confounders but not included in the final multivariable models included alcohol, fiber, education, race, family history of colon cancer, self-reported history of colonic polyps, self-reported screening for colorectal cancer, menopausal hormone therapy use for women, and intake of processed meats, saturated fat, selenium, vitamin D, and total calories. Inclusion of multivitamin use in the dietary folate models or of dietary folate in the supplement-only models did not change the results, and multivitamin use was not included in the total folate analyses because it was the sole source of folic acid in supplement form.
Stratified models were created to evaluate effect modification by sex, race, smoking, BMI, use of aspirin/NSAIDs, dietary methionine, and alcohol intake. Significance of effect modification was tested by adding a multiplicative interaction term to the unstratified multivariable models. The association with folate intake was also evaluated by tumor site. To compare the associations between colon and rectal cancers, for example, we fit 2 separate Cox proportional hazards regression models to the whole cohort, one for each site-specific outcome (27). For the colon model, cancer at the rectum was considered to be a censoring event and vice versa. For each model we used the corresponding scores to compute a robust sandwich estimate for all model parameters that accounted for the repeated use of the same individuals. Differences between association parameters for colon and rectal cancers were then assessed by using a Wald test. The same analysis was used to compare the associations of folate with proximal compared with distal colon cancer. All comparisons used 2-sided P values, and significance was based on α < 0.05. All statistical analyses were performed by using SAS software version 9.1.3 (SAS Institute Inc).
RESULTS
A total of 7212 incident colorectal cancer cases were identified during up to 11.2 y of follow-up (mean: 9.1 y), and 6484 cases were identified on or after 1 July 1997. The mean energy-adjusted intake of dietary folate before fortification was 297 μg/d, and it rose to 391 μg/d after fortification. More than 50% of the study participants (52% of men and 60% of women) reported intake of supplemental folate through use of multivitamins.
The baseline characteristics shown in Table 1 indicate that folate intake was associated with behaviors consistent with a healthy lifestyle. Participants who reported high intakes of both dietary and total folate were more likely to be multivitamin users, to be highly educated, to be nonsmokers, to be physically active, and to report undergoing some type of colorectal cancer screening. They also tended to be slightly leaner and to have higher dietary intakes of calcium, fiber, and fruit and vegetables and lower intakes of alcohol and red meat. Total folate intake was positively associated with daily use of aspirin and NSAIDs and with hormone replacement therapy use among women.
TABLE 1.
Characteristics of study participants by quintile of prefortification dietary and total folate intake in the NIH-AARP Study1
| Dietary folate2 |
Total folate3 |
||||||
| Characteristics | Q1 | Q3 | Q5 | Q1 | Q3 | Q5 | |
| Dietary folate intake (μg/d)2 | |||||||
| Prefortification food values | 168.1 ± 0.14 | 283.0 ± 0.1 | 460.8 ± 0.3 | 196.8 ± 0.1 | 308.9 ± 0.4 | 397.6 ± 0.4 | |
| Postfortification food values | 255.3 ± 0.2 | 379.6 ± 0.1 | 554.0 ± 0.3 | 287.8 ± 0.2 | 401.3 ± 0.4 | 491.5 ± 0.4 | |
| Use of supplement containing folate (%) | 49.1 | 55.9 | 60.1 | 3.5 | 62.5 | 99.1 | |
| Multivitamin use (%) | |||||||
| None | 51.0 | 44.1 | 39.9 | 96.5 | 37.5 | 0.9 | |
| ≤6 times/wk | 12.8 | 12.3 | 10.6 | 3.5 | 32.5 | 2.4 | |
| ≥1 time/d | 36.2 | 43.7 | 49.5 | 0.0 | 30.0 | 96.6 | |
| Supplemental folate (μg/d) | 184.3 ± 0.7 | 218.5 ± 0.7 | 244.9 ± 0.8 | 2.5 ± 0.04 | 184.5 ± 0.5 | 502.4 ± 0.7 | |
| Total folate intake (μg/d)3 | |||||||
| Prefortification food values | 352.4 ± 0.7 | 501.5 ± 0.7 | 705.7 ± 0.8 | 199.3 ± 0.1 | 493.4 ± 0.2 | 900.0 ± 0.6 | |
| Postfortification food values | 439.6 ± 0.7 | 598.1 ± 0.7 | 798.9 ± 0.8 | 290.3 ± 0.2 | 585.8 ± 0.3 | 993.9 ± 0.6 | |
| Age (y) | 61.3 ± 0.02 | 62.2 ± 0.02 | 62.7 ± 0.02 | 61.6 ± 0.02 | 61.9 ± 0.02 | 62.5 ± 0.02 | |
| Person-years of follow-up | 9.0 ± 0.01 | 9.1 ± 0.01 | 9.1 ± 0.01 | 9.0 ± 0.01 | 9.1 ± 0.01 | 9.1 ± 0.01 | |
| Sex, male (%) | 60.7 | 59.4 | 55.7 | 64.5 | 57.2 | 54.0 | |
| Education, college graduate/postgraduate (%) | 30.7 | 39.3 | 44.7 | 31.2 | 39.2 | 44.1 | |
| Race, white, non-Hispanic (%) | 90.8 | 92.4 | 89.8 | 90.9 | 90.4 | 91.5 | |
| Smoking (%) | |||||||
| Never | 28.3 | 35.8 | 40.3 | 29.7 | 35.5 | 38.7 | |
| Former | 46.4 | 50.7 | 49.4 | 47.9 | 47.9 | 50.2 | |
| Current | 21.2 | 9.9 | 6.6 | 18.3 | 12.8 | 7.6 | |
| Vigorous physical activity, ≥5 times/wk (%) | 13.2 | 18.7 | 26.5 | 13.7 | 18.5 | 25.6 | |
| BMI (kg/m2) | 27.4 ± 0.02 | 27.2 ± 0.02 | 26.5 ± 0.02 | 27.7 ± 0.02 | 27.0 ± 0.02 | 26.5 ± 0.02 | |
| Daily use of aspirin/NSAIDs (%)5 | 29.1 | 31.8 | 33.0 | 27.5 | 29.8 | 36.3 | |
| HRT use in women (%) | |||||||
| Never | 49.5 | 45.7 | 47.9 | 53.9 | 47.6 | 43.0 | |
| Current | 40.7 | 44.3 | 42.2 | 36.4 | 42.6 | 46.9 | |
| Former | 9.5 | 9.8 | 9.7 | 9.3 | 9.6 | 9.9 | |
| Family history of colon cancer (% yes) | 8.2 | 8.9 | 9.3 | 8.4 | 8.8 | 9.2 | |
| Self-reported history of polyps (% yes) | 9.2 | 9.7 | 9.1 | 9.1 | 9.5 | 9.6 | |
| Self-reported CRC screening (% yes)5 | 40.5 | 45.2 | 46.5 | 41.7 | 43.7 | 46.8 | |
| Alcohol consumption (%) | |||||||
| Nondrinker | 7.8 | 6.2 | 11.0 | 7.2 | 8.2 | 9.3 | |
| ≤15 g/d | 67.3 | 72.5 | 73.4 | 68.5 | 71.6 | 73.4 | |
| >15 g/d | 24.9 | 21.4 | 15.6 | 24.3 | 20.3 | 17.4 | |
| Dietary calcium (mg/d)2 | 551.4 ± 0.8 | 734.1 ± 0.9 | 882.5 ± 1.0 | 591.4 ± 0.9 | 724.3 ± 1.0 | 842.0 ± 1.0 | |
| Dietary fiber (g/d)2 | 11.9 ± 0.01 | 18.1 ± 0.01 | 25.2 ± 0.02 | 13.4 ± 0.01 | 18.6 ± 0.02 | 23.0 ± 0.02 | |
| Fruit and vegetables (servings/d)2 | 3.9 ± 0.01 | 6.4 ± 0.01 | 9.8 ± 0.01 | 4.5 ± 0.01 | 6.8 ± 0.01 | 8.7 ± 0.01 | |
| Red meat (g/d)2 | 69.6 ± 0.1 | 60.0 ± 0.1 | 41.5 ± 0.1 | 70.2 ± 0.1 | 55.9 ± 0.1 | 45.6 ± 0.1 | |
| Total energy (kcal/d) | 1813 ± 3 | 1836 ± 2 | 1821 ± 2 | 1851 ± 3 | 1807 ± 3 | 1826 ± 2 | |
Frequencies do not add up to 100% because of rounding and missing values. CRC, colorectal cancer risk; HRT, hormone replacement therapy; NSAIDs, nonsteroidal antiinflammatory drugs; Q, quintile.
Adjusted for energy intake using the residual method.
Adjusted dietary folate plus unadjusted supplemental folate.
Mean ± SE (all such values).
Frequencies are for 322,206 participants with data from the supplementary Risk Factor Questionnaire.
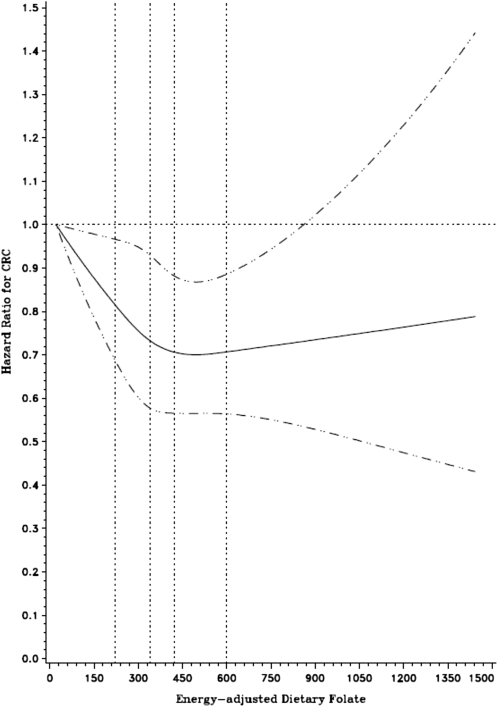
An inverse association was observed between dietary folate intake and risk of colorectal cancer in both the pre- and postfortification periods, although the result was not significant for the prefortification analysis (Table 2). The restricted cubic spline model confirmed a nonlinear relation in the postfortification analysis (P-nonlinearity < 0.01), with a plateau effect at the highest intakes of dietary folate (Figure 1). Alternative categorization with the use of quintiles of folate intake showed a similar pattern of association (HR for postfortification highest compared with lowest quintile: 0.91; 95% CI: 0.83, 0.99) and restricting the analysis to only those who reported no use of multivitamins also did not change the associations (HR for highest compared with lowest quintile: 0.83, 95% CI: 0.73, 0.94). The inverse association in the postfortification period was stronger for synthetic folic acid from foods (HR for 100-μg increase: 0.92; 95% CI: 0.88, 0.95) than for natural folate from foods (HR for 100-μg increase: 1.00; 95% CI: 0.97, 1.03) (data not shown).
TABLE 2.
RRs and 95% CIs of colorectal cancer for dietary, supplemental, and total folate intakes during the pre- and postfortification periods
| Food values |
|||||||||||
| Prefortification period1 |
Postfortification period2 |
||||||||||
| Unadjusted |
Multivariable3 |
Unadjusted |
Multivariable3 |
||||||||
| Folate intake | Cases | RR | 95% CI | RR | 95% CI | Cases | RR | 95% CI | RR | 95% CI | |
| Diet4 | |||||||||||
| <200 μg/d | 140 | 1.00 | Reference | 1.00 | Reference | 243 | 1.00 | Reference | 1.00 | Reference | |
| 200 to <300 μg/d | 301 | 0.87 | 0.71, 1.07 | 0.94 | 0.77, 1.16 | 1359 | 0.89 | 0.78, 1.02 | 0.92 | 0.80, 1.06 | |
| 300 to <400 μg/d | 197 | 0.77 | 0.62, 0.95 | 0.90 | 0.71, 1.14 | 2389 | 0.77 | 0.68, 0.88 | 0.86 | 0.75, 0.98 | |
| 400 to <500 μg/d | 63 | 0.68 | 0.51, 0.92 | 0.84 | 0.61, 1.15 | 1605 | 0.67 | 0.59, 0.77 | 0.81 | 0.70, 0.93 | |
| ≥500 μg/d | 27 | 0.65 | 0.43, 0.97 | 0.81 | 0.52, 1.25 | 629 | 0.67 | 0.58, 0.78 | 0.86 | 0.74, 1.00 | |
| ≥600 μg/d | — | — | — | — | — | 259 | 0.60 | 0.50, 0.71 | 0.81 | 0.67, 0.97 | |
| P-trend | 0.001 | 0.19 | <0.001 | 0.003 | |||||||
| 100-μg Increase5 | 728 | 0.89 | 0.83, 0.96 | 0.95 | 0.88, 1.03 | 6484 | 0.90 | 0.88, 0.92 | 0.97 | 0.94, 0.99 | |
| Supplement | |||||||||||
| 0 μg/d | 341 | 1.00 | Reference | 1.00 | Reference | 3186 | 1.00 | Reference | 1.00 | Reference | |
| <400 μg/d | 82 | 0.97 | 0.76, 1.23 | 1.04 | 0.82, 1.32 | 719 | 0.88 | 0.81, 0.95 | 0.93 | 0.86, 1.01 | |
| 400 μg/d | 271 | 0.92 | 0.78, 1.08 | 1.01 | 0.86, 1.19 | 2307 | 0.83 | 0.79, 0.88 | 0.91 | 0.86, 0.96 | |
| >400 μg/d | 34 | 0.88 | 0.62, 1.25 | 0.97 | 0.68, 1.39 | 272 | 0.74 | 0.66, 0.84 | 0.82 | 0.72, 0.92 | |
| P-trend | 0.26 | 0.93 | <0.001 | <0.001 | |||||||
| Total6 | |||||||||||
| <200 μg/d | 77 | 1.00 | Reference | 1.00 | Reference | 145 | 1.00 | Reference | 1.00 | Reference | |
| 200 to <300 μg/d | 141 | 0.79 | 0.60, 1.04 | 0.85 | 0.64, 1.13 | 773 | 0.89 | 0.74, 1.06 | 0.90 | 0.76, 1.08 | |
| 300 to <400 μg/d | 109 | 0.80 | 0.60, 1.07 | 0.94 | 0.70, 1.28 | 1289 | 0.75 | 0.63, 0.89 | 0.82 | 0.69, 0.98 | |
| 400 to <500 μg/d | 57 | 0.87 | 0.62, 1.23 | 1.06 | 0.75, 1.51 | 885 | 0.64 | 0.53, 0.76 | 0.75 | 0.63, 0.90 | |
| 500 to <600 μg/d | 64 | 0.78 | 0.56, 1.09 | 0.90 | 0.64, 1.26 | 483 | 0.62 | 0.52, 0.75 | 0.75 | 0.62, 0.90 | |
| 600 to <700 μg/d | 133 | 0.86 | 0.65, 1.14 | 1.03 | 0.77, 1.38 | 641 | 0.67 | 0.56, 0.80 | 0.77 | 0.64, 0.92 | |
| 700 to <800 μg/d | 85 | 0.70 | 0.51, 0.95 | 0.90 | 0.65, 1.24 | 949 | 0.63 | 0.52, 0.74 | 0.75 | 0.63, 0.89 | |
| 800 to <900 μg/d | 27 | 0.58 | 0.37, 0.90 | 0.78 | 0.49, 1.23 | 704 | 0.59 | 0.49, 0.70 | 0.75 | 0.62, 0.90 | |
| ≥900 μg/d | 35 | 0.58 | 0.39, 0.87 | 0.74 | 0.49, 1.11 | 615 | 0.54 | 0.45, 0.64 | 0.70 | 0.58, 0.84 | |
| P-trend | 0.01 | 0.47 | <0.001 | <0.001 | |||||||
| 100-μg Increase5 | 728 | 0.97 | 0.94, 0.99 | 0.99 | 0.96, 1.02 | 6484 | 0.95 | 0.94, 0.96 | 0.98 | 0.97, 0.99 | |
The prefortification period was from baseline to 30 June 1997.
The postfortification period began on 1 July 1997.
Cox proportional hazards models adjusted for sex, smoking (never; former, ≤20 cigarettes/d; former, >20 cigarettes/d; current, ≤20 cigarettes/d; current, >20 cigarettes/d), physical activity (never or rarely, 1–3 times/mo, 1–2 times/wk, 3–4 times/wk, ≥5 times/wk), use of aspirin/nonsteroidal antiinflammatory drugs (no use, monthly, weekly, or daily), BMI (in kg/m2; <22.5, 22.5 to <25, 25 to <27.5, 27.5 to <30, 30 to <32, or ≥32), and quintiles of dietary calcium and red meat intakes.
Adjusted for energy intake by the residual method.
RR (95% CI) for a 100-μg increase in folate intake (continuous variable).
Energy-adjusted dietary intake plus unadjusted supplemental intake.
FIGURE 1.
HRs (solid line) on a continuous basis derived from a cubic spline regression model with 4 knots (vertical dotted lines) placed at the 5th, 35th, 65th, and 95th percentiles of dietary folate intake (μg/d). The dashed lines represent the 95% CIs for the spline curve. The model was adjusted for age, sex, smoking, physical activity, use of aspirin/nonsteroidal antiinflammatory drugs, BMI, dietary calcium, and red meat intake. CRC, colorectal cancer.
We observed a significant inverse association between intake of folic acid from supplements and risk of colorectal cancer in the postfortification period only (Table 2). Cohort members taking 400 μg supplemental folic acid, equivalent to taking a multivitamin daily, had a reduced risk (HR: 0.91; 95% CI: 0.86, 0.96) compared with those not taking any supplements. The results for the pre- and postfortification periods were not significantly different (P-heterogeneity = 0.23), and the folic acid content of multivitamins did not change between the pre- and postfortification periods. Initiating follow-up 6 mo after baseline to remove potential prevalent/subclinical cancers did not alter the results of any of our analyses.
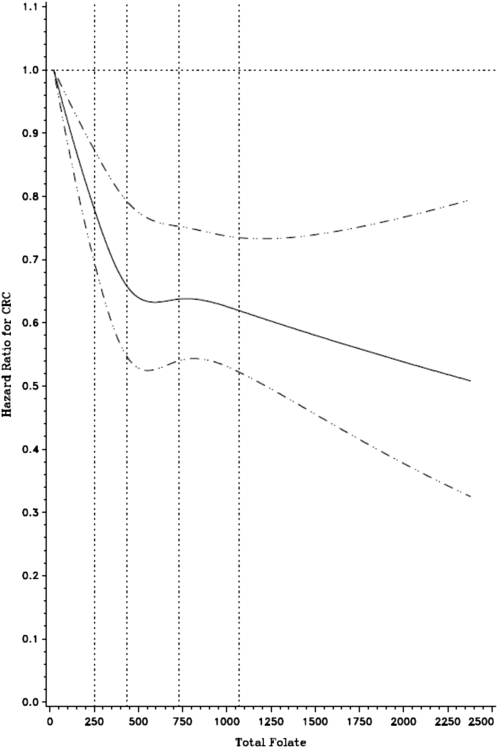
We also observed a nonsignificant inverse association at the highest categories of total folate intake in the prefortification period. For the postfortification period, we observed a significant linear protective association, with a 30% reduction in risk of the highest compared with the lowest intake category. Use of deciles rather than absolute cutoffs yielded similar results (HR for postfortification highest compared with lowest decile: 0.74; 95% CI: 0.66, 0.83). The spline curve in Figure 2 shows the inverse association over the entire range of total folate intake.
FIGURE 2.
HRs (solid line) on a continuous basis derived from a cubic spline regression model with 4 knots (vertical dotted lines) placed at the 5th, 35th, 65th, and 95th percentiles of total folate intake (μg/d). The dashed lines represent the 95% CIs for the spline curve. The model was adjusted for age, sex, smoking, physical activity, use of aspirin/nonsteroidal antiinflammatory drugs, BMI, dietary calcium, and red meat intake. CRC, colorectal cancer.
In stratified analyses (Table 3), similar inverse associations with total folate intake were observed in men and women (P-heterogeneity = 0.70), but for dietary folate we observed a significant interaction with sex (P-heterogeneity = 0.03); a protective association was observed only for men (HR for ≥600 μg/mL compared with <200 μg/mL: 0.71; 95% CI: 0.57, 0.89) and not women (HR: 1.03; 95% CI: 0.76, 1.39). We saw no indication of effect modification by race (Table 3), because the associations observed in African Americans were similar to those in non-Hispanic whites (P-heterogeneity = 0.25). Comparable inverse associations with total folate (Table 3) were observed for cancer of the colon and rectum (P-heterogeneity = 0.08), but the association with cancer of the distal colon was significantly stronger than that for the association of cancer of the proximal colon (P-heterogeneity = 0.004). Similarly, a stronger association between dietary folate and cancer was observed for the distal colon (P-heterogeneity < 0.001) (data not shown).
TABLE 3.
RRs and 95% CIs for colorectal cancer and total folate intake in the postfortification period, by sex, race, and cancer site
| Total folate intake (μg/d) |
||||||
| <300 | 300 to <500 | 500 to <700 | 700 to <900 | ≥900 | P-trend | |
| Sex | ||||||
| Men | ||||||
| Cases | 653 | 1487 | 727 | 1028 | 391 | |
| Multivariable RR (95% CI)1 | 1.00 (reference) | 0.82 (0.75, 0.90) | 0.81 (0.72, 0.90) | 0.78 (0.70, 0.86) | 0.74 (0.65, 0.84) | <0.0001 |
| Women | ||||||
| Cases | 265 | 687 | 397 | 625 | 224 | |
| Multivariable RR (95% CI)1 | 1.00 (reference) | 0.97 (0.84, 1.12) | 0.89 (0.76, 1.05) | 0.91 (0.79, 1.06) | 0.84 (0.69, 1.01) | 0.03 |
| P-heterogeneity2 | 0.70 | |||||
| Race | ||||||
| Non-Hispanic whites | ||||||
| Cases | 834 | 2002 | 1017 | 1557 | 567 | |
| Multivariable RR (95% CI)1 | 1.00 (reference) | 0.87 (0.80, 0.94) | 0.83 (0.75, 0.91) | 0.83 (0.76, 0.91) | 0.78 (0.70, 0.88) | <0.0001 |
| African Americans | ||||||
| Cases | 42 | 76 | 42 | 56 | 15 | |
| Multivariable RR (95% CI)1 | 1.00 (reference) | 0.72 (0.49, 1.08) | 0.66 (0.42, 1.04) | 0.84 (0.54, 1.29) | 0.41 (0.22, 0.77) | 0.08 |
| P-heterogeneity2 | 0.25 | |||||
| Specific tumor sites | ||||||
| Colon | ||||||
| Cases | 677 | 1597 | 840 | 1245 | 449 | |
| Multivariable RR (95% CI)1 | 1.00 (reference) | 0.86 (0.78, 0.94) | 0.84 (0.76, 0.93) | 0.83 (0.75, 0.92) | 0.75 (0.67, 0.86) | 0.0001 |
| Rectum | ||||||
| Cases | 251 | 595 | 294 | 420 | 171 | |
| Multivariable RR (95% CI)1 | 1.00 (reference) | 0.87 (0.75, 1.01) | 0.80 (0.67, 0.95) | 0.77 (0.65, 0.90) | 0.79 (0.64, 0.96) | 0.003 |
| P-heterogeneity2 | 0.08 | |||||
| Proximal colon | ||||||
| Cases | 345 | 912 | 505 | 729 | 276 | |
| Multivariable RR (95% CI)1 | 1.00 (reference) | 0.95 (0.84, 1.08) | 0.97 (0.84, 1.11) | 0.92 (0.81, 1.05) | 0.87 (0.74, 1.03) | 0.12 |
| Distal colon | ||||||
| Cases | 307 | 627 | 301 | 462 | 159 | |
| Multivariable RR (95% CI)1 | 1.00 (reference) | 0.75 (0.65, 0.87) | 0.68 (0.58, 0.80) | 0.71 (0.61, 0.82) | 0.62 (0.51, 0.76) | <0.0001 |
| P-heterogeneity2 | 0.0004 | |||||
Cox proportional hazards models adjusted for sex, smoking, physical activity, use of aspirin/nonsteroidal antiinflammatory drugs, BMI, and quintiles of dietary calcium and red meat intakes.
Determined by using a likelihood ratio test.
The association of total and dietary folate with colorectal cancer risk was modified by alcohol intake (P-heterogeneity < 0.001). We observed significant linear inverse associations (P-trend = 0.0003) with increasing folate intake in participants who reported drinking >15 g alcohol (∼1 drink) per day, but no association in nondrinkers (Table 4). No significant interactions by smoking, BMI, use of aspirin or NSAIDs, or methionine intake were observed.
TABLE 4.
RRs and 95% CIs for colorectal cancer and folate intake in the postfortification period, stratified by alcohol consumption
| Nondrinker |
Drinker, ≤15 g/d |
Drinker, >15 g/d |
||||
| Intake | Cases | RR (95% CI)1 | Cases | RR (95% CI)1 | Cases | RR (95% CI)1 |
| Dietary folate | ||||||
| <200 μg/d | 29 | 1.00 (reference) | 119 | 1.00 (reference) | 95 | 1.00 (reference) |
| 200 to <300 μg/d | 93 | 0.98 (0.65, 1.50) | 791 | 0.87 (0.72, 1.06) | 475 | 1.02 (0.81, 1.27) |
| 300 to <400 μg/d | 139 | 0.96 (0.64, 1.45) | 1632 | 0.85 (0.70, 1.02) | 618 | 0.89 (0.71, 1.11) |
| 400 to <500 μg/d | 127 | 1.06 (0.69, 1.61) | 1175 | 0.81 (0.67, 0.98) | 303 | 0.78 (0.61, 1.00) |
| ≥500 μg/d | 108 | 1.04 (0.67, 1.61) | 653 | 0.86 (0.70, 1.05) | 127 | 0.75 (0.57, 0.99) |
| P-trend | 0.63 | 0.27 | 0.0003 | |||
| Continuous23 | 496 | 1.02 (0.95, 1.09) | 4370 | 0.98 (0.95, 1.01) | 1618 | 0.91 (0.87, 0.96) |
| Total folate | ||||||
| <300 μg/d | 69 | 1.00 (reference) | 518 | 1.00 (reference) | 331 | 1.00 (reference) |
| 300 to <500 μg/d | 147 | 1.02 (0.76, 1.37) | 1520 | 0.91 (0.82, 1.00) | 507 | 0.79 (0.68, 0.91) |
| 500 to <700 μg/d | 95 | 0.96 (0.70, 1.32) | 729 | 0.85 (0.76, 0.95) | 300 | 0.80 (0.68, 0.94) |
| 700 to <900 μg/d | 109 | 0.90 (0.66, 1.24) | 1163 | 0.85 (0.76, 0.95) | 381 | 0.78 (0.67, 0.91) |
| ≥900 μg/d | 76 | 1.06 (0.75, 1.50) | 440 | 0.81 (0.71, 0.93) | 99 | 0.60 (0.47, 0.75) |
| P-trend | 0.87 | 0.001 | 0.0003 | |||
| Continuous24 | 496 | 1.00 (0.97, 1.03) | 4370 | 0.98 (0.97, 0.99) | 1618 | 0.96 (0.95, 0.98) |
Cox proportional hazards models adjusted for sex, smoking, physical activity, use of aspirin/nonsteroidal antiinflammatory drugs, BMI, calcium, and red meat intake.
Values are RRs (95% CIs) for a 100-μg increase in folate intake.
P-heterogeneity ≤0.0001 for dietary folate and alcohol (likelihood ratio test).
P-heterogeneity = 0.001 for total folate and alcohol (likelihood ratio test).
DISCUSSION
We examined the association between pre- and postfortification folate intake and colorectal cancer in the context of a cohort study, with evaluation of important potential confounders such as colorectal cancer screening history and dietary intake. Overall, folate intake was inversely associated with colorectal cancer risk after fortification, with no evidence of increased risk at the levels of intake reported in the study. Increased total folate intake was linearly associated with decreased risk, and supplemental folic acid was inversely associated as well. However, the 8.5 y of postfortification follow-up in our study may not have been sufficient to observe the relevant effects of folic acid fortification due to the latent period involved in development of invasive colorectal carcinoma.
Previous prospective cohort studies have reported inverse associations between folate intake and risk of colorectal cancer or adenomas (28–37), with a suggested 20–40% reduced risk of participants with the highest folate intake (4). Conversely, the AFPPS reported that daily folic acid (1 mg) did not prevent recurrence of colorectal adenomas compared with placebo and appeared to increase the risk of advanced adenomas and multiple adenomas (9). These results have been attributed to the growth-promoting effects of folic acid on existing undiagnosed precancerous lesions (38). Although 2 other trials with shorter follow-up periods (3–6 y compared with 6–8 y in the AFPPS) did not report any increased risk of adenoma recurrence with folic acid supplementation (39, 40), the AFPPS results caused a renewed focus on the issue of mandatory grain fortification with folic acid (11, 17, 18, 41–45). Two ecologic studies observed temporal associations between fortification and increased colon cancer rates in the United States and Chile (18, 46), but these studies are limited because intake was not specifically measured on individuals and linked to a cancer outcome.
Although we observed an inverse association between folate intake and colorectal cancer, with no evidence of increased risk, our results do not preclude a positive association between high folate intake and colorectal cancer risk over a longer time period or in susceptible subgroups, such as those with existing adenomas. When we restricted the analysis to the 9% of the cohort who self-reported a history of colorectal polyps in the 1996 questionnaire, total folate intake was not associated with colorectal cancer risk (HR for highest compared with lowest quintile: 1.01; 95% CI: 0.72, 1.43). It is possible that the 8.5 y of postfortification follow-up time in our study was not long enough to detect an adverse association with folate intake. A mathematical model of colorectal carcinogenesis by Luebeck et al (47) suggested that in individuals who were 60 y of age when folic acid supplementation was initiated, increased colorectal cancer rates might be observed after ∼10 y. Conversely, the temporal associations observed by Mason et al (18) indicated a much shorter time until an increased risk due to fortification might be observed at the population level. The adenoma-carcinoma sequence (48) likely proceeds over ≥10 y, but the precise time frame during which folic acid might accelerate progression and how quickly effects on incident cancer might be detected are not known. The AFPPS detected an increased recurrence of multiple adenomas with folic acid supplementation within 6–8 y of follow-up in the context of secondary prevention, but the time course of adenoma development is considerably shorter than that of colorectal cancer. We found no evidence of positive associations with incident cancer in a population expected to include many individuals with undiagnosed intestinal polyps (49), some of which would be advanced beyond the earliest stages, so it may be informative that we did not observe any evidence of altered cancer risk after fortification. Whereas our study does not support a rapid increase in colorectal cancer risk on increased folic acid consumption due to mandatory fortification, a latent effect based on the full time course of colorectal carcinogenesis may not be evident over the 8.5 y of postfortification follow-up.
Duration of follow-up is also important for interpretation of the inverse association we observed between folate intake and colorectal cancer risk, because some studies have suggested a considerable latency period for a protective effect of folic acid supplementation (50, 51). A recent study by Lee at al (52) that included repeated assessments of folate intake reported an inverse association between total folate intake 12–16 y before diagnosis and colorectal cancer, but no association for more recent folate intake. Consistent with protective effects of folate occurring early in the adenoma-carcinoma sequence, an inverse association was found between recent folate intake and incidence of colorectal adenomas. We observed inverse associations with folate intake assessed more recently than 12–16 y, but our baseline assessment may reflect habitual intake over a longer period of time. Lee et al also reported no evidence of an increased risk of colorectal adenoma or cancer with increased total folate or folic acid intake after 6 y of postfortification follow-up.
Supplemental folic acid in our study originated exclusively from multivitamin use. One study reported a 75% reduction in colon cancer risk of women who were long-term users of multivitamins containing ≥400 μg folic acid (50), but another large study in women reported no association (53). Contrary to our results, some previous cohort studies observed a protective association between dietary folate and colorectal cancer, but not between supplemental folic acid and colorectal cancer (7, 54). For folate intake from dietary sources, we observed a nonlinear inverse association with evidence of a plateau effect at higher intakes, >400 μg/d. Our results are consistent with the hypothesis that inadequate folate intake may increase the risk of colorectal cancer, but increasing dietary intake at high levels may not confer additional benefit. High intake of folic acid, but not naturally occurring folate, can result in detectable levels of unmetabolized folic acid in serum (55), which in one study was associated with a reduction in the number of cancer-protective natural killer cells (56). Another study found both repletion and supplementation with folic acid (1 mg/d) was associated with upregulation of proinflammatory gene pathways in colorectal tissue (57). Thus, concerns of adverse effects are primarily directed at excessive intakes of folic acid rather than intakes of natural folates, but we observed a stronger inverse association with colorectal cancer risk of dietary folic acid than of natural folates.
A potential limitation of our study was the possibility that the observed associations were due to residual confounding rather than to a true folate effect. Baseline folate intake was associated with other “healthy” behaviors that may affect colorectal cancer risk, such as dietary choices and colorectal cancer screening, and it is possible that improvements in these factors over the time period of the study occurred differentially by baseline folate intake. Perhaps most concerning was potential confounding by increased detection and removal of precancerous lesions due to increased colorectal cancer screening over the study period. Restricting the analysis to participants who responded to both the 1996 questionnaire and a follow-up questionnaire administered in 2004, the screening prevalence in both 1996 and 2004 was higher for those with the highest baseline folate intake, although differences in prevalence over time were very similar across folate intake quintiles (41% and 47% in 1996 compared with 53% and 58% in 2004 for quintile 1 and quintile 5 of dietary folate, respectively). No appreciable change from the original multivariable-adjusted folate–colorectal cancer risk estimates (HR for postfortification total folate quintile 5 compared with quintile 1: 0.85; 95% CI: 0.75, 0.98) was observed when we included in the model a variable for “screened in the past 3 y” in 1996, “screened ever/never” in 2004, “screened in the past 5 y” in 2004, or when variables from both time periods were added (HR for postfortification total folate quintile 5 compared with quintile 1: 0.83; 95% CI: 0.72, 0.96). Adjustment for screening also did not affect risk estimates for postfortification dietary folate or supplemental folic acid intake. We did not have follow-up data on the participants’ diets, but differential changes in dietary confounders would likely occur in a pattern similar to the baseline differences, for which adjustment in the multivariable models did not greatly change the folate–colorectal cancer risk associations. In addition, we observed no evidence of changes in the HRs over time; if dietary changes over time differentially affected the cancer risk of those with high compared with low folate intakes, we would expect to observe a deviation from the proportionality assumption. Residual confounding was possible, but our results are consistent with numerous studies in different populations (31–37), and comprehensive attempts to adjust for confounders did not eliminate the observed associations.
Other limitations included possible measurement errors from food frequency questionnaires, although this would likely result in attenuated risk estimates (58), and that supplemental folic acid was based only on multivitamin use and not other supplemental sources. Finally, the NIH-AARP Diet and Health Study did not include the collection of biological samples, so we were unable to examine biomarkers of folate status or the effect of MTHFR (methylene tetrahydrofolate reductase) or other one-carbon metabolism genotypes.
In this prospective cohort study, which included 8.5 y of postfortification follow-up, intakes of dietary, supplemental, or total folate were associated with reduced risks of colorectal cancer and no evidence of increased risk overall or in any subgroups. Our postfortification results are consistent with the large existing body of observational evidence from unfortified populations and with a recent study reporting 6 y of postfortification follow-up, but folate may affect the early stages of colorectal carcinogenesis and therefore the effect of fortification may not be fully evident with <10 y of follow-up. Our large-scale analysis in the NIH-AARP Diet and Health Study examined the longest period of postfortification follow-up yet reported, but further research is needed to assess the effect of folic acid fortification, particularly over longer follow-up times and for high-risk subsets of the population.
Acknowledgments
Cancer incidence data from the Atlanta metropolitan area were collected by the Georgia Center for Cancer Statistics, Department of Epidemiology, Rollins School of Public Health, Emory University. Cancer incidence data from California were collected by the California Department of Health Services, Cancer Surveillance Section. Cancer incidence data from the Detroit metropolitan area were collected by the Michigan Cancer Surveillance Program, Community Health Administration, State of Michigan. The Florida cancer incidence data used in this report were collected by the Florida Cancer Data System under contract with the Florida Department of Health. The views expressed herein are solely those of the authors and do not necessarily reflect those of the Florida Cancer Data System or Florida Department of Health. Cancer incidence data from Louisiana were collected by the Louisiana Tumor Registry, Louisiana State University Medical Center in New Orleans. Cancer incidence data from New Jersey were collected by the New Jersey State Cancer Registry, Cancer Epidemiology Services, New Jersey State Department of Health and Senior Services. Cancer incidence data from North Carolina were collected by the North Carolina Central Cancer Registry. Cancer incidence data from Pennsylvania were supplied by the Division of Health Statistics and Research, Pennsylvania Department of Health, Harrisburg, PA. The Pennsylvania Department of Health specifically disclaims responsibility for any analyses, interpretations or conclusions. Cancer incidence data from Arizona were collected by the Arizona Cancer Registry, Division of Public Health Services, Arizona Department of Health Services. Cancer incidence data from Texas were collected by the Texas Cancer Registry, Cancer Epidemiology and Surveillance Branch, Texas Department of State Health Services.
We are indebted to the participants in the NIH-AARP Diet and Health Study for their outstanding cooperation. We also thank Sigurd Hermansen and Kerry Grace Morrissey from Westat for study outcome ascertainment and management and Leslie Carroll at Information Management Services for data support and analysis. Finally, we thank Yikyung Park of the National Cancer Institute for her helpful comments.
The authors’ responsibilities were as follows—TMG, SJW, STM and RS-S: designed the research; ARH, AFS, and AS: collected the data; TMG, SJW, RMP, and RS-S: analyzed the data and performed the statistical analyses; TMG, SJW, RMP, STM, and RS-S: wrote the manuscript; and TMG: had primary responsibility for the final content. All authors critically reviewed and approved the final manuscript. None of the authors reported any conflicts of interest.
Footnotes
AFPPS, Aspirin-Folate Polyp Prevention Study; DHQ, Diet History Questionnaire; NSAIDs, nonsteroidal antiinflammatory medications.
REFERENCES
- 1.Choi SW, Mason JB. Folate and carcinogenesis: an integrated scheme. J Nutr 2000;130:129–32 [DOI] [PubMed] [Google Scholar]
- 2.Kim YI. Folate and carcinogenesis: evidence, mechanisms, and implications. J Nutr Biochem 1999;10(2):66–88 [DOI] [PubMed] [Google Scholar]
- 3.Zingg JM, Jones PA. Genetic and epigenetic aspects of DNA methylation on genome expression, evolution, mutation and carcinogenesis. Carcinogenesis 1997;18:869–82 [DOI] [PubMed] [Google Scholar]
- 4.Kim YI. Folate and colorectal cancer: an evidence-based critical review. Mol Nutr Food Res 2007;51:267–92 [DOI] [PubMed] [Google Scholar]
- 5.World Cancer Research Fund/American Institute for Cancer Research Food, nutrition, physical activity and the prevention of cancer: a global perspective. Washington, DC: AICR, 2007 [Google Scholar]
- 6.Kim DH, Smith-Warner SA, Spiegelman D, Yaun SS, Colditz GA, Freudenheim JL, Giovannucci E, Goldbohm RA, Graham S, Harnack L, et al. Pooled analyses of 13 prospective cohort studies on folate intake and colon cancer. Cancer Causes Control 2010;21:1919–30 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Roswall N, Olsen A, Christensen J, Dragsted LO, Overvad K, Tjonneland A. Micronutrient intake and risk of colon and rectal cancer in a Danish cohort. Cancer Epidemiol 2010;34(1):40–6 [DOI] [PubMed] [Google Scholar]
- 8.Eussen SJ, Vollset SE, Igland J, Meyer K, Fredriksen A, Ueland PM, Jenab M, Slimani N, Boffetta P, Overvad K, et al. Plasma folate, related genetic variants, and colorectal cancer risk in EPIC. Cancer Epidemiol Biomarkers Prev 2010;19(5):1328–40 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Cole BF, Baron JA, Sandler RS, Haile RW, Ahnen DJ, Bresalier RS, McKeown-Eyssen G, Summers RW, Rothstein RI, Burke CA, et al. Folic acid for the prevention of colorectal adenomas: a randomized clinical trial. JAMA 2007;297(21):2351–9 [DOI] [PubMed] [Google Scholar]
- 10.Kim YI. Folate: a magic bullet or a double edged sword for colorectal cancer prevention? Gut 2006;55(10):1387–9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Kim YI. Folic acid fortification and supplementation—good for some but not so good for others. Nutr Rev 2007;65:504–11 [DOI] [PubMed] [Google Scholar]
- 12.Ulrich CM. Folate and cancer prevention: a closer look at a complex picture. Am J Clin Nutr 2007;86(2):271–3 [DOI] [PubMed] [Google Scholar]
- 13.Quinlivan EP, Gregory JF., III Effect of food fortification on folic acid intake in the United States. Am J Clin Nutr 2003;77:221–5 [DOI] [PubMed] [Google Scholar]
- 14.Jacques PF, Selhub J, Bostom AG, Wilson PW, Rosenberg IH. The effect of folic acid fortification on plasma folate and total homocysteine concentrations. N Engl J Med 1999;340:1449–54 [DOI] [PubMed] [Google Scholar]
- 15.Pfeiffer CM, Caudill SP, Gunter EW, Osterloh J, Sampson EJ. Biochemical indicators of B vitamin status in the US population after folic acid fortification: results from the National Health and Nutrition Examination Survey 1999–2000. Am J Clin Nutr 2005;82(2):442–50 [DOI] [PubMed] [Google Scholar]
- 16.Honein MA, Paulozzi LJ, Mathews TJ, Erickson JD, Wong LY. Impact of folic acid fortification of the US food supply on the occurrence of neural tube defects. JAMA 2001;285(23):2981–6 [DOI] [PubMed] [Google Scholar]
- 17.Ulrich CM, Potter JD. Folate supplementation: too much of a good thing? Cancer Epidemiol Biomarkers Prev 2006;15(2):189–93 [DOI] [PubMed] [Google Scholar]
- 18.Mason JB, Dickstein A, Jacques PF, Haggarty P, Selhub J, Dallal G, Rosenberg IH. A temporal association between folic acid fortification and an increase in colorectal cancer rates may be illuminating important biological principles: a hypothesis. Cancer Epidemiol Biomarkers Prev 2007;16(7):1325–9 [DOI] [PubMed] [Google Scholar]
- 19.Schatzkin A, Subar AF, Thompson FE, Harlan LC, Tangrea J, Hollenbeck AR, Hurwitz PE, Coyle L, Schussler N, Michaud DS, et al. Design and serendipity in establishing a large cohort with wide dietary intake distributions: the National Institutes of Health-American Association of Retired Persons Diet and Health Study. Am J Epidemiol 2001;154:1119–25 [DOI] [PubMed] [Google Scholar]
- 20.Michaud DS, Midthune DD, Hermansen S, Leitzmann M, Harlan LC, Kipnis V, Schatzkin A. Comparison of cancer registry case ascertainment with SEER estimates and self-reporting in a subset of the NIH-AARP Diet and health Study. J Registry Manag 2005;32:70–5 [Google Scholar]
- 21.Fritz AG. International classification of diseases for oncology: ICD-O. 3rd ed Geneva, Switzerland: World Health Organization, 2000 [Google Scholar]
- 22.Subar AF, Midthune D, Kulldorff M, Brown CC, Thompson FE, Kipnis V, Schatzkin A. Evaluation of alternative approaches to assign nutrient values to food groups in food frequency questionnaires. Am J Epidemiol 2000;152:279–86 [DOI] [PubMed] [Google Scholar]
- 23.Dixon LB, Zimmerman TP, Kahle LL, Subar AF. Adding carotenoids to the NCI Diet History Questionnaire Database. J Food Compost Anal 2003;16:269–80 [Google Scholar]
- 24.Thompson FE, Kipnis V, Midthune D, Freedman LS, Carroll RJ, Subar AF, Brown CC, Butcher MS, Mouw T, Leitzmann M, et al. Performance of a food-frequency questionnaire in the US NIH-AARP (National Institutes of Health-American Association of Retired Persons) Diet and Health Study. Public Health Nutr 2008;11(2):183–95 [DOI] [PubMed] [Google Scholar]
- 25.Willett W, Stampfer MJ. Total energy intake: implications for epidemiologic analyses. Am J Epidemiol 1986;124:17–27 [DOI] [PubMed] [Google Scholar]
- 26.Durrleman S, Simon R. Flexible regression models with cubic splines. Stat Med 1989;8:551–61 [DOI] [PubMed] [Google Scholar]
- 27.Pierce DA, Preston DL. Joint analysis of site-specific cancer risks for the atomic bomb survivors. Radiat Res 1993;134:134–42 [PubMed] [Google Scholar]
- 28.Flood A, Caprario L, Chaterjee N, Lacey JV, Jr, Schairer C, Schatzkin A. Folate, methionine, alcohol, and colorectal cancer in a prospective study of women in the United States. Cancer Causes Control 2002;13:551–61 [DOI] [PubMed] [Google Scholar]
- 29.Flood A, Velie EM, Chaterjee N, Subar AF, Thompson FE, Lacey JV, Jr, Schairer C, Troisi R, Schatzkin A. Fruit and vegetable intakes and the risk of colorectal cancer in the Breast Cancer Detection Demonstration Project follow-up cohort. Am J Clin Nutr 2002;75:936–43 [DOI] [PubMed] [Google Scholar]
- 30.Harnack L, Jacobs DR, Jr, Nicodemus K, Lazovich D, Anderson K, Folsom AR. Relationship of folate, vitamin B-6, vitamin B-12, and methionine intake to incidence of colorectal cancers. Nutr Cancer 2002;43:152–8 [DOI] [PubMed] [Google Scholar]
- 31.Konings EJ, Goldbohm RA, Brants HA, Saris WH, van den Brandt PA. Intake of dietary folate vitamers and risk of colorectal carcinoma: results from The Netherlands Cohort Study. Cancer 2002;95:1421–33 [DOI] [PubMed] [Google Scholar]
- 32.Larsson SC, Giovannucci E, Wolk A. A prospective study of dietary folate intake and risk of colorectal cancer: modification by caffeine intake and cigarette smoking. Cancer Epidemiol Biomarkers Prev 2005;14(3):740–3 [DOI] [PubMed] [Google Scholar]
- 33.Terry P, Jain M, Miller AB, Howe GR, Rohan TE. Dietary intake of folic acid and colorectal cancer risk in a cohort of women. Int J Cancer 2002;97:864–7 [DOI] [PubMed] [Google Scholar]
- 34.Wei EK, Giovannucci E, Wu K, Rosner B, Fuchs CS, Willett WC, Colditz GA. Comparison of risk factors for colon and rectal cancer. Int J Cancer 2004;108:433–42 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Giovannucci E, Rimm EB, Ascherio A, Stampfer MJ, Colditz GA, Willett WC. Alcohol, low-methionine–low-folate diets, and risk of colon cancer in men. J Natl Cancer Inst 1995;87:265–73 [DOI] [PubMed] [Google Scholar]
- 36.Giovannucci E, Stampfer MJ, Colditz GA, Rimm EB, Trichopoulos D, Rosner BA, Speizer FE, Willett WC. Folate, methionine, and alcohol intake and risk of colorectal adenoma. J Natl Cancer Inst 1993;85:875–84 [DOI] [PubMed] [Google Scholar]
- 37.Fuchs CS, Willett WC, Colditz GA, Hunter DJ, Stampfer MJ, Speizer FE, Giovannucci EL. The influence of folate and multivitamin use on the familial risk of colon cancer in women. Cancer Epidemiol Biomarkers Prev 2002;11:227–34 [PubMed] [Google Scholar]
- 38.Ulrich CM, Potter JD. Folate and cancer—timing is everything. JAMA 2007;297(21):2408–9 [DOI] [PubMed] [Google Scholar]
- 39.Logan RF, Grainge MJ, Shepherd VC, Armitage NC, Muir KR. Aspirin and folic acid for the prevention of recurrent colorectal adenomas. Gastroenterology 2008;134(1):29–38 [DOI] [PubMed] [Google Scholar]
- 40.Wu K, Platz EA, Willett WC, Fuchs CS, Selhub J, Rosner BA, Hunter DJ, Giovannucci E. A randomized trial on folic acid supplementation and risk of recurrent colorectal adenoma. Am J Clin Nutr 2009;90(6):1623–31 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Kim YI. Folic acid supplementation and cancer risk: point. Cancer Epidemiol Biomarkers Prev 2008;17(9):2220–5 [DOI] [PubMed] [Google Scholar]
- 42.Osterhues A, Holzgreve W, Michels KB. Shall we put the world on folate?. Lancet 2009;374(9694):959–61 [DOI] [PubMed] [Google Scholar]
- 43.Sauer J, Mason JB, Choi SW. Too much folate: a risk factor for cancer and cardiovascular disease? Curr Opin Clin Nutr Metab Care 2009;12:30–6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Solomons NW. Food fortification with folic acid: has the other shoe dropped? Nutr Rev 2007;65:512–5 [DOI] [PubMed] [Google Scholar]
- 45.Ulrich CM. Folate and cancer prevention—where to next? Counterpoint. Cancer Epidemiol Biomarkers Prev 2008;17(9):2226–30 [DOI] [PubMed] [Google Scholar]
- 46.Hirsch S, Sanchez H, Albala C, de la Maza MP, Barrera G, Leiva L, Bunout D. Colon cancer in Chile before and after the start of the flour fortification program with folic acid. Eur J Gastroenterol Hepatol 2009;21:436–9 [DOI] [PubMed] [Google Scholar]
- 47.Luebeck EG, Moolgavkar SH, Liu AY, Boynton A, Ulrich CM. Does folic acid supplementation prevent or promote colorectal cancer? Results from model-based predictions. Cancer Epidemiol Biomarkers Prev 2008;17(6):1360–7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Leslie A, Carey FA, Pratt NR, Steele RJC. The colorectal adenoma-carcinoma sequence. Br J Surg 2002;89:845–60 [DOI] [PubMed] [Google Scholar]
- 49.Cannon-Albright LA, Bishop DT, Samowitz W, DiSario JA, Lee R, Burt RW. Colonic polyps in an unselected population: prevalence, characteristics, and associations. Am J Gastroenterol 1994;89:827–31 [PubMed] [Google Scholar]
- 50.Giovannucci E, Stampfer MJ, Colditz GA, Hunter DJ, Fuchs C, Rosner BA, Speizer FE, Willett WC. Multivitamin use, folate, and colon cancer in women in the Nurses’ Health Study. Ann Intern Med 1998;129:517–24 [DOI] [PubMed] [Google Scholar]
- 51.Jacobs EJ, Connell CJ, Chao A, McCullough ML, Rodriguez C, Thun MJ, Calle EE. Multivitamin use and colorectal cancer incidence in a US cohort: does timing matter? Am J Epidemiol 2003;158:621–8 [DOI] [PubMed] [Google Scholar]
- 52.Lee JE, Willett WC, Fuchs CS, Smith-Warner SA, Wu K, Ma J, Giovannucci E. Folate intake and risk of colorectal cancer and adenoma: modification by time. Am J Clin Nutr 2011;93:817–25 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Neuhouser ML, Wassertheil-Smoller S, Thomson C, Aragaki A, Anderson GL, Manson JE, Patterson RE, Rohan TE, van Horn L, Shikany JM, et al. Multivitamin use and risk of cancer and cardiovascular disease in the Women's Health Initiative cohorts. Arch Intern Med 2009;169(3):294–304 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Sanjoaquin MA, Allen N, Couto E, Roddam AW, Key TJ. Folate intake and colorectal cancer risk: a meta-analytical approach. Int J Cancer 2005;113:825–8 [DOI] [PubMed] [Google Scholar]
- 55.Bailey RL, Mills JL, Yetley EA, Gahche JJ, Pfeiffer CM, Dwyer JT, Dodd KW, Sempos CT, Betz JM, Picciano MF. Unmetabolized serum folic acid and its relation to folic acid intake from diet and supplements in a nationally representative sample of adults aged > or =60 y in the United States. Am J Clin Nutr 2010;92(2):383–9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Troen AM, Mitchell B, Sorensen B, Wener MH, Johnston A, Wood B, Selhub J, McTiernan A, Yasui Y, Oral E, et al. Unmetabolized folic acid in plasma is associated with reduced natural killer cell cytotoxicity among postmenopausal women. J Nutr 2006;136(1):189–94 [DOI] [PubMed] [Google Scholar]
- 57.Protiva P, Mason JB, Liu Z, Hopkins ME, Nelson C, Marshall JR, Lambrecht RW, Pendyala S, Kopelovich L, Kim M, et al. Altered folate availability modifies the molecular environment of the human colorectum: implications for colorectal carcinogenesis. Cancer Prev Res 2011;4:530–43 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Kipnis V, Subar AF, Midthune D, Freedman LS, Ballard-Barbash R, Troiano RP, Bingham S, Schoeller DA, Schatzkin A, Carroll RJ. Structure of dietary measurement error: results of the OPEN biomarker study. Am J Epidemiol 2003;158:14–26 [DOI] [PubMed] [Google Scholar]