Abstract
Background: The relation between consumption of different types of red meats and risk of type 2 diabetes (T2D) remains uncertain.
Objective: We evaluated the association between unprocessed and processed red meat consumption and incident T2D in US adults.
Design: We followed 37,083 men in the Health Professionals Follow-Up Study (1986–2006), 79,570 women in the Nurses’ Health Study I (1980–2008), and 87,504 women in the Nurses’ Health Study II (1991–2005). Diet was assessed by validated food-frequency questionnaires, and data were updated every 4 y. Incident T2D was confirmed by a validated supplementary questionnaire.
Results: During 4,033,322 person-years of follow-up, we documented 13,759 incident T2D cases. After adjustment for age, BMI, and other lifestyle and dietary risk factors, both unprocessed and processed red meat intakes were positively associated with T2D risk in each cohort (all P-trend <0.001). The pooled HRs (95% CIs) for a one serving/d increase in unprocessed, processed, and total red meat consumption were 1.12 (1.08, 1.16), 1.32 (1.25, 1.40), and 1.14 (1.10, 1.18), respectively. The results were confirmed by a meta-analysis (442,101 participants and 28,228 diabetes cases): the RRs (95% CIs) were 1.19 (1.04, 1.37) and 1.51 (1.25, 1.83) for 100 g unprocessed red meat/d and for 50 g processed red meat/d, respectively. We estimated that substitutions of one serving of nuts, low-fat dairy, and whole grains per day for one serving of red meat per day were associated with a 16–35% lower risk of T2D.
Conclusion: Our results suggest that red meat consumption, particularly processed red meat, is associated with an increased risk of T2D.
INTRODUCTION
Diabetes is highly prevalent in the US population. More than 11% of US adults aged ≥20 y (25.6 million persons) have diabetes; the majority (90–95%) suffer from T2D,4 and 1.9 million new cases of diabetes occur each year (1). Although obesity and physical inactivity are major determinants of T2D and account for much of the increase in prevalence (2), dietary factors also play an important role in its development (3).
We have shown previously that processed red meat consumption was associated with an increased risk of T2D in 3 Harvard cohorts (4–6). This was also confirmed by 2 recent meta-analyses (7, 8), but these meta-analyses did not come to an agreement as to whether unprocessed red meat was associated with diabetes risk. No study has so far examined whether substitution of other dietary components for red meat, such as low-fat dairy products, nuts, and whole grains, could lower diabetes risk. These foods are major sources of protein intake and have been related to a lower risk of T2D (9–11). Therefore, we aimed to 1) update our previous analyses of meat consumption and diabetes risk with the use of the same analysis strategy, with longer follow-up years in the 3 large cohorts (HPFS and NHS I and II); 2) conduct an updated meta-analysis of the results from the 3 cohorts and previous literature; and 3) estimate the effects of substitution of low-fat dairy products, nuts, and whole grains for red meat on diabetes risk.
SUBJECTS AND METHODS
Study population
We used data from 3 prospective cohort studies: HPFS, NHS I, and NHS II. The HPFS was initiated in 1986, when 51,529 US male dentists, pharmacists, veterinarians, optometrists, osteopathic physicians, and podiatrists, aged 40–75 y, returned a baseline questionnaire that inquired about detailed medical history, as well as lifestyle and usual diet. The NHS I consisted of 121,700 female registered nurses, aged 30–55 y, who lived in one of 11 states and completed a baseline questionnaire about their lifestyle and medical history in 1976. The NHS II was established in 1989 and was composed of 116,671 younger female registered nurses, aged 25–42 y, who responded to a baseline questionnaire similar to the NHS I questionnaire. Detailed descriptions of the 3 cohorts were introduced elsewhere (4–6). In all 3 cohorts, questionnaires were administered at baseline and biennially thereafter, to collect and update information on lifestyle practice and occurrence of chronic diseases. The follow-up proportions of the participants in these cohorts were all >90%.
In the current analysis, we excluded men and women who had diagnoses of diabetes (including type 1 and type 2 diabetes and gestational diabetes), cardiovascular disease, or cancer at baseline (1986 for HPFS, 1980 for NHS I, and 1991 for NHS II, when we first assessed diet in these cohorts). In addition, we excluded participants who left >70 of the 131 food items blank on the baseline FFQ or who reported unusual total energy intakes (ie, daily energy intake <800 or >4200 kcal/d for men and <500 or >3500 kcal/d for women). We also excluded participants without baseline information on meat consumption or follow-up information on diabetes diagnosis date (detailed information can be found under “Supplemental data” in the online issue). After exclusions, data from 37,083 HPFS participants, 79,570 NHS I participants, and 87,504 NHS II participants were available for analysis. The study protocol was approved by the institutional review boards of Brigham and Women's Hospital and Harvard School of Public Health.
Assessment of meat consumption
In 1980, a 61-item FFQ was administered to the NHS I participants to collect information on their usual intake of foods and beverages in the previous year. In 1984, 1986, 1990, 1994, 1998, and 2002, similar but expanded 131-item FFQs were sent to these participants to update their diet records. With the use of the expanded FFQ used in the NHS I, dietary data were collected in 1986, 1990, 1994, 1998, and 2002 from the HPFS participants, and in 1991, 1995, 1999, and 2003 from the NHS II participants. In all FFQs, we asked the participants how often, on average, they consumed each food of a standard portion size. There were 9 possible responses, which ranged from “never or less than once per month” to “6 or more times per day.” Nutrient intake was calculated by multiplication of the frequency of consumption of each food by the nutrient composition in the standard portion size of that food and then summing up the nutrient intake from all relevant food items. The food composition database was created primarily from USDA sources (12). Questionnaire items on unprocessed red meat consumption included “beef or lamb as main dish,” “pork as main dish,” “hamburger,” and “beef, pork, or lamb as a sandwich or mixed dish,” and items on processed red meat included “bacon,” “hot dogs,” and “sausage, salami, bologna, and other processed red meats.” The standard serving size was 85 g (3 ounces) for unprocessed red meat, 45 g for one hot dog, 28 g for 2 slices of bacon, or 45 g for one piece of other processed red meat. The reproducibility and validity of these FFQs have been shown in detail elsewhere (13–16). The correlation coefficients between FFQ and multiple dietary records ranged from 0.38 to 0.70 for various red meat intakes (16).
Assessment of covariates
In the biennial follow-up questionnaires, we inquired about and updated information on risk factors for chronic diseases, such as body weight, cigarette smoking, physical activity, medication use, and family history of diabetes, as well as history of chronic diseases, including hypertension and hypercholesterolemia. Among NHS I and II participants, we ascertained menopausal status, postmenopausal hormone use, and oral contraceptive use.
Assessment of diabetes
A supplementary questionnaire about symptoms, diagnostic tests, and hypoglycemic therapy was mailed to participants who reported that they had received a diagnosis of diabetes. A case of T2D was considered confirmed if at least one of the following was reported on the supplementary questionnaire [in accordance with National Diabetes Data Group criteria (17)]: 1) one or more classic symptoms (excessive thirst, polyuria, weight loss, hunger) and fasting plasma glucose concentrations ≥7.8 mmol/L or random plasma glucose concentrations ≥11.1 mmol/L; 2) ≥2 elevated plasma glucose concentrations on different occasions (fasting concentrations ≥7.8 mmol/L, random plasma glucose concentrations ≥11.1 mmol/L, and/or concentrations of ≥11.1 mmol/L after ≥2 h shown by oral-glucose-tolerance testing) in the absence of symptoms; or 3) treatment with hypoglycemic medication (insulin or oral hypoglycemic agent). The diagnostic criteria were changed by the American Diabetes Association in June 1998, and the threshold for the diagnosis of diabetes became a fasting plasma glucose of 7.0 mmol/L, instead of 7.8 mmol/L (18). Only cases confirmed by the supplemental questionnaires were included.
The validity of the supplementary questionnaire for the diagnosis of diabetes has been documented previously. Of the 59 T2D cases in HPFS and 62 cases in NHS I who were confirmed by the supplementary questionnaire, 57 (97%) and 61 (98%) were reconfirmed by medical records (19, 20). Deaths were identified by reports from next of kin or postal authorities, or by searching the National Death Index. At least 98% of deaths among the study participants were identified (21).
Statistical analysis
We calculated each individual's person-years from the date of return of the baseline questionnaire to the date of diagnosis of T2D, death, or the end of the follow-up (31 January 2006 for HPFS; 30 June 2008 for NHS I; or 30 June 2007 for NHS II), whichever came first. We used time-dependent Cox proportional hazard regression to estimate the HR for red meat consumption in relation to the risk of T2D. In the multivariate analysis, we simultaneously controlled for various potential confounding factors in addition to age and calendar time with updated information at each 2-y questionnaire cycle, including ethnicity (whites, nonwhites), smoking status [never, past, current (1–14 cigarettes/d), current (15–24 cigarettes/d), or current (>24 cigarettes/d)], alcohol intake (0, 0.1–4.9, 5.0–14.9, or ≥15 g/d in women; 0, 0.1–4.9, 5.0–29.9, or ≥30 g/d in men), physical activity (<3, 3–8.9, 9–17.9, 18–26.9, or ≥27 metabolic equivalent-hours/week), history of hypertension and hypercholesterolemia, family history of diabetes, postmenopausal status and menopausal hormone use (NHS I and II participants only), oral contraceptive use (NHS II participants only), and quintiles of total energy intake and dietary score. We created a low diabetes risk diet score as a diet low in trans fat and glycemic load and high in cereal fiber and the ratio of polyunsaturated to saturated fat. The dietary score summed the quintile values of the 4 components, and 5 represented the lowest-risk quintile in each dietary factor (2). BMI (in kg/m2) was updated every 2 y and was included in the model as a time-varying covariate in an additional model because red meat intake was associated with BMI and weight gain in our cohorts (22), and thus BMI could be considered an intermediate variable between red meat intake and diabetes.
Our primary analysis adjusted for this dietary score and total energy intake. We also conducted a sensitivity analysis with adjustment for other major dietary variables (whole grain, fish, nuts, sugar-sweetened beverages, coffee, egg, potatoes, fruit and vegetables, all in quintiles) instead of the dietary score. We conducted another sensitivity analysis to correct for measurement error (23) in the assessment of red meat intake with the use of data from validation studies conducted in HPFS (13) and NHS I (15). This method used a regression calibration approach to correct RR estimates for measurement error for the time-dependent measures of dietary variable intake (23).
To better represent long-term diet and to minimize within-person variation, we created cumulative averages of food and nutrient intake from baseline to the censoring events (24). We also conducted a sensitivity analysis with the use of only baseline dietary variables to predict the future risk of T2D. To minimize missing values of dietary variables in each follow-up FFQ, we replaced missing values with the cumulative means before the missing values. The last value was carried forward for one 2-y cycle to replace nondietary missing values. We investigated the effect of a “substitution” of a serving of one food for another by including both as continuous variables in the same multivariable model, which contained both nondietary covariates and total energy intake. The difference in their beta coefficients and their variances and covariance were used to estimate the beta coefficient and variance for the substitution effect, which was used to calculate HRs and 95% CIs for the substitution effect (25, 26).
Proportional hazards assumption was tested with a time-dependent variable with the inclusion of an interaction term between the red meat intake and months to events (P > 0.05 for all tests). To test for linear trend, the median value was assigned to each quintile and this value was modeled as a continuous variable. All the analyses were conducted separately in each cohort, and we also conducted meta-analyses to summarize the estimates of association across the 3 studies. No significant heterogeneities were shown when the results were pooled across the 3 cohorts; therefore, fixed-effect models were used. The data were analyzed with the Statistical Analysis Systems software package, version 9.1 (SAS Institute Inc), whereas the meta-analysis was performed with the use of the STATA statistical program, version 9.2 (StataCorp).
Updated meta-analysis on red meat intake and risk of incident T2D
We further conducted an updated meta-analysis that incorporated our new results from the 3 cohorts into the findings of previous studies. The 2 recent meta-analyses involved a search of literature up to December 2008 (7) or March 2009 (8). Thus, we conducted additional literature searches on MEDLINE (http://www.ncbi.nlm.nih.gov/pubmed) and EMBASE (http://www.embase.com/) from March 2009 to April 2011 (details can be found under “Supplemental data” in the online issue).
RESULTS
We documented 2438 incident T2D cases during a maximum of 20 y of follow-up in the HPFS, 8253 cases during a maximum of 28 y in the NHS I, and 3068 cases during a maximum of 16 y in the NHS II. The distribution of baseline characteristics according to intake of total red meat consumption is shown in Table 1. For both men and women, red meat intake was negatively associated with physical activity, but positively associated with BMI and smoking. In addition, a high red meat intake was associated with a high intake of total energy and a worse diabetes dietary score. Unprocessed and processed red meat consumption was moderately correlated (Spearman correlation coefficients from 0.38 to 0.53 in the 3 cohorts). However, red meat consumption was only weakly correlated with intakes of poultry (coefficients from −0.05 to 0.24) or fish (coefficients from −0.20 to 0.05).
TABLE 1.
Baseline age-adjusted characteristics of participants in the 3 cohorts according to quintile of total red meat consumption1
| Characteristics | HPFS (1986) |
NHS I (1980) |
NHS II (1991) |
||||||
| Q1 (n = 7187) | Q3 (n = 7027) | Q5 (n = 7247) | Q1 (n = 15,777) | Q3 (n = 15,579) | Q5 (n = 15,900) | Q1 (n = 17,506) | Q3 (n = 17,542) | Q5 (n = 17,575) | |
| Total red meat intake (servings/d) | 0.24 (0.11–0.37)2 | 1.01 (0.92–1.10) | 2.16 (1.93–2.57) | 0.58 (0.44–0.68) | 1.50 (1.42–1.63) | 2.86 (2.57–3.36) | 0.37 (0.24–0.48) | 1.03 (0.95–1.10) | 2.04 (1.80–2.40) |
| Age (y) | 53.6 ± 9.63 | 52.4 ± 9.5 | 51.9 ± 9.2 | 47.2 ± 7.2 | 45.7 ± 7.2 | 45.8 ± 7.1 | 36.2 ± 4.7 | 36.0 ± 4.7 | 35.9 ± 4.7 |
| Physical activity (MET-h/wk) | 27.8 ± 34.0 | 20.2 ± 28.3 | 17.7 ± 25.2 | 16.9 ± 24.8 | 13.8 ± 20.1 | 12.5 ± 17.3 | 26.4 ± 33.7 | 19.8 ± 25.2 | 18.2 ± 24.7 |
| BMI (kg/m2) | 24.7 ± 3.0 | 25.4 ± 3.1 | 25.9 ± 3.4 | 23.8 ± 4.1 | 24.2 ± 4.3 | 24.5 ± 4.6 | 23.3 ± 4.4 | 24.5 ± 5.1 | 25.7 ± 6.0 |
| Race, white (%) | 93.0 | 95.4 | 96.0 | 96.9 | 97.9 | 97.2 | 95.5 | 97.1 | 96.0 |
| Current smoker (%) | 5.1 | 9.8 | 14.3 | 25.4 | 28.1 | 31.7 | 10.2 | 12.2 | 14.2 |
| Hypertension (%) | 18.7 | 18.3 | 19.0 | 14.3 | 14.5 | 15.2 | 5.1 | 6.1 | 7.3 |
| High cholesterol (%) | 14.5 | 9.4 | 7.7 | 5.7 | 4.9 | 4.2 | 13.9 | 14.4 | 14.5 |
| Family history of diabetes (%) | 18.7 | 18.5 | 18.1 | 25.9 | 27.1 | 28.7 | 31.3 | 33.2 | 36.4 |
| Postmenopausal (%) | NA | NA | NA | 30.6 | 30.3 | 30.5 | 3.0 | 3.1 | 3.4 |
| Current menopausal hormone use (%)4 | NA | NA | NA | 20.9 | 21.1 | 21.0 | 84.4 | 82.9 | 83.6 |
| Current oral conceptive use (%) | NA | NA | NA | NA | NA | NA | 12.3 | 10.8 | 10.2 |
| Total energy (kcal/d) | 1661 ± 507 | 1889 ± 490 | 2400 ± 514 | 1202 ± 396 | 1522 ± 386 | 2027 ± 473 | 1439 ± 466 | 1743 ± 460 | 2240 ± 511 |
| Alcohol (g/d) | 8.5 ± 12.3 | 11.3 ± 14.8 | 13.5 ± 17.5 | 5.9 ± 9.8 | 6.7 ± 10.8 | 6.8 ± 11.4 | 3.3 ± 6.0 | 3.1 ± 5.9 | 3.1 ± 6.3 |
| Diabetes dietary score | 13.7 ± 2.6 | 11.8 ± 2.6 | 10.7 ± 2.2 | 13.0 ± 2.2 | 11.9 ± 2.0 | 11.1 ± 1.8 | 12.8 ± 2.7 | 11.9 ± 2.7 | 11.3 ± 2.6 |
| Cereal fiber (g/d) | 7.5 ± 5.3 | 5.7 ± 3.4 | 4.6 ± 2.5 | 2.9 ± 1.9 | 2.5 ± 1.4 | 1.9 ± 1.1 | 6.8 ± 4.0 | 5.5 ± 2.7 | 4.7 ± 2.1 |
| Glycemic load | 139 ± 29 | 123 ± 23 | 111 ± 22 | 97 ± 27 | 84 ± 23 | 73 ± 22 | 134 ± 24 | 121 ± 19 | 110 ± 18 |
| Polyunsaturated to saturated fat ratio | 0.74 ± 0.27 | 0.55 ± 0.15 | 0.46 ± 0.12 | 0.43 ± 0.18 | 0.34 ± 0.11 | 0.29 ± 0.09 | 0.56 ± 0.21 | 0.50 ± 0.14 | 0.46 ± 0.12 |
| trans Fat (% of total energy) | 0.9 ± 0.5 | 1.3 ± 0.5 | 1.5 ± 0.5 | 1.8 ± 0.8 | 2.3 ± 0.7 | 2.5 ± 0.6 | 1.4 ± 0.7 | 1.7 ± 0.6 | 1.8 ± 0.6 |
| Fruit and vegetables (servings/d) | 6.1 ± 3.2 | 5.1 ± 2.5 | 5.1 ± 2.4 | 4.1 ± 2.3 | 3.9 ± 1.9 | 4.1 ± 2.0 | 4.9 ± 3.1 | 4.9 ± 2.7 | 5.6 ± 2.9 |
| Coffee (cups/d) | 1.5 ± 1.6 | 1.9 ± 1.8 | 2.3 ± 1.9 | 2.0 ± 1.9 | 2.2 ± 1.9 | 2.4 ± 2.0 | 1.5 ± 1.6 | 1.6 ± 1.7 | 1.6 ± 1.8 |
| Egg (servings/d) | 0.19 ± 0.30 | 0.31 ± 0.33 | 0.51 ± 0.51 | 0.37 ± 0.36 | 0.41 ± 0.34 | 0.50 ± 0.43 | 0.12 ± 0.18 | 0.17 ± 0.18 | 0.25 ± 0.25 |
| Soft drinks (servings/d) | 0.61 ± 0.94 | 0.79 ± 1.03 | 0.89 ± 1.05 | 0.61 ± 0.97 | 0.71 ± 1.00 | 0.82 ± 1.09 | 1.20 ± 1.43 | 1.46 ± 1.46 | 1.82 ± 1.61 |
| Dairy (servings/d) | 1.7 ± 1.3 | 1.9 ± 1.3 | 2.1 ± 1.4 | 1.8 ± 1.3 | 1.8 ± 1.2 | 1.8 ± 1.2 | 2.1 ± 1.4 | 2.3 ± 1.4 | 2.5 ± 1.5 |
| Nuts (servings/d) | 0.45 ± 0.41 | 0.45 ± 0.58 | 0.48 ± 0.60 | 0.16 ± 0.33 | 0.13 ± 0.26 | 0.15 ± 0.28 | 0.10 ± 0.26 | 0.08 ± 0.19 | 0.09 ± 0.20 |
| Potato (servings/d) | 0.38 ± 0.32 | 0.53 ± 0.36 | 0.76 ± 0.45 | 0.32 ± 0.31 | 0.47 ± 0.37 | 0.63 ± 0.45 | 0.34 ± 0.29 | 0.51 ± 0.34 | 0.75 ± 0.46 |
| Fish (servings/d) | 0.55 ± 0.48 | 0.38 ± 0.33 | 0.32 ± 0.30 | 0.50 ± 0.57 | 0.39 ± 0.41 | 0.33 ± 0.37 | 0.28 ± 0.32 | 0.28 ± 0.25 | 0.30 ± 0.29 |
| Poultry (servings/d) | 0.63 ± 0.50 | 0.55 ± 0.39 | 0.52 ± 0.39 | 0.63 ± 0.57 | 0.59 ± 0.46 | 0.58 ± 0.53 | 0.54 ± 0.42 | 0.77 ± 0.46 | 0.97 ± 0.67 |
Data were age standardized except for age and red meat intake. HPFS, Health Professionals Follow-Up Study; MET, metabolic equivalent; NA, not available; NHS, Nurses’ Health Study; Q, quintile.
Median; interquartile range in parentheses (all such values).
Mean ± SD (all such values).
Current menopausal hormone users among postmenopausal women.
The HRs of T2D according to unprocessed, processed, and total red meat consumption are shown in Table 2. In age- and multivariate-adjusted models, red meat consumption was positively associated with the risk of development of T2D across the 3 studies (all P for trend: <0.001). After further adjustment for updated BMI status, these associations were substantially attenuated but remained significant. In the pooled analysis of estimates from the 3 studies that used fixed-effect models, a one serving/d increase of unprocessed, processed, and total red meat consumption was associated with a 12% (95% CI: 8%, 16%), 32% (95% CI: 25%, 40%), and 14% (95% CI: 10%, 18%) elevated risk of T2D, respectively. Adjustment for other major dietary variables (whole grain, fish, nuts, sugar-sweetened beverages, coffee, egg, potatoes, and fruit and vegetables) instead of diabetes dietary score in the sensitivity analyses did not materially alter the associations, and the corresponding HRs (95% CIs) for unprocessed, processed, and total red meat consumptions were 1.12 (1.08, 1.16), 1.29 (1.22, 1.37), and 1.14 (1.11, 1.18), respectively. Sensitivity analyses with the use of energy density (serving ∙ 1000 kcal−1 ∙ d−1) or conventional cutoffs were consistent with our main results (see Tables S2 and S3 under “Supplemental data” in the online issue). In a sensitivity analysis with only baseline dietary variables, the corresponding HRs (95% CIs) were 1.07 (0.98, 1.16), 1.16 (1.11, 1.20), and 1.10 (1.02, 1.18), respectively. In another sensitivity analysis, which accounted for measurement error in dietary variables, the results were strengthened, and the corresponding HRs (95% CIs) were 1.66 (1.28, 2.14), 1.53 (1.34, 1.75), and 1.44 (1.10, 1.99), respectively.
TABLE 2.
HRs (95% CI) of type 2 diabetes risk according to quintile of red meat intake in the HPFS, NHS I, and NHS II1
| Frequency of consumption |
P-trend2 | HR (95% CI) for one serving/d | |||||
| Q1 | Q2 | Q3 | Q4 | Q5 | |||
| HPFS | |||||||
| Unprocessed red meat | |||||||
| Servings/d | 0.17 (0.08, 0.24)3 | 0.43 (0.37, 0.47) | 0.65 (0.57, 0.73) | 0.94 (0.86, 1.02) | 1.44 (1.29, 1.65) | ||
| Cases/person-years | 375/129,461 | 394/127,874 | 526/134,407 | 489/128,901 | 654/132,331 | ||
| Age-adjusted model | 1.00 | 1.07 (0.93, 1.24) | 1.41 (1.23, 1.60) | 1.33 (1.16, 1.52) | 1.79 (1.58, 2.04) | <0.001 | 1.38 (1.29, 1.48) |
| Multivariate model 1 | 1.00 | 1.03 (0.89, 1.19) | 1.31 (1.14, 1.50) | 1.23 (1.06, 1.42) | 1.65 (1.42, 1.91) | <0.001 | 1.33 (1.23, 1.44) |
| Multivariate model 2 | 1.00 | 0.95 (0.82, 1.09) | 1.14 (0.99, 1.31) | 1.05 (0.90, 1.21) | 1.29 (1.11, 1.50) | <0.001 | 1.16 (1.06, 1.26) |
| Processed red meat | |||||||
| Servings/d | 0.02 (0, 0.05) | 0.12 (0.09, 0.13) | 0.21 (0.20, 0.25) | 0.38 (0.33, 0.44) | 0.72 (0.60, 0.97) | ||
| Cases/person-years | 340/138,550 | 409/121,238 | 441/131,831 | 593/131,520 | 655/129,834 | ||
| Age-adjusted model | 1.00 | 1.38 (1.20, 1.60) | 1.40 (1.22, 1.62) | 1.88 (1.65, 2.15) | 2.08 (1.82, 2.37) | <0.001 | 1.55 (1.43, 1.68) |
| Multivariate model 1 | 1.00 | 1.32 (1.14, 1.53) | 1.32 (1.14, 1.52) | 1.75 (1.51, 2.01) | 1.96 (1.69, 2.27) | <0.001 | 1.47 (1.34, 1.62) |
| Multivariate model 2 | 1.00 | 1.18 (1.02, 1.37) | 1.12 (0.97, 1.30) | 1.44 (1.25, 1.66) | 1.55 (1.33, 1.79) | <0.001 | 1.34 (1.21, 1.48) |
| Total red meat | |||||||
| Servings/d | 0.25 (0.12, 0.36) | 0.60 (0.52, 0.69) | 0.94 (0.86, 1.03) | 1.34 (1.23, 1.46) | 2.02 (1.78, 2.43) | ||
| Cases/person-years | 346/129,959 | 398/131,492 | 488/130,148 | 545/130,744 | 661/130,631 | ||
| Age-adjusted model | 1.00 | 1.16 (1.01, 1.34) | 1.44 (1.25, 1.65) | 1.60 (1.39, 1.83) | 1.96 (1.72, 2.24) | <0.001 | 1.33 (1.27, 1.39) |
| Multivariate model 1 | 1.00 | 1.11 (0.96, 1.29) | 1.37 (1.18, 1.58) | 1.54 (1.32, 1.79) | 1.92 (1.64, 2.25) | <0.001 | 1.33 (1.25, 1.41) |
| Multivariate model 2 | 1.00 | 1.01 (0.87, 1.17) | 1.17 (1.01, 1.35) | 1.25 (1.08, 1.45) | 1.44 (1.23, 1.68) | <0.001 | 1.19 (1.12, 1.27) |
| NHS I | |||||||
| Unprocessed red meat | |||||||
| Servings/d | 0.37 (0.28, 0.45) | 0.61 (0.56, 0.67) | 0.84 (0.75, 0.98) | 1.09 (0.97, 1.26) | 1.57 (1.33, 1.95) | ||
| Cases/person-years | 1278/404,906 | 1534/402,338 | 1592/403,148 | 1793/394,652 | 2056/409,128 | ||
| Age-adjusted model | 1.00 | 1.23 (1.14, 1.32) | 1.28 (1.19, 1.37) | 1.46 (1.36, 1.57) | 1.64 (1.53, 1.75) | <0.001 | 1.29 (1.24, 1.34) |
| Multivariate model 1 | 1.00 | 1.17 (1.08, 1.26) | 1.18 (1.10, 1.28) | 1.29 (1.19, 1.39) | 1.39 (1.28, 1.50) | <0.001 | 1.15 (1.10, 1.20) |
| Multivariate model 2 | 1.00 | 1.10 (1.02, 1.19) | 1.10 (1.02, 1.18) | 1.16 (1.08, 1.25) | 1.23 (1.14, 1.33) | <0.001 | 1.09 (1.04, 1.14) |
| Processed red meat | |||||||
| Servings/d | 0.05 (0.02, 0.07) | 0.14 (0.13, 0.16) | 0.23 (0.21, 0.27) | 0.35 (0.32, 0.42) | 0.64 (0.52, 0.83) | ||
| Cases/person-years | 1174/403,239 | 1426/381,686 | 1632/420,660 | 1885/404,142 | 2136/404,445 | ||
| Age-adjusted model | 1.00 | 1.26 (1.17, 1.36) | 1.42 (1.32, 1.53) | 1.65 (1.53, 1.77) | 1.93 (1.80, 2.08) | <0.001 | 1.85 (1.74, 1.97) |
| Multivariate model 1 | 1.00 | 1.19 (1.10, 1.29) | 1.32 (1.22, 1.42) | 1.46 (1.36, 1.58) | 1.60 (1.48, 1.72) | <0.001 | 1.54 (1.44, 1.65) |
| Multivariate model 2 | 1.00 | 1.08 (1.00, 1.17) | 1.15 (1.06, 1.24) | 1.23 (1.14, 1.33) | 1.30 (1.20, 1.40) | <0.001 | 1.30 (1.21, 1.41) |
| Total red meat | |||||||
| Servings/d | 0.50 (0.36, 0.61) | 0.83 (0.75, 0.95) | 1.12 (0.99, 1.30) | 1.44 (1.27, 1.68) | 2.07 (1.75, 2.55) | ||
| Cases/person-years | 1212/401,534 | 1444/405,038 | 1586/401,953 | 1863/403,092 | 2148/402,555 | ||
| Age-adjusted model | 1.00 | 1.22 (1.13, 1.31) | 1.35 (1.25, 1.46) | 1.59 (1.48, 1.71) | 1.84 (1.71, 1.97) | <0.001 | 1.32 (1.28, 1.36) |
| Multivariate model 1 | 1.00 | 1.16 (1.07, 1.25) | 1.25 (1.16, 1.35) | 1.42 (1.31, 1.54) | 1.55 (1.43, 1.68) | <0.001 | 1.21 (1.16, 1.25) |
| Multivariate model 2 | 1.00 | 1.08 (1.00, 1.17) | 1.13 (1.04, 1.22) | 1.25 (1.15, 1.35) | 1.31 (1.21, 1.42) | <0.001 | 1.13 (1.08, 1.17) |
| NHS II | |||||||
| Unprocessed red meat | |||||||
| Servings/d | 0.17 (0.07, 0.25) | 0.43 (0.37, 0.47) | 0.61 (0.56, 0.66) | 0.84 (0.76, 0.92) | 1.29 (1.12, 1.53) | ||
| Cases/person-years | 368/271,759 | 462/266,716 | 562/278,014 | 690/276,425 | 986/273,261 | ||
| Age-adjusted model | 1.00 | 1.28 (1.12, 1.47) | 1.54 (1.35, 1.76) | 1.89 (1.66, 2.14) | 2.70 (2.39, 3.04) | <0.001 | 1.88 (1.77, 1.99) |
| Multivariate model 1 | 1.00 | 1.17 (1.01, 1.34) | 1.31 (1.14, 1.50) | 1.39 (1.21, 1.59) | 1.69 (1.47, 1.93) | <0.001 | 1.36 (1.26, 1.47) |
| Multivariate model 2 | 1.00 | 1.01 (0.88, 1.16) | 1.13 (0.99, 1.29) | 1.12 (0.98, 1.28) | 1.27 (1.11, 1.46) | <0.001 | 1.18 (1.09, 1.28) |
| Processed red meat | |||||||
| Servings/d | 0 (0, 0.03) | 0.07 (0.07, 0.10) | 0.14 (0.13, 0.17) | 0.25 (0.21, 0.28) | 0.49 (0.39, 0.64) | ||
| Cases/person-years | 389/273,513 | 464/254,002 | 582/296,610 | 626/262,102 | 1007/279,949 | ||
| Age-adjusted model | 1.00 | 1.29 (1.13, 1.48) | 1.54 (1.35, 1.75) | 1.78 (1.57, 2.02) | 2.81 (2.50, 3.16) | <0.001 | 2.53 (2.34, 2.73) |
| Multivariate model 1 | 1.00 | 1.10 (0.96, 1.26) | 1.24 (1.08, 1.41) | 1.32 (1.16, 1.51) | 1.72 (1.52, 1.96) | <0.001 | 1.76 (1.58, 1.96) |
| Multivariate model 2 | 1.00 | 0.93 (0.81, 1.06) | 1.00 (0.88, 1.14) | 1.02 (0.89, 1.16) | 1.21 (1.07, 1.38) | <0.001 | 1.37 (1.21, 1.55) |
| Total red meat | |||||||
| Servings/d | 0.35 (0.21, 0.45) | 0.67 (0.60, 0.73) | 0.92 (0.85, 1.00) | 1.23 (1.13, 1.34) | 1.80 (1.58, 2.13) | ||
| Cases/person-years | 324/271,508 | 443/272,488 | 564/273,770 | 678/273,938 | 1059/274,471 | ||
| Age-adjusted model | 1.00 | 1.40 (1.21, 1.61) | 1.79 (1.56, 2.05) | 2.16 (1.89, 2.46) | 3.38 (2.98, 3.83) | <0.001 | 1.82 (1.74, 1.91) |
| Multivariate model 1 | 1.00 | 1.23 (1.07, 1.42) | 1.46 (1.27, 1.68) | 1.58 (1.37, 1.81) | 2.07 (1.80, 2.38) | <0.001 | 1.42 (1.34, 1.50) |
| Multivariate model 2 | 1.00 | 1.05 (0.91, 1.21) | 1.16 (1.01, 1.34) | 1.17 (1.02, 1.35) | 1.37 (1.19, 1.57) | <0.001 | 1.18 (1.11, 1.26) |
| Pooled results4 | |||||||
| Unprocessed red meat | 1.00 | 1.06 (1.00, 1.12) | 1.11 (1.05, 1.18) | 1.13 (1.07, 1.20) | 1.25 (1.17, 1.33) | <0.001 | 1.12 (1.08, 1.16) |
| Processed red meat | 1.00 | 1.06 (1.00, 1.13) | 1.11 (1.05, 1.18) | 1.22 (1.15, 1.29) | 1.32 (1.24, 1.40) | <0.001 | 1.32 (1.25, 1.40) |
| Total red meat | 1.00 | 1.06 (1.00, 1.13) | 1.14 (1.07, 1.21) | 1.23 (1.16, 1.31) | 1.34 (1.26, 1.43) | <0.001 | 1.14 (1.10, 1.18) |
One serving of unprocessed red meat equals 85 g (3 ounces) pork, beef, or lamb; one serving of processed red meat equals 28 g bacon or 45 g hot dog, sausage, salami, bologna, or other processed red meats. Multivariate model 1 was adjusted for age (continuous), alcohol consumption (0, 0.1–4.9, 5.0–14.9, ≥15 g/d), physical activity level (<3, 3–8.9, 9–17.9, 18–26.9, ≥27 metabolic equivalent task hours/wk), smoking status (never; past; current: 1–14, 15–24, or >24 cigarettes/d), race (white, nonwhite), menopausal status and hormone use in women (premenopausal, postmenopausal never users, postmenopausal past users, postmenopausal current users), family history of diabetes, history of hypertension and hypercholesterolemia, quintiles of total calories, and dietary score. Multivariate model 2 was the same as model 1 with the addition of a BMI category (in kg/m2; <23, 23–24.9, 25–29.9, 30–34.9, ≥35). HPFS, Health Professionals Follow-Up Study; NHS, Nurses’ Health Study; Q, quintile.
P-trend was calculated by assigning median values to each quintile and was treated as continuous variable.
Median; interquartile range in parentheses (all such values).
Results from multivariate model 2 were combined with the use of a fixed-effects model given that the Egger's tests for heterogeneity were not significant for all 3 meta-analyses (all P values > 0.15).
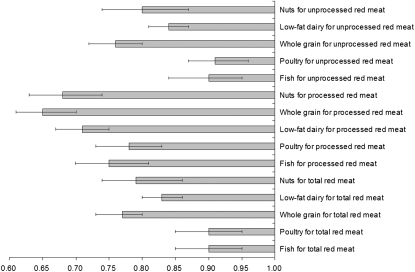
When compared with one daily serving of red meat, one daily serving of nuts (28 g), low-fat dairy products (240 mL milk, 28 g cheese, or 120 mL yogurt), and whole grains [32 g (1 slice) of bread or 200 g (1 cup) of cooked brown rice or cereals] was associated with a lower risk of T2D (Figure 1). One serving of nuts per day was associated with a 21% (95% CI: 14%, 26%) lower risk of T2D when compared with one serving total red meat/d. Similarly, when compared with one serving total red meat/d, the risk reductions associated with low-fat dairy products and whole grain were 17% (14%, 20%) and 23% (20%, 27%), respectively. The corresponding risk reductions associated with nuts, low-fat dairy products, and whole grains were 20% (13%, 26%), 16% (13%, 19%), and 24% (20%, 28%) for unprocessed red meat, and 32% (26%, 37%), 29% (25%, 33%), and 35% (30%, 39%) for processed red meat, respectively. Lower risks of T2D were also shown when substitutions of one serving of total red meat/d with one serving of poultry/d (85 g, 3 ounces; 10%; 95% CI: 5%, 15%) and one serving of fish/d (85 g, 3 ounces; 10%; 95% CI: 5%, 15%) were made.
FIGURE 1.
HRs and 95% CIs for diabetes associated with replacement of other food groups for red meat intake. Adjusted for age (continuous), BMI category (in kg/m2; <23, 23–24.9, 25–29.9, 30–34.9, or ≥35), alcohol consumption (0, 0.1–4.9, 5.0–14.9, or ≥15 g/d), physical activity level (<3, 3–8.9, 9–17.9, 18–26.9, or ≥27 metabolic task hours/wk), smoking status (never; past; current: 1–14, 15–24, or >24 cigarettes/d), race (white or nonwhite), menopausal status and hormone use in women (premenopausal, postmenopausal never hormone users, postmenopausal past hormone users, or postmenopausal current hormone users), family history of diabetes, history of hypertension and hypercholesterolemia, and quintile of total energy intake.
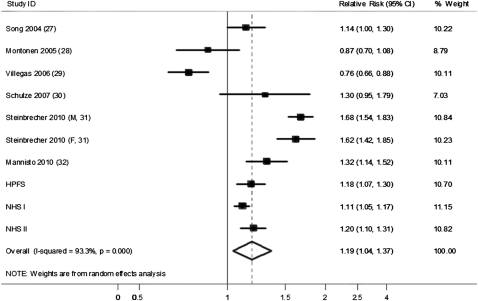
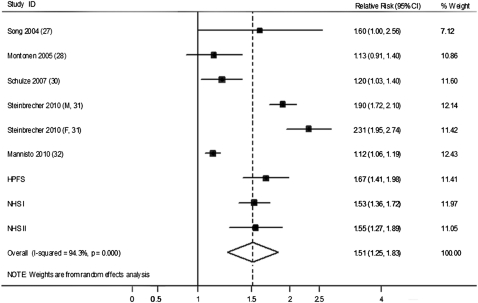
We further conducted an updated meta-analysis incorporating our new results from the 3 cohorts together with the findings of previous studies. Our updated search on MEDLINE and EMBASE found 300 potential citations, of which 2 studies met the inclusion criteria, in addition to the citations in the 2 previous meta-analyses. Therefore, a total of 6 prospective studies (27–32) were included in our updated meta-analysis, along with results from our current analysis (see Table S4 under “Supplemental data” in the online issue). The detailed characteristics of the included studies are shown in Table S5 under “Supplemental data” in the online issue. Unprocessed and processed red meat intakes were both associated with a significantly increased risk of T2D, as shown in Figures 2 and 3. The RRs (95% CIs) from the random-effects model for 100 g/d of unprocessed red meat (3.5 ounces/d, 1.2 serving/d), and 50 g/d of processed red meat (1.8 ounces/d, 1.8 serving/d) were 1.19 (1.04, 1.37), and 1.51 (1.25, 1.83), respectively. No significant publication bias was shown for the association between unprocessed red meat and risk of T2D (see Figure S1 under “Supplemental data” in the online issue), although a significant publication bias was detected for processed red meat (see Figure S2 under “Supplemental data” in the online issue). With the use of the trim and fill method, the RR for processed red meat was 1.23 (1.01, 1.52) (see Figure S3 under “Supplemental data” in the online issue). Significant heterogeneity was shown for both unprocessed and processed red meat (I2 = 93.3% and 94.3%, respectively; both P < 0.001; Figures 2 and 3).
FIGURE 2.
HRs for 100 g unprocessed red meat consumption per day and type 2 diabetes. The RR of each study is represented by a square, and the size of the square represents the weight of each study to the overall estimate. The 95% CIs are represented by the horizontal lines, and the diamond represents the overall estimate and its 95% CI. HPFS, Health Professionals Follow-Up Study; NHS, Nurses’ Health Study.
FIGURE 3.
HRs for 50 g processed red meat consumption per day and type 2 diabetes. The RR of each study is represented by a square, and the size of the square represents the weight of each study to the overall estimate. The 95% CIs are represented by the horizontal lines, and the diamond represents the overall estimate and its 95% CI. HPFS, Health Professionals Follow-Up Study; NHS, Nurses’ Health Study.
DISCUSSION
In these 3 large prospective cohorts of US men and women, we observed that red meat consumption was positively associated with the risk of T2D, and this association was observed for both unprocessed and processed red meat, with a relatively higher risk for the latter. Substitution of nuts, low-fat dairy products, and whole grains for red meat was associated with a significantly lower risk of diabetes.
Red meat is a major food source of protein and fat, and its health effects have attracted much attention with regard to its association with cardiovascular disease and diabetes (8). Our results are largely consistent with our previously published results in these 3 cohorts. Processed red meat consumption has been associated consistently with a higher risk of T2D in our previous investigations in HPFS (4) and NHS I (5) and II (6). The positive association between processed red meat intake and T2D has also been reported by other cohort studies (27, 31, 32). For unprocessed red meat consumption, inconsistent results were reported in our previous analyses (4–6). In the current updated analysis with longer follow-up years, we observed that a one serving/d increase of unprocessed red meat consumption was associated with a 16%, 9%, and 18% higher risk of T2D in HPFS, NHS I, and NHS II, respectively. The 2 meta-analyses reported similar risk estimates of development of T2D associated with red meat consumption: an RR of 1.20 (95% CI: 1.04, 1.38) for a 120-g/d red meat increase in consumption in Aune et al's (7) meta-analysis from 9 cohorts, and 1.16 (95% CI: 0.92, 1.46) for a 100-g/d red meat increase in consumption in Micha et al's (8) meta-analysis from 5 cohorts. The 2 meta-analyses differed in the quantity of red meat intake (120 g/d compared with 100 g/d), and also in the number of included studies (9 compared with 5). Our updated meta-analysis suggested that a 100-g/d unprocessed red meat increase was associated with a 19% (95% CI: 4%, 37%) increased risk.
Several mechanisms may explain the adverse effect of red meat intake on T2D. First, the association may be mediated through the effects of heme-iron derived from red meats (33, 34). Iron is a strong prooxidant that catalyses several cellular reactions in the production of reactive oxygen species, and increases the level of oxidative stress (35). This can cause damage to tissues, in particular the pancreatic beta cells, and high body iron stores have been shown to be associated with an elevated risk of T2D (35). In our analysis, red meat consumption was highly correlated with heme-iron intake (correlation coefficients ranged from 0.53 to 0.66), and the risk estimates for diabetes were further attenuated after adjustment for dietary heme-iron intake (data not shown). Second, although unprocessed and processed meats contain similar amounts of saturated fat, other constituents in processed meat, particularly sodium and nitrites, might partially explain the higher RR associated with processed red meats. A prospective study in Finland suggested that the association between processed meat and diabetes was largely explained by sodium (32). Nitrites and nitrates are used frequently in the preservation of processed meat, and they can be converted into nitrosamines through interaction with amino compounds either in the stomach or within the food product. Nitrosamines have been shown to be toxic to pancreatic beta cells and to increase the risk of diabetes in animal studies (36), and blood nitrite concentrations in adults have been related to endothelial dysfunction (37) and impaired insulin response (38). Other potential mechanisms may involve advanced glycation end-products (39) or increased concentrations of inflammatory mediators (40) and gamma-glutamyltransferase (41) with high red meat intake. Lastly, in the present study, adjustment for updated BMI somewhat attenuated the association between red meat intake and diabetes risk, which suggests that the association between red meat intake and diabetes risk may be partly mediated through weight gain and obesity. In our cohorts (22) and a large European cohort (42), red meat intake was positively associated with future risk of weight gain. For example, an increase in meat intake of 250 g/d would lead to a 2-kg greater weight gain after 5 y (42).
The strengths of the current study include a large sample size, the high proportions of follow-up, and repeated assessments of dietary and lifestyle variables. The consistency of the results across all 3 cohorts indicates that our findings are unlikely to be due to chance. The current study was subject to a few limitations as well. First, our study populations primarily consisted of working health professionals with European ancestry. Although the homogeneity of socioeconomic status helps reduce confounding, the observed associations may not be generalizable to other populations. However, in a secondary analysis, we did not find any significant differences between whites and nonwhites on all the associations (data not shown). Second, because diet was assessed by FFQs, some measurement error of meat intake assessment is inevitable. However, the FFQs used in these studies were validated against multiple diet records, and reasonable correlation coefficients between these assessments of meat intake were observed (13–16). Because we used a prospective study design, any measurement errors of meat intake are independent of study outcome ascertainment, and, therefore, are more likely to attenuate the associations toward the null. In a sensitivity analysis, correction for measurement errors strengthened the associations somewhat. Moreover, we calculated cumulative averages for dietary variables to minimize the random measurement error caused by within-person variation and to accommodate diet changes over time. Lastly, because of the observational nature of our cohorts, the observed associations do not necessarily mean causation; although we adjusted for established and potential risk factors for T2D, unmeasured and residual confounding is still possible.
In conclusion, we showed that a greater consumption of unprocessed and processed red meat is consistently associated with a higher risk of T2D. Compared with red meat, other dietary components, such as nuts, dairy products, and whole grains, were associated with lower risks. Therefore, from a public health point of view, reduction of red meat consumption, particularly processed red meat, and replacement of it with other healthy dietary components, should be considered to decrease T2D risk.
Supplementary Material
Acknowledgments
We are indebted to the participants in the Health Professionals Follow-Up Study and the Nurses’ Health Study I and II for their continuing outstanding support and to our colleagues working in these studies for their valuable help. We also thank Rong Chen and Tricia Li for their help with the statistical analysis and programming. We are grateful to Janine Kroeger for providing unpublished data from the EPIC-Potsdam study.
The authors’ responsibilities were as follows—AP and FBH: designed and conducted the analysis; JEM, WCW, and FBH: obtained funding; AP, QS, AMB, MBS, JEM, WCW, and FBH: interpreted the data; AP: wrote the manuscript; and QS, AMB, MBS, JEM, WCW, and FBH: edited the manuscript. The sponsors had no role in the study design, data collection and analysis, decision to publish, or preparation of the manuscript. None of the authors had a conflict of interest.
Footnotes
Abbreviations used: FFQ, food-frequency questionnaire; HPFS, Health Professionals Follow-Up Study; NHS, Nurses’ Health Study; T2D, type 2 diabetes.
REFERENCES
- 1.Centers for Disease Control and Prevention National diabetes fact sheet: general information and national estimates on diabetes in the United States, 2011. Available from: http://www.cdc.gov/diabetes/pubs/factsheet11.htm (cited 28 May 2011)
- 2.Hu FB, Manson JE, Stampfer MJ, Colditz G, Liu S, Solomon CG, Willett WC. Diet, lifestyle, and the risk of type 2 diabetes mellitus in women. N Engl J Med 2001;345:790–7 [DOI] [PubMed] [Google Scholar]
- 3.Hu FB, van Dam RM, Liu S. Diet and risk of type II diabetes: the role of types of fat and carbohydrate. Diabetologia 2001;44:805–17 [DOI] [PubMed] [Google Scholar]
- 4.van Dam RM, Willett WC, Rimm EB, Stampfer MJ, Hu FB. Dietary fat and meat intake in relation to risk of type 2 diabetes in men. Diabetes Care 2002;25:417–24 [DOI] [PubMed] [Google Scholar]
- 5.Fung TT, Schulze M, Manson JE, Willett WC, Hu FB. Dietary patterns, meat intake, and the risk of type 2 diabetes in women. Arch Intern Med 2004;164:2235–40 [DOI] [PubMed] [Google Scholar]
- 6.Schulze MB, Manson JE, Willett WC, Hu FB. Processed meat intake and incidence of Type 2 diabetes in younger and middle-aged women. Diabetologia 2003;46:1465–73 [DOI] [PubMed] [Google Scholar]
- 7.Aune D, Ursin G, Veierod MB. Meat consumption and the risk of type 2 diabetes: a systematic review and meta-analysis of cohort studies. Diabetologia 2009;52:2277–87 [DOI] [PubMed] [Google Scholar]
- 8.Micha R, Wallace SK, Mozaffarian D. Red and processed meat consumption and risk of incident coronary heart disease, stroke, and diabetes mellitus: a systematic review and meta-analysis. Circulation 2010;121:2271–83 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Jiang R, Manson JE, Stampfer MJ, Liu S, Willett WC, Hu FB. Nut and peanut butter consumption and risk of type 2 diabetes in women. JAMA 2002;288:2554–60 [DOI] [PubMed] [Google Scholar]
- 10.Choi HK, Willett WC, Stampfer MJ, Rimm E, Hu FB. Dairy consumption and risk of type 2 diabetes mellitus in men: a prospective study. Arch Intern Med 2005;165:997–1003 [DOI] [PubMed] [Google Scholar]
- 11.de Munter JS, Hu FB, Spiegelman D, Franz M, van Dam RM. Whole grain, bran, and germ intake and risk of type 2 diabetes: a prospective cohort study and systematic review. PLoS Med 2007;4:e261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.US Department of Agriculture Composition of foods: raw, processed, prepared, 1963-1991. Washington, DC: US Government Printing Office, 1992 [Google Scholar]
- 13.Feskanich D, Rimm EB, Giovannucci EL, Colditz GA, Stampfer MJ, Litin LB, Willett WC. Reproducibility and validity of food intake measurements from a semiquantitative food frequency questionnaire. J Am Diet Assoc 1993;93:790–6 [DOI] [PubMed] [Google Scholar]
- 14.Rimm EB, Giovannucci EL, Stampfer MJ, Colditz GA, Litin LB, Willett WC. Reproducibility and validity of an expanded self-administered semiquantitative food frequency questionnaire among male health professionals. Am J Epidemiol 1992;135:1114–26 [DOI] [PubMed] [Google Scholar]
- 15.Willett WC, Sampson L, Stampfer MJ, Rosner B, Bain C, Witschi J, Hennekens CH, Speizer FE. Reproducibility and validity of a semiquantitative food frequency questionnaire. Am J Epidemiol 1985;122:51–65 [DOI] [PubMed] [Google Scholar]
- 16.Salvini S, Hunter DJ, Sampson L, Stampfer MJ, Colditz GA, Rosner B, Willett WC. Food-based validation of a dietary questionnaire: the effects of week-to-week variation in food consumption. Int J Epidemiol 1989;18:858–67 [DOI] [PubMed] [Google Scholar]
- 17.National Diabetes Data Group Classification and diagnosis of diabetes mellitus and other categories of glucose intolerance. Diabetes 1979;28:1039–57 [DOI] [PubMed] [Google Scholar]
- 18.Report of the Expert Committee on the Diagnosis and Classification of Diabetes Mellitus. Diabetes Care 1997;20:1183–97 [DOI] [PubMed] [Google Scholar]
- 19.Manson JE, Rimm EB, Stampfer MJ, Colditz GA, Willett WC, Krolewski AS, Rosner B, Hennekens CH, Speizer FE. Physical activity and incidence of non-insulin-dependent diabetes mellitus in women. Lancet 1991;338:774–8 [DOI] [PubMed] [Google Scholar]
- 20.Hu FB, Leitzmann MF, Stampfer MJ, Colditz GA, Willett WC, Rimm EB. Physical activity and television watching in relation to risk for type 2 diabetes mellitus in men. Arch Intern Med 2001;161:1542–8 [DOI] [PubMed] [Google Scholar]
- 21.Rich-Edwards JW, Corsano KA, Stampfer MJ. Test of the National Death Index and Equifax Nationwide Death Search. Am J Epidemiol 1994;140:1016–9 [DOI] [PubMed] [Google Scholar]
- 22.Mozaffarian D, Hao T, Rimm EB, Willett WC, Hu FB. Changes in diet and lifestyle and long-term weight gain in women and men. N Engl J Med 2011;364:2392–404 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Qiu W, Rosner B. Measurement error correction for the cumulative average model in the survival analysis of nutritional data: application to Nurses’ Health Study. Lifetime Data Anal 2010;16:136–53 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Hu FB, Stampfer MJ, Rimm E, Ascherio A, Rosner BA, Spiegelman D, Willett WC. Dietary fat and coronary heart disease: a comparison of approaches for adjusting for total energy intake and modeling repeated dietary measurements. Am J Epidemiol 1999;149:531–40 [DOI] [PubMed] [Google Scholar]
- 25.Halton TL, Willett WC, Liu S, Manson JE, Stampfer MJ, Hu FB. Potato and french fry consumption and risk of type 2 diabetes in women. Am J Clin Nutr 2006;83:284–90 [DOI] [PubMed] [Google Scholar]
- 26.Bernstein AM, Sun Q, Hu FB, Stampfer MJ, Manson JE, Willett WC. Major dietary protein sources and risk of coronary heart disease in women. Circulation 2010;122:876–83 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Song Y, Manson JE, Buring JE, Liu S. A prospective study of red meat consumption and type 2 diabetes in middle-aged and elderly women: the Women's Health Study. Diabetes Care 2004;27:2108–15 [DOI] [PubMed] [Google Scholar]
- 28.Montonen J, Jarvinen R, Heliovaara M, Reunanen A, Aromaa A, Knekt P. Food consumption and the incidence of type II diabetes mellitus. Eur J Clin Nutr 2005;59:441–8 [DOI] [PubMed] [Google Scholar]
- 29.Villegas R, Shu XO, Gao YT, Yang G, Cai H, Li H, Zheng W. The association of meat intake and the risk of type 2 diabetes may be modified by body weight. Int J Med Sci 2006;3:152–9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Schulze MB, Hoffmann K, Boeing H, Linseisen J, Rohrmann S, Möhlig M, Pfeiffer AF, Spranger J, Thamer C, Häring HU, et al. An accurate risk score based on anthropometric, dietary, and lifestyle factors to predict the development of type 2 diabetes. Diabetes Care 2007;30:510–5 [DOI] [PubMed] [Google Scholar]
- 31.Steinbrecher A, Erber E, Grandinetti A, Kolonel L, Maskarinec G. Meat consumption and risk of type 2 diabetes: the multiethnic cohort. Public Health Nutr 2010;14:568–74 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Männistö S, Kontto J, Kataja-Tuomola M, Albanes D, Virtamo J. High processed meat consumption is a risk factor of type 2 diabetes in the Alpha-Tocopherol, Beta-Carotene Cancer Prevention study. Br J Nutr 2010;103:1817–22 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Jiang R, Ma J, Ascherio A, Stampfer MJ, Willett WC, Hu FB. Dietary iron intake and blood donations in relation to risk of type 2 diabetes in men: a prospective cohort study. Am J Clin Nutr 2004;79:70–5 [DOI] [PubMed] [Google Scholar]
- 34.Rajpathak S, Ma J, Manson J, Willett WC, Hu FB. Iron intake and the risk of type 2 diabetes in women: a prospective cohort study. Diabetes Care 2006;29:1370–6 [DOI] [PubMed] [Google Scholar]
- 35.Rajpathak SN, Crandall JP, Wylie-Rosett J, Kabat GC, Rohan TE, Hu FB. The role of iron in type 2 diabetes in humans. Biochim Biophys Acta 2009;1790:671–81 [DOI] [PubMed] [Google Scholar]
- 36.Tong M, Neusner A, Longato L, Lawton M, Wands JR, de la Monte SM. Nitrosamine exposure causes insulin resistance diseases: relevance to type 2 diabetes mellitus, non-alcoholic steatohepatitis, and Alzheimer's disease. J Alzheimers Dis 2009;17:827–44 [PMC free article] [PubMed] [Google Scholar]
- 37.Kleinbongard P, Dejam A, Lauer T, Jax T, Kerber S, Gharini P, Balzer J, Zotz RB, Scharf RE, Willers R, et al. Plasma nitrite concentrations reflect the degree of endothelial dysfunction in humans. Free Radic Biol Med 2006;40:295–302 [DOI] [PubMed] [Google Scholar]
- 38.Pereira EC, Ferderbar S, Bertolami MC, Faludi AA, Monte O, Xavier HT, Pereira TV, Abdalla DS. Biomarkers of oxidative stress and endothelial dysfunction in glucose intolerance and diabetes mellitus. Clin Biochem 2008;41:1454–60 [DOI] [PubMed] [Google Scholar]
- 39.Peppa M, Goldberg T, Cai W, Rayfield E, Vlassara H. Glycotoxins: a missing link in the “relationship of dietary fat and meat intake in relation to risk of type 2 diabetes in men”. Diabetes Care 2002;25:1898–9 [DOI] [PubMed] [Google Scholar]
- 40.Azadbakht L, Esmaillzadeh A. Red meat intake is associated with metabolic syndrome and the plasma C-reactive protein concentration in women. J Nutr 2009;139:335–9 [DOI] [PubMed] [Google Scholar]
- 41.Lee DH, Steffen LM, Jacobs DR., Jr Association between serum gamma-glutamyltransferase and dietary factors: the Coronary Artery Risk Development in Young Adults (CARDIA) Study. Am J Clin Nutr 2004;79:600–5 [DOI] [PubMed] [Google Scholar]
- 42.Vergnaud AC, Norat T, Romaguera D, Mouw T, May AM, Travier N, Luan J, Wareham N, Slimani N, Rinaldi S, et al. Meat consumption and prospective weight change in participants of the EPIC-PANACEA study. Am J Clin Nutr 2010;92:398–407 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.